Abstract
Background
Short internodes contribute to plant dwarfism, which is exceedingly beneficial for crop production. However, the underlying mechanisms of internode elongation are complicated and have been not fully understood.
Results
Here, we report a maize dwarf mutant, dwarf2014 (d2014), which displays shortened lower internodes. Map-based cloning revealed that the d2014 gene is a novel br2 allele with a splicing variation, resulting in a higher expression of BR2-T02 instead of normal BR2-T01. Then, we found that the internode elongation in d2014/br2 exhibited a pattern of inhibition-normality-inhibition (transient for the ear-internode), correspondingly, at the 6-leaf, 12-leaf and 14-leaf stages. Indeed, BR2 encodes a P-glycoprotein1 (PGP1) protein that functions in auxin efflux, and our in situ hybridization assay showed that BR2 was mainly expressed in vascular bundles of the node and internode. Furthermore, significantly higher auxin concentration was detected in the stem apex of d2014 at the 6-leaf stage and strictly in the node region for the ear-internode at the 14-leaf stage. In such context, we propose that BR2/PGP1 transports auxin from node to internode through the vascular bundles, and excessive auxin accumulation in the node (immediately next to the intercalary meristem) region suppresses internode elongation of d2014.
Conclusions
These findings suggest that low auxin levels mediated by BR2/PGP1 in the intercalary meristem region are crucial for internode elongation.
Similar content being viewed by others
Background
Dwarfism or semi-dwarfism confers a number of advantages to crop varieties, such as increased lodging resistance, denser growth, and higher harvest index, which are extraordinarily beneficial for crop production [1,2,3]. For crops, height is usually determined by the internodes’ number and length [4]. Numerous dwarf mutants have been characterized by short internodes, which have been attributed to impaired internode elongation. Therefore, increasing our knowledge of internode elongation will aid the improvement of crop yield.
Stem/internode elongation is mainly controlled by several plant hormones, including gibberellins (GAs), brassinosteroids (BRs), strigolactones (SLs), and auxins [5]. Defects in the biosynthesis or the signaling of these hormones can cause dwarf phenotypes [6,7,8,9,10], although each hormone might play a different role in stem/internode elongation, irregularities of which will lead to various mutant phenotypes. In maize, there are several GA-related mutants that display extremely reduced height with uniformed short internodes, such as dwarf1(d1), d3, d5 and anther ear1 (an1), as well as dominant mutants D8 and D9 [11,12,13], all of which influence internode elongation throughout the growth period, accompanied by a certain degree of yield loss. Most BR-associated mutants exhibit multiple defective phenotypes in addition to dwarfism [14,15,16,17]. For example, the maize nana plant2 (na2) mutant displays suppression of tillers, altered leaf morphology, and andromonoecy [18]. Unlike GAs and BRs, the reduction in plant height of SL mutants may be an indirect effect of increased tillers because the deficiency in SLs enhances cell division in axillary meristems (AMs) [19, 20], which ultimately leads to a redirection of the nutrition toward tiller growth instead of internode elongation.
Auxin biosynthesis is performed through multiple Trp-dependent or Trp-independent pathways, mainly involving YUCCA (YUC) and TAA1/TAR1/TAR2 genes [21]. Quadruple yuc1 yuc4 yuc10 yuc11 mutants do not develop a hypocotyl and root meristem [22]. Similarly, taa1 mutants in Arabidopsis displaye a defective hypocotyl and root [23] and taa1 tar1 tar2 triple mutants lacked roots and were seedling lethal [24]. In maize, the vanishing tassel2 (vt2) gene is an ortholog of Arabidopsis TAA1, and vt2 mutants exhibit no tassel branches or spikelets, as well as a semi-dwarf phenotype with fewer leaves [25]. Many organs, including the stem/internode, are damaged in auxin biosynthesis mutants. The SCFTIR1/AFB-mediated proteolysis of Aux/IAA proteins is the major auxin signaling pathway, which is clearly responsible for many auxin actions [26, 27]. Most mutants in these components have a similar seedling lethal phenotype [28,29,30]. In addition, synthesized auxin is often directionally transported by auxin transporters to specific tissues, where it acts a potent signal that triggers a plethora of developmental responses [31]. The maize br2 and sorghum orthologue dwarf3 (dw3) exhibit reduced auxin transport and shortened statures [32], and auxin transport is crucial for regulating internode elongation.
Actually, several dwarf mutants described above exhibited various abnormal patterns of internode elongation and other morphological characteristics, which suggests that their underlying mechanisms should be discriminating and be worth pursuing further. In this study, we identified a novel br2 allelic mutant, d2014, that exhibited shortened lower internodes but nearly normal upper parts, indicating that br2 has a unique regulation on plant height development. The br2 is a very famous dwarf gene (first cloned in 2003), which was considered ideal for shortening maize’s height due to its unique phenotype (mild dwarf, shorter lower internodes yet nearly normal upper internodes) [32, 33]. It was well-suited to dissect the mechanism of plant height development for maize improvement. Here, in order to reveal the effects of the d2014/br2 mutation on internode elongation, we performed a dynamic comparison of internode elongation at several stages between d2014 and wild type (WT) plant. Furthermore, we explored the specific location of BR2 expression in the stem and detected the dynamic variation of auxin concentration so as to reveal the mechanism of internode elongation by BR2/PGP1-mediated auxin transport.
Results
Characterization of the maize d2014 dwarf mutant
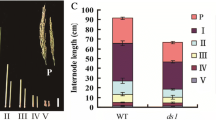
A maize dwarf mutant, d2014, arose spontaneously from the HL9047 (WT) inbred line in 2014, and its self-crossed progenies steadily presented with uniform short stature with erect leaves (Fig. 1a). The plant height of d2014 was reduced by 58.61 cm, whereas its ear height was 44.59 cm less than that of WT (Table 1). This showed that the reduced height of lower internodes mainly contributes to the d2014 dwarf phenotype. Additionally, other traits of d2014, such as ear length, ear width, ear weight, hundred-grain weight, total tassel length, tassel branch number, tasseling stage, and total leaf number were altered slightly in comparison with WT (Fig. 1b; Table 1). Especially for the ear traits, small variations were not enough to lead to yield loss. Therefore, the d2014 mutant might be useful in maize breeding programs.
To ascertain the genetic basis of dwarfism for d2014, three F2 and three BC1 populations derived from d2014 × HL5038, d2014 × HL5054 and d2014 × F19 were generated. F2 and BC1 populations presented tall or short individual separation. Through χ2 tests, the plant height segregated in a 3:1 ratio (tall/short) in all three F2 populations and in a 1:1 ratio (tall/short) in three BC1 populations (Additional file 2: Table S2). These results indicated that this dwarf phenotype of d2014 was genetically controlled by a single recessive gene.
