Abstract
Background
Maize production in lowland agro-ecologies in West and Central Africa is constrained by the fungus Exserohilum turcicum, causal agent of Northern Corn Leaf Blight (NCLB). Breeding for resistance to NCLB is considered the most effective management strategy. The strategy would be even more effective if there is adequate knowledge of the characteristics of E. turcicum in a target region. Maize leaves showing NCLB symptoms were collected during field surveys in three major maize growing areas in Nigeria: Ikenne, Ile-Ife, and Zaria during 2018/2019 and 2019/2020 growing seasons to characterize E. turcicum populations interacting with maize using morphological and molecular criteria.
Results
A total of 217 E. turcicum isolates were recovered. Most of the isolates (47%) were recovered from the Ikenne samples while the least were obtained from Zaria. All isolates were morphologically characterized. A subset of 124 isolates was analyzed for virulence effector profiles using three primers: SIX13-like, SIX5-like, and Ecp6. Inter- and intra-location variations among isolates was found in sporulation, growth patterns, and presence of the effectors. Candidate effector genes that condition pathogenicity and virulence in E. turcicum were found but not all isolates expressed the three effectors.
Conclusion
Morphological and genetic variation among E. turcicum isolates was found within and across locations. The variability observed suggests that breeding for resistance to NCLB in Nigeria requires selection for quantitative resistance to sustain the breeding efforts.
Similar content being viewed by others
Background
Maize (Zea mays L.) is a source of carbohydrates, vitamins, minerals, and proteins. Over 4.5 billion people in developing countries obtain ~ 30% of their calories from maize. In sub-Saharan Africa (SSA), maize is the most widely grown and consumed staple food with an estimated 50% of the populace depending on maize as the primary staple [1, 2]. Maize ranks first in the world cereal production (1.15 billion tons) with the United States of America as the largest producer (~ 50% of the total production) [3]. In Africa, South Africa is the highest maize producer (11.3 m tons), followed by Nigeria (11.0 m tons), and Ethiopia (9.6 m tons) [3].
Production and productivity of maize in SSA are confronted with myriads of biotic and abiotic stresses [4]. These include low soil fertility; drought, heat stresses; combined heat and drought stresses; parasitic weeds [Striga hermonthica (Del.) Benth]; insect pests; and several plant pathogenic fungi, bacteria, viruses, and nematodes [1, 5, 6]. Globally, fungi are prominent causal agents of plant diseases and pose a major constraint to maize production [7, 8]. Foliar diseases by fungi can be destructive, and one of the most damaging is the Northern Corn Leaf Blight (NCLB) incited by Exserohilum turcicum (Passerini) Leonard and Suggs. The disease is prevalent in highland regions [9]. However, the spread to lowlands has been documented [4, 10].
The fungus E. turcicum, a hemi-biotrophic ascomycete [11], survives in humid conditions and moderately low (17–28 °C) temperatures [12]. As a biotroph, the fungus penetrates the host either through suppression or evasion of the host defense structures while maintaining the viability of the host [13, 14]. This is immediately followed by the necrotrophic phase, characterized by increased production of cell wall-degrading enzymes (CWDEs), secondary metabolites, and fungal biomass leading to necrosis [15, 16]. CWDEs aid penetration and aggressive colonization of host cells [17]. Fungal effectors are required for penetration and subsequent host colonization [18, 19]. Lo Presti et al. and Jones and Dangl reported that fungal pathogens produce effectors that modulate host defense structures and physiology to penetrate, evade detection, and acquire nutrients [18, 20]. However, an immune response can be elicited if the corresponding resistance gene (R-gene) of the host is recognized by the corresponding effector gene [21].
The photosynthetic potential of maize is significantly reduced by E. turcicum infection and this invariably affects grain yield [22]. The premature death of infected plants has been reported when the disease epidemic begins at an early stage [11, 23]. This is consequent upon the alteration of the physiological activities of the infected host [24]. Additionally, increases in temperature and rate of transpiration have been associated with infected plant parts as the severity increases. The progress of infection from the lower leaves to the leaves above the ear before tasseling or silking becomes very severe, especially, when the environment favors the establishment of the pathogen [11, 25].
NCLB can be managed through cultural methods (e.g., crop rotation with non-host crops or complete burying of disease crop residues through tillage operations), chemical control, and host plant resistance (HPR) [6, 26]. HPR is an economically feasible, safe, durable, sustainable, and eco-friendly control method for managing plant diseases [27, 28]. However, understanding variations among and within populations of causal agents of a disease is important for developing appropriate breeding strategies [29]. Diversity within communities of plant pathogenic fungi is shaped by sexual reproduction, clonality, and/or mutation. A mixed system of reproduction, i.e., sexual and clonal, has been reported in E. turcicum populations. Although the sexual stage of E. turcicum is relatively uncommon in nature, substantial evidence of recombination in tropical populations exists [30,31,32].
