Abstract
Background
In ipecac (Carapichea ipecacuanha (Brot.) L. Andersson), adventitious shoots can be induced simply by placing internodal segments on phytohormone-free culture medium. The shoots form locally on the epidermis of the apical region of the segments, but not the basal region. Levels of endogenous auxin and cytokinin transiently increase in the segments after 1 week of culture.
Results
Here, we conducted RNA-seq analysis to compare gene expression patterns in apical and basal regions of segments before culture and after 1 week of culture for adventitious shoot formation. The results revealed 8987 differentially expressed genes in a de novo assembly of 76,684 genes. Among them, 276 genes were upregulated in the apical region after 1 week of culture relative to before culture and the basal region after 1 week of culture. These genes include 18 phytohormone-response genes and shoot-formation-related genes. Validation of the gene expression by quantitative real-time PCR assay confirmed that the expression patterns were similar to those of the RNA-seq data.
Conclusions
The transcriptome data show that expression of cytokinin biosynthesis genes is induced along with the acquisition of cellular pluripotency and the initiation of cell division by wounding in the apical region of internodal segments, that trigger adventitious shoot formation without callusing.
Similar content being viewed by others
Background
In 1902, Gottlieb Haberlandt proposed that plant cells can divide and differentiate to form the whole plant body [1]. The concept is called ‘totipotency’. Since whole plants were regenerated from carrot tissue segments [2], plant tissue culture has been used as a fundamental technique to regenerate plantlets from plant cells, tissues, or organs isolated from mother plants, on culture media [3]. Nowadays, it is an essential technique for production of virus-free plants, rapid multiplication of rare plant genotypes, genetic transformation, and production of commercially valuable plant-derived secondary metabolites, as well as for basic and applied research in plant science [4]. The balance of exogenously applied auxin and cytokinin (CK) concentrations affects organogenesis: a high ratio of auxin to CK induces adventitious roots, a low ratio induces adventitious shoots, and a high concentration of both hormones induces callus [5]. In general, de novo shoot and root organogenesis require the addition of phytohormones, such as auxin and CK, into the culture media in plant tissue culture [6].
Understanding the molecular mechanism of de novo plant organogenesis is important because it addresses many fundamental questions in developmental biology. So far, the molecular mechanism of plant regeneration has been studied widely in a genetically tractable model plant, Arabidopsis thaliana L. [7]. In the Arabidopsis culture system, phytohormone treatment is critical to inducing plant regeneration via two steps: callus induction of root or hypocotyl segments on auxin-rich medium, and subsequent shoot regeneration from the callus on CK-rich medium. In the first step, auxin promotes callus formation via AUXIN RESPONSE FACTOR (ARF)-mediated activation of LATERAL ORGAN BOUNDARIES DOMAIN proteins (LBDs) [8]. Auxin also promotes the acquisition of cellular pluripotency via activation of LBD16, PLETHORA proteins (PLTs), and CUP-SHAPED COTYLEDON proteins (CUCs) [9, 10]. In the second step, CKs promote shoot meristem formation via ARABIDOPSIS RESPONSE REGULATOR (ARR)-mediated activation of WUSCHEL (WUS) expression [11,12,13,14]. Other transcription factors, SHOOT MERISTEMLESS (STM) and RAP2.6 L, also promote shoot meristem formation independent of CK signaling [15,16,17]. In addition, wounding can induce callus formation and shoot regeneration. Wounding promotes expression of CK biosynthesis genes such as ISOPENTENYL TRANSFERASE 3 (IPT3) and LONELY GUYs (LOGs) [18], and AP2/ERF transcription factors such as WOUND-INDUCED DEDIFFERENTATION 1 (WIND1) and its homologs [19] in callus formation at the wounding site. Expression of these genes activates CK signaling mediated by type-B ARRs, inducing cell division via upregulation of CYCLIN D3;1 (CYCD3;1). Wounding upregulates other AP2/ERF transcription factors, including PLTs [18]. In shoot regeneration, ENHANCER OF SHOOT REGENERATION 1 (ESR1) and ESR2 work downstream of WIND1 and CK signaling [20].
On the other hand, adventitious shoots can be induced by placing explants on culture media without phytohormones in some plant species, such as Dianthus caryophyllus L. [21], Aegle marmelos L. Corrêa [22], Bacopa monnieri (L.) Wettst [23]., Celastrus paniculatus Willd [24]., Kalanchoë blossfeldiana Poelln [25]., and Carapichea ipecacuanha (Brot.) L. Andersson (ipecac) [26]. Ipecac is a medicinal plant that contains alkaloids such as emetine and cephaeline, which are used in expectorants, emetics, and amebicides, in its roots [27, 28]. Adventitious shoot formation of ipecac can be observed 4 weeks after internodal segments are placed on phytohormone-free culture media [26]. This rare characteristic allows us to analyze the dynamics and effects of endogenous phytohormones during adventitious shoot formation, and to easily evaluate the direct effects of exogenously applied chemicals. Adventitious shoots are locally formed on the epidermis of the apical region of internodal segments, but not in basal region [29]. Endogenous levels of indole-3-acetic acid (IAA) are low in the apical region of internodal segments and high in the basal region [29]. These data indicate a negative relation between the position of adventitious shoots formed and IAA distribution in internodal segments. By using inhibitors of polar auxin transport, we found that endogenous IAA is transported from the apical to basal region in the internodal segments, maintaining the IAA levels in the apical region at a low concentration appropriate for adventitious shoot formation [30]. However, the molecular basis for how ipecac cells initiate shoot meristem formation is still undiscovered.