The d2014 gene is a br2 allele
To clone the dwarf d2014 gene, we carried out positional cloning using the (d2014 × HL5054) × d2014 BC1 population. Firstly, 300 BC1 individuals that had a similar dwarf phenotype as d2014 were identified and genotyped by 150 pleomorphic simple sequence repeat (SSR) makers, and then the d2014 gene locus was defined to a 40.05 Mb genetic interval between marker umc1281 and umc1278 (Fig. 2a). Subsequently, 768 dwarf plants were genotyped for fine mapping by newly developed InDel molecular makers, and the d2014 gene was narrowed to a smaller segment flagged by the two markers a4 and a15, which are 2.85 Mb apart (Fig. 2a). Finally, 2000 recessive individuals were used to determine the candidate region of about 510 Kb that contained the d2014 gene (Fig. 2a).
Identification of the d2014 gene. a Positional cloning of the d2014 gene: Firstly, the d2014 locus was mapped between the markers umc1281 and umc1278 on the chromosome 1 using 300 dwarf individuals from (d2014 × HL5054) × d2014 BC1 population. Secondly, we enlarged the population and developed new Indel molecular markers. The location of the d2014 gene was then narrowed down to a smaller segment flanked by the markers b4 and b15. Finally, using 2000 recessive plants, we locate the candidate region in about 470 Kb between the markers c5 and c7. The numbers below the vertical lines indicate the recombinants. b The BR2 and other six predicted genes in the 470 Kb region, and and the sequence alignment of BR2 in d2014 and WT. c Variation of the BR2 protein sequence between d2014 and WT
Database analysis showed that BR2 and six other candidate genes are within this 470 Kb region (http://ensembl.gramene.org/biomart/martview/; Fig. 2b). Genomic DNA sequences of these genes from the d2014 mutant and WT were compared, and the results showed that only the BR2 sequences in d2014 had two variant regions, including a 546 bp insertion in intron 3 (C2073) and a 233 bp deletion (A6568 to C6800) in exon 5 (Fig. 2b). The inserted sequence in intron 3 seemed not to be characterized as transposable elements, while the missing region in exon 5 would result in the deletion of 92 amino acids and a frameshift affecting the translation of 29 additional amino acids (Fig. 2c). Moreover, a known br2 mutant (114F) was crossed with d2014 and WT, respectively. Significant differences in plant growth and development between the two hybrids were observed. At the 14-leaf stage, the morphological height of hybrid 114F × d2014 was significantly lower than that of hybrid 114F × WT, and the internode length of the former was strongly shortened in comparison with hybrid 114F × WT (Additional file 3: Figure S1). Over all, these results confirmed that the d2014 gene was a br2 allele with a unique variation.
The d2014/br2 mutation alters the normal splicing of BR2 gene
Generally, a genetic mutation would cause the variation of natural expression pattern or protein function, resulting in phenotypic defects. Database analysis showed that BR2 had two transcripts: BR2-T01 (4672 bp) and BR2-T02 (2061 bp) (https://www.maizegdb.org/). Actually, BR2-T01 is composed of 5 exons, while BR2-T02 only has 4 exons excluding the exon 5 (Fig. 3a). In addition to the 5’UTR, 3’UTR and exon 5, a vital region with an additional 15 bp in BR2-T02 exon 4 is different from that in BR2-T01 exon 4 (Fig. 3b). To verify the existence of these two transcripts in vivo, their cDNA sequences were amplified and compared. For BR2-T01, the band size of d2014 was distinctly less than that of WT (Fig. 3c), which was in agreement with a fragment deletion in d2014. Sequencing the band revealed the previously predicted 233 bp deletion. Meanwhile, there was also a similar band of about 2000 bp for BR2-T02 in d2014 and WT (Fig. 3c), the sequences of which were identical. This suggested that the two transcripts truly existed in vivo.
Identification of the two transcripts of BR2. a Distribution of the exons of BR2-T01 and BR2-T02. b Differences in exon4 between BR2-T01 and BR2-T02. c Molecular identification of BR2-T01 and BR2-T02. The red arrow indicates the differences of the band size between mutant d2014 and WT for BR2-T01. d The expression pattern of BR2-T01 and BR2-T02 in d2014 and WT. ** P ≤ 0.01 (Student’s t-test); ns, not significant
In view of this, it was necessary to analyze the effect of d2014/br2 mutation on the two transcripts. To define the expression patterns of BR2-T01 and BR2-T02, qRT-PCR was carried out through specific quantitative primers. During the three stages of 6-leaf, 10-leaf and 15-leaf, the expression levels of BR2-T01 in d2014 were all lower than in WT, whereas BR2-T02 was significantly up-regulated in d2014 during the three stages (Fig. 3d). These data suggested that the natural expression of BR2-T01 is crucial for WT normal growth, and the down-regulated BR2-T01 leads to the defective traits of d2014. Interestingly, the total BR2 expression level was roughly similar between d2014 and WT (Fig. 3d). These results revealed that the d2014 mutation alters the normal splicing of BR2 gene, causing a higher expression of BR2-T02 instead of normal BR2-T01.
Analysis of BR2/PGP1 and auxin efflux in yeast
In maize, BR2 encodes an auxin transporter, PGP1, which functions in auxin export from intercalary meristems [34]. In order to investigate whether the protein BR2/PGP1 (T01 and/or T02) is responsible for auxin transport at the cellular level, we functionally expressed these two variants in yeast. Fluorinated indole derivate (5-FI), a toxic analog of indole-3-acetic acid (IAA, namely auxin), is cytotoxic to yeast and has been used to investigate auxin transport [35, 36]. Yeast mutant strain gef1 lacks endogenous chloride channel protein [37], which results in 5-FI accumulation in gef1 cytoplasm after the undissociated 5-FI molecules entering cells by passive diffusion, leading to gef1’s hypersensitivity to 5-FI. Using the yeast mutant strain gef1, we found that both of WT-PGP1-T01 and d2014-PGP1-T01 provided certain resistance against 5-FI compared to the vector control (Fig. 4a). The result indicated that the protein PGP1-T01 exported 5-FI to the outside of the yeast cell, namely, PGP1-T01 was responsible for auxin export. Interestingly, PGP1-T02 also exhibited slight but significant resistance against 5-FI in the yeast mutant strain gef1 (Fig. 4b), indicating its functions in auxin export. In terms of alternative splicing, there are mainly two types: front mutation (T01 and T02, both defective) and back mutation (T01, defective; T02, normal). When the two proteins, PGP1-T01 and PGP1-T02, play similar roles in auxin’s export, the second type of mutation would display a mild phenotype, which opens the possibility that there is a variation of br2 mutant with diverse defects.
Functional analysis of protein PGP1-T01 and PGP1-T02. a, b Heterologous expression of PGP1-T01 and PGP1-T02 for detoxification assays in 5-FI hypersensitive gef1 mutant strain, respectively. Represents that 1-, 10-, 100-, and 1000-fold dilutions (from right to left) of yeast cells were orderly spotted on YPDA medium containing 0 μM or 250 μM 5-FI, including the original strain gef1, the vector control and the transformed strains
Defects of internode elongation in the d2014 mutant
A typical characteristic of br2 mutants is that dwarf stature derives from shortened lower internodes [32, 34]. Of the d2014/br2 mutant, the length of certain internodes below the ear was dramatically reduced; additionally, the ear-internode was also shortened, whereas the internodes above the ear were approximately normal (Fig. 5a). Meanwhile, we found that other br2 mutants, br2-114F and br2-117A, also displayed an abnormal ear-internode phenotype similar to d2014 (Fig. 5b). These data showed that the loss-of-function alleles of br2 specifically regulated the elongation of certain internodes.