Genetic diversity in E. turcicum has been studied via different methods [30,31,32,33,34]. Haasbroek et al. developed 13 microsatellite markers to study the genetic variability of E. turcicum in South Africa and were able to distinguish isolates with specificity to either maize or sorghum [35]. In addition, identification of genes facilitating host colonization during the biotrophic and necrotrophic stages has been effective for the classification of different strains [16]. Also, formae speciales of Setosphaeria turcica in China were identified and their genetic diversity studied using ‘universally primed polymerase chain reaction (PCR)’ [36]. Human et al. employed a time-course RNA sequencing method and identified several enzymes, secondary metabolites, effectors, and candidate genes that moderate the pathogenicity of E. turcicum [16]. The effector gene Ecp6, has been characterized for its role in pathogenicity [3, 16]. In addition, secreted in xylem (SIX) genes (SIX5-like and SIX13-like) modulate host responses and can interact with the R-genes when recognized by the host [37]. The expressions of Ecp6 and SIX13-like genes are associated with the establishment of the pathogen during the biotrophic stage [16] while SIX5-like genes facilitate the passage of the Avr2 effector genes to adjacent cells [38].
Adequate knowledge of both the genetic basis of resistance and diversity within communities of the causal agents of the disease are of prime importance in breeding for HPR. However, in Nigeria, although significant work to elucidate the genetic basis of the resistance in early-maturing and extra-early-maturing maize germplasm has been done [10, 23, 39], there is a lacuna in information regarding the genetic diversity among E. turcicum populations interacting with maize. Therefore, the present study investigated variability among E. turcicum populations associated with maize grown in three agro-ecologies of Nigeria during two seasons. In addition to morphological characterization, a subset of isolates of E. turcicum were subjected to molecular assays to determine the presence or absence of plant disease effectors. The results could contribute to refined breeding efforts for the development of resistance in maize to various pathotypes of E. turcicum interacting with maize in Nigeria.
Results
Morphological characterization of Exserohilum turcicum isolates
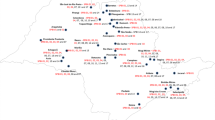
A total of 217 isolates of E. turcicum were recovered from Ikenne (102), Ile-Ife (61), and Zaria (54) (Fig. 1). Morphological variations based on color, radial mycelia growth (RMG), and growth rate were observed among and within locations (Fig. 2). Isolates grew radially on PDA with black fluffy but irregularly interspersed with whitish mycelia and aerial hyphae in some isolates (e.g., ETDS 5 from Ile-Ife; Fig. 2). Generally, the isolates produced black pigmentation on potato dextrose agar (PDA) (Fig. 2).
Generally, the conidia were cylindrical, tapering from the middle with transverse septations and conspicuously protruded hilum (Fig. 3). The conidiophores were cylindrical, septate, olivaceous brown, and bear single conidia. The conidia varied in the number of transverse septations, sporulation, and size (Fig. 3). The number of septation of the conidia in Ikenne isolates, ranged from 2 to 10 (Table 1), while in Ile-Ife and Zaria isolates it ranged from 2 to 4 (Tables 2 and 3). The maximum number of transverse septations across locations ranged from 8 to 13. The highest number of septation (13) was recorded among Ikenne isolates.
Morphology of Exserohilum turcicum isolates grown on PDA. Colony (A) and spore (B) of NGETIB16-13; colony (C) and spore (D) of NGETIK16-6; colony (E) and spore(F) of ET-IK-7-7B-3; colony (G) and spore (H) of ETDS 46; colony (I) and spore (J) of ET-AB-1A2; colony (K) and spore (L) of 10-IK-20G. All spores magnified at ×40
The number of spores per microscopic field (SPMF) is presented in Fig. 4. Isolates from Ikenne (102) had representatives in each category. However, only five had excellent SPMF. Thirty-five isolates had very good SPMF, 42 had good SPMF, and only seven and 13 isolates had fair and poor SPMF, respectively (Fig. 4). Similar distribution in terms of sporulation was observed for Ile-Ife isolates. Although none of the Zaria isolates had excellent sporulation, the other classes were observed (Fig. 4).