Since the adventitious shoots of ipecac are formed only in the apical region of internode segments, we expect that gene expression patterns differ between the apical and basal regions, so differential expression analysis should reveal the genes that control adventitious shoot formation in ipecac. We consider that excision of the internode section is the first trigger for adventitious shoot formation in ipecac, and cause dynamic gene reprogramming in the section. In addition, endogenous IAA and CKs transiently increase in the internodal segments after 1 week of culture [29]. Thus, we hypothesized that the expression of genes related to adventitious shoot formation is induced in the apical region of internodal segments after 1 week of culture. Here, we conducted RNA-seq analysis to compare gene expression patterns in apical and basal regions of internodal segments before and after 1 week of culture.
Results
Sequence generation by RNA-seq and de novo assembly of transcripts
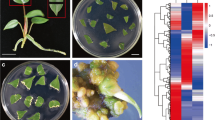
To explore genes controlling adventitious shoot formation in ipecac, we analyzed the RNA-seq transcriptome of internodal segments. RNA was isolated from four sample types of internodal segments: apical region before culture (0a), basal region before culture (0b), apical region after 1 week of culture (1a), and basal region after 1 week of culture (1b) (Fig. 1). RNA of leaves and roots was used as reference. A total of 16 samples (3 each of 0a, 0b, 1a, and 1b; 2 each of leaves and roots) were sequenced by RNA-seq to generate comprehensive transcriptome sequences. Sequencing of the 16 libraries generated 113,423,893 paired-end reads with good quality (Table 1). Multi-dimensional scaling classified gene expression patterns in the segments into three clusters: 0a + 0b, 1a, and 1b (Fig. 2).
Sample preparation for RNA-seq analysis. Internodal segments (5 mm in length) were cut and divided into apical and basal regions before or after 1 week of culture on hormone-free B5 medium. 0a, apical region without culture; 0b, basal region without culture; 1a, apical region after 1 week of culture; 1b, basal region after 1 week of culture. n = 12 in 0a and 0b and 8 in 1a and 1b. Bar, 5 mm
All RNA-seq reads were de novo assembled with Trinity software, resulting in 189,603 contigs that grouped into 76,684 isoform clusters (i.e., unigenes). From the transcriptome sequences, we extracted and selected 62,491 non-redundant (nr) open reading frames (ORFs) with N50 of 1122 bp. The completeness of the predicted ORFs were evaluated using BUSCOs. The ORF sets contained 92.1 and 86.3% of eukaryote and embryophyte BUSCOs (ver. odb9), respectively. All translated sequences of the ORF set were compared with the NCBI nr protein database using BLASTP. Among the ORFs, 35,298 (56.4%) hit protein sequences in the NCBI nr database. The most frequent BLASTP top-hit species was Coffea canephora (20,169, 32.3%), which belongs to the Rubiaceae along with ipecac.
Profiling of differential gene expression in internodal segments
To compare spatiotemporal gene expression patterns in internodal segments, we analyzed abundances of differentially expressed genes (DEGs) mapped on the assembled transcriptome as reference sequences. We compared transcripts of apical and basal regions of internodal segments and transcripts before and after culture in the same internodal region. Among 8987 differentially expressed genes, we found 6 genes upregulated in 0a relative to 0b (0.0096%), 423 in 1a relative to 1b (0.68%), 1927 in 1a relative to 0a (3.1%), and 2249 in 1b relative to 0b (3.6%) (Fig. 3a, b). We found 2 genes downregulated in 0a relative to 0b (0.0032%), 1135 in 1a relative to 1b (1.8%), 1831 in 1a relative to 0a (2.9%), and 1414 in 1b relative to 0b (2.3%) (Fig. 3c, d). To find region-specific genes, we narrowed down the number of genes whose expression changed in 1a and 1b. In 1a, 276 genes were upregulated (defined as “1a-up”) and 274 genes were downregulated (“1a-down”) relative to both 0a and 1b (Fig. 4a, b; Tables S2, S3). In 1b, 715 genes were upregulated (“1b-up”) and 79 genes were downregulated (“1b-down”) relative to both 0b and 1a (Fig. 4c, d; Tables S4, S5).
MA plots of differentially expressed genes (DEGs) between (a) 0a and 0b, (b) 1a and 1b, (c) 1a and 0a, and (d) 1a and 0a. 0a, apical region without culture; 0b, basal region without culture; 1a, apical region after 1 week of culture; 1b, basal region after 1 week of culture. Colored numbers indicate numbers of (red) upregulated and (blue) downregulated genes (log FC > |1|, FDR < 0.01)
Venn diagrams of numbers of DEGs. a Upregulated in 1a relative to 0a and 1b (1a-up). b Downregulated in 1a relative to 0a and 1b (1a-down). c Upregulated in 1b relative to 0b and 1a (1b-up). d Downregulated in 1b relative to 0b and 1a (1b-down). 0b, basal region without culture; 1a, apical region after 1 week of culture; 1b, basal region after 1 week of culture (log FC > |1|, FDR < 0.01)
Enrichment analysis of gene ontology (GO)
Next, we investigated GO terms enriched in each of the 1a-up, 1a-down, 1b-up, and 1b-down groups (Figs. 5, 6). The most enriched GO term in 1a-up was shoot system morphogenesis (GO: 0010016; Fig. 5a). In addition, plant organ formation (GO: 1905393), chromosome organization (GO: 0051276), regulation of cell cycle (GO: 0051726), pattern specification process (GO: 0007389), plant organ morphogenesis (GO: 1905392), and regulation of cell population proliferation (GO: 0042127) were detected as terms associated with organogenesis and cell division. Group 1a-up also included terms related to phytohormone signaling: cytokinin metabolism process (GO: 0009690), response to auxin (GO: 0009733), response to brassinosteroid (GO: 0009741), and hormone-mediated signaling pathway (GO: 0009755). In 1a-down, GO terms related mainly to primary cell wall decomposition and secondary cell wall biosynthesis were enriched: cell wall biogenesis (GO: 0042546), cell wall modification (GO: 0042545), lignin biosynthetic process (GO: 0009809), sterol metabolic process (GO: 0016125), and pectin metabolic process (GO: 0045488) (Fig. 5b). In 1b-up, GO terms related mainly to plant immunity and wounding response were detected: systemic acquired resistance (GO: 0009627), response to bacterium (GO: 0009617), immune system process (GO: 0002376), response to wounding (GO: 0009611), and immune effector process (GO: 0002252) (Fig. 6a). In 1b-down, only two GO terms were detected: post-embryonic plant organ morphogenesis (GO: 0090697) and fatty acid metabolism process (GO: 0006631) (Fig. 6b). These GO terms were all categorized into biological process.
