Abstract
Background
Tea, the second largest consumer beverage in the world after water, is widely cultivated in tropical and subtropical areas. However, the effect of environmental factors on the distribution of wild tea plants is unclear.
Results
A total of 159 wild tea plants were collected from different altitudes and geological types of the Guizhou Plateau. Using the genotyping-by-sequencing method, 98,241 high-quality single nucleotide polymorphisms were identified. Genetic diversity, population structure analysis, principal component analysis, phylogenetic analysis, and linkage disequilibrium were performed. The genetic diversity of the wild tea plant population from the Silicate Rock Classes of Camellia gymnogyna was higher than that from the Carbonate Rock Classes of Camellia tachangensis. In addition, the genetic diversity of wild tea plants from the second altitude gradient was significantly higher than that of wild tea plants from the third and first altitude gradients. Two inferred pure groups (GP01 and GP02) and one inferred admixture group (GP03) were identified by population structure analysis and were verified by principal component and phylogenetic analyses. The highest differentiation coefficients were determined for GP01 vs. GP02, while the lowest differentiation coefficients were determined for GP01 vs. GP03.
Conclusions
This study revealed the genetic diversity and geographical distribution characteristics of wild tea plants in the Guizhou Plateau. There are significant differences in genetic diversity and evolutionary direction between Camellia tachangensis with Carbonate Rock Classes at the first altitude gradient and Camellia gymnogyna with Silicate Rock Classes at the third altitude gradient. Geological environment, soil mineral element content, soil pH, and altitude markedly contributed to the genetic differentiation between Camellia tachangensis and Camellia gymnogyna.
Similar content being viewed by others
Background
Tea (Camellia sinensis (L.) O. Kuntze) is the second largest consumer beverage in the world after water, and is widely cultivated in tropical and subtropical areas [1, 2]. China is the origin of tea plants and has the broadest genetic variation globally, especially in the Guizhou Plateau. The altitude of Guizhou Plateau increases from approximately 200 m in the east to approximately 2,800 m in the west, and the plateau is predominantly composed of Carbonate Rock (CR) and Silicate Rock (SR) undergoing complicated and extensive folding, faulting, and stream erosion [3]. Altitude is known to play an important role in plant diversity [4, 5]. Further analysis of the effects of different geological characteristics, altitudes and soils formed at different altitudes on the genetic diversity of wild tea plants is of great significance to the genetic breeding of tea plants.
Molecular markers are essential for breeding major crops in current agriculture industry, and many molecular marker techniques have been developed and applied to the detection of genetic diversity and environmental adaptability of various crops [6,7,8]. Single nucleotide polymorphisms (SNPs) are the most effective marker system for determining DNA sequence variations and are responsible for specific traits or are used to track the evolutionary history of species. In addition, SNPs are the most common source of genetic variation in eukaryotes, and have become an important symbol of plant genetic research. The extensive SNP variation can be captured using genotyping-by-sequencing, which not only offers a cost-effective solution but also narrows the genotyping gap between local or specific populations [9, 10]. For example, a total of 3.6 million SNPs were identified in 517 rice varieties using next-generation sequencing, revealing the genetic basis of agronomic traits of rice to adapt to climate change [11]. Taranto et al. [12] identified 32,950 high-quality SNPs in Capsicum annuum germplasm through sequencing (genotyping-by-sequencing, GBS) and evaluated the genetic diversity in C. annuum germplasm. Meanwhile, Niu et al. [13] recognized 79,016 high-quality SNPs in 415 tea materials using the GBS method and explored the genetic diversity of tea germplasm resources in Guizhou Plateau.
Local adaptation is the basis of segregation of most phenotypic variations within species, with various environmental factors shaping the patterns of genetic variation and differentiation of wild germplasm resources [14]. Environmental factors, including light, temperature, water content, and especially altitude and geological rock, play an important role in plant genetic differentiation. Soils formed by different rocks exhibit significant differences in fertility, thus forming distinct suitable growth areas for plants [3]. Altitude also affects soil development owing to differences in soil moisture content, soil temperature, light, and other conditions. For instance, several granite endemic species in Western Australia have low diversity and high population differentiation [15]. In China, soil is a major evolutionary factor in the species richness model of subtropical plants (Camelliaceae) [16]. Differences in altitude cause differences of temperature, rainfall, and light in the region and subsequently the difference of population distribution and genetic diversity of plants. Such as, the overall evolution speed of azaleas growing at high altitudes is faster than that at low altitudes. Rhododendron growing at high altitude often resists the environmental heterogeneity caused by low temperature and altitude by improving its own carbohydrates, fatty acids, amino acids, and flavonoids. Rhododendron at low altitude usually adapts to low-altitude nematode, nitrogen, water deficiency, hypoxia, and acidic pH conditions by releasing the hormones salicylic acid (SA) and jasmonic acid (JA) [17, 18]. The Tibetan poplar (Populus szechuanica var. tibetica Schneid) was found to have the highest genetic diversity at low altitude and the lowest genetic diversity at high altitude [19]. Therefore, altitude and geological environment have an important impact on plant genetic diversity.
In our previous study, we investigated the agronomic characteristics, soil nutrient status, and ecological environment of wild tea plants in Guizhou, China and discovered significant differences in the altitude and ecological environment regarding growth of Camellia tachangensis and Camellia gymnogyna. C. tachangensis was predominantly distributed in the high-altitude carbonate rock area in the southwest of the Guizhou Plateau, while C. gymnogyna was predominantly distributed in the low-altitude silicate rock area in the north of the Plateau. C. tachangensis and C. gymnogyna have obvious differences in tree type, leaves, flowers, and fruits [20,21,22]. In the current study, we sought to further explore C. tachangensis and C. gymnogyna adapt to the evolution in different altitudes and geological environments of the Guizhou Plateau. Wild tea plants (159 in total) were collected from different altitudes and geological environments in Guizhou Plateau, and GBS was used to identify the significant SNPs of these plant materials. Subsequently, population structure analysis, principal component analysis (PCA), and phylogenetic analysis were conducted, and the 159 wild tea plants were divided into three groups (GP01, GP02, and GP03). In addition, linkage disequilibrium (LD), genetic diversity, and soil nutrient characteristics of the different populations were analyzed. Findings from the study provide valuable information for future tea breeding and genetic analysis.
Results
Sequencing and variant discovery
To explore the genetic variation of the wild tea plant population of Guizhou Plateau, 159 wild tea plants, comprising 101 C. tachangensis plants and 58 C. gymnogyna plants, were collected based on the different altitudes and geological types of Guizhou Plateau (Additional file 1: Table S1). The geographical characteristics and distribution of the 159 wild tea plants in Guizhou Plateau are shown in Fig. 1. Subsequently, 162.9 Gb clean sequencing data with an average of 1.02 Gb per accession were obtained (Additional file 1: Table S2), which were further mapped to the reference genome of tea (http://tpia.tea.plant.org/). SNPs were then detected and genotyped by GATK (version 3.7.0) based on the reference genome [23]. In total, 29,393,327 SNPs were identified and the heterozygosity value was calculated. The average heterozygosity rate per accession was 8.16% (Additional file 1: Table S3). The filter left 98,241 high-quality SNPs, which were unevenly distributed on 15 chromosomes. The smallest and largest SNP density was detected on chromosome 15 and chromosome 1, respectively (Fig. 2; Additional file 1: Table S4), while the average number of SNPs per chromosome was 9,162. Analysis of nucleotide substitutions showed that the 98,241 SNPs were classified into two types: transition and transversion. There were 911,455 (78.10%) transitions and 255,518 (21.90%) transversions. The substitution frequencies were 79,404 (6.80%) A/T, 63,029 (5.40%) A/C, 66,018 (5.66%) G/T, 47,067 (4.03%) C/G, 456,661 (39.13%) C/T, and 454,794 (38.97%) A/G. The transition to transversion ratio was 3.57 (Table 1).
