Abstract
Background
As a vital osmoticum, proline has an important role in enhancing the tolerance of plants to environmental stress. It is unclear whether the application of exogenous proline can improve the tolerance of Brassica juncea to cadmium (Cd).
Results
This study investigated the effects of different concentrations of proline (20, 40, 60, 80, and 100 mg/L) under Cd stress at different times (0 d, 2 d, and 7 d) on the growth and physiology of B. juncea. Treatment with exogenous proline not only increased the content of proline in B. juncea but also alleviated Cd-induced seedling growth inhibition via the maintenance of higher photosynthetic pigment content and cell viability and a decrease in the content of Cd. Moreover, it increased the activities of antioxidant enzymes and the glutathione/glutathione disulfide ratio to reduce the accumulation of reactive oxygen species. Compared with other concentrations, 60 mg/L of exogenous proline was the most effective at mitigating Cd toxicity in B. juncea.
Conclusions
Exogenous proline treatment enhanced the tolerance to Cd via a decrease in Cd accumulation and reestablishment of the redox homeostasis in B. juncea.
Similar content being viewed by others
Background
Cadmium (Cd) is a toxic metal element, which is difficult to degrade [1]. It is one of the most aggressive and persistent heavy metals in the natural environment. The dangers of Cd include two aspects: (1) Cd reduces the yield by interfering with the lifecycle of the plant; and (2) Cd is easily absorbed and accumulated by plants, which enables it to enter the food chain and deleteriously affects animals and humans [2]. The National Soil Pollution Status Survey Bulletin shows that the rate of Cd in China exceeded 7.0%, ranking it first among inorganic pollutants [3]. Cd can hinder plant growth, inhibit chlorophyll synthesis, and decrease plant biomass, gas exchange attributes, nitrogen utilization, and the activities of assimilation enzymes, as well as interfere with the antioxidant systems [4,5,6,7].
A burst of reactive oxygen species (ROS) is induced in plants under abiotic stresses, thereby hindering their normal growth and development [8, 9]. Plants protect the cell mechanisms against oxidative stress by accumulating osmotic compounds. Proline is the most common osmolyte, which stabilizes the osmotic difference between the extracellular environment and the cytoplasm [10]. In addition, it can protect plant cells from oxidative stress by inhibiting the production of ROS [11]. The accumulation of proline is regarded as an adaptive strategy in response to heavy metal stress in plants [12, 13]. It has been reported that treatment with proline significantly reduced the loss of photosynthetic pigments and counteracted mercury (Hg)-triggered oxidative stress in coriander (Coriandrum sativum L.). seedlings [14]. Proline can reduce the lipid peroxidation of rice (Oryza sativa) seedlings under Cr (VI) stress, which manifests as a decrease in the content of malondialdehyde (MDA) and root cell activity [15]. The foliar spraying of proline significantly improved the growth, net photosynthetic rate (Pn), the content of chlorophyll, leaf carbonic anhydrase activity, and quantum yield of photosystem II, as well as the activities of antioxidant enzymes in Brassica juncea [16]. Exogenous proline alleviated the adverse effects of Cd on date palm (Phoenix dactylifera L.) and olive (Olea europaea L. cv. Chemlali). It reduced the oxidative damage caused by Cd accumulation, established a better level of plant growth, water status, and photosynthetic activity, and resulted in higher activities of antioxidant enzymes in the roots and leaves [17, 18].
B. juncea has been identified as a metal accumulator that is ecologically and economically important, and it is a large biomass crop plant within the Brassicaceae family [19]. Increasing amounts of evidence suggest that it has a remarkable capacity to take up a large number of Cd molecules from soils contaminated with Cd [20, 21]. However, whether treatment with exogenous proline alleviates the toxicity of Cd in B. juncea remains unknown. Thus, the aim of this study was to investigate the effects of exogenous proline on plant growth, biomass, contents of Cd and proline, cell viability, the activities of antioxidant enzymes, including superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), catalase (CAT), and the contents of non-enzymatic products, including chlorophyll, glutathione (GSH), and GSH/glutathione disulfide (GSSG), by exploring the potential ameliorative efficacy of proline on Cd toxicity in B. juncea.
Results
Effects of exogenous proline on the endogenous proline content in seedlings
The application of exogenous proline significantly increased (P < 0.05) the content of endogenous proline in B. juncea plants stressed with Cd (Fig. 1). At an exogenous proline concentration of 60 mg/L, the increase in endogenous proline content was the most significant in B. juncea leaves and roots. The plants were subjected to Cd stress for 2 d and 7 d, and the concentration of endogenous proline increased 2.8- and 2.4-fold in the leaves, whereas it increased 4.5- and 3.7- fold in the roots compared with the control group, respectively.
Effect of exogenous proline on the proline content in leaves (A) and roots (B) of Brassica juncea seedlings under Cd stress. All the analyses were performed with three replicates. Error bars represent the standard deviations (SD). The different letters in the group indicate significantly different values between treatments (P < 0.05)
Effect of exogenous proline on plant growth
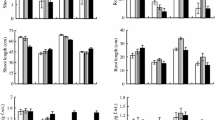
As shown in Fig. 2 A, B, and E, supplementation with exogenous proline mitigated the inhibitory effect of Cd stress at different times on the plant height and root length. The plants treated with 60 and 80 mg/L proline grew remarkably higher (P < 0.05) compared with those in the control group following Cd treatment for 7 days, whereas the root length of plants treated with 40, 60, and 80 mg/L proline increased significantly (P < 0.05). The 60 mg/L treatment had the most obvious effect on the growth of Cd-stressed B. juncea seedlings, which increased by 38.4% and 36.79% in plant height and root length, respectively, compared with the control group.
Effect of exogenous proline on the plant height (A), root length (B), shoot dry weight (C), root dry weight (D), and plants (E) of Brassica juncea under Cd stress. All the analyses were performed with three replicates. Error bars represent the standard deviations (SD). The different letters in the group indicate significantly different values between treatments (P < 0.05)
Figure 2 C and D show that treatment with exogenous proline ameliorated the loss of shoot and root dry weight under Cd stress at 2 d and 7 d, respectively. In comparison with the control plants, the treatment of plants that had been subjected to 2 d of Cd stress with exogenous proline (40, 60, and 80 mg/L) significantly alleviated the reduction in shoot dry weight (P < 0.05). After Cd treatment for 7 days, the shoot and the root dry weights of B. juncea seedlings were the most clearly affected after 40 mg/L and 60 mg/L proline treatment, respectively (P < 0.05). Compared with the control group, the shoot and root dry weight increased by 46.69% and 119.58%, respectively. Exogenous proline significantly increased the plant height, root length, and shoot and root dry weight, indicating that it alleviated the stress of Cd on the growth of B. juncea seedlings.
As shown in Fig. S1, the plant height and dry weight of the seedlings treated solely with 60 mg/L proline for 7 days were higher than those of the control. This illustrates that 60 mg/L proline is also beneficial for the normal growth of B. juncea.