Classification of Exserohilum turcicum isolates recovered from Ikenne, Ile-Ife and Zaria in 2018/2019 based on spore count per microscopic field. Excellent: ≥ 25 spores per microscopic field at ×25 (SPMF); very good: 20 to 24 SPMF; good: 15 to 19 SPMF; fair: 10 to 14 SPMF; and poor: 1 to 9 SPMF (Harlapur et al., 2007)
Significant differences were observed for spore length and RMG of the isolates from each location. The conidia length and the RMG of Ikenne isolates is presented in Table 1. IK-7-7B-3 had the largest spore length while 10-IK-20G had the shortest (Table 1). The RMG of Ikenne isolates 14 days after plating (DAP) ranged from 24.0 cm2 for NGETIK-16-8 to 72.3 cm2 for ET-IK-24. Similar results were observed for Ile-Ife isolates. There were significant differences among isolates for spore length and RMG at 14 DAP (Table 2). ETDS 134 had the shortest conidia length while ETDS 140 had the largest (Table 2). In addition, the RMG at 14 DAP ranged from 20.4 cm2 for ETDS 8 to 71.8 cm2 for ETDS 97. The morphological characteristics of Zaria isolates are presented in Table 3. The spore length of AB19-21A1 was 3.0 μm and was significantly shorter than AB19-3A1 which had the largest spore. AB19-11A1 had the lowest RMG at 14 DAP while AB19-4-A1 had the largest (Table 3). The correlation analysis showed no significant relationship between the spore count and the RMG for all the isolates across locations, however, positive (r = 0.41, P < 0.05) significant relationships were observed for spore count and spore length as well as for septation and spore length (r = 0.84, P < 0.05) (Table 4). For example, IK-5-1 A-4 (from Ikenne) and ETDS 46 (from Ile-Ife) had excellent sporulation but poor RMG, while ET-IK-24 had very good sporulation and the highest RMG. ETDS 97 had poor sporulation and the highest RMG among Ile-Ife isolates.
The growth rate of selected E. turcicum isolates is presented in Fig. 5. For Ikenne isolates, NGETIK-16-8 had the least growth rate, followed by NGETIK-16-11. However, the highest growth rate was recorded for ET-IK-24. Similarly, significant (P < 0.05) differences were found for growth rates of Ile-Ife isolates. For example, the least growth rate was recorded for ETDS 8 while ETDS 97 had the highest, a poor sporulating isolate. Furthermore, the Zaria isolates also had significant variations in relation to growth rate. AB19-4 A-1, classified as a good sporulating isolate, had the fastest growth compared to other isolates.
Molecular characterization of a subset of Exserohilum turcicum isolates
Of the E. turcicum isolates (217) identified and characterized morphologically, only 56, 47, and 21 from Ikenne, Ile-Ife, and Zaria, respectively, were characterized molecularly. The isolates were selected for representative groups based on morphological features such as RMG, sporulation, conidia size, and mycelial pattern. All selected isolates amplified the reference EF1-α primer pair (Fig. 6a). On the other hand, amplification of segments of the three E. turcicum-specific primer pairs for effector genes varied. Overall, 89% of the isolates amplified the SIX13-like primer pair: 43 of the 56 Ikenne isolates amplified the SIX13-like primer pair; all except one of the 47 Ile-Ife isolates amplified it while all Zaria isolates amplified it (Fig. 6b). There were notable variations for the SIX5-like amplification compared to SIX13-like. Overall, 59% of the isolates amplified the SIX5-like primers: 27/29 Ikenne isolates, 27/47 Ile-Ife isolates, and 19/21 Zaria isolates (Fig. 6c). For Ecp6 primer pair there were amplifications for 42/56 Ikenne isolates, 34/47 Ile-Ife isolates, and 20/21 Zaria isolates. Overall, 77% of the isolates amplified the Ecp6 primer. Ecp6 amplification bands of selected isolates is presented in Fig. 6d. Of the 124 isolates characterized, 57 amplified the three effectors. Most of the Zaria isolates amplified the three effectors. A Venn diagram showing number of isolates amplifying 1, 2, or 3 effectors is presented in Fig. 7.
Discussion
The present study is the first survey of E. turcicum associated with maize in different agro-ecologies in Nigeria. The study was conducted to determine whether morphological and molecular variability among isolates interacting with maize in Nigeria exists. A total of 217 isolates were recovered in three locations: Ikenne (102), Ile-Ife (61), and Zaria (54). The results of the characterization of the isolates using morphological and molecular criteria revealed variations within and among the locations. The relative humidity and annual rainfall of the study locations are higher in Ikenne compared to Zaria [10]. These climatic conditions might have contributed to the higher incidence of the disease and, hence the numerous isolates of E. turcicum recovered from Ikenne compared to Ile-Ife and Zaria.