Morphological comparison of internodes at the mature stage. a Morphology of the internodes below the ear or above the ear between d2014 and WT. The white box indicates ear-internode. Bar = 10 cm. b Morphology of the ear-internodes of the three br2 mutants: d2014, br2-114F, and br2-117A. The arrows indicate the ear-internodes. Bar = 2 cm
Since the total leaf numbers and tasseling stage of WT and d2014 (Table 1) are nearly the same, thus, their developmental phase should be paralleled. To clarify how the mutation of br2 affects internode elongation, we carried out a dynamic comparison of stem growth between d2014 and WT. Plenty of individuals were planted and leaf numbers were labeled to ensure the same developmental stage. Before the 12-leaf stage, the 6th, 7th, 8th, 9th, 10th, and 11th internodes in d2014 were scarcely elongated (Fig. 6a, b, c, d), and the stem height of all these internodes was significantly lower than that in WT (Additional file 4: Figure S2A). From the 12-leaf stage to the 14-leaf stage, the11th, and 12th internodes in d2014 grew rapidly compared to previous patterns, and their lengths increased (Fig. 6d, e). Moreover, the length of the 11th, 12th and 13th internodes (ear-internode) in d2014 was similar to that of WT at the 14-leaf stage (Fig. 6e). Namely, the 5 lowest internodes in d2014 were shortened seriously (Additional file 4: Figure S2B). Strangely, after the 14-leaf stage, the 11th, 12th and 13th internodes in d2014 grew slowly in comparison with WT (Fig. 6e, f), and the elongation of the ear-internode was sharply restrained (Fig. 6g). Nevertheless, the patterns of upper internode elongation in both d2014 and WT were exactly alike after the 16-leaf stage (Fig. 6g, h), which led to nearly paralleled length of the upper internodes (Additional file 4: Figure S2C). All these results revealed that defects of internode elongation in mutant d2014 are disparate at different developmental stages; especially, at the 6-leaf, 12-leaf and 14-leaf stages, the internode elongation in d2014 exhibits variation pattern of inhibited-normal-inhibited transiently.
Dynamic observation of internode elongation. a, b, c, d, e, f, g, h The length of each internode at the 6-, 8-, 10-, 12-, 14-, 16-, 18-, 20-leaf stages, respectively. IN represents internode. At the 6-leaf stage, the first internode (6th internode) above ground is visible and then more internodes are elongated subsequently at other stages. The arrows in e and g indicate the ear-internode (13th internode)
Effect of the d2014 mutation on cell morphology in shortened internodes
At an early developmental stage (before 12-leaf stage), the elongation of the lower internodes in d2014 was seriously inhibited. At the 6-leaf stage, the first over-ground internode (the 6th internode) was elongating. Though it seemed to be very short (Fig. 6a), there was a trending difference between WT and d2014. In order to understand the cell morphology of these shortened internodes in d2014, we observed the young 6th internode at the 6-leaf stage. Cross-sections showed that d2014 had regular parenchyma cells similar to WT, and their cell sizes were comparable, whereas the numbers of vascular bundles in d2014 had been significantly reduced (Fig. 7a, c). Longitudinal sections showed that the length of parenchyma cells was not changed between d2014 and WT, but the interval of two adjacent vascular bundles in d2014 was larger than that in WT (Fig. 7b), which meant that there was a reduced vascular bundle number for d2014. Thus, the d2014 mutation originally affects the formation of vascular bundle in shortened internode cells.
Morphological observation of the 6th internode cells of d2014 and WT at the 6-leaf stage. a Cross-section of cells of d2014 and WT. The arrows point to the vascular bundle (VB); N represents the total VB numbers. The magnification times are 20 ×. Bar = 150 μm. b Longitudinal section of cells of d2014 and WT. The magnification times are 20 ×. Bar = 200 μm. c The statistics of VB number for the fixed center cross section. ** indicates the VB numbers in d2014 were significantly reduced at P ≤ 0.01 level
Location of BR2 expression in the stem
Since the mutant d2014/br2 displays defects in internode elongation, we predicted that BR2 is expressed in these tissues. However, the expression pattern of BR2 has not been precisely defined previously. To figure out the location of BR2 expression in the stem, we performed in situ RNA hybridization at the 10-leaf stage. In longitudinal sections of the stem apex, BR2 expression was clearly localized in each node (Fig. 8a). The upper and lower nodes revealed clear signals in the single internode on the ear (Fig. 8b). In cross sections of the longest internode, BR2 mRNAs were observed in a mass of vascular bundles (Fig. 8c). A single internode was selected for control experiments, in which the sense probe produced no signal (Fig. 8d, e). In maize stems, the vascular bundles interlace in nodes and then tilt into internodes with multiple branches, which finally form a network pipe from top to bottom. Thus, BR2 expression was mainly localized in vascular bundles of the node and internode. Xing indicated that the subcellular localization of QPH1 (BR2) is exclusively in membrane [33]. There is no variation in the signal peptide region of d2014/br2, thus, mutation should not influence the subcellular localization of d2014/br2. In WT, the expression level, localization and transport activity of BR2 are normal, relatively; while under other circumstances (for example br2 mutation), developmental defects occur. Overall, these data indicated that PGP1 functions in auxin export from vascular bundles in stem, then regulating internode elongation.
In situ localization of BR2 mRNA in stem at the 10-leaf stage. a Longitudinal section of stem apex. The arrows represent each node in stem apex. Bar = 0.1 cm. b Longitudinal section of an intact internode. IN, internode; N, node. Bar = 0.3 cm. c Transverse section of longest internode. VB, vascular bundle. Bar = 0.2 cm. d, e Hybridization of a single internode with a BR2 sense probe for longitudinal section and transverse section, respectively. Bar = 0.2 cm
Quantitative analysis of auxin in the d2014 stem
Auxin transport influences the distribution and local concentration of auxin [38]. In the prior studies, br2 mutation influences auxin transport and local concentration, which are responsible for the dwarf phenotype [32,33,34]. The defective auxin transport in d2014 stems, mediated by BR2/PGP1, would cause the auxin concentration variation in the stem and then influence the internode elongation. To investigate the links between the two, we detected the auxin concentration in several key periods of internode elongation variation (as mentioned above). At the 6-leaf stage, auxin concentration in the d2014 stem apex was significantly higher than that in WT (Fig. 9a). We also measured the auxin concentration in the stem apex of d2014 and WT at the 12-leaf and 14-leaf stages. However, auxin concentration was gradually reduced compared with the former period (Fig. 9a). Moreover, little difference was observed between d2014 and WT at these two stages (Fig. 9a). These showed that the d2014/br2 caused higher auxin level in the stem apex at the early development stages, at least at the 6-leaf stage, and correspondingly, the internode elongation of d2014 was suppressed. In addition, we examined the auxin levels of the single ear-internode at the 14-leaf stage, including two parts: the node and the internode region. Interestingly, the auxin level of the nodes in d2014 was significantly higher than the nodes in WT, as well as the internode region itself (Fig. 9b), while in WT, there was no significant difference (Fig. 9b). Furthermore, the auxin levels of the two parts exceeded the stem apex at the 14-leaf stage (Fig. 9a; b). These data revealed that auxin at the 14-leaf stage was increased for ear-internode and was detained in the node region of d2014. Notably, the ear-internode in d2014 began to grow rather slowly after the 14-leaf stage and finally was extremely shortened. Taken together, these data showed that the d2014/br2 caused increasing in auxin levels in the shortened lower internodes (stem apex at the 6-leaf stage) and ear internodes (node region at the 14-leaf stage), which were responsible for the defects of internode elongation.