Effects of exogenous proline on chlorophyll and carotenoid contents, net photosynthetic rate, stomatal conductance, intercellular carbon dioxide concentration, and transpiration rate
As shown in Fig. 3, with the increase of exogenous proline, similar trends were found in the contents of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids in Cd-induced B. juncea leaves for 2 d and 7 d, showing a trend of first increasing and then decreasing. These results indicated that 60 mg/L of exogenous proline was most effective at maintaining the contents of photosynthetic pigments in B. juncea leaves under 2 d and 7 d of Cd stress (P < 0.05). Compared with the control group, the contents of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids following treatment with 60 mg/L of exogenous proline increased by 46.77%, 11.26%, 34.06%, and 18.98% after 2 d of treatment with Cd, respectively, whereas it increased by 55.44%, 44.49%, 50.04% and 22.17% after 7 d of treatment with Cd, respectively.
Effect of exogenous proline on the chlorophyll a content (A), chlorophyll b content (B), total chlorophyll content (C), and carotenoid content (D) of Brassica juncea seedlings under Cd stress. All the analyses were performed with three replicates. Error bars represent the standard deviations (SD). The different letters in the group indicate significantly different values between treatments (P < 0.05)
As shown in Fig. 4, the addition of exogenous proline significantly increased the Pn of B. juncea leaves with the increase in Cd treatment time (P < 0.05) and reached a maximum at a concentration of 60 mg/L. The stomatal conductance (Gs), intercellular carbon dioxide concentration (Ci), and transpiration rate (Tr) of B. juncea decreased with increasing Cd treatment times, but at a concentration of 60 mg/L, the Gs and Tr were significantly higher than those of the control, and the Ci was significantly lower than that of the control (P < 0.05).
Effect of exogenous proline on the net photosynthetic rate (Pn) (A), stomatal conductance (Gs) (B), intercellular carbon dioxide concentration (Ci) (C) and transpiration rate (Tr) (D) of Brassica juncea seedlings under Cd stress. All the analyses were performed with three replicates. Error bars represent the standard deviations (SD). The different letters in the group indicate significantly different values between treatments (P < 0.05)
Effect of exogenous proline on cell viability in seedlings
Figure 5 A shows that the relative cell viability of B. juncea leaves treated with exogenous proline was significantly higher than that of the control. After Cd treatment for 2 d and 7 d, the relative cell viability of B. juncea leaves reached its maximum when the proline concentration was 60 mg/L, which were 96.73% and 91.34%, respectively. The results indicate that adding an appropriate amount of proline is favorable to the relative cell viability of B. juncea under Cd stress.
As shown in Fig. 5B, as the concentration of exogenous proline increases, the root tip changes from dark to bright, and then dark. When the concentration of exogenous proline was 60 mg/L, the relative cell viability reached its maximum.
Effect of exogenous proline on Cd content in seedlings
As shown in Fig. 6, the Cd content of B. juncea seedlings increased significantly with the increase in time of treatment with Cd. After Cd stress for 7 days, the belowground contents of Cd in the B. juncea seedlings treated with different concentrations of exogenous proline decreased significantly compared with the control (P < 0.05), whereas the aboveground contents decreased significantly in treatments with 40, 60, and 80 mg/L proline. Among them, treatment with 60 mg/L proline treatment reached the lowest value, a decrease of 33.47% and 16.72% compared with those of the control group, respectively. No significant differences in translocation coefficients (the ratio of the element’s presence in the plant’s aboveground parts compared to that in the plant’s belowground parts) were observed in the treatments following Cd stress for 2 d. Following Cd stress for 7 days, exogenous proline (40, 60, and 80 mg/L) significantly decreased the translocation coefficients compared with the control (P < 0.05).
Effect of exogenous proline on the aboveground Cd content (A), belowground Cd content (B), and translocation coefficients (C) of Brassica juncea seedlings under Cd stress. All the analyses were performed with three replicates. Error bars represent the standard deviations (SD). The different letters in the group indicate significantly different values between treatments (P < 0.05)
Histochemical staining of ROS
The presence of dark blue and dark brown spots indicates serious oxidative rupture under heavy metal stress [22]. As shown in Fig. 7, the leaves and roots of B. juncea stained with 3,3′-diaminobenzidine (DAB) and nitro blue tetrazolium (NBT) became deeper with the increase in Cd stress time, indicating that the oxidative rupture became increasingly serious. Following exogenous treatment with proline, the color of Cd-stressed B. juncea leaves and roots by NBT and DAB staining were significantly lighter than those of the control. Exogenous proline, at a concentration of 60 mg/L, was the most effective at mitigating Cd toxicity in the leaves and roots. Therefore, the accumulation of hydrogen peroxide (H2O2) and superoxide anions (O2−) decreased significantly after Cd-stressed B. juncea seedlings were treated with exogenous proline.
Effects of exogenous proline on antioxidant enzyme activities and MDA content in seedlings
As illustrated in Fig. 8, under 2 d of Cd stress, compared with the control, treatment with 60 mg/L exogenous proline was the most effective at increasing the activities of SOD, POD, APX, and CAT in the leaves by 44.38%, 252.56%, 73.88%, and 59.52% respectively, whereas they increased by 14.88%, 41.75%, 33.53%, and 58.82% in the roots compared with the control group, respectively (P < 0.05). Following Cd treatment for 7 d, they increased by 153.75%, 206.97%, 334.48%, and 75.24% in the leaves, respectively, whereas they increased by 53.35%, 16.96%, 42.32%, and 63.64% in the roots, respectively. Therefore, the application of the appropriate amount of exogenous proline can enhance the activities of SOD, POD, APX, and CAT in B. juncea seedlings, which can effectively alleviate Cd stress. Consistent with the enhancement of activities of antioxidant enzymes, the content of MDA, a product of the peroxidation of membranes, was reduced.
Effects of exogenous proline on antioxidation enzymes activities of SOD (A, B), POD (C, D), APX (E, F) and CAT (G, H) and the content of MDA (I, J) in the leaves and roots of Brassica juncea under Cd stress, respectively. All the analyses were performed with three replicates. Error bars represent the standard deviations (SD). The different letters in the group indicate significantly different values between treatments (P < 0.05). APX, ascorbate peroxidase; CAT, catalase; MDA, malondialdehyde; POD, peroxidase; SOD, superoxide dismutase
Effects of exogenous proline on the contents of reduced glutathione, oxidized glutathione, and phytochelatins and the activities of glutathione reductase in seedlings
As shown in Fig. 9, with the extension of Cd treatment, the contents of GSH and GSSG in B. juncea leaves and roots increased, whereas the ratio of GSH/GSSG decreased and then increased. The GSH/GSSG ratio first increased and then decreased as the concentration of exogenous proline increased. Under different times of Cd stress, exogenous proline remarkably increased the contents of GSH and GSSG and the GSH/GSSG ratio in the B. juncea leaves and roots (P < 0.05), and reached its maximum value when the concentration of exogenous proline was 60 mg/L.