The recovered isolates varied significantly in morphology including colony color, growth pattern, conidia structures, size, and RMG. Several colony colors of E. turcicum have been reported on PDA: from white to green-white, olivaceous gray to brown with black pigmentation [10, 40, 41]. The range of shades of colony morphology colors observed in the present study were similar to those previously reported. The conidia of all isolates had a conspicuous protruded hilum, as expected [12]. The number of septation and conidia length conformed with those of previous reports [41]. Some isolates had a high spore reproduction rate, which could be used as a proxy for survival and spread of plant pathogens [42]. Different categories of SPMF following a modified scale [43] were recorded. Most isolates across locations had > 15 SPMF indicating their rapid spore production under laboratory conditions. Badu-Apraku et al. recorded high incidence and severity of NCLB in the study locations which could be linked to the rapid growth and multiplication of E. turcicum in the study area [10]. Generally, pathogens spread by rapid production and dissemination of spores [43]. Therefore, the different growth rates and SPMF recorded in the present study suggest that the pathogen has the potential to rapidly spread and this could be a challenge to NCLB management in the study areas. The observed morphological variation of E. turcicum could be a result of varying environmental conditions of the study area and the survival strategy of the pathogen. Anwer et al. [41] and Abebe and Singburaudom [44] reported that environmental conditions could result in morphological variations in E. turcicum populations.
In the present study, there was variability among and within locations in isolate expression of effector genes conditioning pathogenicity and virulence. The choice of the method and the target genes adopted was based on the study of Human et al. [16]. They identified several enzymes, secondary metabolites, effectors, and candidate genes moderating E. turcicum pathogenicity and reported that the expression of Ecp6, SIX13-like, and SIX5-like effector genes were associated with the establishment of the pathogen during the biotrophic stage. Xue et al. [45], Niu et al. [37], and Cao et al. [38] reported similar results. However, in the present study, the expression of effector genes differed among the isolates. The number of isolates expressing only one of the following three effector genes are in parenthesis: SIX13-like (13), SIX5-like (1), and Ecp6 (7). There were 28 isolates producing both SIX13-like and Ecp6 effector genes, 11 producing both SIX13-like and SIX5-like, and five producing both Ecp6 and SIX5-like (Fig. 7). Isolates from the current study that produced the three effector genes are more virulent compared to the others. In our previous studies, E. turcicum isolate NGETIB16-13, which amplified the three effector genes, was the most virulent isolate and therefore was used in resistance screening studies in the field [10, 23, 28, 39].
Several studies have reported the genetic relatedness of E. turcicum isolates from different locations. For example, McDonald and Linde reported a high potential of gene flow among E. turcicum isolates which portend genetic variations due to sexual reproduction [46]. Similarly, Borchardt et al. reported high genetic diversity, weak linkage disequilibrium and near-equal mating-type genes ratio for E. turcicum haplotypes, which provided evidence for frequent sexual recombination in populations from tropical regions of Kenya, Mexico, and China [47]. However, Human et al. provided evidence for clonal lineages of E. turcicum races among isolates recovered from locations that were separated by > 700 km in South Africa [16]. It is unclear which phenomenon conditions sexual reproduction in E. turcicum. The varying morphological and effector profiles found in the current study across and within locations suggest that the examined populations are not clonal. Therefore, further research is needed to determine the effects of mutation, marker loss, parasexuality, and/or recombination on the observed variations in effector production.
Conclusion
This is the first report on E. turcicum associated with maize fields in different agro-ecologies in Nigeria. From three test locations —Ikenne, Ile-Ife and Zaria—217 isolates of E. turcicum were recovered and characterized using morphological and molecular traits. Morphological and genetic variation among E. turcicum isolates were found within and across locations. Candidate effector genes that condition pathogenicity and virulence in E. turcicum were found but not all isolates possessed the three genes, SIX5-like, SIX13-like, and Ecp6. The variations observed among the different isolates of E. turcicum evaluated in the present study calls for the review of the breeding methods for developing NCLB-resistant maize genotypes. These include the development of maize genotypes with quantitative resistance or multi-lines with specific resistant genes to different isolates of the pathogen. Relying on qualitative or monogenic resistance would not be enough for the management of the disease because it could easily be overcome by new strains of the pathogen.