The change of auxin concentrations in the stem at different stages. a Auxin levels of stem apex in d2014 and WT at the 6-, 12-, and 14-leaf stages. b Auxin levels of a single ear-internode including node and internode region at the 14-leaf stage. ** indicates significant difference at 0.01 level by Student’s t-test. ns, not significant
Discussion
d2014 gene, with a splicing variation, is a potentially useful br2 allele
In maize, there have been many reports on the identification of br2 mutants, which involved a variety of the mutation loci (Additional file 5: Figure S3). At first, the BR2 gene were cloned and confirmed by transposon tagging with Mutator (Mu), such as br2–6, br2–7 and br2–9 with Mu insertion in exon 1, as well as br2–3 with Mu insertion in intron 4 [32]. Subsequently, several other br2 alleles, such as one SNP variant (G/T5295) in exon 5 [33] and a 241 bp deletion (G6367 to C6617) in exon 5 [39], were identified to result in the partial loss of BR2/PGP1 protein function. All the above br2 mutants were derived from multiple inbred lines with a different genetic background. Nevertheless, they all presented shorter lower internodes, but nearly normal upper internodes. Without exception, our d2014br2 displayed similar features, which were verified by allelism test with br2-114F. Apart from the above phenotypes, we also found that the br2 mutants (d2014, br2-114F and br2-117A) displayed a shortened ear-internode. Therefore, it is certain that this br2 allele is responsible for the shortened internodes.
In the maize B73 reference genome, BR2 has two predicted splicing variants: BR2-T01 and BR2-T02. In this study, we identified a new br2 allele (d2014), which contained a 546 bp insertion in BR2 intron 3 (C2073) and a 233 bp deletion (A6568 to C6800) in BR2 exon 5. Sequencing of the cDNA confirmed the presence of the two BR2 splicing variants, and BR2-T02 was highly expressed in d2014 while BR2-T01 expression level was very low, which was contrary to that in WT. Similarly, the two BR2 splicing variants have been verified molecularly in a dwarf br2-NC238 allele mutant [40]. The insertion of a novel transposon in BR2 intron 4 alters the normal splicing of the gene, which results in abundant expression of BR2-T02 in br2-NC238 instead of BR2-T01 in tall NC238 plants. Generally, insertion, deletion, or single-base replacement of the exon region may result in the defective protein with altered function; while the variation of the intron region might affect gene expression pattern through changing the mRNA splicing. Therefore, we concluded that the insertion of 546 bp in intron 3 altered the normal splicing of BR2 gene, which was the main reason for the dwarfing of d2014. Overall, d2014 with a splicing variants has the enriched types of br2 allele.
Some studies have shown that some different splicing variants perform equivalent functions to the constitutive form, even when lacking amino acid sequences or protein domains [41]. The BR2 gene encodes an auxin transport protein, PGP1, which is an ATP-binding cassette (ABC) transporter [42]. In general, full-size ABC transporters consist of two similar halves, each containing a transmembrane domain (TMD) and a nucleotide binding domain (NBD) [43]. The so-called half-size ABC transporters only have a half (one TMD and one NBD) and require dimerization for their transport activity [43]. An example is ECERIFERUM5, which is a half-size ABC protein and functions in wax export to the plant cuticle [44]. Here, through the analysis of protein domains by (http://smart.embl-heidelberg.de/smart), we found that PGP1-T01 was a full-size ABC transporter, while PGP1-T02 contained exactly a half, making it a half-size ABC transporter (Additional file 6: Figure S4). Diana Santelia and Markus Geisler examined the auxin transport properties (influx or efflux) of ABCB protein by heterogeneous expression in yeast [35, 36]. Since the strong promoter was used to induce the overexpression of ABCB protein, it would not accurately evaluate the auxin transport activity. Similarly, our heterogeneous expression assays using strong promoter identified the transport properties of the two proteins PGP1-T01/T02, which indicated that PGP1-T01 (even including the defective d2014-PGP1-T01) and PGP1-T02 have played similar roles in auxin efflux. Previously, Multani and Knöller have confirmed that br2 mutation leads to the decrease of auxin flow from top to bottom, which is responsible for the dwarf phenotype [32, 34]. In our study, the expression of br2-T01 in d2014 was significantly lower than that of BR2-T01 in WT (normal status), indicating that the normal auxin transport activity in d2014 was impaired. Nevertheless, our phenotypic analysis showed that d2014 was a mild dwarf mutant with nearly no other undesirable traits, which should be attributable to the compensation of functional PGP1-T02. Actually, in the F2 population of br2-qph1 and br2-117A, plant height and ear height segregated in a 3:1 ratio (br2-qph1/− plants to br2-117A/br2-117A plants), which indicated that br2-qph1 was dominant to br2-117A [33]. Namely, different br2 alleles would give rise to certain defects in varying degrees. Therefore, our work shows a useful br2 allele that has beneficial potential for maize improvement by moderately reducing the plant height while not affecting the yield.
BR2/PGP1 functions in auxin efflux from the vascular bundles of the node and internode
Auxin is often synthesized in the apex region and is directionally transported to the lower region [45, 46]. This directional auxin flow is attributable to membranous auxin transporters. So far, three major families of auxin transporters have been identified: AUXIN-RESISTANT1 (AUX1)/AUX1-LIKEs (LAXs) for auxin influx and PINFORMEDs (PINs) and several PGP proteins for auxin efflux [47]. Some studies indicated that ABCB efflux transporters might limit auxin reuptake at efflux sites for assisting PIN-oriented auxin flow [48]. In addition, several studies suggested that ABCBs may play a role in local auxin loading of long-distance auxin transport [49, 50].