Geographic distribution of 159 tea accessions. Note: Geographic distribution of accessions are represented by triangles on the altitude map of Guizhou. The red triangle represents C. gymnogyna and the blue triangle represents C. tachangensis. I Dolomite sub-suitable area, II Dolomitic limestone suitable area, III Clastic rock most suitable area, IV Purple clastic rock suitable area. The altitude is rising from pure green to purple
Genetic diversity estimation
Nucleotide diversity (Pi), observed heterozygosity (Ho), minor allele frequency (MAF), and inbreeding coefficient (Fis) are recognized indicators of genetic diversity. In this study, Pi, Ho, MAF, and Fis of the 159 wild tea plants were 0.230, 0.085, 0.149, and 0.644, respectively (Table 2). Further analysis of the genetic diversity of tea plant populations from two different species showed that Ho and MAF of the C. gymnogyna tea plant population were significantly higher than those of the C. tachangensis population. Fis was higher for the C. tachangensis population compared with the C. gymnogyna population. Analysis of the genetic diversity of tea plant populations from three different altitude gradients revealed that Pi, Ho, and MAF of the tea plant population from H2 were significantly higher compared with those from H3 and H1, and Pi, Ho, and MAF of the tea plant population from H3 was significantly higher than that of the population from H1. Comparison of the genetic diversity of tea plant populations in the CR and SR Classes of Guizhou Plateau revealed that Ho and MAF of the tea plant population in SR were significantly higher compared with those for the tea plant population in CR (Table 2). Among the four geological areas, Pi and MAF of the tea plant population in I were significantly higher compared with those in III, IV, and II (Table 2).
Positive values of Tajima’s D arise from an excess of intermediate frequency alleles and can result from population bottlenecks, structure, and/or balancing selection [24]. In this study, a positive value of Tajima’s D was detected in the wild tea plant population, suggesting that the wild tea plant population underwent population bottlenecks and/or balancing selection (Table 2). Fst (differentiation coefficient) is used as a measure of population structure; Fst values of 0–0.05 and 0.05–0.15 indicate little and moderate divergence, respectively [25, 26]. In this study, the Fst value of C. gymnogyna and C. tachangensis was 0.075, suggesting that the moderate divergence occurred in the wild tea plant population between C. gymnogyna and C. tachangensis (Table 3). In addition, the Fst values of geological areas I and III, I and IV, II and III, and II and IV were 0.056, 0.063, 0.126, and 0.145, respectively, suggesting that the moderate divergence occurred in the wild tea plant population between I and III between I and IV, between II and III, and between II and IV. The Fst values of geological areas I and II, III and IV were 0.036 and 0.003, respectively, suggesting that little divergence occurred in the wild tea plant population between I and II, and between III and IV. The highest pairwise genetic distance (GD) was for II vs. IV, while the lowest pairwise GD was for III vs. IV (Table 4). The Fst values in H1 vs. H2 were 0.032, indicating that little divergence occurred in H1 vs. H2. Meanwhile, the Fst values in H2 vs. H3 and H1 vs. H3 were 0.071 and 0.149, respectively, suggesting that moderate divergence occurred in H2 vs. H3 and in H1 vs. H3. The highest pairwise GD was for H1 vs. H3, while the lowest pairwise GD was for H1 vs. H2 (Table 5).
Population structure, PCA, and phylogenetic analysis
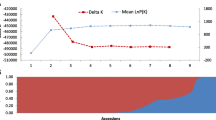
To further explore the relationship of the 159 wild tea plant populations, the 98,241 high-quality SNPs were used to perform population structure analysis, PCA, and phylogenetic analysis. Dynamic changes in population structure were further estimated under different K values (K = 1–9). Analysis of cross-validation error (CV error) revealed that the CV error changed little as K increased from 1 to 9, and CV error was minimal at K = 2 (Additional file 2: Fig. S2). Accessions with membership coefficients > 0.80 were assigned to the corresponding pure groups, while those with coefficients < 0.80 were assigned to the admixture group (Additional file 1: Table S1) [11]. This resulted in the 159 wild tea plants being further classified into three population groups—two ancestral groups and one admixture group (Additional file 1: Table S1). The first ancestral group (referred to as ‘III and H3 of C. gymnogyna (III H3 C. gymnogyna)’ or ‘GP01’ from now on) contained 67 accessions, including 55 (82.0%) C. gymnogyna and 12 (18.0%) C. tachangensis, which all came from the SR Classes (46 (68.6%) tea accessions from area III, and 21 (31.4%) tea plants from area IV) (Additional file 1: Table S5), and these accessions were also divided into two clusters based on altitude (17 (25.3%) tea accessions from H2 and 50 (74.7%) tea accessions from H3) (Additional file 1: Table S6). The second ancestral group (referred to as ‘II and H1 of C. tachangensis’ (II H1 C. tachangensis) or ‘GP02’ from now on) contained 45 C. tachangensis, which came from the CR Classes (including 11 (24.4%) accessions from area I and 34 (75.6%) accessions from area II), with 39 (86.6%) plants from H1 and 6 (13.4%) plants from H2. The remaining 47 accessions formed an admixed group (referred to as ‘I and H2 of C. tachangensis’ (I H2 C. tachangensis) or ‘GP03’ from now on) that, comprised 44 (93.6%) C. tachangensis and 3 (6.4%) C. gymnogyna, with 37 (78.7%) plants collected from area I and 10 (21.3%) individuals collected from area II, and 42 (89.3%) and 5 (10.7%) of the accessions were distributed in H2 and H3, respectively (Additional file 1: Table S6). To identify potential population stratification, PCA and a maximum-likelihood phylogenetic tree (ML tree) were used to investigate dendrogram relationships among the 159 wild tea accessions using the 98,241 SNPs (Fig. 3C and Additional file 2: Fig S3). PCA and the ML tree revealed three major clusters corresponding to groups GP01, GP02, and GP03 from the population structure analysis, which further confirmed the accuracy of the population grouping (Fig. 3A and B).