Effect of exogenous proline on the leaf GSH content (A), leaf GSSG content (B), leaf GSH/GSSG ratio (C), root GSH content (D), root GSSG content (E), and root GSH/GSSG ratio (F) of Brassica juncea seedlings under Cd stress. All the analyses were performed with three replicates. Error bars represent standard deviations (SD). The different letters in the group indicate significantly different values between treatments (P < 0.05). GSH, glutathione; GSH/GSSG, ratio of glutathione/glutathione disulfide
The application of exogenous proline did not affect the contents of phytochelatins (PCs) in plants that were not subjected to Cd stress (Fig. S2). Compared with the control group, Cd stress significantly increased the content of PCs in the leaves and roots, especially in the roots (P < 0.05). After 7 days of Cd stress, the contents of PCs in the roots were elevated by 79.39% and 120.79%, respectively. In addition, 60 mg/L proline treatment resulted in an increase in the contents of PCs in roots by 60.19% and 18.25%, respectively, compared with Cd stress alone.
As shown in Fig. S3, after 7 days of Cd treatment, compared with the control, the activities of glutathione reductase (GR) of the leaves and roots increased significantly by 21.49% and 47.97%, respectively (P < 0.05). Compared with Cd stress alone, the GR activities in the leaves and roots of B. juncea treated with Cd and 60 mg/L proline were significantly increased by 10.20% and 16.71%, respectively (P < 0.05).
Discussion
Exogenous proline mitigated the toxic effects of Cd in B. juncea
In this study, Cd significantly inhibited the growth of B. juncea, while the application of exogenous proline increased the proline content (Fig. 1), protected the photosynthetic pigments (Fig. 3), and maintained photosynthesis (Fig. 4) and cell viability (Fig. 5), thus alleviating the inhibition of Cd on growth (Fig. 2). The results also showed that 60 mg/L was the optimal concentration of exogenous proline to alleviate the toxic effects of Cd in B. juncea. Similar results were observed in chickpea (Cicer arietinum) [23], olive plants (Olea europaea L. cv. Chemlali) [17], and wheat (Triticum aestivum L.) [12] under Cd stress, as well as arsenate-stressed eggplant (Solanum melongena) [24]. Interestingly, the alleviating effects of exogenous proline on cd toxicity in B. juncea showed an approximate inverted U curve. Namely, 60 mg/L proline was most effective in alleviating cadmium toxicity, and the effect was weaker when it was lower or higher than 60 mg/L. The reason may be that excessive proline is detrimental to plants [25].
Cd stress can lead to the excessive accumulation of ROS, which will affect cell elongation and cell division, thus, hindering plant growth and development [12, 26]. Therefore, the inhibition of plant growth and development is considered as one of the vital indices to evaluate the toxic effects of Cd [24]. Proline plays multifarious roles, including adaptation, recovery, and signaling, when it comes to combating stress in plants [27]. The promotion of exogenous proline on plant growth and development may be related to its potential to stabilize subcellular compartments through the detoxification of free radicals and maintenance of the redox potential under Cd stress [12].
Previous studies have also reported substantial reductions in chlorophyll content and photosynthesis rates under Cd toxicity [28,29,30]. This could be explained by the effects on the synthesis of chloroplasts in cells and reduction in the content of chlorophyll, which hampers photosynthesis in Cd-stressed plants [31]. In our study, the addition of exogenous proline increased the content of chlorophyll in plant leaves. Similar studies showed that the addition of exogenous proline alleviated the loss of total chlorophyll and carotenoids in coriander under Hg stress [14]. Wani et al. [16] also reported that foliar spraying different concentrations of proline could improve the chlorophyll and photosynthetic characteristics in the two varieties of B. juncea (‘Varuna’ and ‘RH-30’). This can be explained by the ability of proline to protect the photosynthetic organelle of plants under abiotic stress [32]. The positive role of proline in protecting plants under stress is related to the electron transfer of proline between stable cell membranes and mitochondria [33].
Cd stress seriously affects the overall photosynthetic capacity of plants and destroys the photosynthetic machinery [34]. In this study, Cd inhibited the Pn and Gs of B. juncea leaves, while the Ci increased. The Ci levels at 2 d and 7 d were higher than those at 0 d (Fig. 4), which indicates that the decrease of Pn might be due to the non-stomatal limitation caused by the reduction in the fixation of CO2 by RuBisCo [35]. The Tr levels in the B. juncea leaves are reduced under Cd stress, possibly due to increased cellular stomatal resistance or even closure. This result is similar to the effect of Cd on the photosynthetic rate of hybrid Pennisetum [36] and mung bean [Vigna radiata (L.) Wilczek] seedlings [35]. The addition of exogenous proline mitigated the photosynthetic inhibition of leaves. This is similar to the studies of Cd on pigeon pea (Cajanus cajan L.) [37], and other stresses on chili (Capsicum annuum) [38] and sorghum (Sorghum bicolor) [39]. Stressed plants with exogenously added proline were more effective at assimilating CO2 compared to stressed plants, which may be associated with the increased levels of Gs and Tr. The enhancement of Tr was mainly due to the increase in Gs levels, which triggered enhanced CO2 diffusion within the leaf tissues [40]. The Ci increased through the enhancement of Gs, which, in turn, affected the water content of plant tissues under Cd stress. Therefore, exogenous proline may play an effective mechanism to mitigate photosynthesis inhibition in Cd-stressed B. juncea.
Exogenous proline reduced the Cd content in both the belowground part and the aboveground part of B. juncea exposed to Cd stress. In addition, the translocation coefficient of Cd content decreased following exogenous proline treatment (Fig. 6). This suggests that the translocation of Cd to the aboveground part was restricted, thus, protecting the photosynthetic apparatus of the leaves. Similar findings were reported in the application of exogenous proline in tobacco (Nicotiana benthianum) [41], olive [17], and date palm [18] under Cd stress. In vitro experiments showed that proline could form complexes with Cd [42]. Exogenous proline treatment also elevated the levels of GSH (Fig. 9) and PCs (Fig.S2), chelators of Cd. PCs form chelates with heavy metal ions in the cytosol and are subsequently transported into the vacuole [43]. It is worth considering that these chelators may play roles in the retention of Cd in the roots, thereby reducing the translocation of Cd to the aboveground part. Therefore, the application of exogenous proline can effectively limit the contents of Cd and alleviate the toxicity caused by Cd to B. juncea.
Exogenous proline maintained the redox homeostasis in B. juncea under Cd stress
DAB and NBT staining showed that exogenous proline had a positive effect at ameliorating the accumulation of ROS in the response of B. juncea to Cd exposure, thus, protecting the plants from Cd toxicity (Fig. 7). A similar result was reported by Yu et al. [15], in which exogenous proline positively affected the amelioration of the lipid peroxidation of rice seedlings exposed to chromium.