Methodology
Sample collection and fungal isolation
Maize leaves with NCLB symptoms were collected from three test locations within contrasting agro-ecologies in Nigeria: Ikenne (6°53′ N, 3°42′ E), Ile-Ife (7°18̕′ N, 4°33̕′ E), and Zaria (11°7̕′ N, 7°45̕′ E), (Fig. 1). Using a purposive sampling method, leaves were collected, kept in labelled sample bags, and transferred to the laboratory for isolation of the causal agent. Five maize fields per location were sampled. Each field was divided into five plots and five leaf samples were obtained per plot. One to three sections per leaf showing NCLB symptoms were used for fungal isolations. Diseased tissues were surface sterilized for 30 s in 0.5% NaOCl and rinsed twice in sterile distilled water. The excised NCLB lesions were placed on sterile, moist filter paper in a Petri dish and incubated for 4 to 7 d at 25 °C to induce sporulation. Spores from each lesion were axenically transferred to potato dextrose agar (PDA), and incubated for 10 to 15 d. To obtain single spore cultures of each isolate, a plug of the sporulating fungal mycelia (14-d-old) was placed in a 40-ml vial containing 10 ml sterile distilled water. The mixture was briefly vortexed, serially diluted, and 10 µl of a 10− 5 suspension was spread using a sterile plastic spreader on water agar and incubated for 24 h. Spore germination was then examined with the aid of a dissecting microscope. A single germinating spore was isolated using a sterile pin, placed on PDA, and incubated at room temperature (25 °C) in the laboratory for 14 d.
Morphological characteristics of Exserohilum turcicum
The characteristics (diameter, shape, size, and spore septation) of the spores of E. turcicum were determined by staining microscope mounts with lactophenol cotton blue stain and using a compound Olympus BX51 microscope at ×25 magnification. After taking the measurements with the calibrated ocular micrometer, photomicrographs of the spores were taken.
The radial mycelia growth (RMG) was calculated according to Harlapur et al. [43], with minor modifications. Briefly, plugs of the pure culture of each isolate were plated on PDA calibrated with a plastic ruler. Vertical and horizontal length growths (cm) of the mycelia of each isolate were measured for 14 d (at two days intervals). The RMG was calculated by multiplying the vertical and horizontal lengths with ∏ = 3.1416, assuming the shape of the Petri dish. The RMG and average spore length was subjected to analysis of variance (ANOVA) and isolates with rapid mycelial growth were identified. Production of spores by isolates was classified based on the number of spores per microscopic field (SPMF) as excellent: ≥ 25 SPMF; very good: 20 to 24 SPMF; good: 15 to 19 SPMF; fair: 10 to 14 SPMF; and poor: 1 to 9 SPMF at ×25 magnification [43]. The mean ± standard error (SE) for spore count and spore length were calculated while the relationships between spore length, spore count, and RMG were determined using Pearson correlation analysis. Mean ± SE, ANOVA, and correlation analysis were estimated using SAS v.9.1 (SAS Institute, Cary, NC, USA).
Genomic DNA extraction
The methods of Sambrook et al. [48] and Callicott and Cotty [49] were employed for DNA extraction [48, 49]. Briefly, spores of pure cultures of 7-d-old E. turcicum grown in PDA were harvested by adding 1.5 ml 0.1% TWEEN®80 to the culture. Then, 1.2 ml of the suspension was transferred into a sterile 1.5 ml Eppendorf tube in a laminar flow hood. The suspension was centrifuged at 8,000× g for 5 min. Afterwards, the supernatant was carefully removed without disturbing the precipitate. Lysis Buffer (450 µl: 270 mM Tris, 90 mM EDTA, 1% SDS, pH 8.0) was added to each tube and vortexed briefly to resuspend the precipitate. The tubes were placed in an Eppendorf ThermoMixer® C at 60 °C and 8,000 × g for 60 min, and thereafter, centrifuged at 14,000 × g for 30 min. Then, 340 µl of the supernatant were transferred into a new, labelled sterile tube and 340 µl of refrigerated 4 M ammonium acetate were added. The suspension was thoroughly mixed. Afterwards, 680 µl ice-cold absolute ethanol was added, the content mixed, and the tube placed in a freezer at -20 °C for 30 min. The mixture was then centrifuged at 14,000 × g for 5 min and the supernatant was carefully removed. The tube was left to dry after draining the ethanol and the pellet re-suspended in 50 µl sterile nuclease-free water and gently mixed. The DNA concentration was determined using a Nanodrop spectrophotometer (Thermo Scientific™) and recorded.
PCR primer design
Three E. turcicum specific primers that target effector genes (Ecp6, SIX13-like, and SIX5-like) were designed for the present study. Available sequences of these regions were collected from NCBI repositories, aligned and primers designed using Geneious Prime (v2020.2.4). A reference elongation factor primer (EF1-688 F/EF1-125R; [50]) was also used. The details of the four primer pairs are presented in Table 5.