Population structure and LD of 159 accessions. A Inferred population structure of 159 accessions. Bar plot of individual membership coefficients for the genetic clusters was inferred using ADMIXTURE (K = 2) based on 98,241 SNPs. Individual membership coefficients (Q) were sorted within each cluster. GP01 and GP02 are shown in red and blue, respectively. B Principal component analysis (PCA). The three PCA scatter diagram was made by the first and second principal components. Three inferred populations were identified in ADMIXTURE, GP01 is shown in red, GP02 in blue, and GP03 in yellow. C ML tree comparing the classification of inferred populations, Species C. tachangensis and C. gymnogyna, altitude: H1, H2, and H3 corresponding to the first (> 1400 m), second (1400–1100 m), and third (< 1100 m) altitude gradient, respectively. D LD decay plot of 159 accessions and three inferred populations
LD analysis
LD analysis is used to clarify domestication and breeding history. LD was estimated for the population of 159 wild tea accessions by using 29,393,327 non-pruned LD SNPs. The LD (r2) rapidly decayed with increasing physical distance. The maximum r2 value was 0.226 for the LD decay of all 159 accessions. As r2 decayed to half maximum (0.113), the corresponding physical distance was 0.116 Kb (Fig. 3D). Moreover, the slowest LD was identified in GP02, with LD decay of 0.260 Kb at r2 = 0.113. The physical distance for GP01 was 0.135 Kb at r2 = 0.113. The fastest LD decay was for GP03 with 0.120 Kb at r2 = 0.113.
Genetic differentiation analysis of the inferred populations
To further investigate the genetic diversity of the inferred populations, the Tajima’s D, Pi, Ho, and MAF of GP01, GP02 and GP03 were calculated. Ho, Pi, and MAF were significantly higher for GP03 compared with those of GP01 and GP02, and Pi, Ho, and MAF were significantly higher for GP01 than for GP02 (Fig. 4). The three groups had positive Tajima’s D values, indicating that all three groups underwent population bottlenecks, and/or balancing selection (Fig. 4).
Genetic diversity of three inferred populations of 159 accessions Note: Pi, nucleotide diversity; Ho, observed heterozygosity; MAF, minor allele frequency; Fis, inbreeding coefficient; GD, genetic distance; Fst, differentiation coefficient; Nm, gene flow. Different letters indicate a significant difference in p = 0.05 levels by t-test. GP01 and GP02 are pure groups and GP03 is the admixture group base on ADMIXTURE software at K = 2
Previous studies showed that Fst in the range of 0.00 to 0.05 indicate little divergence, Fst in the range of 0.05 to 0.15 indicate moderate divergence and 0.15 to 0.25 indicate large divergence [26, 27]. The pairwise Fst was analyzed among three inferred groups (Fig. 4). The Fst between GP01 and GP03, and GP02 and GP03 was 0.05 and 0.07, respectively, indicating moderate divergence, while the mean Fst between GP01 and GP02 was 0.154, indicating large divergence. The highest level of difference was detected between GP01 and GP02, and the lowest level of difference was detected between GP01 and GP03, while an intermediate differentiation was observed between GP02 and GP03 (Fig. 4). A lowest GD was detected on GP01 vs GP03 and GP02 vs GP03, while the highest GD was detected on GP01 vs GP02. The higher gene flow (Nm) was detected for GP01 vs GP03 and GP02 vs GP03, while the lowest Nm was detected for GP01 vs GP02. Therefore, there are more gene exchanges between GP01 and GP03, GP02 and GP03 and less gene exchange between GP01 and GP02 (Fig. 4).
In the analysis of molecular variance (AMOVA), no variants were detected in other populations such as GP01, GP02, and GP03, indicating that these populations have the same genetic basis. Most of the variants were detected within population (91.5%) and 8.5% were found among population (Table 6).
Characteristics of soil nutrients and altitude factors in different populations
Statistical analyses were conducted on soil nutrient content, pH, mean annual temperature (MAT) and mean annual rainfall (MAR) of different species, altitude, rock types, and groups (Table 7). The analysis of variance showed that soil organic matter (SOM), alkaline nitrogen (AN), total phosphorus (TP), available potassium (AK), exchangeable magnesium (Mg), exchangeable calcium (Ca) content, pH and MAR of C. tachangensis were significantly higher compared with those of C. gymnogyna, while the exchangeable aluminum (Al) content and MAT of C. gymnogyna were significantly higher than those of C. tachangensis. The total nitrogen (TN), exchangeable Ca content and pH of H1 were significantly higher than those of H2 and H3, while the exchangeable Al content and MAT were significantly lower compared with those of H2 and H3. The SOM, AN, TP, AK, exchangeable Mg, pH, and MAR of H1 and H2 were significantly higher than those of H3. The SOM, TN, AN, TP, AK, exchangeable Mg, exchangeable Ca, pH, and MAR of CR were significantly higher compared with those of SR, while the contents of AP, exchangeable Al and MAT were significantly lower compared with those of SR. The content of exchangeable Al in soil I was significantly lower than that in soils II, III, and IV, and the content of AK was significantly higher than that in soils II, III, and IV. The SOM, AN, TP, exchangeable Mg, exchangeable Ca and MAR in soils I and II were significantly higher compared with those in soils III and IV. In addition, the SOM, TN and pH of GP02 were significantly higher than those of GP03 and GP01, and the SOM, TN, AN, TP, exchangeable Mg, exchangeable Ca content, pH, and MAR of GP02 and GP03 were significantly higher than those of GP01.
Discussion
Prior research has highlighted the influence of environmental factors on plant genetic differentiation [28,29,30]. In the current investigation, factors such as altitude and geological type emerge as key drivers in the evolutionary process of wild tea plants.
Genetic diversity
In this study, 29,393,327 initial SNPs and 98,241 high-quality SNPs after applying filtering criteria were used to detect the genetic diversity of 159 wild tea plants from the Guizhou Plateau. In our previous report, 1,001,372 initial SNPs and 79,016 high-quality SNPs were obtained from 415 tea plants, which are lower than the high-quality SNPs in our material study under the same filtering conditions [13]. This demonstrates that it is feasible to analyze the genetic diversity and genetic differentiation of 159 wild tea plants with 98,241 high-quality SNPs by the GBS method.
Life-history or geographic traits play an important role in genetic diversity [31]. In general, less genetic diversity exists in an endemic species that is not widely distributed compared with that found in a widespread species [32], usually because their population numbers are limited, and as they are isolated from other populations they adapt to their particular habitat [33]. In this study, the Ho and MAF of the C. gymnogyna population were significantly higher than that of the C. tachangensis population, while Fis of the C. tachangensis population was significantly higher than that for C. gymnogyn, suggesting that there was rich genetic diversity in the C. gymnogyn population. The SOM, AN, TP, AK, exchangeable Mg and Ca contents, and pH of the C. tachangensis population were significantly higher compared with those of the C. gymnogyn population. Previous studies have revealed that C. gymnogyn may have evolved from C. tachangensis [34]. C. tachangensis is relatively primitive, which may adapt to the natural environment with high soil nutrient content, pH and low temperature in Guizhou Plateau for a long time. C. tachangensis has conducted frequent inbreeding, reduced communication with other tea plants, and has low genetic diversity.