Proline is an osmotic substance, which has an osmotic adjustment function and antioxidant activity [44]. It stabilizes the osmotic differences between the cell’s surroundings and cytoplasm, protects the plant cells from oxidative stress, and maintains the intracellular redox equilibrium [11, 45, 46]. Previous studies have shown that Cd stress can aggravate the degree of plasma membrane peroxidation, destroy the structure of the cell membrane, and lead to the accumulation of a large number of ROS in plants [30, 47, 48]. This can be explained by the direct or indirect involvement of proline in the process of ROS elimination, which may reduce the accumulation of ROS and the oxidative damage of Cd to the plasma membrane to some extent, thereby helping to maintain the normal physiological processes of B. juncea.
As shown in Fig. 8, the activities of antioxidant enzymes, including SOD, POD, APX, and CAT, in B. juncea seedlings leaves and roots treated with 60 mg/L proline were significantly higher than those of the control (P < 0.05). Thus, exogenous proline, particularly at a concentration of 60 mg/L, could improve the activities of these antioxidant enzymes in B. juncea seedlings under Cd stress. Xu et al. [49] had similar findings that proline could reduce the toxicity of Cd to black nightshade (Solanum nigrum) by detoxifying ROS and increasing the activities of antioxidant enzymes. In addition, Islam et al. [41] found that exogenous proline supplements could improve the activities of antioxidant enzymes, thus, enhancing the tolerance of tobacco to Cd stress. Heavy metal stress can impact the activities of some antioxidant enzymes, including SOD, POD, APX, and CAT in plants [50]. SOD is a defensive enzyme system that scavenges ROS, and the addition of exogenous proline could ensure that there is sufficient SOD to degrade H2O2 [33]. POD catalyzes the oxidation of several compounds using H2O2 as the electron acceptor [51]. APX acts a pivotal part in antioxidant defense by involving the fine regulation of ROS [52]. CAT catalyzes the conversion of H2O2 to water and molecular oxygen [50]. Therefore, the addition of exogenous proline may act as a protectant of antioxidative capacities to mitigate the toxicity of Cd.
In this study, the contents of GSH and GSSG in the leaves and roots of B. juncea under Cd stress increased, and the ratio of GSH/GSSG decreased. After the addition of exogenous proline, the GSH, GSSG, and GSH/GSSG ratios in the leaves and roots of B. juncea increased compared with the control (Fig. 9). GSH plays a critical role in the response of plants to oxidative stress [53, 54]. As an antioxidant, it can remove ROS, such as H2O2 and O2− [55]. GSH has a high reducing ability and plays a multifunctional role in many biological processes, such as cell growth and division, sulfate transport, signal transduction, protein and nucleic acid synthesis, and the detoxification of exogenous drugs. In addition, the balance between GSH and GSSG actively maintains cellular redox homeostasis [56, 57]. It has been reported that GSH combines with Cd to reduce its toxicity [43]. The toxic effect of Hg on sea purslane (Halimione portulacoides L.) increased the content of GSH and decreased the ratio of GSH/GSSG [58]. Arsenate (AsV), zinc, and nickel stress significantly reduced the GSH/GSSG ratio in rice [59] and seep monkeyflower (Mimulus guttatus) [60]. This could be owing to the ability of plant chelates derived from GSH to form complexes with heavy metal ions to alleviate heavy metal stress [13, 61]. When the plants were stressed, the GSH/GSSG ratio decreased owing to the consumption of GSH in the detoxification and metabolism of ROS, which led to a change of the redox state. Additionally, the GSH/GSSG signal activated various defense mechanisms in plants through the redox signal pathway [62].
In this study, Cd stress led to the accumulation of PCs in B. juncea. The induction of PCs reduces the damage caused by heavy metals by complexing excess heavy metals via thiol (-SH) and avoiding their intracellular circulation in the form of free ions [63]. Other studies also found that Cd stress caused a significant accumulation of PCs in plants [64,65,66]. Our study suggested that the addition of exogenous proline increased the GSH content, thereby increasing the synthesis of PCs, which improved the chelation of Cd and reduced the oxidative damage caused by Cd.
In the present experiment, the GR activity was enhanced by the addition of exogenous proline under Cd treatment (Fig. S3). Similarly, other studies have shown that the addition of exogenous proline significantly increased the level of GR under various environmental stresses [67,68,69]. GR uses NADPH as the electron donor to catalyze the reduction of GSSG to GSH [70], thus, playing a key role in maintaining the content of GSH and the redox state (GSSG/GSH) of the glutathione pool in plants [62]. Our results suggest that exogenous proline may maintain the GSH pool by enhancing GR activity, thereby maintaining higher GSH/GSSH ratios and consequently redox homeostasis.
Both GSH and proline belong to the α-Ketoglutarate family and synthesized from the same precursor glutamate [71]. Excess proline within plant cells can also be degraded to glutamate [72]. Therefore, it is possible that exogenous proline addition under Cd stress reduced the demand for endogenous proline synthesis, thereby reducing the consumption of L-glutamate for proline synthesis, which was beneficial for GSH synthesis. This may also be part of the reason why exogenous proline treatment increased the content of GSH. The relationship between proline metabolism and GSH metabolism under Cd stress needs further investigation.
Conclusions
Exogenous proline protected the photosynthetic pigments, maintained photosynthesis and cell viability, and reduced the uptake and translocation of Cd, thus, alleviating the inhibition of Cd on growth in B. juncea. Exogenous proline treatment reestablished redox homeostasis by elevating the activities of antioxidant enzymes and nonenzymatic antioxidant contents, which, in turn, mitigated the toxicity of Cd to B. juncea (Fig. 10). Compared with other concentrations, 60 mg/L exogenous proline was the most effective at mitigating Cd toxicity in B. juncea. However, the molecular mechanism and related signaling pathway of proline in alleviating Cd toxicity in B. juncea merit further study.
Materials and methods
Plant growing conditions and treatment
B. juncea ‘Purple-leaf Mustard’, a rapeseed cultivar planted in Hunan, China, was kindly provided by Prof. Liu of Hunan Agricultural University [73] and preserved at the Central South University of Forestry and Technology. The seeds were sterilized with 70% ethanol and germinated for 2 days at 25 °C. The seedlings grown in one-half Hoagland nutrient solution were transferred to a climate chamber with a 14 h/10 h day/night alternate cycle, 200 µmol/m− 2s− 1 light intensity, and relative humidity of 55–75% at 25 °C.
After 40 days, seedlings of similar sizes were subjected to the following treatments: (1) Cd 80 µM + proline 0 mg/L (Control), (2) Cd 80 µM + proline 20 mg/L (T1), (3) Cd 80 µM + proline 40 mg/L (T2), (4) Cd 80 µM + proline 60 mg/L(T3), (5) Cd 80 µM + proline 80 mg/L (T4), and (6) Cd 80 µM + proline 100 mg/L (T5). In addition, to further compare the effects of control condition and the treatment of proline alone on plant growth and PCs content, and activities of GR, another four treatments were set up as follows: (1) normal growth (Control), (2) 80 µM Cd treatment (Cd), (3) 60 mg/L proline treatment (Pro), and 80 µM Cd + 60 mg/L proline treatment (Cd + Pro). Samples were taken at 0 d, 2 d, and 7 d for analysis. Previous studies have shown that 80 µM Cd in one-half Hoagland nutrient solution can cause significant toxic effects on B. juncea [74, 75]. The nutrient solution was changed every two days. Three replicates were established for each treatment.