Quantification of DEGs selected in 1a-up by qRT-PCR
To validate the DEG data obtained by RNA-seq, we analyzed 18 genes selected from 1a-up: for auxin metabolism (GRETCHEN HAGEN 3.6 (GH3.6)), auxin signaling (ARF5), CK biosynthesis (IPT3, LOG7), CK metabolism (ADENINE PHOSPHORIBOSYLTRANSFERASE 1 (APT1), APT5, CYTOKININE OXIDASE 6 (CKX6)), CK signaling (Arabidopsis Thaliana HISTIDINE-CONTAINING PHOSPHOTRANSFER PROTEIN 1 (AHP1)), strigolactone (SL) biosynthesis (CYP711A), plastochron control (CYP78A5), cyclin (CYCD3;1), and organogenesis (PLT2, CUC2, ESR2, DOF3.4, LBD18, LBD21, LBD25) (Table 2). The expression of all of these genes was confirmed by qRT-PCR in 0a, 0b, 1a, and 1b of newly collected internodal segments that were different from RNA-seq samples. Expression of all genes was highest in 1a (Fig. 7). However, there was no significant difference among 0a, 0b, 1a, and 1b in APT1 (P = 0.254) or CKX6 (P = 0.268).
Relative expression of phytohormone-response genes and shoot-formation-related genes. Data are means ± SE (n = 4). Eight segments were used in each experiment. EF-1 was used as an internal standard. 0a, apical region without culture; 0b, basal region without culture; 1a, apical region after 1 week of culture; 1b, basal region after 1 week of culture. Different letters indicate significant differences among segments (Tukey’s HSD, P < 0.05)
Discussion
We performed RNA-seq of the apical and basal regions of internodal segments of ipecac before and after 1 week of culture. Our results show that the expression of phytohormone- and organogenesis-related genes increased in the apical region of the segments after 1 week of culture (Fig. 5a), indicating that de novo meristem formation had already started. Here we provide a hypothetical model of gene expression at the early stage of adventitious shoot formation in ipecac based on our RNA-seq data (Fig. 8).
Hypothetical model of adventitious shoot formation in ipecac. In the early stage (after 1 week of culture), phytohormone biosynthesis genes, phytohormone-response genes, and shoot-formation-related genes are expressed in the apical region of internodal segments. The appropriate expression triggers the meristem formation of adventitious shoots in the late stage (after 4 or 5 weeks of culture)
CK biosynthesis starts from binding of dimethyl allyl pyrophosphate and an adenine nucleotide (ATP or ADP) through IPT to form the N6-(∆2-isopentenyl)adenine (2iP) nucleotides [31, 32]. The isoprene side chain of 2iP nucleotides is hydroxylated by CYP735A, resulting in the formation of trans-zeatin (tZ) nucleotides [33]. The 2iP and tZ nucleotides become the bioactive 2iP and tZ through the action of 5′-monophosphate phosphoribohydrolase, encoded by LOG [34, 35]. In internode culture of ipecac in our previous work, amounts of tZ and trans-zeatin riboside greatly increased in the middle region of internodal segments after 1 week of culture, and tZ-type CKs were widely distributed throughout the segments [29]. Thus, we presumed that tZ-type CKs are mainly involved in the induction of ipecac adventitious shoots. However, we found CK biosynthesis genes IPT3 and LOG7 in the 1a-up group, but did not find CYP735A in our gene expression analysis (Table 2; Fig. 7), indicating that the change of 2iP-CK level, but not tZ-type CKs, has an important role in adventitious shoot formation of ipecac. For CK homeostasis, CK metabolism genes such as APT1, APT5, and CKX6 were also induced, but their expression was relatively weak compared with that of CK biosynthesis genes (Table 2; Fig. 7), indicating the activation of CK biosynthesis, rather than CK degradation.
The 1a-up group also included an SL biosynthesis gene, CYP711A, which encodes an enzyme catalyzing oxidation of an SL precursor, carlactone (Table 2; Fig. 7) [36]. Elevation of CYP711A expression in rice produced SLs, which induced CK degradation by CKX9 [37]. Our recent study showed that exogenously applied SL suppressed adventitious shoot formation of ipecac, whereas application of SL biosynthesis inhibitors (TIS108 and KK5) or an antagonist (KK094) stimulated it by inducing biosynthesis of 2iP-type CKs [38]. Because TIS108 and KK5 both inhibited enzymatic activity of CYP711A [39, 40], reduction of SL levels might downregulate CKX expression, allowing endogenous CK levels to increase and thus stimulating adventitious shoot formation. This explanation also supports an idea that a change of 2iP-type CKs levels directly affects adventitious shoot formation of ipecac.