Earlier research has uncovered that a suitable environment will promote gene exchange between plants and increase the genetic diversity of plants. The favorable and mild environmental conditions at mid-altitude mean plant populations at this altitude exhibit greater diversity compared with those at low and high altitudes [19, 35, 36]. In this study, genetic diversity was significantly higher for the wild tea population at H2 than for the wild tea populations at H3 and H1. In addition, the genetic diversity was significantly higher for the wild tea populations in the SR Classes compared with those in the CR Classes. Among the four suitable areas, the genetic diversity of wild tea plant populations was significantly higher in area I compared with those in areas III, IV, and II (Table 2). The MAT of H1 was significantly lower than that of H2 and H3, and the SOM, AN, TP and AK contents of H2 were significantly higher than those of H3. The SOM, TN, AN, TP, AK, exchangeable Mg and Ca contents, and pH of CR were significantly higher than those of SR. In addition, the exchangeable Al content of area I was significantly lower compared with that in areas, II, III, and IV. A reasonable explanation for the results of this study is that the low MAT in the H1 areas delayed the flowering time of wild tea plants, hindered the natural hybridization between tea plants, and ultimately reduced the genetic diversity of wild tea plants [37]. In contrast, H2 areas with a suitable temperature and high soil nutrient content promote the natural hybridization of wild tea plants, which accounts for wild tea plants in H2 area exhibiting the highest genetic diversity [38]. The CR region has suitable soil nutrients, but it is possible that wild tea plants have reduced genetic diversity due to long-term adaptation to local suitable environments. Studies have shown that species can adapt to the selection pressure on their own growth by improving their genetic diversity [39, 40]. On the other hand, unfavorable soil or environment in general may kill off a lot of plants in the populations thus reducing the genetic diversity. Our results show that the genetic diversity of the wild tea plant population in I areas is higher than that in III, IV and II areas, and the exchangeable Al in I areas is significantly lower than that in III, IV, and II areas, indicating that the high diversity of wild tea plants in I areas may be more affected by the selection pressure of the low Al soil environment.
In addition, it has been shown that positive Tajima’s D indicated population bottlenecks and/or balancing selection [24]. In our study, positive Tajima’s D values were observed in all populations, suggesting that population bottlenecks and/or balancing selection occurred in wild tea plant populations (Table 2). Fst has been widely used as a measure of population structure. The Fst values for wild tea plant populations of C. tachangensis and C. gymnogyn, H1 and H3, H2 and H3, areas II and III, and areas II and IV were 0.05–0.15, indicating that moderate divergence occurred in wild tea plant populations between C. tachangensis and C. gymnogyn, between H1 and H3, between H2 and H3, between areas II and III, and between areas II and IV [25, 26].
Population structure
Environmental factors, including geological environment, altitude, temperature, rainfall, and light, significantly affect the growth of tea plants. In particular, altitude and geological environment played important roles in the distribution, population structure, and evolutionary direction of germplasm resources of wild plants [41, 42]. Zhao et al. [26] divided cultivated tea plants in the Guizhou Plateau into four groups with reference to the river basin distribution. Our results showed that 159 wild tea plants from the Guizhou Plateau were divided into three groups—two pure groups (GP01 and GP02) and one mixed group (GP03)—and this grouping was consistent with that of the PCA and phylogenetic analysis.
Geological heterogeneity is known to impact species diversity [43]. The mineral content of soil is instrumental in the genetic differentiation of species populations [44]. In this study, the GP01 population was III H3 C. gymnogyn distributed in the SR Classes, while the GP02 and GP03 populations were II H1 C. tachangensis and I H2 C. tachangensis, respectively, distributed in the CR Classes. CR soil is rich in calcium and has a high content of organic matter (calcium-humus), including soil TN, AN, TP, AK and exchangeable Mg, while SR soil is rich in AP and exchangeable Al [45]. We observed higher gene flow, lower genetic distance and genetic differentiation between II H1 C. tachangensis and I H2 C. tachangensis, while the lowest gene flow and the highest genetic distance and genetic differentiation were observed between III H3 C. gymnogyn and II H1 C. tachangensis. Therefore, III H3 C. gymnogyn and II H1 C. tachangensis have the least gene exchange, and they assume different evolutionary directions due to different soil nutrient contents. With the spread of wild tea plants in the Guizhou Plateau, the primitive II H1 C. tachangensis gradually differentiated into III H3 C. gymnogyn in the process of transmission from CR to SR, so as to better adapt to the lower soil nutrients and meet the demand for P and Al. It indicated that rock types and soil nutrients developed from rock played an important role in the genetic differentiation of C. tachangensis and C. gymnogyn.
The pH values of soil developed from different rocks are distinct. The pH of soil developed from rocks with high calcium and magnesium contents was higher than that from rocks with high aluminum content [46, 47]. Moreover, the pH of soil from dolomitic limestone, dolomite, and clastic rock gradually decreased [48]. In this study, the II H1 C. tachangensis population was predominantly distributed in area II of the CR Classes, the I H2 C. tachangensis population was mainly distributed in area I of the CR Classes, and the III H3 C. gymnogyn population was predominantly distributed in area III of the SR Classes. The II H1 C. tachangensis were relatively primitive tea plants. In this study, the II H1 C. tachangensis growing area II has evolved into I H2 C. tachangensis growing area I, and then evolved into the H3 III C. gymnogyn growing area III in the process of communication by spreading. The II H1 C. tachangensis were growing in relatively higher pH soil from dolomitic limestone, the I H2 C. tachangensis were growing in relatively medium pH soil developed from dolomite, and the III H3 C. gymnogyn were growing in relatively lower pH soil from clastic rock. Ca and Mg ions alkalize soil, while Al ions acidify soil [47]. Our observations suggest that high pH II H1 C. tachangensis and medium pH I H2 C. tachangensis have a moderate of differentiation, while high pH II H1 C. tachangensis and low pH III H3 C. gymnogyn have a higher of genetic differentiation. Soil pH plays an important role in the propagation and differentiation of wild tea plants in the Guizhou Plateau and maintains the different evolutionary directions of the plant populations.
The II H1 C. tachangensis populations were mainly distributed at higher altitude with low temperature, the I H2 C. tachangensis populations were predominantly distributed at middle altitudes with high temperature, and the III H3 C. gymnogyn populations were mainly distributed at lower altitudes with high temperature. We observed the highest gene flow and the lowest genetic distance and genetic differentiation between III H3 C. gymnogyn and I H2 C. tachangensis, higher gene flow and lower genetic distance and genetic differentiation between II H1 C. tachangensis and I H2 C. tachangensis, and the lowest gene flow and the highest genetic distance and genetic differentiation between III H3 C. gymnogyn and II H1 C. tachangensis. Thus, the genetic differentiation between III H3 C. gymnogyn and II H1 C. tachangensis is the largest. Due to the difference of temperature at different altitudes, the genetic differentiation between C. tachangensis and C. gymnogyn was caused, which was congruent with previous studies [49,50,51]. Loricaria populations will differentiate from high to low altitude to form distinct genetic populations [52]. In this study, II H1 C. tachangensis distributed in higher altitude areas gradually spread to middle altitude areas, and then evolved into I H2 C. tachangensis under the adaptation of suitable temperature and soil rich in soil nutrients developed by dolomite. Subsequently, continued spreading of the I H2 C. tachangensis to the lower altitude resulted in their evolution into III H3 C. gymnogyn by natural selection on the clastic rock most suitable area to meet the demand for Al. We detected the highest and lowest genetic diversity in I H2 C. tachangensis and II H1 C. tachangensis, respectively. It is possible that the I area at medium altitude may be due to the high content of soil nutrients, suitable temperature, and frequent genetic exchanges among wild tea plants, which have increased their genetic diversity. In contrast, the II area at higher altitude may be suitable for local adaptation of tea plants owing to the lower temperature and rich soil nutrients, thereby reducing genetic diversity of the wild tea plants.