Detection of proline content
The contents of proline were determined as described by Bates et al. [76]. A total of 0.5 g of fresh leaves were ground in 5 mL of sulfosalicylic acid (3%, v/v) and then boiled for 10 min. The extract (2 mL) was added to 2 mL of acidic ninhydrin and boiled for 30 min. After cooling, 4 mL of toluene was added to the mixture and centrifuged at 3000 g for 5 min. The absorbance was measured at a wavelength of 520 nm with toluene as a blank. The proline content in the sample was calculated using the linear correlation equation of the standard curve of L-proline (Abs = 0.0406 C + 0.2327, r2 = 0.9825).
Determination of plant height, root length, and dry weight
Plant height and root length were measured with a ruler. The fresh plants were placed in an oven at 65 °C and dried to a constant weight to measure the dry weight of shoots and roots.
Measurement of chlorophyll and carotenoid content, net photosynthetic rate, stomatal conductance, intercellular carbon dioxide concentration, and transpiration rate
The contents of photosynthetic pigments were assayed as described by Petrovic and Krivokapic [77]. Fresh leaves (0.2 g) were ground in calcium carbonate (0.1 g) and 95% ethanol and measured the absorbances at 665 nm, 649 nm, and 470 nm wavelengths were measured using 95% ethanol as a blank.
Ca: chlorophyll a; Cb: chlorophyll b; Cx,c: carotenoid content; C: pigment concentration (mg/L); V: volume of extract (mL); N: dilution ratio; m: sample mass (g).
The Pn, Gs, Ci, and Tr of B. juncea were measured by a portable photosynthetic system (LI-6400XT; LI-COR, Lincoln, NE, USA) with photosynthetic effective radiation of 100 µmol/m− 2s− 1, room temperature of 25 °C, and relative humidity of 70%.
Analysis of cell viability
The cell viabilities of plant leaf and root were determined by the Evans blue staining method [78, 79]. The area of leaves that was dyed was calculated using Image J (NIH, Bethesda, MD, USA). The calculation formula is as follows:
Determination of Cd content
The content of Cd was measured as described by Yang. et al. [47]. A total of 0.3 g of dry B. juncea leaf or root samples were ground into a powder digested with a diacid mixture of (HNO3/HClO4) (9:1, v/v), and then diluted to 50 mL with ultrapure water. The content of Cd was determined using a flame atomic absorption spectrophotometer (ICE3500; Thermo Scientific, Waltham, MA, USA) after filtration. The Cd standard curve (Abs = 0.46900 C + 0.019858, R2 = 0.997) was plotted using the Cd standard solution (China national standard sample No. GSB04-1721-2004) obtained from Guobiao (Beijing) Testing & Certification Co., Ltd. (Beijing, China).
Histochemical detection of ROS
The presence of H2O2 and O2− in leaves was detected by the DAB and NBT staining methods, respectively [80]. The leaves and roots were submerged in a solution of DAB or NBT and subjected to vacuum slightly, then shaken for 4 h under dark conditions before they were decolorized and photographed. In the presence of endogenous peroxidase, DAB polymerized at the sites of H2O2 accumulation, which formed a brown DAB polymer. The blue formazan precipitate produced by the reduction of NBT by O2− became visible.
Determination of antioxidant enzyme activities and MDA content
Fresh leaves (0.1 g) were homogenized in an ice bath with 1 mL of 0.1 M sodium phosphate buffer (pH 7.8). The homogenate was centrifuged at 8000 g for 10 min at 4 °C, and the supernatant was collected for subsequent analysis. The whole extraction procedure was conducted at 4 °C. The activities of the antioxidant enzymes (i.e., SOD, POD, APX, and CAT) were determined by kits purchased from Shanghai Sinobest Biotechnology Co., Ltd. (Shanghai, China). The content of MDA was assayed by the thiobarbituric acid (TBA) method [81].
Measurements of contents of glutathione, glutathione disulfide, and phytochelatins
The contents of glutathione (GSH) and glutathione disulfide (GSSG) were measured as described by Griffith [82]. The fresh leaves and roots (0.5 g) were extracted with 2.5 mL of 5% sulfosalicylic acid and centrifuged at 12,000 g for 20 min at 4 °C. A volume of 50 µL of supernatant was mixed with 50 µL of 5% sulfosalicylic acid, 24 µL of 1.84 mol/L triethanolamine, and 50 µL of 10% vinylpyridine, and incubated at 25 °C for 1 h to remove the GSH. A volume of 706 µL of 50 mmol/L phosphate buffer (pH 7.5, containing 2.5 mmol/L EDTA), 20 µL 10mmol/L EDTA, 20 µL of 10 mmol/L NADPH, and 80 µL of 12.5 mmol/L 5,5’-dithio-bis(2-nitrobenzoic acid) (DTNB) were mixed and incubated at 25 °C for 10 min, and then 20 µL of 50 U/mL GR was added. A mixture of extracts without DTNB and phosphate buffer was used as a blank, and the absorbance at 412 nm at 3 min was measured. The content of GSSG in the plant tissue was calculated using a standard curve of GSSG.
The content of total glutathione (GSH + GSSG) was determined by replacing the vinyl pyridine with distilled water of equal volume. The content of GSH was determined by subtracting the content of GSSG from the total glutathione.
The content of phytochelatins (PCs) was measured as described by Keltjens and van Beusichem [83]. The contents of non-protein thiol (NPT) were first determined as described by Nagalakshmi and Prasad [84], and then the contents of PCs are calculated as follows:
Determination of glutathione reductase activities
The GR activities were determined as described by Foyer and Halliwell [85] with some modifications. Fresh leaves and roots (0.1 g) were homogenized in phosphate buffer solution (containing 1 mmol/L EDTA) and centrifuged at 12,000 g for 30 min at 4 °C. The supernatant was the enzyme extract. A volume of 1.35 mL of phosphate buffer (containing 1 mmol/L EDTA), 0.05 mL of 5 mmol/L GSSG solution, and 0.1 mL of enzyme solution were added to a centrifuge tube in sequence. Finally, 20 µL of 4 mmol/ L NADPH solution was added to initiate the enzymatic reaction. The absorbance values of the reaction mixture at 340 nm were recorded at 30 s intervals, starting 15 s after initiation and measured continuously to obtain data for at least six points. The decrease at 340 nm was read from 0.5 to 3.5 min to calculate the enzyme activity.
