Abstract
Sensory processing in the auditory brainstem can be studied with auditory brainstem responses (ABRs) across species. There is, however, a limited understanding of ABRs as tools to assess the effect of pharmacological interventions. Therefore, we set out to understand how pharmacological agents that target key transmitter systems of the auditory brainstem circuitry affect ABRs in rats. Given previous studies, demonstrating that Nrxn1α KO Sprague Dawley rats show substantial auditory processing deficits and altered sensitivity to GABAergic modulators, we used both Nrxn1α KO and wild-type littermates in our study. First, we probed how different commonly used anesthetics (isoflurane, ketamine/xylazine, medetomidine) affect ABRs. In the next step, we assessed the effects of different pharmacological compounds (diazepam, gaboxadol, retigabine, nicotine, baclofen, and bitopertin) either under isoflurane or medetomidine anesthesia. We found that under our experimental conditions, ABRs are largely unaffected by diverse pharmacological modulation. Significant modulation was observed with (i) nicotine, affecting the late ABRs components at 90 dB stimulus intensity under isoflurane anesthesia in both genotypes and (ii) retigabine, showing a slight decrease in late ABRs deflections at 80 dB stimulus intensity, mainly in isoflurane anesthetized Nrxn1α KO rats. Our study suggests that ABRs in anesthetized rats are resistant to a wide range of pharmacological modulators, which has important implications for the applicability of ABRs to study auditory brainstem physiology.
Similar content being viewed by others
Introduction
Auditory brainstem responses (ABRs), also known as brainstem auditory evoked potentials, are electrical potentials commonly evoked by click sounds, which can be measured non-invasively and that speak to synaptic transmission within the auditory brainstem circuits. ABRs are widely used for assessing hearing thresholds [1], intraoperative neuromonitoring [2], screening for sensory abnormalities in neurodevelopmental disorders [3], or testing ototoxicity in drug development [4].
In both humans and rodents, ABRs consist of distinct deflections (also referred to as ‘waves’), that are generated by the activation of specific neuronal nuclei within the auditory pathway [5,6,7]. We can differentiate between four to five waves, with a temporal separation of about 0.8–1.0 ms each [8]. Wave I is generated by the distal part of the auditory nerve (AN). Wave II reflects the projection of the cochlear nucleus (CN); Wave III is generated by the superior olivary complex (SOC), wave IV by the lateral lemniscus and inferior colliculus (IC), and lastly wave V reflects signal transmission from the thalamus to the auditory cortex (AC) [9,10,11].
The neurotransmitter systems in the auditory brainstem circuitry are mainly glutamatergic, GABAergic, glycinergic, and cholinergic [12,13,14]. The ventral part of the cochlear nucleus sends glutamatergic projections to the lateral superior olive (LSO), the medial superior olive, and the medial nucleus of the trapezoid body (MNTB), while the dorsal cochlear nucleus send glutamatergic projections to the IC. MNTB neurons make glycinergic inhibitory synapses with the LSO neurons. SOC neurons send glutamatergic projections to the lateral lemniscus and the IC, targeting the medial geniculate body in the thalamus, which sends glutamatergic projections again to the AC [12, 13]. The descending auditory projections start from the AC and terminate in subcortical auditory centers, such as the IC in the auditory brainstem [15, 16].
While ABRs have been used extensively to assess auditory brainstem physiology [17] and its abnormalities [18, 19], the capacity of ABRs to be modulated by pharmacological agents remains poorly understood. Therefore, we set out to test the effects of various pharmacological modulators on rodent ABRs. Here we used acute pharmacological treatments prior to the ABR measurements. We tested the effects of enhancing the GABAergic neurotransmission in the auditory brainstem via injecting diazepam (a γ2-containing GABAA receptor enhancer), gaboxadol (a δ-containing GABAA receptor agonist) or baclofen (a GABAB receptor agonist). Both GABAA and GABAB receptors are widely expressed along the different nuclei in the auditory brainstem [20,21,22,23,24]. A previous study emphasized the role of baclofen and diazepam as potent modulators of both the excitability of neurons in the ascending auditory pathway and the processing of auditory information by IC neurons [25]. Moreover, we used bitopertin (a non-competitive selective inhibitor of glycine transporter 1 (GlyT-1) [26]) to investigate the role of increased glycinergic neurotransmission on ABRs. GlyT-1 is one of the two glycine transporters family, which work as an endogenous regulator of glycine, but also play a crucial role in maintaining glycine neurotransmission homeostasis and modulating glycine levels at N-methyl-D-aspartic acid (NMDA) sites [26]. GlyT-1 is widely expressed in neuronal and glial cells [27], among the different brain regions including the auditory brainstem [28]. We also used retigabine (a broad Kv7 enhancer), which is well known to increase neuronal hyperpolarization [29] and thus may reduce synaptic outputs in the auditory brainstem by acting on the Kv7.4 channels of the outer hair cells in the inner ear [30]. In addition, we used nicotine, a nicotinic acetylcholine receptor (nAChR) agonist, to inhibit excitatory output of the outer hair cells in the cochlea [31].