Wang [53] believed that most of the high frequency alleles of the plants bred outside (such as cross-pollinated wind-borne plants) appeared in each population, with high similarity, less differences, and small genetic variation among populations. Our results demonstrate that most of the genetic variation for wild tea plants exists within populations (91.5%) and not among populations (8.5%), which is consistent with previous reports [54, 55]. Tea plant is a woody plant with cross-pollination and a long life. Through continuous cross-pollination, tea plants distributed in the Guizhou Plateau have repeatedly carried out gene exchange between different species and populations, resulting in small differences between populations. This may verify the hypothesis that tea plants in Guizhou Plateau originated from the same species.
Conclusions
The highest genetic diversity was observed for the I H2 C. tachangensis group (GP03) growing in I areas at middle altitude, while the II H1 C. tachangensis group (GP02) growing in the II areas at high altitude had the lowest genetic diversity. In the Guizhou Plateau, the suitable temperature in the middle altitude and the higher nutrient content of soil developed from dolomite promote the formation of high genetic diversity, while the low temperature at high altitude and the high pH of soil developed from limestone with dolomite promote the formation of low genetic diversity. Temperature and, soil nutrients and pH are the main factors affecting the genetic diversity of wild tea plants in the Guizhou Plateau. Findings from this study will facilitate understanding of the adaptive evolutionary characteristics of wild tea plants in the Guizhou Plateau, and will provide reference suggestions for further research on wild tea germplasm resources.
Methods
Plant materials
In total, 159 wild tea germplasm were used in this study (Additional file 1: Table S1). Of these, 101 were identified as Camellia tachangensis (F.C.Zhang) and 58 were Camellia gymnogyna Chang according to the classification system reported by Chen [34] and Min [56]. Based on the combined information of the agricultural climate zoning map of Guizhou Plateau, the suitable tea-planting areas in Guizhou Plateau, and the field survey growth altitude of wild tea plants [57, 58], the altitude of the wild tea-growing areas in Guizhou was divided into three gradients: > 1400 m was divided into the first altitude gradient (H1), 1400–1100 m was the second altitude gradient (H2), and < 1100 m was the third altitude gradient (H3). Among the 159 materials in the study, 39 were located at H1, 65 were located at H2, and 55 were located at H3 (Additional file 2: Fig S1). Based on the stratigraphic regionalization and the development history of the geological environment, the rock type of Guizhou Plateau where tea plants grew was divided into the Carbonate Rock (CR) and Silicate Rock (SR) Classes (Additional file 1: Table S1) [3]. The CR Class contained the Dolomite sub-suitable area (I) and the Dolomitic limestone suitable area (II), while the SR Classes contained the Clastic rock most suitable area (III) and the Purple clastic rock suitable area (IV). Among the 159 tea accessions, 92 were distributed in the CR Classes, including 48 samples from a Dolomite sub-suitable area (I) and 44 samples from a Dolomitic limestone suitable area (II). There were 67 tea accessions in the SR Class, of which 46 samples were from a Clastic rock most suitable area (III) and 21 samples were from a Purple clastic rock suitable area (IV).
Meteorological data of the distribution areas of wild tea germplasm resources were collected from the meteorological departments of all cities and counties in Guizhou Province. Specifically, these data comprised that from meteorological observation stations of all towns and townships, historical meteorological data recorded in the city annals, and the average values of the mean annual temperature (MAT) and mean annual rainfall (MAR) over 5 years (Additional file 1: Table S7). Geographical and altitude information were acquired with GPS, and rock types were judged according to a lithological map of Guizhou Province (Additional file 1: Table S1). The altitude data of Guizhou Plateau was from the geospatial data cloud (http://www.gscloud.cn/sources/index?pid=1&rootid=1) and is mapped using GIS techniques. Professor Suzhen Niu from the Key Laboratory of Plant Resources Conservation and Germplasm Innovation in Mountainous Region, Guizhou University, Ministry of Education, Institute of Agro-Bioengineering, associate professor Jie Yin and lecturer Qinfei Song of the College of Tea Science of Guizhou University, and researcher Zhengwu Chen of the Institute of Tea Science, Guizhou Academy of Agricultural Sciences conducted morphological identification on all sampled tea plants, and stored them in the tea germplasm resource garden of Guizhou University (Additional file 3). This field study and experimental research complied with local legislation, and national and international guidelines. The authors also complied with the Convention on the Trade in Endangered Species of Wild Fauna and Flora and Regulations of Guizhou Province on the protection of ancient tea plants.
DNA extraction, library construction, and sequencing
Genomic DNA was extracted from tea plants using the Rapid Extraction Kit of Plant Genomic DNA (Beijing Biomed Gene Technology Co. Ltd., Beijing, China) according to the manufacturer’s instructions. The genomic DNA was digested with the restriction endonucleases SacI and MseI (5 U; New England Biological Laboratory (NEB), Ipswich, USA), then the adapters “SacAD and MseAD”, which have unique barcodes, were ligated to the DNA fragments. Fragments of 500-–550 bp were selected for amplification and sequenced on an Illumina Hi Seq platform (Illumina, San Diego, CA, USA). The length of the original paired-end sequence is 150 bp [13, 59].
Sequence alignment and SNP identification
Barcodes were used to multiplex raw DNA reads, and the adapters were removed using custom Perl scripts. Only 5 read quality values > were retained. The reads were mapped to the reference genome (http://tpia.TeaPlant.org/) using BWA-MEM v. 0.7.10 (https://sourceforge.net/projects/bio-bwa/files/) with default parameters [26]. SNPs were filtered according to the methods of Niu et al. [13] and McKenna et al. [23]. The SNPs of MAF > 0.05 or the rate of missing data < 20% were saved with VCF tools v. 0.1.160 (https://github.com/vcftools/vcftools) [60]. The SNP density plot was drawn in CM plot v. 3.7.0 (https://rdrr.io/cran/CMplot/) [61]. A total of 98,241 SNPs were selected from 159 tea samples and subsequent analysis was conducted (Additional file 4: Table S1).