ESR2, DNA-BINDING PROTEIN WITH ONE FACTOR3.4 (DOF3.4), and CYCD3;1 were included in the 1a-up group (Table 2; Fig. 7). Arabidopsis has two ESR genes (ESR1 and ESR2) encoding transcription factors belonging to the AP2/ERF family, and ESR2 mutation decreased adventitious shoot formation more strongly than ESR1 mutation, indicating that ESR2 has a more important role than ESR1 in regulating shoot regeneration [41]. DOF proteins are a family of plant-specific transcription factors with a conserved zinc finger DNA-binding domain. Among the DOF proteins, DOF3.4 (also named OBP1) is induced by ESR2 and can directly bind to the promoter of a D-type cyclin CYCD3;3 (probably also CYCD3;1) during G1/S transition and the action of DOF2.3 in S-phase [42, 43]. CYCD3;1 dominantly promotes the G1/S transition of the cell cycle, and expression of its gene is also regulated by CK signals in the shoot apical meristem [44, 45]. Arabidopsis has three CYCD3 members; CYCD3 is required for normal shoot meristem formation, because Arabidopsis cycd3 triple mutants cannot form adventitious shoots on callus derived from hypocotyls cultured on CK-rich medium [46].
IAA is a major endogenous auxin and is biosynthesized by the conversion of l-Trp to indole-3-pyruvic acid by aminotransferase encoded by TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1 / TRYPTOPHAN AMINOTRANSFERASE RELATED, and the conversion of indole-3-pyruvic acid to IAA through flavin monooxygenase encoded by YUCCA [47, 48]. In ipecac, IAA is transiently produced in the early stage (after 1 week of culture) of adventitious shoot formation [29] and induces expression of LBD genes, PLT2, and CUC2 (Table 2; Fig. 7). Among the 43 Arabidopsis LBDs [49], LBD18 (in particular), LBD21, and LBD25 were upregulated in 1a (Table 2; Fig. 7). Both LBD18 and PLT2 act as regulators of root stem cells to establish pluripotency [50, 51]. However, the pluripotent cells start de novo shoot formation by actions of shoot-formation-promoting factors such as CUC2 and ARF5 (also named MONOPTEROS) [9, 10, 52]. CYP78A5 (also named KLUH) regulates leaf initiation rate and organ size [53, 54]. Activation of CYP78A requires CUC1 and CUC2 expression and counteracts STM in the promotion of meristem activity [55]. STM and RAP2.6 L are also important genes activated in de novo shoot formation, but they are not present in 1a-up. ESR is also thought to regulate the commitment of root cells to shoot differentiation in Arabidopsis, in that ESR2 changes root stem cells induced by PLT2 into shoot meristem cells [56]. In internodal segments of ipecac, IAA is immediately transported from the apical to the basal region through PIN proteins for the homeostasis of endogenous auxin levels [30]. IAA is also inactivated by GH3, which is an acyl amidosynthetase that conjugates amino acids to IAA [57], in the apical region of the segments (Table 2; Fig. 7). Maintenance of low auxin levels in the apical region produces relatively high CK conditions, which might help adventitious shoot formation.
Furthermore, the GO analysis indicates that the biosynthesis of primary and secondary cell walls is suppressed in the apical region of internodal segments after 1 week of culture (Fig. 5b). Cellulose levels and transcripts of secondary cell wall biosynthesis genes were low in the shoot apical meristem [58]. It would be beneficial to reduce cellulose and lignin levels for adventitious meristem formation. In 1b-up, GO terms involved in immune response and wounding response were included (Fig. 6a). When plants are injured physically, they rapidly activate defense responses to protect themselves from pathogenic infections, and they repair the wound site through callus formation [18]. In ipecac, callus is formed at the wound site of the basal region of internodal segments because IAA is accumulated in the basal region [29]. Thus, detection of these GO terms in 1b-up is reasonable, but they were not found in 1a-up (Fig. 6a). In 1b-down, terms for post-embryonic plant organ morphogenesis were detected (Fig. 6b), indicating conditions in which it is difficult to form adventitious shoots.
In ipecac, adventitious shoot formation is observed in apical region of internodal segments but not in basal region. In the apical region, endogenous CK levels are increased by the expression of CK biosynthesis genes such as IPT3 and LOG7, IAA inactivation is induced by GH3 expression, and IAA is transported to basal region by polar auxin transport (Koike et al. 2020), inducing the conditions with a low auxin-to-CK ratio in the apical region. It probably triggers the expression of genes associated with shoot system morphogenesis and plant organ formation. In contrast, highly accumulated IAA suppresses CK biosynthesis and embryonic plant organ morphogenesis in the basal region, resulting in suppression of adventitious shoot formation.
Conclusion
Ipecac can form adventitious shoots only in the apical region of internodal segments without callusing on phytohormone-free medium. We performed RNA-seq analysis to understand gene expression patterns in the early stage of de novo shoot regeneration. Expression patterns during adventitious shoot formation of ipecac were similar to those of shoot regeneration on callus in Arabidopsis. However, not all events showed the same patterns. We propose that CK biosynthesis, acquisition of cellular pluripotency, and initiation of cell division spontaneously start at almost the same time following wounding, and elevated endogenous CKs induce adventitious shoot formation without callusing on internodal segments. When we compare endogenous phytohormone levels in some plant species, auxin levels are lower and CK levels are higher in ipecac than in Arabidopsis, rapeseed, and tomato, which cannot form adventitious shoots without phytohormone treatment [18, 38, 59, 60]. Thus, endogenous CKs (especially 2iP-type CK) is a key regulator of adventitious shoot formation of ipecac. Although transcription of IPT and LOG is well known to be regulated in response to nitrate ion levels [61], wounding-inducible transcription factors are still unknown. In the future, it will be important to elucidate the mechanism of transcription of CK biosynthesis genes induced by wounding in ipecac.