PCR conditions and gel electrophoresis
The target fragment of each region was amplified using the following reagent concentrations: 6.0 µl OneTaq Quick-Load 2 × Master Mix, 0.24 µl 10 mM of each primer, 2 µl 20 ng/µl DNA, and 3.52 µl nuclease-free water to make a final volume of 12 µl for PCR. Initial denaturation was at 95 °C for 2 min, followed by 35 cycles of 95 °C for 30 s, annealing at 50 °C for 3 min, and extension at 72 °C for 2 min, with a final extension step at 72 °C for 25 min. The amplified fragments were separated on 1% agarose gel, which contained 1 × Tris-acetate-EDTA (TAE) buffer. The 1% agarose gel was prepared by dissolving 1 g agarose in 100 ml TAE. The solution was melted in the microwave for 5 min and allowed to cool. On cooling, 5 µl of loading dye (SafeView™ classic) was added to the solution before pouring into the electrophoresis tray and afterwards placed in a tank containing 1 × TAE for electrophoresis for 40 min at 110 V. The gel was visualized for the amplified band patterns using a gel documentation system.
Data Availability
The datasets used in the present study have been deposited in the IITA CKANN Data Repository: https://doi.org/10.25502/kkcw-v956/d.
Abbreviations
- NCLB:
-
Northern Corn Leaf Blight
- SSA:
-
sub-Saharan Africa
- CWDEs:
-
cell wall-degrading enzymes
- HPR:
-
host plant resistance
- PCR:
-
polymerase chain reaction
- SIX:
-
secreted in xylem
- RMG:
-
radial mycelia growth
- PDA:
-
potato dextrose agar
- SPMF:
-
spores per microscopic field
- EDTA:
-
ethylenediaminetetraacetic acid
- SDS:
-
sodium dodecyl sulfate
- SAS:
-
Statistical Analysis Software
- SE:
-
standard error
- IITA:
-
International Institute of Tropical Agriculture
References
Badu-Apraku B, Fakorede M, Menkir A, Sanogo D. Conduct and management of maize field trials. IITA. 2012.
Bhadmus OA, Badu-Apraku B, Adeyemo OA, Ogunkanmi AL. Genetic analysis of early white quality protein maize inbreds and derived hybrids under low-nitrogen and combined drought and heat stress environments. Plants. 2021;10(12):2596.
Food and Agriculture Organization of the United Nations. Statistical databases. Rome, Italy: Food and Agriculture Organization of the United Nations; 2020. http://www.fao.org/faostat/en/#compare.
Akinwale RO, Oyelakin AO. Field assessment of disease resistance status of some newly-developed early and extra-early maize varieties under humid rainforest conditions of Nigeria. J Plant Breed Crop Sci. 2018;10(3):69–79.
Badu-Apraku B, Fakorede MAB. Advances in genetic enhancement of early and extra-early maize for sub-saharan Africa. Switzerland: Springer; 2017.
Munkvold GP, White DG. Compendium of Corn Diseases. 4th ed. St. Paul, MN, U.S.A.: American Phytopathological Society; 2016.
Pataky J, Williams M, Warsaw B, Meyer M, Moody J. Midwest vegetable trial report for 2007. In: Lafayette W, editor, Sweet corn hybrid disease nursery, ed. West Lafayette. 2007,91–104.
Doehlemann G, Ökmen B, Zhu W, Sharon A. Plant pathogenic fungi. In: Heitman J, Howlett BJ, Crous PW, Stukenbrock EH, James TY, Gow NAR, eds, The Fungal Kingdom. 2017,7013–726.
Bashir K, Kamaruzaman S, Khairulmazmi A. First report of northern corn leaf blight disease caused by Exserohilum turcicum on Zea mays in Malaysia. J Mol Gen Med. 2018;12(4):1–2.
Badu-Apraku B, Bankole FA, Ajayo BS, Fakorede MAB, Akinwale RO, Talabi AO, et al. Identification of early and extra-early maturing tropical maize inbred lines resistant to Exserohilum turcicum in sub-Saharan Africa. Crop Prot. 2021a;139:105386.
Hooda KS, Khokhar MK, Shekhar M, Karjagi CG, Kumar B, Mallikarjuna N, et al. Turcicum leaf blight—sustainable management of a re-emerging maize disease. J Plant Dis Prot. 2017;124(2):101–13.
Carson LM. Appendix. In White DG, Munkvold GP, editor, Compendium of Corn Diseases 4th Ed (4th edition, pp. 29–31). American Phytopathological Society, St. Paul. 2016.
Ohm RA, Feau N, Henrissat B, Schoch CL, Horwitz BA, Barry KW, et al. Diverse lifestyles and strategies of plant pathogenesis encoded in the genomes of eighteen Dothideomycetes fungi. PLOS Path. 2012;8(12):1–26.
Hurni S, Scheuermann D, Krattinger SG, Kessel B, Wicker T, Herren G et al. The maize disease resistance gene Htn1 against northern corn leaf blight encodes a wall-associated receptor-like kinase. Proc. Natl. Acad. Sci. 2015;112(28):8780–8785.