Population structure and LD
ADMIXTURE v. 1.30 (http://dalexander.github.io/admixture/download.html) was used to speculate the proportions of admixtures among the wild tea populations, and the number of ancestries (k) was in the range of 1–9. The score threshold was set to 0.8 to distinguish between pure and admixture groups [61]. PCA was conducted in TASSEL v. 5.2.72 (https://tassel.bitbucket.io) [62]. The maximum likelihood (ML) phylogenetic tree was reconstructed by using MEGA v. 10.2.4 (https://www.megasoftware.net/dload_win_gui) to run 500 bootstrap replicates. The phylogenetic tree was displayed by iTOL [63]. Using PopLDdecay v. 3.29 (https://github.com/BGI-shenzhen/PopLDdecay) with default parameters, the LD was calculated statistically based on the correlation coefficient (R2) of the unpruned pairwise SNPs throughout the genome [64]. The VCF file was converted to a pedigree file using VCFtools v. 0.1.160 [64].
Genetic diversity
The genetic diversity indexes included Ho, Pi, MAF, Fis, and Tajima’s D. Ho, MAF, and Fis of each inferred population were calculated using Plink v. 1.90 (https://www.cog-genomics.org/plink2/) [65]. VCFtools was employed to calculate Pi and Tajima’s D of each inferred population and Fst of the paired inferred population [60]. Fst was then used to calculate Nm according to the formula Nm = (1 − Fst)/4Fst [26]. MEGA v. 10.2.4 was used to calculate GD for the pairwise inferred populations. Significant differences between these indexes and the single factor analysis of variance (ANOVA) of soil nutrients in different groups were calculated using SPSS v.25 (IBM Corp., Armonk, NY, USA) [66]. Analysis of molecular variance (AMOVA [67]) was conducted in Arlequin v.2.000 [68] to determine the division of the overall genetic variation at two levels: within the population and between species.
Soil chemical characteristics
In the distribution area of wild tea germplasm resources, select the area with the largest population density of wild tea plants and set it at 600m2 (20 m × 30 m). Next, five points were set on the diagonal of each sample plot, and one soil profile was dug at each point by removing 0–5 cm dead leaves from the surface layer and then collecting 10–60 cm of the soil layer. The soil samples collected at the five points were mixed and impurities and stones were removed. Approximately 1 kg of soil samples were subjected to the quartering method, spread on white paper indoors, and allowed to dry, naturally, then plant roots and stones were picked out. The resulting samples were ground with wooden sticks and passed through a nylon sieve [21]. The soil samples were analyzed for soil organic matter (SOM), total nitrogen (TN), available nitrogen (AN), total phosphorus (TP), available phosphorus (AP), available potassium (AK), exchangeable Ca, exchangeable Al, exchangeable Mg, and pH based on standard methods (Additional file 1: Table S7) [69]. Briefly, SOM was determined by the wet combustion process of potassium dichromate. TN and AN were analyzed by the Kjeldahl method and basic nitrogen diffusion method respectively. TP was determined by the HClO4-H2SO4 method. AK and AP were determined by flame emission spectrometry and the Olsen method, respectively. Exchangeable Ca and exchangeable Mg were measured by continuous source atomic absorption spectrometry. The pH of the soil was tested with a glass electrode (ratio of soil to water = 1:2.5).
Availability of data and materials
The plant materials are growing in our resource nursery and are available from the corresponding author on reasonable request. The raw sequence data reported in this study have been deposited in the Genome Sequence Archive [70] in BIG Data Center, Beijing Institute of Genomics (BIG), Chinese Academy of Sciences, under accession number CRA001438, and are publicly accessible at http://bigd.big.ac.cn/gsa.
Abbreviations
- CV error:
-
Cross-validation error
- Fis:
-
Inbreeding coefficient
- Fst:
-
Differentiation coefficients
- GBS:
-
Genotyping-by-sequencing
- GD:
-
Genetic distance
- GDR:
-
Genetic distance range
- GWAS:
-
Genome-wide association studies
- Ho:
-
Observed heterozygosity
- LD:
-
Linkage disequilibrium
- MAF:
-
Minor allele frequency
- ML tree:
-
Maximum-Likelihood tree
- Nm:
-
Gene flow
- PCA:
-
Principal component analyses
- Pi :
-
Nucleotide diversity
- SNPs:
-
Nucleotide polymorphisms
- C.Tachangensis :
-
Camellia tachangensis (F.C.Zhang)
- C. Gymnogyna :
-
Camellia gymnogyna Chang
- CR:
-
Carbonate Rock
- SR:
-
Silicate Rock
References
Xia EH, Tong W, Wu Q, Wei S, Zhao J, Zhang ZZ, Wei CL, Wan XC. Tea plant genomics: achievements, challenges and perspectives. Hortic Res. 2020;7:7.
Zhang L, Pan JR, Zhu C. Determination of the geographical origin of Chinese teas based on stable carbon and nitrogen isotope ratios. J Zhejiang Univ Sci B. 2012;13(10):824–30.
Bi K. On the Relationship between Tea-leaf Quality and Geological Environments of Guizhou. Guizhou Geol. 1997;02:105–20.
Cheng L, Dong X, Liu Q, Wang R, Li Y, Huang X, Zhao Y. SLAF-Seq Technology-Based Genome-Wide Association and Population Structure Analyses of Ancient Camellia sinensis (L.) Kuntze in Sandu County. Forests. 13(11):1885.
Hongwei ANQS. Study on genetic diversity, population structure and genetic differentiation of tea germplasm in Guizhou. Acta Agriculturae Zhejiangensis. 2021;33(07):1234–43.
Babu KN, Sheeja TE, Minoo D, Rajesh MK, Samsudeen K, Suraby EJ, Kumar IPV. Random Amplified Polymorphic DNA (RAPD) and Derived Techniques. Methods Mol Biol. 2021;2222:219–47.
Danilevicz MF, Tay Fernandez CG, Marsh JI, Bayer PE, Edwards D: High-Throughput Genotyping Technologies in Plant Taxonomy. Methods Mol Biol (Clifton, N.J.) 2021, 2222:149–166.
Tuvesson SD, Larsson CT, Ordon F. Use of Molecular Markers for Doubled Haploid Technology: From Academia to Plant Breeding Companies. Methods Mol Biol. 2021;2288:49–72.
De Donato M, Peters SO, Mitchell SE, Hussain T, Imumorin IG. Genotyping-by-sequencing (GBS): a novel, efficient and cost-effective genotyping method for cattle using next-generation sequencing. PLoS ONE. 2013;8(5): e62137.
Torkamaneh D, Laroche J, Belzile F. Fast-GBS v2.0: an analysis toolkit for genotyping-by-sequencing data. Genome. 2020;63(11):577-81.
Huang X, Wei X, Sang T, Zhao Q, Feng Q, Zhao Y, Li C, Zhu C, Lu T, et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet. 2010;42(11):961-7.
Taranto F, D Agostino N, Greco B, Cardi T, Tripodi P. Genome-wide SNP discovery and population structure analysis in pepper (Capsicum annuum) using genotyping by sequencing. BMC Genomics. 2016;17(1):943.
Niu S, Song Q, Koiwa H, Qiao D, Zhao D, Chen Z, Liu X, Wen X. Genetic diversity, linkage disequilibrium, and population structure analysis of the tea plant (Camellia sinensis) from an origin center, Guizhou plateau, using genome-wide SNPs developed by genotyping-by-sequencing. BMC Plant Biol. 2019;19(1):328.