Initially, we tested these compounds under the application of isoflurane, a frequently used anesthesia method for rodent ABRs [1]. In a second step, we also tested a subset of compounds under medetomidine anesthesia that may better preserve the dynamics of neural circuits and therefore could reveal compound effects different from those under isoflurane. Furthermore, by using three well-regarded anesthesia methods (isoflurane, ketamine/xylazine, and medetomidine), we compared the ABRs between Nrxn1α KO rats and wildtype littermates under the most frequently used anesthetic conditions. In humans, a 2p16.3 (NRXN1) deletion is associated with intellectual disability, autism spectrum disorder, and schizophrenia [32]. Previously, we showed that auditory processing is substantially impaired in Nrxn1α KO rats, and that cortical auditory responses are impacted differently by GABAergic modulation compared to their wild-type littermates [33]. Therefore, the inclusion of Nrxn1α KO Sprague Dawley rats allowed us to test if functional alterations of auditory brainstem circuits could explain some of our previous results.
Materials and methods
Animals
Experiments were conducted on adult Nrxn1α KO rats and wild-type littermates (strain: Sprague Dawley (SD)-Nrxn1 < tm1sage > bred by Charles River, France. Only male rats were used. Rats were housed in groups of two, in a temperature-controlled room on a 12 h light/dark cycle with ad libitum food and water. Overall, four animal cohorts have been used, since is not feasible to run all tests in a single cohort given limitations from age-effects and animal welfare perspective, as Table 1 shows.
Anesthesia
Isoflurane-based anesthesia started with inducing unconsciousness via isoflurane inhalation (Isofluran Baxter, Cat. no.: hdg9623, Baxter, GER), in a chamber filled with 5% isoflurane for 3 min and maintained throughout the ABR recordings at 2.5% isoflurane in medical air.
For medetomidine-based anesthesia, animals were first anesthetized via isoflurane inhalation (4% isoflurane for 4 min), and then injected with a bolus of medetomidine (0.1 mg/kg, s.c., Dorbene, Graeub, CH), followed by 1 min isoflurane inhalation at 4% to maintain anesthesia until the effect of medetomidine fully unfolded. Before starting the ABR measurements, isoflurane inhalation was stopped for 5 min to ensure isoflurane washout. At the end of the recording, Atipamezoli (0.1 mg/kg, s.c., Alzane, Graeub, CH) was injected to reverse the sedative and analgesic effects of medetomidine. Ketamine-based anesthesia was performed by i.p injection of a ketamine/xylazine mixture (80 mg/kg ketamine mixed with 5 mg/kg xylazine, i.p., Ketasol 100 with Xylasol, Graeub, CH). ABR measurements were started 10 min after injection.