BR2/PGP1 is an orthologue of Arabidopsis thaliana ABCB1/PGP1, which is expressed in shoot and root apices and functions primarily in auxin efflux from meristematic cells into long-range auxin transport streams [34, 51, 52]. Previously, Knöller applied [3H]-IAA to the upper stem of maize (B73 vs br2), and found that several [3H]-IAA accumulation peaks were detected near the nodes; meanwhile, the moving front was reduced and free auxin levels in the node of br2 mutant are significantly higher than in adjacent internodes [34]. Consistently, in our d2014/br2, higher auxin levels were detected in the stem apex (including multiple nodes) at the 6-leaf stage and in the node region of the ear-internode at the 14-leaf stage. In a word, the br2 mutation caused auxin accumulation in the node regions. Notably, our in situ hybridization assay showed that BR2 is primarily expressed in vascular bundles (VBs) of the nodes and internodes. In line with this, Anne Sophie Knoller indicated that BR2 is mainly expressed in the nodal region (dense VBs) [34]. In this context, we propose that BR2/PGP1 functions in auxin efflux from the node to the internode through vascular bundles. Thus, auxin efflux is reduced from the nodes to the internodes in br2 mutant, and auxin level is higher in br2 node regions.
Excessive auxin levels in the intercalary meristem region suppress internode elongation
Internode elongation is controlled by several factors, mainly the plant hormones. In this study, we found that the variation of auxin level in d2014 stem influences internode elongation. At the 6-leaf stage, the elongation of the lower internodes began to be severely suppressed in d2014, along with higher auxin levels in the stem apex compared with WT. As the auxin levels of the stem apex gradually decreased at the 12-leaf and 14-leaf stages, internodes in d2014 grew rapidly. At the 14-leaf stage, additionally, the auxin level of the node region next to the ear in d2014 was much more than that in WT, corresponding to the irregular and shortened ear-internode. In short, d2014/br2 and WT have the same genetic background except the br2 locus, causing the shortened internodes, as well as higher auxin levels in their node regions, which suggested that higher auxin levels in d2014 stems (node regions) suppress the internode elongation.
Generally, auxin has fundamental roles in rapid stimulation of cell expansion for promoting growth [53, 54]. This seems to be contradictory with what we found in this study. Nevertheless, similar scene that auxin inhibits growth is fit for apical dominance in plant. Growing shoot apexes produce an inhibitory hormone, auxin, which moves downwards within the stem and inhibits the growth of axillary buds (including the AM) next to the node [55, 56]. Recent studies have revealed that a local auxin minimum is critical for AM formation or initiation [57,58,59]. Correspondingly, the stem of some plants, including maize, contains intercalary meristems that support stem growth independently of the shoot apex [5, 60]. Intercalary meristems are located in the internodes immediately above a node, by which the internode can elongate [61]. Moreover, our cytological analysis showed that the d2014 mutation causes a reduced number of vascular bundles, which should be related to intercalary meristem functions. In concordance with this, Multani has also observed the cellular architecture of the br2 internodes, and their data showed that the VB numbers of 1th over-ground internode (6-week-old) are reduced [32]; at the same time, Xing found that the VB numbers of 6th over-ground internode (19-leaf stage) are altered (reduced without specific statistics) in br2 mutants [33], which indicated that less-elongated internodes in br2 have reduced VB numbers. Some studies have shown that cell division or differentiation dependent on auxin transport flow is crucial for the formation of vascular bundles in the leaf [62]. Therefore, we speculate that a low auxin level is indispensable for intercalary meristem functions and, in reverse, internode elongation is suppressed when excessive auxin is arrested in intercalary meristem region.
All the br2 mutants presented shortened lower internodes, as well as ear-internode, but nearly normal upper internodes. Through dynamic observation of internode elongation, we found that the variation of internode elongation in d2014 is closely related to the 6-leaf, 12-leaf and 14-leaf stages. Notably, at the 6-leaf stage, the first internode (6th internode) above the ground was visible, and more internodes elongated subsequently (Additional file 7: Figure S5A); whereas, the juvenile tassel came into view at the 12-leaf stage (Additional file 7: Figure S5B); in addition, the ear-internode, at the 14-leaf stage, was undetectable until the upmost AM emerged (Additional file 7: Figure S5C). These three stages involve the growth and development of shoot apical meristem (SAM), inflorescence meristem (IM) and AM, which is associated with the synthesis of auxin. In context, we present a model to illuminate how loss-of-function of br2 alleles uniquely regulates internode elongation (Fig. 10). At early stages, vigorous auxin is synthesized in the SAM region, and then excessive auxin is arrested in the intercalary meristem region of the lower internodes, and finally, elongation of the lower internodes is suppressed. Along with SAM translating to IM, declining auxin is generated in the apex region, and the upper internodes grow normally. For the severely shortened ear-internode, the chief culprit is a new auxin source derived from the developmental AM, which promotes abundant auxin being restricted in the intercalary meristem region of the ear-internode. Overall, the BR2/PGP1-dependent low auxin level in the intercalary meristem region is crucial for internode elongation.
Conclusions
Plant height is one of most important agronomic traits in crop breeding. Maize BR2/PGP1 regulates the plant height by promoting auxin efflux from node to internode and avoiding excessive auxin accumulation in the intercalary meristem region to suppress the internode elongation. These findings are of great significance to the decryption of genetic mechanism of plant height and the improvement of maize yield.
Methods
Plant materials
The d2014 is a dwarf mutant derived from the HL9047 inbred line (as WT), which was bred by our pedigree method after 9-generation selection. Three different tall stature inbred lines (HL5038, HL5054 and F19) were selected from our high generation breeding materials to construct F2 and BC1 populations with d2014. In addition, the br2 mutants were requested from the Maize Genetic Cooperation Stock Center (stock number: 114F and 117A), the br2-114F and br2-117A were identified and amplified by our group for br2 allelism test. All the plants were permissively cultivated in the standard experimental field of Sichuan Agricultural University in Sichuan during the summer and in Yunnan during the winter.
Positional cloning of the d2014 gene
The BC1 population (HL5054 × d2014) × d2014 was used, and 6000 individuals were prepared to define the d2014 gene locus. Indeed, using the simple sequence repeat (SSR) makers obtained from the MaizeGDB Database and Indel molecular makers acquired from Liu Jian research group in Sichuan Agricultural University, d2014 gene was mapped to chromosome 1 bin 1.06, a ~ 510 Kb region with BR2 and six other predicted genes. To identify the mutation site(s) of d2014 gene, we amplified the DNA sequences of these genes and compared them between d2014 and WT. Meanwhile, we used the br2 mutant 114F to examine the allelism between d2014 gene and BR2.