Gibson M, Moyle LC. Regional differences in the abiotic environment contribute to genomic divergence within a wild tomato species. Mol Ecol. 2020;29(12):2204–17.
Butcher PA, Bradbury D, Krauss SL: Limited pollen-mediated dispersal and partial self-incompatibility in the rare ironstone endemic Tetratheca paynterae subsp. paynterae increase the risks associated with habitat loss. Conserv Genet. 2011; 12(6):1603–1618.
Rao M, Steinbauer MJ, Xiang X, Zhang M, Mi X, Zhang J, Ma K, Svenning J: Environmental and evolutionary drivers of diversity patterns in the tea family (Theaceae s.s.) across China. Ecol Evol. 2018; 8(23):11663–11676.
Liu X, Wang Y, Shen S. Transcriptomic and metabolomic analyses reveal the altitude adaptability and evolution of different-colored flowers in alpineRhododendron species. Tree Physiol. 2022;42(5):1100–13.
Wang X, Gao Y, Wu X, Wen X, Li D, Zhou H, Li Z, Liu B, Wei J, Chen F, et al. High-quality evergreen azalea genome reveals tandem duplication-facilitated low-altitude adaptability and floral scent evolution. Plant Biotechnol J. 2021;19(12):2544–60.
Shen D, Bo W, Xu F, Wu R: Genetic diversity and population structure of the Tibetan poplar (Populus szechuanica var. tibetica) along an altitude gradient. BMC Genet. 2014; 15 Suppl 1(Suppl 1): S11.
Niu S, Zhao Z, Song Q, Chen Z. Eco-environmental diversity of wild tea germplasm resources in Guizhou Province. Zhejiang Agric J. 2020;32(07):1223–32.
Suzhen N, Qinfei S, Hongwei An, et al. Study on diversity of ancient tea germplasm resources in Guizhou based on morphological characteristics. Zhejiang Agric J. 2019;31(10):1689–99.
He L, Yu H, Chen Y, Bai D, Niu S. Characteristics of soil fertility and its influencing factors in the main production areas of Wild tea plants in Guizhou. Mol Plant Breed. 2022:1–17.
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303.
Pandey J, Scheuring DC, Koym JW, Coombs J, Novy RG, Thompson AL, Holm DG, Douches DS, Miller JJ, Vales MI. Genetic diversity and population structure of advanced clones selected over forty years by a potato breeding program in the USA. Sci Rep. 2021;11(1):8344.
Shu G, Cao G, Li N, Wang A, Wei F, Li T, Yi L, Xu Y, Wang Y. Genetic variation and population structure in China summer maize germplasm. Sci Rep. 2021;11(1):8012.
Zhao Z, Song Q, Bai D, Niu S, He Y, Qiao D, Chen Z, Li C, Luo J, Li F. Population structure analysis to explore genetic diversity and geographical distribution characteristics of cultivated-type tea plant in Guizhou Plateau. BMC Plant Biol. 2022;22(1):55.
Ochoa A, Storey JD. Estimating FST and kinship for arbitrary population structures. PLoS Genet. 2021;17(1):e1009241.
Gai Z, Zhai J, Chen X, Jiao P, Zhang S, Sun J, Qin R, Liu H, Wu Z, Li Z: Phylogeography Reveals Geographic and Environmental Factors Driving Genetic Differentiation of Populus sect. Turanga in Northwest China. Front Plant Sci. 2021; 12:705083.
Portella RO, Cordeiro EMG, Marques APS, Ming LC, Zucchi MI, Lima MP, Martins ER, Hantao LW, Sawaya ACHF, Semir J, et al. Evidence of altitudinal gradient modifying genomic and chemical diversity in populations of Lychnophora pinaster Mart. Phytochemistry. 2021;192: 112898.
Ye H, Wang Z, Hou H, Wu J, Gao Y, Han W, Ru W, Sun G, Wang Y. Localized environmental heterogeneity drives the population differentiation of two endangered and endemic Opisthopappus Shih species. BMC Ecol Evol. 2021;21(1):56.
Garnier J, Lafontaine P. Life history traits and dispersal shape neutral genetic diversity in metapopulations. J Math Biol. 2022;84(6):45.
Huh M K, Huh H W. Patterns of genetic diversity and population structure of the clonal herb, Potentilla fragarioides var. sprengeliana (Rosaceae) in Korea. Acta Botanica Sinica. 2000;42(1):64-70.
Barrett SCH, Kohn JR, Falk DA, Holsinger KE. Genetic and evolutionary consequences of small population size in plants: implications for conservation. Genetics Conserv Rare Plants. 1991.
Chen LYFTQ. Discussion on the classification and evolution of tea group plants. Tea Science. 2000;02:89–94.
Taira H. Regeneration system and genetic diversity of Cryptomeria japonica growing at different altitudes. Can J Forest Res. 1997;27(4):447–52.
Tackett M, Berg C, Simmonds T, Lopez O, Brown J, Ruggiero R, Weber J. Breeding system and geospatial variation shape the population genetics of Triodanis perfoliata. Ecol Evol. 2022;12(10):e9382.
Hirao AS, Kudo G. Landscape genetics of alpine-snowbed plants: comparisons along geographic and snowmelt gradients. Heredity (Edinb). 2004;93(3):290–8.
Halbritter AH, Fior S, Keller I, Billeter R, Edwards PJ, Holderegger R, Karrenberg S, Pluess AR, Widmer A, Alexander JM. Trait differentiation and adaptation of plants along elevation gradients. J Evol Biol. 2018;31(6):784–800.
Yang L, Wen K, Ruan X, Zhao Y, Wei F, Wang Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules. 2018;23(4):762.
Audigeos D, Buonamici A, Belkadi L, Rymer P, Boshier D, Scotti-Saintagne C, Vendramin GG, Scotti I. Aquaporins in the wild: natural genetic diversity and selective pressure in the PIP gene family in five Neotropical tree species. BMC Evol Biol. 2010;10:202.
Bemmels JB, Title PO, Ortego J, Knowles LL. Tests of species-specific models reveal the importance of drought in postglacial range shifts of a Mediterranean-climate tree: insights from integrative distributional, demographic and coalescent modelling and ABC model selection. Mol Ecol. 2016;25(19):4889–906.
Yan X, Zhang F. Plant Nutrition Genetics Agricultural Press: Agricultural Publishing House; 1997. pp. 62–9.
Rahbek C, Borregaard MK, Antonelli A, Colwell RK, Holt BG, Nogues-Bravo D, Rasmussen C, Richardson K, Rosing MT, Whittaker RJ, et al. Building mountain biodiversity: Geological and evolutionary processes. Science. 2019;365(6458):1114–9.
Li S, Wang Z, Su Y, Wang T. EST-SSR-based landscape genetics of Pseudotaxus chienii, a tertiary relict conifer endemic to China. Ecol Evol. 2021;11(14):9498–515.
Bai Y. Zhou Y, Zhou X, Zhang C. Differentiating Karst Soil and Soil in Karst Region—a case study of Houzhai River Watershed in Puding County of Guizhou Province. Soils. 2020;52(02):414-20.