Pharmacology
Doses and pre-treatment times were chosen according to previously established pharmacokinetic/pharmacodynamics profiles [33, 35,36,37,38,39]. Treatment conditions were randomized using a Latin-based square design (also referred to as “William’s design”), in which each animal received every compound (or vehicle) in a randomized fashion. The randomization controls for putative day-to-day variability and allows within-subject comparison strengthening statistical power, in line with previous neuropharmacological studies [33]. No blinding was performed. The duration of the washout phase between dosing was at least 48 h. The control condition was represented by the administration of an equal volume of the vehicle solution (0.9% saline + 0.3% Tween20: Cat. no.: 11332465001, Sigma-Aldrich, GER). Animals were injected with diazepam (3 mg/kg, Roche Pharmaceuticals, CH), gaboxadol (10 mg/kg, Cat. no.: T101, Sigma-Aldrich, GER), retigabine (3 mg/kg, Roche Pharmaceuticals, CH), nicotine (5 mg/kg, ( −) nicotine hydrogen tartrate salt, Cat. no.: SML1236, Sigma–Aldrich, GER), baclofen (5 mg/kg, Cat. no.: B5399, Sigma–Aldrich, GER), bitopertin (10 mg/kg, Roche Pharmaceuticals, CH) or vehicle solution. Intraperitoneal injection was performed 15 min before starting the ABR measurements for all compounds, except for bitopertin, which reaches maximal exposures at around 60 min after application. Given the time necessary for the preparation (anesthesia, placing of the animal in to the recording device and positioning the electrodes) the actual ABR recordings happened about 30 min post-dosing (or at 75 min in case of bitopertin).
Electrophysiological recording and acoustic stimulation
Prior to the ABR measurements, sound volume calibration was performed following the RZ6 Open Field Calibration Setup (Tucker-Davis Technologies, FL), including a signal conditioner and a 1/4-inch Prepolarized Free-field microphone (model nr. 480c02, ICP® SENSOR, PCB, NY, USA). The acoustic stimuli used in the ABRs assessment consisted of 512 click sounds, generated at 200 kHz sampling rate. Each click sound is a broadband mono-phasic square wave signal (0.1 ms). The click sounds were presented at a rate of 21 clicks/s, at different sound levels (90, 80, 70, 60, 50, 40, 30, 20, 10 dB SPL), starting with the highest stimulus intensities, in line with established protocols [33, 40]. The ABR measurements were conducted in a sound-attenuating and electrostatically grounded chamber. Body temperature of anesthetized animals (see above) was maintained at 37◦ C using a thermic heating pad (Kent Scientific Corporation, CN, USA). Click sounds were generated with a multi-field speaker (MF1, Tucker-Davis Technologies, FL, USA) connected to a RZ6-A-1 input/output processor (Tucker-Davis Technologies, FL, USA). The speaker was positioned 10 cm from the animal’s right ear. ABR signals were recorded with 13 mm subdermal needle electrodes (Cat. no.: NS-s83018-r9-10, Rochester, Coral Springs, FL, USA), with the signal electrodes placed on the vertex and reference and ground electrodes placed under the ipsi- and contralateral ear, respectively, connected to a RA4PA preamplifier/digitizer and RA4LI low impedance head stage (Tucker-Davis Technologies, FL, USA). Signals were acquired using the following settings: 12 kHz sampling rate, 5 kHz low pass, 100 Hz high pass, 50 Hz notch, using the BioSigRZ software (version 5.5, TDT, FL, USA).
Euthanasia
At the end of the experimental producers, animals received terminal anesthesia; 150 mg/kg pentobarbital (Eskonarkon, Switzerland), i.p.,1:20 dilution with NaCl, followed by decapitation, after confirming a lack of reflexes by paw pinching.
Data processing and analysis
Data analysis was performed as previously described [33]. In brief, in a pre-processing step ABR data were normalized to its pre-stimulus baseline. Resulting ABR waveforms were statistically tested for differences between conditions (see Statistical testing).
Statistical testing
Statistical testing was performed with paired or unpaired cluster-based permutation tests (CBPT) depending on the condition, using custom Python scripts. In brief, first CBPT performs individual t-tests (two-tailed, significance level set to p < 0.05) for each data point. The resulting clusters are then tested for significance by comparing the summed t-values of the initial clusters with summed t-values of clusters obtained from permuted data (here, shuffling over the time domain) over many iterations (N = 1000 permutations, significance threshold: p < 0.05), thereby correcting for multiple comparisons. We visualize both cluster types (with permutation testing: black bars above graphs; and w/o permutation: grey bars, indicating statistical trends). Given that qualitatively no apparent outliers were present, no specific test was performed for outlier detection. No exclusion criteria were predetermined, and no animals were excluded from the statistical analysis. For one animal under one condition in the pharmacology study (nicotine, 5.0 mg/kg), missing vehicle data were input by averaging the respective data points of all other animals under this condition, to allow for paired analyses.