RNA isolation and quantitative real-time PCR (qRT-PCR) analysis
At the 6-leaf, 10-leaf and 15-leaf stages, stem apexes (about 0.5 cm) in d2014 and WT were flash-frozen by liquid nitrogen and stored at − 70 °C. Thereinto, the stem apexes are the top internodes except the juvenile tassel at the 10-leaf and 15-leaf stages. Total RNA was extracted using Trizol reagent, and the quantity and purity of RNA was determined by a NanoDrop 2000 and 1% agarose gel electrophoresis. Then, RNA was reverse-transcribed to cDNA using the PrimeScript™ RT-PCR Kit with gDNA Eraser (TaKaRa). The database showed that BR2 has two splicing forms corresponding to two transcripts: BR2-T01 and BR2-T02 (https://www.maizegdb.org/). PCR amplification of these CDSs was performed, and the PCR products were detected by electrophoresis and then sequenced. In addition, qRT-PCR was performed in a Bio-Rad CFX96 Real-Time PCR System with SYBR Green PCR Master Mix (TaKaRa). Specific primers were designed for distinguishing BR2-T01 and BR2-T02. The primer Q-BR2-T01 was designed to span exons 4 and 5, while the primer Q-BR2-T02 covered exon 3, exon 4, and the 3’UTR region of BR2-T02. In addition, we designed the primer Q-BR2-both, which covered exons 3 and 4, for detecting the total BR2 expression level. After normalization to ACTIN1 expression as an internal control, the relative expression levels were measured through the 2–ΔΔCt method. All primer sequences are listed in Additional file 1: Table S1.
Heterogeneous expression assays in yeast
The yeast mutant strain gef1, which is hypersensitive to the IAA toxic analog 5-Fluoroindole (5-FI), was used to explore auxin transport properties [35, 36]. Yeast gef1, ATCC®4036838™, was ordered from the American Type Culture Collection (ATCC). Firstly, yeast shuttle vector pGADT7, which has two HindIII restriction enzyme cutting sites, was digested by HindIII to remove the nuclear localization signal (NLS) sequence. In addition, the expression of the transcript on vector pGADT7 is driven by a strong promoter PADH1, so the yeast transgenics are similar to the overexpressed lines. Then, the CDSs of BR2-T01 and BR2-T02 were acquired and inserted into pGADT7-HindIII by homologous recombination (single- or multi-fragment), generating pYHE-d2014-PGP1-T01, pYHE-d2014-PGP1-T02, pYHE-WT-PGP1-T01 and pYHE-WT-PGP1-T02. All the recombinant vectors were introduced into the yeast mutant strain gef1. For leucine-deficient type gef1, single colonies were screened in synthetic minimal medium without leucine (SD-Leu−). Lastly, transformants grown in SD-Leu− to OD600 = 0.8 were washed and resuspended in water to OD600 = 1.0. Cells were 10-fold, 100-fold, 1000-fold diluted, and 2.5 μl of each was spotted on YPDA medium containing 0 μM or 250 μM 5-FI [36] with the corresponding vector control and the yeast mutant strain itself as controls. Pictures were taken after 3–6 days of growth at 28 °C. All primer sequences in homologous recombinant are listed in Additional file 1: Table S1.
Dynamic observation of internode elongation
Each 700 d2014 and WT individuals were planted in a specially designed plot. The plot consisted of 3-m-long rows separated by 1 m space between each row. Five-row interval planting was performed for d2014 and WT with 10 replicates, and 14 individuals were planted in each row. The 5th leaf and 10th leaf of uniform individuals were labelled in the field. In general, the first internode above the ground is visible when the 6th leaf is unfolded at the 6-leaf stage. Thus, we measured each visible internode length from the 6-leaf stage to the maturation stage, including the 6, 8, 10, 12, 14, 16, 18, and 20-leaf stages. At each stage, 10 leaf-labelled individuals in adjacent rows were investigated, and the average value was the phenotypic value of internode corresponding to the leaf number.
Histocytological analysis
A plenty of WT and mutant individuals were planted (as described above). At the 6-leaf stage, the first over-ground internode (the 6th internode) elongated. We randomly picked three individuals of WT and mutant, respectively, as three biological replicates for cytological observation of the internodes. The whole 6th internode was cut except for two terminals (the node regions) to make a paraffin section. All samples were vacuumed quickly and fixed in FAA composed of 5% of formaldehyde (40% v/v), 5% of acetate, and 90% of 75% alcohol (v/v) overnight. The paraffin sections were made as described previously [67]. Firstly, the transection was made from the middle part (about 0.1 cm from the upper part). Next, the longitudinal section was made from the rest of the upper part. The cell morphology was observed through an optical microscopy. Based on the same area (the middle region) and the same magnification (20 ×), we counted all the vascular bundles (VBs) for the transection.
In situ hybridization analysis
At the 10-leaf stage, tissues of WT were prepared, including the stem apex (about 0.5 cm segment from the tip), longest internode and single internode (about 1 cm). In situ hybridization for BR2 was performed as described previously [63]. An antisense RNA probe labeled by the Digoxin marker was uniquely designed in the BR2 exon 1 region, and sequences are listed in Additional file 1: Table S1. To reduce the disturbance of the background signal, pilot experiment was conducted to optimize the probe dosage. In addition, a single internode was selected for the control test with a sense probe (Additional file 1: Table S1).
Endogenous auxin concentration analysis
The stem apex segment (about 0.5 cm) was prepared in several periods, including 6-, 12-, and 14-leaf stages. All tissues were flash-frozen by liquid nitrogen and stored at − 70 °C. The measurements of endogenous auxin were performed through liquid chromatography tandem mass spectrometry (LC-MS). Three repeats with three plants in each replicate were performed. Furthermore, at the 14-leaf stage, the ear-internode was divided into two parts, including the basal node segment (about 0.2 cm) and the middle internode segment (about 0.2 cm), the auxin concentration of which was also measured as above.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional files.
Abbreviations
- 5-FI:
-
5-Fluoroindole
- ABC:
-
ATP-binding cassette
- AMs:
-
Axillary meristems
- an1 :
-
Anther ear1
- ATCC:
-
American Type Culture Collection
- AUX1:
-
AUXIN-RESISTANT1
- br2 :
-
Brachytic2
- BRs:
-
Brassinosteroids
- d1 :
-
Dwarf1
- d2014 :
-
Dwarf2014
- dw3 :
-
Dwarf3
- GAs:
-
Gibberellins
- IM:
-
Inflorescence meristem
- LAXs:
-
AUX1-LIKEs
- LC-MS:
-
Liquid chromatography tandem mass spectrometry
- Mu :
-
Mutator
- na2 :
-
Nana plant2
- NBD:
-
Nucleotide binding domain
- NLS:
-
Nuclear localization signal
- PGP1:
-
P-glycoprotein1
- PINs:
-
PINFORMEDs
- qRT-PCR:
-
Quantitative real-time PCR
- SAM:
-
Shoot apical meristem
- SLs:
-
Strigolactones
- SSR:
-
Simple sequence repeat
- TMD:
-
Transmembrane domain
- VBs:
-
Vascular bundles
- vt2 :
-
Vanishing tassel2
- WT:
-
Wild type
References
Li H, Wang Y, Li X, Gao Y, Wang Z, Zhao Y, Wang M. A GA-insensitive dwarf mutant of Brassica napus L. correlated with mutation in pyrimidine box in the promoter of GID1. Mol Biol Rep. 2011;38(1):191–7.
Salamini F. Hormones and the green revolution. Science. 2003;302(5642):71–2.