Palma-Guerrero J, Ma X, Torriani SFF, Zala M, Francisco CS, Hartmann FE, et al. Comparative transcriptome analyses in Zymoseptoria tritici reveal significant differences in gene expression among strains during plant infection. Mol Plant Microbe Interact. 2017;30(3):231–44.
Human MP, Berger DK, Crampton BG. Time-course RNAseq reveals Exserohilum turcicum effectors and pathogenicity determinants. Front Microbiol. 2020;11:1–23.
Choi J, Kim K, Jeon J, Lee Y. Fungal plant cell wall-degrading enzyme database: a platform for comparative and evolutionary genomics in fungi and oomycetes. BMC Genom. 2013;14:1–10.
Lo Presti L, Lanver D, Schweizer G, Tanaka S, Liang L, Tollot M, et al. Fungal effectors and plant susceptibility. Annu Rev Plant Biol. 2015;66:513–45.
Selin C, Kievit TR, Belmonte MF, Fernando WGD, Monaghan J. Elucidating the role of effectors in plant-fungal interactions: progress and challenges. Front Microbiol. 2016;7:1–21.
Jones JDG, Dangl JL. The plant immune system. Nat Rev Genet. 2006;444:323–9.
Flor HH. The complementary genic systems in flax and flax rust. Adv Genet. 1956;8:29–54.
Weems JD, Bradley CA. Exserohilum turcicum race population distribution in the north central United States. Plant Dis. 2018;102(2):292–9.
Badu-Apraku B, Bankole FA, Fakorede MAB, Ayinde O, Ortega‐Beltran A. Genetic analysis of grain yield and resistance of extra‐early maturing maize inbreds to northern corn leaf blight. Crop Sci. 2021b;61:1864–80.
Pant SK, Pramod K, Chauhan VS. Effect of turcicum leaf blight on photosynthesis in maize. Indian Phytopathol. 2001;54:251–2.
Price T, Purvis M, Pruitt H. A quantitative disease severity rating scale for northern corn leaf blight. Plant Health Brief. 2016;17(1):49–50.
Begum H, Raj RB, Satyanarayana E. Field evaluation of five fungicides to control turcicum leaf blight in maize. Indian J Plant Prot. 1993;21:110–1.
Galiano-Carneiro AL, Miedaner T. Genetics of resistance and pathogenicity in the maize/Setosphaeria turcica pathosystem and implications for breeding. Front. Plant Sci. 2017;8:1–13.
Bankole FA, Badu-Apraku B, Salami AO, Falade TDO, Bandyopadhyay R, Ortega-Beltran A. Identification of early and extra-early maturing tropical maize inbred lines with multiple disease resistance for enhanced maize production and productivity in sub-Saharan Africa. Plant Dis. 2022;106:2638–47.
Wiesner-Hanks T, Nelson R. Multiple disease resistance in plants. Annu Rev Phytopathol. 2016;54(1):229–52.
Simcox KD, Bennetzen JL. The use of molecular markers to study Setosphaeria turcica resistance in maize. Phytopathology. 1993;83:1326–30.
Ferguson LM, Carson ML. Spatial diversity of Setosphaeria turcica sampled from the Eastern United States. Phytopathology. 2004;94(8):892–900.
Human MP, Barnes I, Craven M, Crampton BG. Lack of population structure and mixed reproduction modes in Exserohilum turcicum from South Africa. Phytopathology. 2016;106(11):1386–92.
Ferguson LM, Carson ML. Temporal variation in Setosphaeria turcica between 1974 and 1994 and origin of races 1, 23, and 23 N in the United States. Phytopathology. 2007;97(11):1501–11.
Muiru WM, Koopmann B, Mutitu EW, Kimenju JW. Production, purification and separation of phytotoxins from Exserohilum turcicum isolates of Kenyan, German and Austrian origin. Botany. 2011. http://erepository.uonbi.ac.ke:8080/xmlui/handle/123456789/15206.
Haasbroek MP, Craven M, Barnes I, Crampton BG. Microsatellite and mating type primers for the maize and sorghum pathogen, Exserohilum turcicum. Australas Plant Path. 2014;43(5):577–81.
Tang L, Gao ZG, Yao Y, Liu X. Identification and genetic diversity of formae speciales of Setosphaeria turcica in China. Plant Dis. 2014;99(4):482–7.
Niu X, Zhao X, Ling KS, Levi A, Sun Y, Fan M. The FonSIX6 gene acts as an avirulence effector in the Fusarium oxysporum f. sp. niveum-watermelon pathosystem. Sci Rep. 2016;6:1–7.