Results
Auditory brainstem responses are similar for adult Nrxn1α KO Sprague Dawley rats and wild-type littermates under different anesthetics
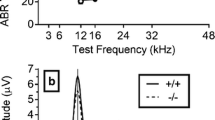
First, we asked whether Nrxn1α KO Sprague Dawley rats show alterations in their ABRs compared to wild-type littermates. To mitigate the risk that putative genotypic differences are missed due to the effects of anesthesia, we performed ABR recordings under three different types of anesthesia. We found that under all conditions, ABRs of Nrxn1α KO animals largely resembled those of their wild-type littermates (Fig. 1 and Additional file 1: Figs. S1 and S2). Except for statistically significant differences in the very late components of the ABRs elicited at 80 dB under medetomidine (Additional file 1: Fig. S2B; time window 5.4–6.5 ms, d = − 1.23, p = 0.028 and time window 7 – 8.5 ms, d = 1.12, p = 0.012).
Comparison of auditory brainstem responses between Nrxn1α KO Sprague Dawley and wild-type littermates rats. ABR waveforms across different stimulus intensities (90, 70, 50 dB) under A isoflurane, B ketamine/xylazine and C medetomidine anesthesia. Recordings from the WT are in blue (N = 12) and Nrxn1α KO in red (N = 12). Data displayed as mean ± SEM, was tested with unpaired CBPT. No robust significant differences were found between genotypes across anesthesia methods. Grey bars above the graphs indicate clusters of significant differences before CBPT-based correction for multiple comparisons, i.e., indicating statistical trends
ABRs are largely resistant to pharmacological modulators under isoflurane anesthesia
Next, we assessed how pharmacological agents that modulate distinct neurotransmitter systems impact ABRs in both wild-type (Fig. 2 and Additional file 1: Fig. S3) and Nrxn1α KO Sprague Dawley rats (Fig. 3 and Additional file 1: Fig. S4). In our first set of experiments, we used isoflurane anesthesia, as it is arguably the most-widely used choice for rodent ABR measurements. In order to investigate the effects of increasing GABAergic neurotransmission, we tested diazepam at 3 mg/kg (a γ2-containing GABAA receptor enhancer; Fig. 2A and 3A), gaboxadol at 10 mg/kg (α4/6δ-containing GABAA receptor agonist; Fig. 2B and 3B) and baclofen at 5 mg/kg (a GABAB receptor agonist; Fig. 2C and 3C). To augment glycinergic neurotransmission we used bitopertin at 10 mg/kg (a GlyT-1 inhibitor; Fig. 2D and 3D). We used retigabine at 3 mg/kg (a pan-Kv7 enhancer; Fig. 2E and 3E) to increase neuronal hyperpolarization and, therefore, to overall reduce synaptic outputs. Nicotine was used at 5 mg/kg (a nAChR agonist; Fig. 2F and 3F) in order to inhibit output of outer hair cells of the cochlea. Interestingly, we found that, compared to the vehicle control, none of the applied pharmacological agents clearly impacted ABRs in either wild-type or Nrxn1α KO Sprague Dawley rats. The only statistically significant effects were observed with nicotine on ABRs elicited at 90 dB and with retigabine on ABRs elicited at 80 dB. nicotine showed a modulation of the very late components of the ABRs in both wild-type (Fig. 2F; time window 5.4–6.25 ms time window, d = − 1.27, p = 0.037) and Nrxn1α KO Sprague Dawley rats (Fig. 3F; time window 6.6–7.9 ms; d = 0.97, p = 0.009), while retigabine only affected ABRs of Nrxn1α KO Sprague Dawley rats (Additional file 1: Fig. S4C, time window 3.6–5.9 ms, d =− 0.84, p = 0.01).