Hedden P. The genes of the green revolution. Trends Genet. 2003;19(1):5–9.
Lv H, Zheng J, Wang T, Fu J, Huai J, Min H, Zhang X, Tian B, Shi Y, Wang G. The maize d2003, a novel allele of VP8, is required for maize internode elongation. Plant Mol Biol. 2014;84(3):243–57.
Wang B, Smith S, Li J. Genetic gegulation of shoot architecture. Annu Rev Plant Biol. 2018;69:437–68.
Evans MMS, Poethig RS. Gibberellins promote vegetative phase change and reproductive maturity in maize. Plant Physiol. 1995;108(2):475–87.
Peng J, Richards D, Hartley N, et al. 'Green revolution' genes encode mutant gibberellin response modulators. Nature. 1999;400(6741):256–61.
Lin H, Wang R, Qian Q, Yan M, Meng X, Fu Z, Yan C, Jiang B, Su Z, Li J, Wang Y. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell. 2009;21(5):1512–25.
Wang Z, Bai M, Oh E, Zhu J. Brassinosteroid signaling network and regulation of photomorphogenesis. Annu Rev Genet. 2012;46:701–24.
Guo H, Li L, Aluru M, Aluru S, Yin Y. Mechanisms and networks for brassinosteroid regulated gene expression. Curr Opin Plant Biol. 2013;16(5):545–53.
Bensen R, Johal G, Crane V, Tossberg J, Schnable P, Meeley R, Briggs S. Cloning and characterization of the maize an1 gene. Plant Cell. 1995;7(1):75–84.
Winkler R, Helentjaris T. The maize Dwarf3 gene encodes a cytochrome P450-mediated early step in gibberellin biosynthesis. Plant Cell. 1995;7(8):1307–17.
Lawit S, Wych H, Xu D, Kundu S, Tomes D. Maize DELLA proteins dwarf plant8 and dwarf plant9 as modulators of plant development. Plant Cell Physiol. 2010;51(11):1854–68.
Bishop G, Nomura T, Yokota T, Harrison K, Noguchi T, Fujioka S, Takatsuto S, Jones J, Kamiya Y. The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proc Natl Acad Sci U S A. 1999;96(4):1761–6.
Schultz L, Kerckhoffs L, Klahre U, Yokota T, Reid J. Molecular characterization of the brassinosteroid-deficient lkb mutant in pea. Plant Mol Biol. 2001;47(4):491–8.
Shimada Y, Fujioka S, Miyauchi N, Kushiro M, Takatsuto S, Nomura T, Yokota T, Kamiya Y, Bishop G, Yoshida S. Brassinosteroid-6-oxidases from Arabidopsis and tomato catalyze multiple C-6 oxidations in brassinosteroid biosynthesis. Plant Physiol. 2001;126(2):770–9.
Hong Z, Ueguchi-Tanaka M, Shimizu-Sato S, Inukai Y, Fujioka S, Shimada Y, Takatsuto S, Agetsuma M, Yoshida S, Watanabe Y, Uozu S, Kitano H, Ashikari M, Matsuoka M. Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J. 2002;32(4):495–508.
Best N, Hartwig T, Budka J, Fujioka S, Johal G, Schulz B, Dilkes B. mEncodes a maize ortholog of the Arabidopsis brassinosteroid biosynthesis gene DWARF1, identifying developmental interactions between brassinosteroids and gibberellins. Plant Physiol. 2016;171(4):2633–47.
Hu Z, Yan H, Yang J, Yamaguchi S, Maekawa M, Takamure I, Tsutsumi N, Kyozuka J, Nakazono M. Strigolactones negatively regulate mesocotyl elongation in rice during germination and growth in darkness. Plant Cell Physiol. 2010;51(7):1136–42.
Benjamins R, Scheres B. Auxin: the looping star in plant development. Annu Rev Plant Biol. 2008;59:443–65.
Cheng Y, Dai X, Zhao Y. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell. 2007;19(8):2430–9.
Yamada M, Greenham K, Prigge M, Jensen P, Estelle M. The transport inhibitor response2 gene is required for auxin synthesis and diverse aspects of plant development. Plant Physiol. 2009;151(1):168–79.
Stepanova A, Robertson-Hoyt J, Yun J, Benavente L, Xie D, Dolezal K, Schlereth A, Jürgens G, Alonso J. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 2008;133(1):177–91.
Phillips K, Skirpan A, Liu X, Christensen A, Slewinski T, Hudson C, Barazesh S, Cohen J, Malcomber S, McSteen P. vanishing tassel2 encodes a grass-specific tryptophan aminotransferase required for vegetative and reproductive development in maize. Plant Cell. 2011;23(2):550–66.
Kepinski S, Leyser O. Auxin-induced SCFTIR1-aux/IAA interaction involves stable modification of the SCFTIR1 complex. Proc Natl Acad Sci U S A. 2004;101(33):12381–6.
Quint M, Gray W. Auxin signaling. Curr Opin Plant Biol. 2006;9(5):448–53.
Hamann T, Benkova E, Bäurle I, Kientz M, Jürgens G. The Arabidopsis bodenlos gene encodes an auxin response protein inhibiting monopteros-mediated embryo patterning. Genes Dev. 2002;16(13):1610–5.
Hardtke C, Ckurshumova W, Vidaurre D, Singh S, Stamatiou G, Tiwari S, Hagen G, Guilfoyle T, Berleth T. Overlapping and non-redundant functions of the Arabidopsis auxin response factors monopteros and nonphototropic hypocotyl 4. Development. 2004;131(5):1089–100.
Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435(7041):441–5.
Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann J, Jürgens G, Estelle M. Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell. 2005b;9(1):109–19.
Grieneisen V, Xu J, Marée A, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449(7165):1008–13.
Multani DS, Briggs SP, Chamberlin MA, Blakeslee JJ, Murphy AS, Johal GS. Loss of an MDR transporter in compact stalks of maize br2 and sorghum dw3 mutants. Science. 2003;302(5642):81–4.
Xing A, Gao Y, Ye L, Zhang W, Cai L, Ching A, Llaca V, Johnson B, Liu L, Yang X, Kang D, Yan J, Li J. A rare SNP mutation in Brachytic2 moderately reduces plant height and increases yield potential in maize. J Exp Bot. 2015;66(13):3791–802.
Knöller A, Blakeslee J, Richards E, Peer W, Murphy A. Brachytic2/ZmABCB1 functions in IAA export from intercalary meristems. J Exp Bot. 2010;61(13):3689–96.
Geisler M, Blakeslee J, Bouchard R, et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 2005;44(2):179–94.
Santelia D, Vincenzetti V, Azzarello E, Bovet L, Fukao Y, Düchtig P, Mancuso S, Martinoia E, Geisler M. MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development. FEBS Lett. 2005;579(24):5399–406.
Greene J, Brown N, DiDomenico B, Kaplan J, Eide D. The GEF1 gene of saccharomyces cerevisiae encodes an integral membrane protein; mutations in which have effects on respiration and iron-limited growth. Mol Gen Genet. 1993;241(5–6):542–53.