Cao L, Blekemolen MC, Tintor N, Cornelissen BJC, Takken FLW. The Fusarium oxysporum Avr2-Six5 effector pair alters plasmodesmatal exclusion selectivity to facilitate cell-to-cell movement of Avr2. Mol. Plant. 2018;11(5):691–705.
Badu-Apraku B, Bankole FA, Fakorede MAB, Ogbe G, Bandyopadhyay R, Ortega-Beltran A. Combining ability and heterotic grouping of turcicum-resistant early-maturing maize inbreds. Agron J. 2021c;113(4):3560–77.
Reddy R, Narayan R, Ranga RR, Sokka S. Cultural and morphological variability among Exserohilum turcicum isolates causing Turcicum leaf blight of maize. Environ Ecol. 2014;32(1):16–21.
Anwer MA, Niwas R, Ranjan T, Mandal SS, Ansar M, Srivastava JN, et al. Molecular and morphological characterization of Exserohilum turcicum (Passerini) Leonard and Suggs causing northern corn leaf blight of maize in Bihar. Bioengineering. 2022;9(8):403.
Pusztahelyi T, Holb IJ, Pócsi I. Secondary metabolites in fungus-plant interactions. Front Plant Sci. 2015;6:1–23.
Harlapur SI, Kulkarni MS, Hegde Y, Kulkarni S. Variability in Exserohilum turcicum (pass.) Leonard and Suggs., causal agent of turcicum leaf blight of maize. Karnataka J Agric Sci. 2007;20:541–4.
Abebe D, Singburaudom N. Morphological, cultural and pathogenicity variation of Exserohilum turcicum (Pass) Leonard and Suggs isolates in maize (Zea mays L). Witthayasan Kasetsat. 2006;40(2):341–52.
Xue C, Wu D, Condon BJ, Bi Q, Wang W, Turgeon BG. Efficient gene knockout in the maize pathogen Setosphaeria turcica using Agrobacterium tumefaciens-mediated transformation. Phytopathology. 2013;103(6):641–7.
McDonald BA, Linde C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu Rev Phytopathol. 2002;40(1):349–79.
Borchardt DS, Welz HG, Geiger HH. Genetic structure of Setosphaeria turcica populations in temperate and tropical climates. Phytopathology. 1998;88:322–9.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1989.
Callicott KA, Cotty PJ. Method for monitoring deletions in the aflatoxin biosynthesis gene cluster of Aspergillus flavus with multiplex PCR. Lett Appl Microbiol. 2015;60(1):60–5.
Alves A, Crous PW, Correia A, Phillips AJL. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008;28:1–13.
Acknowledgements
This study was part of the first author’s doctoral dissertation and was presented during the SPECIAL SESSION of the Annual Meeting of the American Phytopathological Society (APS): Plant Pathologists of the Future: Showcasing Graduate Student Presentation Winners from APS Division Meetings – Plant Health 2022, Pittsburgh. The authors appreciate the staff of IITA Maize Improvement Program—Stress Tolerant Maize for Africa (STMA) Project and the IITA Pathology and Mycotoxin Unit for their contributions to the execution of this research. The authors are grateful for the funding support of the Bill & Melinda Gates Foundation through the Stress Tolerant Maize for Africa [STMA] and the Accelerated Genetic Gains in Maize and Wheat (AGG) Projects as well as the CGIAR Research Programs on Agriculture for Nutrition and Health (A4NH) and MAIZE supported by CGIAR Fund Donors (https://www.cgiar.org/funders/).
Funding
This research was supported by the Bill & Melinda Gates Foundation [OPP1134248], and the CGIAR Research Programs on Agriculture for Nutrition and Health (A4NH) and MAIZE supported by CGIAR Fund Donors (https://www.cgiar.org/funders/). However, the funding bodies played no role in the design of the study and collection, analysis, interpretation of data and the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
F.A.B., B.B.A., and A.O.-B. designed the sampling strategy. F.A.B. and A.O.-B. designed the experiments. F.A.B. performed the experiments. B.B.A., T.D.O.F., R.B., and A.O.-B. provided materials and supervision. F.A.B. wrote the manuscript. B.B.A, A.O.S., T.D.O.F., R.B., and A.O.-B. edited the manuscript. All authors gave final approval to publish the manuscript.
Corresponding author
Ethics declarations
Declarations
The laboratory experiments that were conducted complied with all relevant institutional, national, and international guidelines and legislations.
Competing interests
The authors declare no competing interests.
Competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bankole, F.A., Badu-Apraku, B., Salami, A.O. et al. Variation in the morphology and effector profiles of Exserohilum turcicum isolates associated with the Northern Corn Leaf Blight of maize in Nigeria. BMC Plant Biol 23, 386 (2023). https://doi.org/10.1186/s12870-023-04385-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04385-7