Statistical analysis
The effects of treatments were compared with a one-way analysis of variance (ANOVA) using SPSS 26 (IBM, Inc., Armonk, NY, USA). The means were compared using Duncan’s multiple range test at P < 0.05. All the figures were produced using Origin 2018 (OriginLab, Northampton, MA, USA).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Cd:
-
cadmium
- GSH:
-
glutathione
- GSSH:
-
glutathione disulfide
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
- POD:
-
peroxidase
- APX:
-
ascorbate peroxidase
- CAT:
-
catalase
- MDA:
-
malondialdehyde
- GR:
-
glutathione reductase
- Pn:
-
net photosynthetic rate
- Gs:
-
stomatal conductance
- Ci:
-
intercellular carbon dioxide concentration
- Tr:
-
transpiration rate
- DAB:
-
3,3′-diaminobenzidine
- NBT:
-
nitro blue tetrazolium
- H2O2 :
-
hydrogen peroxide
- O2 − :
-
superoxide anions
- PCs:
-
phytochelatins
- DTNB:
-
5,5’-dithio-bis(2-nitrobenzoic acid)
References
Yang S, Zu Y, Li B, Bi Y, Jia L, He Y, et al. Response and intraspecific differences in nitrogen metabolism of alfalfa (Medicago sativa L.) under cadmium stress. Chemosphere. 2019;220:69–76.
Dalcorso G, Fadnati S, Maistd S, Furini A. How plants cope with cadmium: staking all on metabolism and gene expression. J Integr Plant Biol. 2010;50(10):1268–1280.
Chen N, Zheng Y, He X, Li X, Zhang X. National soil pollution status survey bulletin. J Agro-Environ Sci. 2014;(5):1689–1692.
Li M, Qi XF, Wang XH, Li YY, Wang LL. The effects of Cd2+ stress on photosystem ii functioning of rice (Oryza sativaL.) leaves under elevated CO2 level. Appl Ecol Env Res. 2021;19(1):739–750.
Janeeshma E, Kalaji HM, Puthur JT. Differential responses in the photosynthetic efficiency of Oryza sativa and Zea mays on exposure to Cd and Zn toxicity. Acta Physiol Plant. 2021;43(1):12.
Imran M, Hussain S, Rana MS, Saleem MH, Rasul F, Ali KH, et al. Molybdenum improves 2-acetyl-1-pyrroline, grain quality traits and yield attributes in fragrant rice through efficient nitrogen assimilation under cadmium toxicity. Ecotoxicol Environ Saf. 2021;211:111911.
Liu H, Yang L, Li N, Zhou C, Feng H, Yang J, et al. Cadmium toxicity reduction in rice (Oryza sativa L.) through iron addition during primary reaction of photosynthesis. Ecotoxicol Environ Saf. 2020;200:110746.
Rasheed R, Ashraf MA, Arshad A, Iqbal M, Hussain I. Interactive effects of chitosan and cadmium on growth, secondary metabolism, oxidative defense, and element uptake in pea (Pisum sativum L.). Arab J Geosci. 2020;13(17):847.
Gong W, Dunn BL, Chen Y, Shen Y. Acclimatization of photosynthetic apparatus and antioxidant metabolism to excess soil cadmium in Buddleja spp. Sci Rep. 2020;10(1):21439.
Per TS, Khan NA, Reddy PS, Masood A, Hasanuzzaman M, Khan MIR, et al. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol Biochem. 2017;115:126–140.
Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, et al. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9(7):285.
Rasheed R, Ashraf MA, Hussain I, Haider MZ, Iqbal M. Exogenous proline and glycinebetaine mitigate cadmium stress in two genetically different spring wheat (Triticum aestivum L.) cultivars. Braz J Bot. 2014;(37):399–406.
Hasanuzzaman M, Bhuyan M, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, et al. Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants (Basel). 2020;9(8):681.
Kapoor D, Kavani K, Rattan A, Landi M, Sharma A. Ameliorative role of pre-sowing proline treatment in Coriandrum sativum L. seedlings under mercury toxicity. Phyton. 2021;90(2):489–501.
Yu XZ, Lin YJ, Fan WJ, Lu MR. The role of exogenous proline in amelioration of lipid peroxidation in rice seedlings exposed to Cr (VI). Int Biodeter Biodegr. 2017;123:106–112.
Wani AS, Faraz A, Faizan M, Ahmad A, Hayat S, Tahir I. Foliar spray of proline enhanced the photosynthetic efficiency and antioxidant system in Brassica juncea. Not Bot Horti Agrobo. 2017;45(1):112.
Zouari M, Ben Ahmed C, Elloumi N, Bellassoued K, Delmail D, Labrousse P, et al. Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defense system of Olea europaea L. cv Chemlali exposed to cadmium stress. Ecotoxicol Environ Saf. 2016;128:195–205.
Zouari M, Ahmed CB, Zorrig W, Elloumi N, Rabhi M, Delmail D, et al. Exogenous proline mediates alleviation of cadmium stress by promoting photosynthetic activity, water status and antioxidative enzymes activities of young date palm (Phoenix dactylifera L.). Ecotoxicol Environ Saf. 2016;128:100–108.
Nouairi I, Ben Ammar W, Ben Youssef N, Ben Miled DD, Ghorbal MH, Zarrouk M. Antioxidant defense system in leaves of Indian mustard (Brassica juncea) and rape (Brassica napus) under cadmium stress. Acta Physiol Plant. 2009;31(2):237–247.
Goswami S, Das S. A study on cadmium phytoremediation potential of indian mustard, Brassica juncea. Int J Phytoremediat. 2015;17(1–6):583–588.
Ahmad J, Qamar S, Nida, Khan F, Haq I, Al-Huqail A, et al. Differential impact of some metal(loid)s on oxidative stress, antioxidant system, sulfur compounds, and protein profile of Indian mustard (Brassica juncea L.). Protoplasma. 2020;257(6):1667–1683.
Ghosh S, Shaw AK, Azahar I, Adhikari S, Jana S, Roy S, et al. Arsenate (AsV) stress response in maize (Zea mays L.). Environ Exp Bot. 2016;130:53–67.
Hayat S, Hayat Q, Alyemeni MN, Ahmad A. Proline enhances antioxidative enzyme activity, photosynthesis and yield of Cicer arietinum L. exposed to cadmium stress. Acta Bot Croat. 2013;72(2):323–335.
Singh M, Pratap Singh V, Dubey G, Mohan Prasad S. Exogenous proline application ameliorates toxic effects of arsenate in Solanum melongena L. seedlings. Ecotoxicol Environ Saf. 2015;117:164–173.
Deuschle K, Funck D, Forlani G, Stransky H, Biehl A, Leister D, et al. The role of [Delta]1-pyrroline-5-carboxylate dehydrogenase in proline degradation. Plant Cell. 2004;16(12):3413–3425.
Riaz S, Iqbal M, Hussain I, Rasheed R, Ashraf MA, Mahmood S, et al. Chronic cadmium induced oxidative stress not the DNA fragmentation modulates growth in spring wheat (Triticum aestivum). Int J Agric Biol. 2014;16(4):789–794.