Materials and methods
Plant materials and culture condition
We used the ipecac culture system established by Yoshimatsu and Shimomura [26]. Sterile plants propagated from shoot tips, nodes, and internodes were maintained at Toyo University. The explants were placed on phytohormone-free B5 culture medium (25 mL) [62] solidified with 0.2% Gelrite in a Petri dish (90 mm × 20 mm) and cultured at 24 °C under a 14-h light / 10-h dark photoperiod (10–20 μmol photons m− 2 s− 1). Internodal segments (5 mm length) were divided into two sections (apical and basal regions, 2.5 mm each) before culture (12 samples/set) or after 1 week of culture (8 samples/set) on hormone-free B5 medium for RNA-seq analysis (Fig. 1). Leaves and roots were also collected from sterile plants as reference samples. All experiments were carried out in a completely randomized design.
RNA isolation and transcriptome sequencing
The samples for RNA-seq were frozen in liquid nitrogen and crushed with a mortar and pestle. Total RNA was isolated by using an RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) following the supplied instructions. RNA quantity was measured with a Nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), a Qubit fluorometer (Thermo Fisher Scientific), and a 2100 Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA, USA), and an RNA integrity number was determined with the 2100 Bioanalyzer. cDNA libraries were prepared according to the Low Sample Protocol of the Illumina TruSeq Stranded mRNA Sample Preparation Guide (Illumina, San Diego, CA, USA). RNA was diluted with RNase-free ultrapure water to 40 ng μL− 1. The quality of purified cDNA libraries was confirmed on the 2100 Bioanalyzer. The quantity of purified cDNA was determined by quantitative real-time PCR (qRT-PCR) according to the Kapa Library Quantification Kit platform (Illumina) using a 7500 Real-Time PCR System (Thermo Fisher Scientific). Paired-end reads (106 bp length) were sequenced on a HighSeq 1500 system (Illumina) in rapid-run mode. Sequenced reads and assembled sequences are registered with the DNA Data Bank of Japan (DDBJ).
Analysis of differential gene expression and gene ontology (GO)
By using Cutadapt v. 2.3 software [63], low-quality sequences (quality value < 30) and adapter sequences were trimmed and reads shorter than 25 bp were filtered out. The quality of trimmed reads was evaluated with FastQC v. 0.11.9 software (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). To build a reference transcript, we performed de novo assembly by using Trinity v. 2.6.5 software [64, 65]. Open reading frames (ORFs) on de novo assembly sequences were predicted by TransDecoder. The resulting assembly was evaluated in Benchmarking Universal Single-Copy Ortholog (BUSCO) v. 3 software using the eukaryota_odb9 and embryophyta_odb9 datasets [66]. The de novo assembly of the transcripts is available in supplemental information. The trimmed reads were mapped to the reference transcripts with Bowtie2 v. 2.3.5.1 software [67], and the abundance was estimated with eXpress v. 1.5.1 software [68]. Differential expression was analyzed with the edgeR v. 3.26.8 package [69] in R v. 3.6.1 software using the “trimmed mean of M values” method [70] to calculate normalization values. We defined DEGs with log fold-change (FC) > |1| and false discovery rate (FDR) < 0.01.
GO is a unification tool used to describe the properties of an organism’s genes and their products, categorized into biological processes, cellular components, and molecular functions [71]. Sequences were functionally annotated in OmicsBox v. 1.4 Blast2GO software (BioBam, Spain, Valencia) [72]. GO enrichment analysis was performed in Metascape v. 3.5 software [73] using Arabidopsis gene IDs obtained from the Uniprot database (https://www.uniprot.org/).
Validation for RNA-seq using quantitative real-time reverse-transcription PCR
Internodal segments (8 samples/set; Fig. 1) were placed in a 2.0-mL tube with a zirconia bead (diameter, 5 mm), frozen in liquid nitrogen, and crushed in a TissueLyser II (Qiagen). Total RNA was extracted with an RNeasy Plant Mini Kit (Qiagen) following the supplied instructions. cDNA was synthesized from 100 ng of total RNA using ReverTra Ace qPCR RT Master Mix (Toyobo, Osaka, Japan). Transcript levels were determined by qPCR using a Thunderbird NEXT SYBR qPCR Mix kit (Toyobo). qRT-PCR was performed with a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific). The expression of ELONGATION FACTOR1 (EF1) was used as a reference. The primer sets used are listed in Table S1. Statistical analysis of relative expression was carried out in IBM SPSS Statistics 26.0 software (IBM SPSS Inc., Armonk, NY, USA). Following assessment of the equality of variances by ANOVA, multiple comparison was conducted by Tukey’s honestly significant difference (Tukey’s HSD). P values less than 0.05 were considered statistically significant.
Availability of data and materials
New illumina sequence reads generated during the current study are available from DDBJ Sequence Read Archive (DRA) under accession number DRA013731 (https://ddbj.nig.ac.jp/resource/sra-submission/DRA013731). Other data are included in this published article and its supplementary information files.