Dong LHTLY. Analysis on the differences of main physical and chemical properties of soils developed from different parent materials (rocks) in karst mountain areas. Soil Bulletin. 2008;03:471–4.
Jiang X: Soil Science. Beijing: China Agricultural Publishing House; 2020. pp. 56–63.
Luo M, Zhou Y, Tang F. Study on soil properties of carbonate rock development under different vegetation. Karst in China. 2022:1–16.
Cui D, Tang C, Li J, A X, Yu T, Ma X, Zhang E, Wang Y, Cao G, Xu F et al: Genetic structure and isolation by altitude in rice landraces of Yunnan, China revealed by nucleotide and microsatellite marker polymorphisms. PLOS One. 2017; 12(4):e175731.
Zaborowska J, Łabiszak B, Perry A, Cavers S, Wachowiak W. Candidate Genes for the High-Altitude Adaptations of Two Mountain Pine Taxa. Int J Mol Sci. 2021;22(7):3477.
Zhao RM, Zhang H, An LZ. Thylacospermum caespitosum population structure and cushion species community diversity along an altitudinal gradient. Environ Sci Pollut R. 2018;25(29):28998–9005.
Kolář F, Dušková E, Sklenář P. Niche shifts and range expansions along cordilleras drove diversification in a high-elevation endemic plant genus in the tropical Andes. Mol Ecol. 2016;25(18):4593–610.
Zhong RW. Plant allozyme analysis. China: Science Press; 1996. pp. 103–6.
Yao M, Ma C, Qiao T, et al. Diversity distribution and population structure of tea germplasms in China revealed by EST-SSR markers. Tree Genetics & Genomes. 2012;8(1):205–20.
Zhao D, Yang J, Yang S, Kato K, Luo J. Genetic diversity and domestication origin of tea plant Camellia taliensis (Theaceae) as revealed by microsatellite markers. BMC Plant Biol. 2014;14:14.
Min T. Revision of Camellia sect. Yunnan Bot Res. 1992;02:115–32.
Hu JGXXY. Advantages of climate resources and development suggestions for tea planting in Guizhou. Guizhou Meteorol. 2008;03:19–21.
Zoning CGoGAC. Agroclimatic regionalization in Guizhou province. Guizhou: Guizhou People's Publishing House; 1989. pp. 55–62.
Brown GR, Gill GP, Kuntz RJ, Langley CH, Neale DB. Nucleotide diversity and linkage disequilibrium in loblolly pine. Proc Natl Acad Sci U S A. 2004;101(42):15255–60.
Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, et al. The variant call format and VCFtools. Bioinformatics. 2011;27(15):2156–8.
Muñoz-Pérez JM, Cañas GP, López L, Arias T. Genome-wide diversity analysis to infer population structure and linkage disequilibrium among Colombian coconut germplasm. Sci Rep. 2022;12(1):2958.
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23(19):2633–5.
Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–6.
Zhang C, Dong S, Xu J, He W, Yang T. PopLDdecay: a fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics. 2019;35(10):1786–8.
Slifer, Susan H: PLINK: Key Functions for Data Analysis. Curr Protocols Hum Genetics. 2018; 97(1): e59.
Rosconi F, Rudmann E, Li J, Surujon D, Anthony J, Frank M, Jones DS, Rock C, Rosch JW, Johnston CD, et al. A bacterial pan-genome makes gene essentiality strain-dependent and evolvable. Nat Microbiol. 2022;7(10):1580–92.
Coppi A, Baker AJM, Bettarini I, Colzi I, Echevarria G, Pazzagli L, Gonnelli C, Selvi F. Population Genetics of Odontarrhena (Brassicaceae) from Albania: The Effects of Anthropic Habitat Disturbance, Soil, and Altitude on a Ni-Hyperaccumulator Plant Group from a Major Serpentine Hotspot. Plants (Basel). 2020;9(12):1686.
Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992;131(2):479–91.
Lu RK. Chemical analysis method of agricultural soil. China: China agricultural science press; 2000. pp. 106–7.
Wang Y, Song F, Zhu J, Zhang S, Yang Y, Chen T, et al. GSA: genome sequence archive*. Proteom & Bioinf. 2017;15(1):14–8.
Acknowledgements
We thank College of tea science of Guizhou University for providing research facilities and computing facilities. We thank S.Z. N, J. Y, Q.F. S and Z.W. C for his identification of materials We thank D.C. B for software suggestions in data processing, D.J. H, X.L. D, Y.H. W and Y.J. C for their management of the tea germplasm gardens, and Y.H. H for Provide altitude data of Guizhou Plateau. We thank J. L for her analysis of the earlier data of the article. We thank S.Z. N, X.J. W and X.M. Y for providing suggestions for revising the article.
Funding
This work was funded by Project of the National key R & D plan (2021YFD1200203-1) for design of the study, Project of the National Science Foundation, in RP China (32060700) for design of the study, Project of Guiyang City Science and Technology Plan ([2023] 48-21), Science and Technology Plan Project of Guizhou province, in RP China ([2021] General Project 126), Project of the key field project of Natural Science Foundation of Guizhou Provincial Department of education (KY [2021] 042) for data analysis.
Author information
Authors and Affiliations
Contributions
L.M.H and S.Z.N conceived and supervised the study. J.L analyzed and interpreted the genetic diversity and population structure. D.C.B processed and analyzed the sequencing data. Y.J.C reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We have permission to collect Camellia tachangensis and Camellia gymnogyna are allowed by the Convention on the Trade in Endangered Species of Wild Fauna and Flora and Regulations of Guizhou Province on the protection of ancient tea plants.
Statement specifying permissions
The plants (either cultivated or wild) including the collection of plant material, are complied with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Information of 159 wild tea accessions used in this study. Table S2. The quality control data of 159 wild tea accessions. Table S3. Statistics of Heterozygosity Rate of 98,241 SNPs in 159 wild tea accessions. Table S4. SNP density. Table S5. Statistics of the number and ratio of the accessions of species, rock classes and geologically suitable area in three inferred populations. Table S6. Statistics of the number and ratio of the accessions of altitude gradient in three inferred populations. Table S7. The soil nutrient content and altitude factor of 159 wild tea accessions.
Additional file 2: Figure S1.
Geographic distribution of 159 materials collected at different altitudes and rock types. Note: (A) Geographical position. (B) Distribution map of altitude, rock type and sample point in Guizhou Plateau. Figure S2. Graph for CV error in the range of K=1-9 of 159 wild tea accessions. Figure S3. ML tree of four geologically suitable areas. Note: I Dolomite sub-suitable area, II Dolomitic limestone suitable area, III Clastic rock most suitable area, IV Purple clastic rock suitable area.
Additional file 3: Table S1.
Sampling localities of 159 wild tea accessions.
Additional file 4: Table S1.
Genotyping of 98,241 SNPs based on GBS in 159 wild tea accessions.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, L., Luo, J., Niu, S. et al. Population structure analysis to explore genetic diversity and geographical distribution characteristics of wild tea plant in Guizhou Plateau. BMC Plant Biol 23, 255 (2023). https://doi.org/10.1186/s12870-023-04239-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04239-2