Kaur G, Asthir B. Proline: a key player in plant abiotic stress tolerance. Biol Plantarum. 2015;59(4):609–619.
Sun S, Zhou X, Cui X, Liu C, Fan Y, Mcbride M, et al. Exogenous plant growth regulators improved phytoextraction efficiency by Amaranths hypochondriacus L. in cadmium contaminated soil. Plant Growth Regul. 2020;90(1):29–40.
Rocha GS, Parrish CC, Espíndola E. Shifts in photosynthetic parameters and lipid production of the freshwater microalga Selenastrum gracile (Chlorophyceae) under cadmium exposure. J Appl Phycol. 2020;32:4047–4055.
Wang F, Tan H, Huang L, Cai C, Ding Y, Bao H, et al. Application of exogenous salicylic acid reduces Cd toxicity and Cd accumulation in rice. Ecotoxicol Environ Saf. 2021;207:111198.
Küpper H, Küpper F, Spiller M. Environmental relevance of heavy metal-substituted chlorophylls using the example of water plants. J Exp Bot. 1996;47(2):259–266.
Altuntaş C, Demiralay M, Sezgin Muslu A, Terzi R. Proline-stimulated signaling primarily targets the chlorophyll degradation pathway and photosynthesis associated processes to cope with short-term water deficit in maize. Photosynth Res. 2020;144(1):35–48.
Ben Ahmed C, Ben Rouina B, Sensoy S, Boukhriss M, Ben Abdullah F. Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J Agric Food Chem. 2010;58(7):4216–4222.
Parmar P, Kumari N, Sharma V. Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot Stud. 2013;54(1):45.
Wahid A, Ghani A, Ali I, Ashraf M. Effects of cadmium on carbon and nitrogen assimilation in shoots of mungbean [Vigna radiata (L.) Wilczek] seedlings. J Agron Crop Sci. 2007;193:357–365.
Song X, Yue X, Chen W, Jiang H, Han Y, Li X. Detection of cadmium risk to the photosynthetic performance of hybrid pennisetum. Front Plant Sci. 2019;10:798.
Hayat K, Khan J, Khan A, Ullah S, Ali S, Salahuddin, et al. Ameliorative effects of exogenous proline on photosynthetic attributes, nutrients uptake, and oxidative stresses under cadmium in pigeon pea (Cajanus cajan L.). Plants (Basel). 2021;10(4):796.
Butt M, Sattar A, Abbas T, Sher A, Ijaz M, Ul-Allah S, et al. Foliage applied proline induces salt tolerance in chili genotypes by regulating photosynthetic attributes, ionic homeostasis, and antioxidant defense mechanisms. Hortic Environ Biote. 2020;61(4):693–702.
de Freitas PAF, de Carvalho HH, Costa JH, Miranda RdS, Saraiva KDdC, de Oliveira FDB, et al. Salt acclimation in sorghum plants by exogenous proline: physiological and biochemical changes and regulation of proline metabolism. Plant Cell Rep. 2019;38(3):403–416.
Sharkey T, Bernacchi C, Farquhar G, Singsaas E. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007;30:1035–1040.
Islam MM, Hoque MA, Okuma E, Banu M, Shimoishi Y, Nakamura Y, et al. Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J Plant Physiol. 2009;166(15):1587–1597.
Sharma SS, Schat H, Vooijs R. In vitro alleviation of heavy metal-induced enzyme inhibition by proline. Phytochemistry. 1998;49(6):1531–1535.
Yadav SK. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot. 2010;76(2):167–179.
Chen C, Dickman MB. Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. PNAS. 2005;102(9):3459–3464.
Santiago S. The fermentation analogy: a point of view for understanding the intriguing role of proline accumulation in stressed plants. Front Plant Sci. 2016;7:1339.
He G, Zhang H, Liu S, Li H, Huo Y, Guo K, et al. Exogenous γ-glutamic acid (GABA) induces proline and glutathione synthesis in alleviating Cd-induced photosynthetic inhibition and oxidative damage in tobacco leaves. J Plant Interact. 2021;16(1):296–306.
Yang. S, Zhang. J, Chen L. Growth and physiological responses of Pennisetum sp. to cadmium stress under three different soils. Environ Sci Pollut Res. 2021;28(12):14867–14881.
Zhang H, Heal K, Zhu X, Tigabu M, Xue Y, Zhou C. Tolerance and detoxification mechanisms to cadmium stress by hyperaccumulator Erigeron annuus include molecule synthesis in root exudate. Ecotoxicol Environ Saf. 2021;219:112359.
Xu J, Yin HX, Li X. Protective effects of proline against cadmium toxicity in micropropagated hyperaccumulator, Solanum nigrum L. Plant Cell Rep. 2009;28(2):325–333.
Singh V, Tripathi BN, Sharma V. Interaction of Mg with heavy metals (Cu, Cd) in T. aestivum with special reference to oxidative and proline metabolism. J Plant Res. 2016;129(3):487–497.
Yıldız M, Terzi H. Exogenous cysteine alleviates chromium stress via reducing its uptake and regulating proteome in roots of Brassica napus L. seedlings. S Afr J Bot. 2021;139:114–121.
Abogadallah GM. Insights into the significance of antioxidative defense under salt stress. Plant Signal Behav. 2010;5(4):369–374.
Lin TH, Rao MY, Lu HW, Chiou CW, Lin ST, Chao HW, et al. A role for glutathione reductase and glutathione in the tolerance of Chlamydomonas reinhardtii to photo-oxidative stress. Physiol Plant. 2018;162(1):35–48.
Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7(9):405–410.
Yousuf PY, Hakeem K, Chandna R, Ahmad P: Role of glutathione reductase in plant abiotic stress. Abiotic Stress Responses in Plants. Springer, New York, NY; 2012:149–158.
Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155(1):2–18.
Potters G, Horemans N, Jansen MAK. The cellular redox state in plant stress biology–A charging concept. Plant Physiol Biochem. 2010;48(5):292–300.
Anjum NA, Israr M, Duarte AC, Pereira ME, Ahmad I. Halimione portulacoides (L.) physiological/biochemical characterization for its adaptive responses to environmental mercury exposure. Environ Res. 2014;131:39–49.
Singh AP, Dixit G, Kumar A, Mishra S, Singh PK, Dwivedi S, et al. Nitric oxide alleviated arsenic toxicity by modulation of antioxidants and thiol metabolism in rice (Oryza sativa L.). Front Plant Sci. 2015;6:1272.
Bashmakova EB, Pashkovskiy PP, Radyukina NL, Kuznetsov VV. Interactive effects of zinc and nickel on the glutathione system state in Mimulus guttatus plants. Russ J Plant Physiol. 2016;63(5):626–635.
Blum R, Beck A, Korte A, Stengel A, Letzel T, Lendzian K, et al. Function of phytochelatin synthase in catabolism of glutathione-conjugates. Plant J. 2007;49(4):740–749.
Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, et al. Glutathione in plants: an integrated overview. Plant Cell Environ. 2012;35(2):454–484.
Serrano N, Díaz-Cruz JM, Ariño C, Esteban M. Recent contributions to the study of phytochelatins with an analytical approach. TrAC Trend Anal Chem. 2015;73:129–145.
de Knecht JA, Van Dillen M, Koevoets P, Schat H, Verkleij J, Ernst WHO. Phytochelatins in cadmium-sensitive and cadmium-tolerant Silene vulgaris (chain length distribution and sulfide incorporation). Plant Physiol. 1994;104(1):255–261.
Huang Y, Chen J, Sun Y, Wang H, Zhan J, Huang Y, et al. Mechanisms of calcium sulfate in alleviating cadmium toxicity and accumulation in pak choi seedlings. Sci Total Environ. 2022;805:150115.
Wei W, Peng H, Xie Y, Wang X, Huang R, Chen H, et al. The role of silicon in cadmium alleviation by rice root cell wall retention and vacuole compartmentalization under different durations of Cd exposure. Ecotoxicol Environ Saf. 2021;226:112810.
Hasanuzzaman M, Alam MM, Rahman A, Hasanuzzaman M, Nahar K, Fujita M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. Biomed Res Int. 2014;2014:757219.
Yan ZM, Guo SR, Shu S, Sun J, Tezuka T. Effects of proline on photosynthesis, root reactive oxygen species (ROS) metabolism in two melon cultivars (Cucumis melo L.) under NaCl stress. Afr J Biotechnol. 2011;10(80):18381–18390.
Aggarwal M, Sharma S, Kaur N, Pathania D, Bhandhari K, Kaushal N, et al. Exogenous proline application reduces phytotoxic effects of selenium by minimising oxidative stress and improves growth in bean (Phaseolus vulgaris L.) seedlings. Biol Trace Elem Res. 2011;140(3):354–367.
Jin Y, Tao D, Hao Z, Ye J, Du Y, Liu H, et al. Environmental stresses and redox status of ascorbate. Acta Bot Sin. 2003;45(7):795–800.
Anjum NA, Aref IM, Duarte AC, Pereira E, Ahmad I, Iqbal M. Glutathione and proline can coordinately make plants withstand the joint attack of metal (loid) and salinity stresses. Front Plant Sci. 2014;5:662.
Ghosh UK, Islam MN, Siddiqui MN, Cao X, Khan MAR. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: understanding the physiological mechanisms. Plant Biol. 2022;24(2):227–239.
Yan ML, Liu XJ, Guan CY, Chen XB, Liu ZS. Cloning and expression analysis of an anthocyanidin synthase gene homolog from Brassica juncea. Mol Breeding. 2011;28(3):313–322.
Tan P, Zeng C, Wan C, Liu Z, Dong X, Peng J, et al. Metabolic profiles of Brassica juncea roots in response to cadmium stress. Metabolites. 2021;11(6):383.
Seth CS, Misra V, Chauhan LKS. Accumulation, detoxification, and genotoxicity of heavy metals in Indian mustard (Brassica juncea L.). Int J Phytoremediat. 2012;14(1):1–13.
Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207.
Petrovic D, Krivokapic S. The Effect of Cu, Zn, Cd, and Pb accumulation on biochemical parameters (proline, chlorophyll) in the water caltrop (Trapa natans L.), Lake Skadar, Montenegro. Plants. 2020;9(10):1287.
Maestri E, Marmiroli M, Visioli G, Marmiroli N. Metal tolerance and hyperaccumulation: Costs and trade-offs between traits and environment. Environ Exp Bot. 2010;68(1):1–13.
Eapen S, D’Souza SF. Prospects of genetic engineering of plants for phytoremediation of toxic metals. Biotechnol Adv. 2005;23(2):97–114.
Sekulska-Nalewajko J, Gocławski J, Chojak-Koźniewska J, Kuźniak E. Automated image analysis for quantification of reactive oxygen species in plant leaves. Methods. 2016;109:114–122.
Hao J, Li X, Xu G, Huo Y, Yang H. Exogenous progesterone treatment alleviates chilling injury in postharvest banana fruit associated with induction of alternative oxidase and antioxidant defense. Food Chem. 2019;286:329–337.
Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpiridine. Anal Biochem. 1980;106(1):207–212.
Keltjens WG, van Beusichem ML. Phytochelatins as biomarkers for heavy metal stress in maize (Zea mays L.) and wheat (Triticum aestivum L.): combined effects of copper and cadmium. Plant Soil. 1998;203(1):119–126.
Nagalakshmi N, Prasad MNV. Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci. 2001;160(2):291–299.
Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta. 1976;133(1):21–25.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31671601), the Project of Hunan Provincial Key Laboratory of Crop Germplasm Innovation and Utilization (Grant No. 17KFXM08), the Natural Science Foundation of Hunan Province, China (Grant No. 2021JJ31139), and the Planned Science and Technology Project of Changsha City (kq2004091).
Author information
Authors and Affiliations
Contributions
Z.L., M.Y., and X.D. conceived and designed the experiments. Y.W., P.T., L.C., and Z.Y. performed the experiments. X.D., M.Y., and M.L. contributed reagents/materials/analysis tools. Z.L., Y.W., X.D., and C.Z. analyzed the data. Y.W., C.Z., M.Y., and Z.L. wrote the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The experimental research on plants (including seeds) performed in this study complies with institutional, national and international guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file
1: Fig. S1. The plant height (A), root length (B), shoot dry weight (C), and root dry weight (D) of Brassica juncea under normal growth (Control), 80 µM Cd treatment (Cd), 60 mg/L proline treatment (Pro), and 80 µM Cd + 60 mg/L proline treatment (Cd + Pro). All the analyses were performed with three replicates. Error bars represent the standard deviations (SD). The different letters in the group indicate significantly different values between treatments (P < 0.05).
Additional file 2:
Fig. S2. Effects of exogenous proline on contents of PCs in leaves (A) and roots (B) of Brassica juncea seedlings under normal growth (Control), 80 µM Cd treatment (Cd), 60 mg/L proline treatment (Pro), and 80 µM Cd + 60 mg/L proline treatment (Cd + Pro). All the analyses were performed with three replicates. Error bars represent the standard deviations (SD). The different letters in the group indicate significantly different values between treatments (P < 0.05). PCs, phytochelatins.
Additional file 3:
Fig. S3. Effects of exogenous proline on GR activity in leaves (A) and roots (B) of Brassica juncea seedlings under Cd stress. All the analyses were performed with three replicates. Error bars represent the standard deviations (SD). The different letters in the group indicate significantly different values between treatments (P < 0.05). GR, glutathione reductase.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Y., Tan, P., Chang, L. et al. Exogenous proline mitigates toxic effects of cadmium via the decrease of cadmium accumulation and reestablishment of redox homeostasis in Brassica juncea. BMC Plant Biol 22, 182 (2022). https://doi.org/10.1186/s12870-022-03538-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03538-4