Abbreviations
- 2iP:
-
N 6-(∆2-isopentenyl)adenine
- AHP:
-
Arabidopsis thaliana histidine-containing phosphotransfer protein
- ARF:
-
Auxin response factor
- APT:
-
Adenine phosphoribosyltransferase
- ARR:
-
Arabidopsis response regulator
- CK:
-
Cytokinin
- CKX6:
-
Cytokinine oxidase 6
- CUC:
-
Cup-shaped cotyledon
- CYCD3;1:
-
Cyclin D3;1
- DEG:
-
Differentially expressed gene
- DOF:
-
DNA-binding protein with one factor
- EF1:
-
Elongation factor 1
- ESR1:
-
Enhancer of shoot regeneration 1
- FC:
-
Fold-change
- GH3:
-
Gretchen hagen 3
- GO:
-
Gene ontology
- IAA:
-
Indole-3-acetic acid
- IPT3:
-
Isopentenyl transferase 3
- LBD:
-
Lateral organ boundaries domain
- LOG:
-
Lonely guy
- nr:
-
Non-redundant
- ORF:
-
Open reading frame
- PLT:
-
Plethora
- qRT-PCR:
-
Quantitative real-time PCR
- WUS:
-
Wuschel
- STM:
-
Shoot meristemless
- SL:
-
Strigolactone
- tZ:
-
Trans-zeatin
- WIND1:
-
Wound-induced dedifferentation 1
References
Haberlandt G. Kulturversuche mit isolierten Pflanzenzellen. Sitzungsber Math-Naturwiss Kl Akad Wiss Wien. 1902;111:69–92.
Steward FC, Mapes MO, Mears K. Growth and organized development of cultured cells. II. Organization in cultures grown from freely suspended cell. Am J Bot. 1958;45(10):705–8.
George EF. Plant tissue culture procedure - background. In: George EF, Hall MA, De Klerk GJ, editors. Plant propagation by tissue culture, vol. 1. 3rd ed. Dordrecht: Springer; 2008. p. 1–28.
Espinosa-Leal CA, Puente-Garza CA, García-Lara S. In vitro plant tissue culture: means for production of biological active compounds. Planta. 2018;248(1):1–18.
Skoog F, Miller CO. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–30.
Raspor M, Motyka V, Kaleri AR, Ninković S, Tubić L, Cingel A, Ćosić T. Integrating the roles for cytokinin and auxin in de novo shoot organogenesis: from hormone uptake to signaling outputs. Int J Mol Sci. 2021;22(16):8554.
Ikeuchi M, Favero DS, Sakamoto Y, Iwase A, Coleman D, Rymen B, et al. Molecular mechanisms of plant regeneration. Annu Rev Plant Biol. 2019;70(1):377–406.
Fan M, Xu C, Xu K, Hu Y. LATERAL ORGAN BOUNDARIES DOMAIN transcription factors direct callus formation in Arabidopsis regeneration. Cell Res. 2012;22(7):1169–80.
Daimon Y, Takabe K, Tasaka M. The CUP-SHAPED COTYLEDON genes promote adventitious shoot formation on calli. Plant Cell Physiol. 2003;44(2):113–21.
Kareem A, Durgaprasad K, Sugimoto K, Du Y, Pulianmackal Ajai J, Trivedi Zankhana B, et al. PLETHORA genes control regeneration by a two-step mechanism. Curr Biol. 2015;25(8):1017–30.
Meng WJ, Cheng ZJ, Sang YL, Zhang MM, Rong XF, Wang ZW, et al. Type-B ARABIDOPSIS RESPONSE REGULATORs specify the shoot stem cell niche by dual regulation of WUSCHEL. Plant Cell. 2017;29(6):1357–72.
Wang J, Tian C, Zhang C, Shi B, Cao X, Zhang T-Q, et al. Cytokinin signaling activates WUSCHEL expression during axillary meristem initiation. Plant Cell. 2017;29(6):1373–87.
Zhang T-Q, Lian H, Zhou C-M, Xu L, Jiao Y, Wang J-W. A two-step model for de novo activation of WUSCHEL during plant shoot regeneration. Plant Cell. 2017;29(5):1073–87.
Zubo YO, Blakley IC, Yamburenko MV, Worthen JM, Street IH, Franco-Zorrilla JM, et al. Cytokinin induces genome-wide binding of the type-B response regulator ARR10 to regulate growth and development in Arabidopsis. Proc Natl Acad Sci U S A. 2017;114(29):E5995–6004.
Shi B, Zhang C, Tian C, Wang J, Wang Q, Xu T, et al. Two-step regulation of a meristematic cell population acting in shoot branching in Arabidopsis. PLoS Genet. 2016;12(7):e1006168.
Che P, Lall S, Nettleton D, Howell SH. Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture. Plant Physiol. 2006;141(2):620–37.
Yang S, Poretska O, Sieberer T. ALTERED MERISTEM PROGRAM1 restricts shoot meristem proliferation and regeneration by limiting HD-ZIP III-mediated expression of RAP2.6L. Plant Physiol. 2018;177(4):1580–94.
Ikeuchi M, Iwase A, Rymen B, Lambolez A, Kojima M, Takebayashi Y, et al. Wounding triggers callus formation via dynamic hormonal and transcriptional changes. Plant Physiol. 2017;175(3):1158–74.
Iwase A, Mitsuda N, Koyama T, Hiratsu K, Kojima M, Arai T, et al. The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr Biol. 2011;21(6):508–14.
Iwase A, Harashima H, Ikeuchi M, Rymen B, Ohnuma M, Komaki S, et al. WIND1 promotes shoot regeneration through transcriptional activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis. Plant Cell. 2017;29(1):54–69.