Petrásek J, Friml J. Auxin transport routes in plant development. Development. 2009;136(16):2675–88.
Wei L, Zhang X, Zhang Z, Liu H, Lin Z. A new allele of the Brachytic2 gene in maize can efficiently modify plant architecture. Heredity. 2018;121(1):75.
Balzan S, Carraro N, Salleres B, Cortivo CD, Tuinstra MR, Johal G, Varotto S. Genetic and phenotypic characterization of a novel brachytic2 allele of maize. Plant Growth Regul. 2018;86(1):81–92.
Li C, Zheng L, Zhang J, Lv Y, Liu J, Wang X, Palfalvi G, Wang G, Zhang Y. Characterization and functional analysis of four HYH splicing variants in Arabidopsis hypocotyl elongation. Gene. 2017;619:44–9.
Geisler M, Murphy A. The ABC of auxin transport: the role of p-glycoproteins in plant development. FEBS Lett. 2006;580(4):1094–102.
Hellsberg E, Montanari F, Ecker G. The ABC of phytohormone translocation. Planta Med. 2015;81(6):474–87.
Pighin J, Zheng H, Balakshin L, Goodman I, Western T, Jetter R, Kunst L, Samuels A. Plant cuticular lipid export requires an ABC transporter. Science. 2004;306(5696):702–4.
Nishimura T, Mori Y, Furukawa T, Kadota A, Koshiba T. Red light causes a reduction in IAA levels at the apical tip by inhibiting de novo biosynthesis from tryptophan in maize coleoptiles. Planta. 2006;224(6):1427–35.
Nishimura T, Koshiba T. Auxin biosynthesis site and polar transport in maize coleoptiles. Plant Signal Behav. 2010;5(5):573–5.
Grunewald W, Friml J. The march of the PINs: developmental plasticity by dynamic polar targeting in plant cells. EMBO J. 2010;29(16):2700–14.
Mravec J, Kubes M, Bielach A, Gaykova V, Petrásek J, Skůpa P, Chand S, Benková E, Zazímalová E, Friml J. Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development. 2008;135(20):3345–54.
Bandyopadhyay A, Blakeslee J, Lee O, Mravec J, Sauer M, Titapiwatanakun B, Makam S, Bouchard R, Geisler M, Martinoia E, Friml J, Peer W, Murphy A. Interactions of PIN and PGP auxin transport mechanisms. Biochem Soc Trans. 2007;35(1):137–41.
Blakeslee J, Bandyopadhyay A, Lee O, et al. Interactions among PIN-formed and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell. 2007;19(1):131–47.
Sidler M, Hassa P, Hasan S, Ringli C, Dudler R. Involvement of an ABC transporter in a developmental pathway regulating hypocotyl cell elongation in the light. Plant Cell. 2008;10(10):1623–36.
Noh B, Murphy A, Spalding E. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell. 2001;13(11):2441–54.
Weijers D, Schlereth A, Ehrismann J, Schwank G, Kientz M, Jürgens G. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Dev Cell. 2006;10(2):265–70.
Schenck D, Christian M, Jones A, Lüthen H. Rapid auxin-induced cell expansion and gene expression: a four-decade-old question revisited. Plant Physiol. 2010;152(3):1183–5.
Barbier F, Dun E, Beveridge C. Apical dominance. Curr Biol. 2017;27(17):864–5.
Kebrom T. A growing stem inhibits bud outgrowth-the overlooked theory of apical dominance. Front Plant Sci. 2017;8:1874.
Wang Q, Kohlen W, Rossmann S, Vernoux T, Theres K. Auxin depletion from the leaf axil conditions competence for axillary meristem formation in Arabidopsis and tomato. Plant Cell. 2014a;26(5):2068–79.
Wang Y, Wang J, Shi B, Yu T, Qi J, Meyerowitz E, Jiao Y. The stem cell niche in leaf axils is established by auxin and cytokinin in Arabidopsis. Plant Cell. 2014b;26(5):2055–67.
Wang Y, Jiao Y. Auxin and above-ground meristems. J Exp Bot. 2018;69(2):147–54.
Tsuda K, Abraham-Juarez M, Maeno A, Dong Z, Aromdee D, Meeley R, Shiroishi T, Nonomura K, Hake S. KNOTTED1 cofactors, BLH12 and BLH14, regulate internode patterning and vein anastomosis in maize. Plant Cell. 2017;29(5):1105–18.
Sato-Izawa K, Nakaba S, Tamura K, Yamagishi Y, Nakano Y, Nishikubo N, Kawai S, Kajita S, Ashikari M, Funada R, Katayama Y, Kitano H. DWARF50 (D50), a rice (Oryza sativa L.) gene encoding inositol polyphosphate 5-phosphatase, is required for proper development of intercalary meristem. Plant Cell Environ. 2012;35(11):2031–44.
Biedroń M, Banasiak A. Auxin-mediated regulation of vascular patterning in Arabidopsis thaliana leaves. Plant Cell Rep. 2018;3(9):1215–29.
Long J, Barton M. The development of apical embryonic pattern in Arabidopsis. Development. 1998;125(16):3027–35.
Acknowledgements
This work was financially supported in part by National Key Laboratory of Wheat and Maize Crop Science in Henan Agricultural University and the National Natural Science Foundation of China (No.31571684). We thank Chengdu Diversity Co. Lit (Chengdu Sichuan) for the measurement of the endogenous hormone auxin.
Funding
This work was financially supported in part by National Key Laboratory of Wheat and Maize Crop Science in Henan Agricultural University and the National Natural Science Foundation of China (No.31571684). The funding body was involved in the material creation and designing the study.
Author information
Authors and Affiliations
Contributions
YBH and JHT designed the research. XGZ, XBH, YHL, LJZ, QY, HJZ, XRH, FLZ, and LC performed the experiments. XGZ and XBH wrote the manuscript, JJZ, YFH, HML, YPL, GWY and HHH revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Probe sequences for BR2 in situ hybridization and primers used for sequencing and qRT-PCR as well as homologous recombinant.
Additional file 2: Table S2.
Plant height separation performance in F2 and BC1 populations.
Additional file 3: Figure S1.
The br2 allelism test for d2014.
Additional file 4: Figure S2.
Comparison of certain internode length between d2014 and WT at the 12-leaf, 14-leaf, and 20-leaf stages.
Additional file 5: Figure S3.
Different mutation sites of br2 alleles.
Additional file 6: Figure S4.
The analysis of protein domains of PGP1-T01 and PGP1-T02.
Additional file 7: Figure S5.
The morphological characteristics of the stems of d2014 and WT.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, X., Hou, X., Liu, Y. et al. Maize brachytic2 (br2) suppresses the elongation of lower internodes for excessive auxin accumulation in the intercalary meristem region. BMC Plant Biol 19, 589 (2019). https://doi.org/10.1186/s12870-019-2200-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-019-2200-5