Watad AA, Ahroni A, Zuker A, Shejtman H, Nissim A, Vainstein A. Adventitious shoot formation from carnation stem segments: a comparison of different culture procedures. Scientia Hortic. 1996;65(4):313–20.
Ajithkumar D, Seeni S. Rapid clonal multiplication through in vitro axillary shoot proliferation of Aegle marmelos (L.) Corr., a medicinal tree. Plant Cell Rep. 1998;17(5):422–6.
Tiwari V, Tiwari KN, Singh BD. Comparative studies of cytokinins on in vitro propagation of Bacopa monniera. Plant Cell Tissue Organ Cult. 2001;66(1):9–16.
Rao MS, Purohit SD. In vitro shoot bud differentiation and plantlet regeneration in Celastrus paniculatus Willd. Biol Plant. 2006;50(4):501–6.
Sanikhani M, Frello S, Serek M. TDZ induces shoot regeneration in various Kalanchoë blossfeldiana Poelln. Cultivars in the absence of auxin. Plant Cell Tissue Organ Cult. 2006;85(1):75–82.
Yoshimatsu K, Shimomura K. Efficient shoot formation on internodal segments and alkaloid formation in the regenerates of Cephaelis ipecacuanha A. Richard. Plant Cell Rep. 1991;9(10):567–70.
Chatterjee SK, Nandi RP, Ghosh NC. Cultivation and utilization of ipecac in west Bengal. In: Atal CK, Kapur BM, editors. Cultivation and utilization of medicinal plants. Jammu-Tawi: Regional Research Laboratory, Council of Scientific and Industrial Research; 1982. p. 295–301.
Trease GE, Evans WC. Phamacognosy. 13th ed. London: Bailliere Tindall; 1989.
Koike I, Taniguchi K, Shimomura K, Umehara M. Dynamics of endogenous indole-3-acetic acid and cytokinins during adventitious shoot formation in ipecac. J Plant Growth Regul. 2017;36(4):805–13.
Koike I, Watanabe S, Okazaki K, Hayashi KI, Kasahara H, Shimomura K, et al. Endogenous auxin determines the pattern of adventitious shoot formation on internodal segments of ipecac. Planta. 2020;251(3):73.
Kakimoto T. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate:ATP/ADP isopentenyltransferases. Plant Cell Physiol. 2001;42(7):677–85.
Takei K, Sakakibara H, Sugiyama T. Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J Biol Chem. 2001;276(28):26405–10.
Takei K, Yamaya T, Sakakibara H. Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-Zeatin. J Biol Chem. 2004;279(40):41866–72.
Kuroha T, Tokunaga H, Kojima M, Ueda N, Ishida T, Nagawa S, et al. Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. Plant Cell. 2009;21(10):3152–69.
Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, et al. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature. 2007;445(7128):652–5.
Yoneyama K, Mori N, Sato T, Yoda A, Xie X, Okamoto M, et al. Conversion of carlactone to carlactonoic acid is a conserved function of MAX1 homologs in strigolactone biosynthesis. New Phytol. 2018;218(4):1522–33.
Duan J, Yu H, Yuan K, Liao Z, Meng X, Jing Y, et al. Strigolactone promotes cytokinin degradation through transcriptional activation of CYTOKININ OXIDASE/DEHYDROGENASE 9 in rice. Proc Natl Acad Sci U S A. 2019;116(28):14319–24.
Okazaki K, Watanabe S, Koike I, Kawada K, Ito S, Nakamura H, et al. Strigolactone signaling inhibition increases adventitious shoot formation on internodal segments of ipecac. Planta. 2021;253(6):123.
Ito S, Umehara M, Hanada A, Kitahata N, Hayase H, Yamaguchi S, et al. Effects of triazole derivatives on strigolactone levels and growth retardation in rice. PLoS One. 2011;6(7):e21723.
Kawada K, Takahashi I, Arai M, Sasaki Y, Asami T, Yajima S, et al. Synthesis and biological evaluation of novel triazole derivatives as strigolactone biosynthesis inhibitors. J Agric Food Chem. 2019;67(22):6143–9.
Matsuo N, Makino M, Banno H. Arabidopsis ENHANCER OF SHOOT REGENERATION (ESR)1 and ESR2 regulate in vitro shoot regeneration and their expressions are differentially regulated. Plant Sci. 2011;181(1):39–46.
Skirycz A, Radziejwoski A, Busch W, Hannah MA, Czeszejko J, Kwaśniewski M, et al. The DOF transcription factor OBP1 is involved in cell cycle regulation in Arabidopsis thaliana. Plant J. 2008;56(5):779–92.
Moghaddas Sani H, Hamzeh-Mivehroud M, Silva AP, Walshe JL, Mohammadi SA, Rahbar-Shahrouziasl M, et al. Expression, purification and DNA-binding properties of zinc finger domains of DOF proteins from Arabidopsis thaliana. Bioimpacts. 2018;8(3):167–76.
Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JAH. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science. 1999;283(5407):1541–4.
Menges M, Samland AK. Planchais Sv, Murray JAH: the D-type cyclin CYCD3;1 is limiting for the G1-to-S-phase transition in Arabidopsis. Plant Cell. 2006;18(4):893–906.
Dewitte W, Scofield S, Alcasabas AA, Maughan SC, Menges M, Braun N, et al. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc Natl Acad Sci U S A. 2007;104(36):14537–42.
Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, et al. The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci U S A. 2011;108(45):18512–7.
Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, et al. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc Natl Acad Sci U S A. 2011;108(45):18518–23.
Shuai B, Reynaga-Peña CG, Springer PS. The lateral organ Boundaries gene defines a novel, plant-specific gene family. Plant Physiol. 2002;129(2):747–61.
Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, et al. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119(1):109–20.
Lee HW, Kim NY, Lee DJ, Kim J. LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol. 2009;151(3):1377–89.
Ckurshumova W, Smirnova T, Marcos D, Zayed Y, Berleth T. Irrepressible MONOPTEROS/ARF5 promotes de novo shoot formation. New Phytol. 2014;204(3):556–66.
Anastasiou E, Kenz S, Gerstung M, MacLean D, Timmer J, Fleck C, et al. Control of plant organ size by KLUH/CYP78A5-dependent intercellular signaling. Dev Cell. 2007;13(6):843–56.
Wang J-W, Schwab R, Czech B, Mica E, Weigel D. Dual effects of miR156-targeted SPL genes and CYP78A5/KLUH on plastochron length and organ size in Arabidopsis thaliana. Plant Cell. 2008;20(5):1231–43.
Aida M, Tsubakimoto Y, Shimizu S, Ogisu H, Kamiya M, Iwamoto R, et al. Establishment of the embryonic shoot meristem involves activation of two classes of genes with opposing functions for meristem activities. Int J Mol Sci. 2020;21(16):5864.
Banno H, Ikeda Y, Niu Q-W, Chua N-H. Overexpression of Arabidopsis ESR1 induces initiation of shoot regeneration. Plant Cell. 2001;13(12):2609–18.
Staswick PE, Serban B, Rowe M, Tiryaki I. Maldonado MnT, Maldonado MC, Suza W: characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell. 2005;17(2):616–27.
Yang W, Schuster C, Beahan CT, Charoensawan V, Peaucelle A, Bacic A, et al. Regulation of meristem morphogenesis by cell wall synthases in Arabidopsis. Curr Biol. 2016;26(11):1404–15.
Iwase A, Mita K, Favero DS, Mitsuda N, Sasaki R, Kobayshi M, et al. WIND1 induces dynamic metabolomic reprogramming during regeneration in Brassica napus. Dev Biol. 2018;442(1):40–52.
Larriba E, Sánchez-García AB, Justamante MS, Martínez-Andújar C, Albacete A, Pérez-Pérez JM. Dynamic hormone gradients regulate wound-induced de novo organ formation in tomato hypocotyl explants. Int J Mol Sci. 2021;22(21):11843.
Maeda Y, Konishi M, Kiba T, Sakuraba Y, Sawaki N, Kurai T, et al. A NIGT1-centred transcriptional cascade regulates nitrate signalling and incorporates phosphorus starvation signals in Arabidopsis. Nat Commun. 2018;9(1):1376.
Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50(1):151–8.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet journal. 2011;17(1):10–2.
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–52.
Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, et al. De novo transcript sequence reconstruction from RNA-seq using the trinity platform for reference generation and analysis. Nat Protoc. 2013;8(8):1494–512.
Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–2.
Langmead B, Salzberg SL. Fast gapped-read alignment with bowtie 2. Nat Methods. 2012;9(4):357–9.
Roberts A, Pachter L. Streaming fragment assignment for real-time analysis of sequencing experiments. Nat Methods. 2013;10(1):71–3.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26(1):139–40.
Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11(3):R25.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–9.
Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–6.
Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523.
Acknowledgements
We thank Ms. Miwako Matsumoto and Mr. Takahiro Bino, National Institute for Basic Biology (NIBB), for their technical support in NGS sequencing. We thank Prof. Hirobumi Yamamoto and Dr. Genki Horiguchi, Toyo University, for their constructive comments on this study.
Funding
This study was in part supported by the Inoue Enryo Memorial Foundation for Promoting Science from Toyo University to KO, the Sasagawa Scientific Research Grant from The Japan Science Society to KO (2022–4085), JST SPRING to KO (JPMJSP2159), the 30th and 31st Botanical Research Grant of Ichimura Foundation for New Technology to MU, and the NIBB Collaborative Research Program (Nos. 19–416, 20–418, 21–313, 22NIBB408) to MU.
Author information
Authors and Affiliations
Contributions
KO, IK, and MU designed and coordinated the research. KS provided sterile ipecac plants. KO and IK conducted the ipecac tissue culture and library preparation for NGS sequencing. KY and SS handled NGS sequencing, de novo assembly, and sequence annotation. KO conducted DEG analysis, GO enrichment analysis, and validation of RNA-seq data by qRT-PCR. SK supported the qRT-PCR analysis. KO and MU analyzed all the data. KO, KS, and MU wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In 2001, regenerated plants of ipecac were kindly provided from the Tsukuba Division, Research Center for Medicinal Plant Resources, National Institutes of Biomedical Innovation, Health and Nutrition in Japan. Ipecac is not a wild or endangered plant. The plant materials collection was carried out with permission of the Tsukuba Division, Research Center for Medicinal Plant Resources, National Institutes of Biomedical Innovation, Health and Nutrition, and complied with national or international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Primer list for qRT-PCR. Table S2. Genes upregulated in apical region of internodal segments. Table S3. Genes downregulated in apical region of internodal segments. Table S4. Genes upregulated in basal region of internodal segments. Table S5. Genes downregulated in basal region of internodal segments.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Okazaki, K., Koike, I., Kera, S. et al. Gene expression profiling before and after internode culture for adventitious shoot formation in ipecac. BMC Plant Biol 22, 361 (2022). https://doi.org/10.1186/s12870-022-03756-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03756-w