Auditory brainstem responses post pharmacological treatment in WT Sprague Dawley rats under isoflurane anesthesia. ABR waveforms across different stimulus intensities (90, 70, 50 dB) post intraperitoneal injection with diazepam (3 mg/kg) in magenta; (N = 14), gaboxadol (10 mg/kg) in teal; (N = 14), baclofen (5 mg/kg) in blue; (N = 14), bitopertin (10 mg/kg) in purple, retigabine (3 mg/kg) in red; (N = 18), nicotine (5 mg/kg) in yellow; (N = 14),; (N = 14), or vehicle solution in black (0.9% saline + 0.3% Tween). Within each experimental block, dosing was counterbalanced, and applied 15 min prior to the ABR recordings for all compounds, except for bitopertin (60 min pre-treatment time). The Black bars above the graphs indicate clusters of significant differences between conditions. The Gray bars indicate clusters that have not reached significance threshold post-permutations. Data displayed as mean ± SEM
Auditory brainstem responses post pharmacological treatments in Nrxn1α Sprague Dawley rats under isoflurane anesthesia. ABR waveforms across different stimulus intensities (90, 70, 50 dB) post intraperitoneal injection with diazepam (3 mg/kg) in magenta; (N = 14), gaboxadol (10 mg/kg) in teal; (N = 14), baclofen (5 mg/kg) in blue; (N = 13), bitopertin (10 mg/kg) in purple; (N = 11), retigabine (3 mg/kg) in red; (N = 16), nicotine (5 mg/kg) in yellow; (N = 14), or vehicle solution in black (0.9% saline + 0.3% Tween). Within each experimental block, dosing was counterbalanced, and applied 15 min prior to the ABR recordings for all compounds, except for in bitopertin (60 min pre-treatment time). The Black bars above the graphs indicated CBPT clusters of significant differences within subjects, i.e., between conditions. The Gray bars indicate clusters that have not reached significance threshold post-permutations. Data displayed as mean ± SEM
ABRs are largely resistant to pharmacological modulations under medetomidine anesthesia
With the lack of pharmacological modulation observed under isoflurane, we next tested if ABRs could be modulated more clearly under medetomidine, a widely used anesthetic in functional imaging that is considered to preserve better network dynamics as compared to isoflurane or ketamine. To test this hypothesis, we focused on the three compounds diazepam (Fig. 4A and 5A), bitopertin (Fig. 4B and 5B) and retigabine (Fig. 4C and 5C). Like our observations under isoflurane, pharmacological modulation did not alter ABRs of both wild-type (Fig. 4 and Additional file 1: Fig. S5) and Nrxn1α KO Sprague Dawley rats (Fig. 5 and Additional file 1: Fig. S6) under medetomidine. The only statistically significant difference was found for retigabine in wild-type animals, reducing the amplitude of late components of ABRs elicited at 40 dB (Additional file 1: Fig. S5C; time window 3.8–5.5 ms, d = -1.44, p = 0.012; and time window 5.6–7 ms, d = -1.68, p = 0.013).
Auditory brainstem responses post pharmacological treatment in WT Sprague Dawley rats under medetomidine anesthesia. ABR waveforms across different stimulus intensities (90, 70, 50 dB) post intraperitoneal injection with diazepam (3 mg/kg) in magenta; (N = 12), bitopertin (10 mg/kg) in purple; (N = 12), retigabine (3 mg/kg) in red; (N = 12), or vehicle solution in black (0.9% saline + 0.3% Tween). Within each experimental block, dosing was counterbalanced, and applied 15 min prior to the ABR recordings for all compounds, except for in bitopertin (60 min pre-treatment time). Data displayed as mean ± SEM, was tested with unpaired CBPT. No robust significant differences were found between genotypes across anesthesia methods. Grey bars above the graphs indicate clusters of significant differences before CBPT-based correction for multiple comparisons, i.e., indicating statistical trends
Auditory brainstem responses post pharmacological treatment in Nrxn1α KO Sprague Dawley rats under medetomidine anesthesia. ABR waveforms across different stimulus intensities (90, 70, 50 dB) post intraperitoneal injection with diazepam (3 mg/kg) in magenta; (N = 12), bitopertin (10 mg/kg) in purple; (N = 12), retigabine (3 mg/kg) in red; (N = 12), or vehicle solution in black (0.9% saline + 0.3% Tween). Within each experimental block, dosing was counterbalanced, and applied 15 min prior to the ABR recordings for all compounds, except for in bitopertin (60 min pre-treatment time). Data displayed as mean ± SEM, was tested with unpaired CBPT. No robust significant differences were found between genotypes across anesthesia methods. Grey bars above the graphs indicate clusters of significant differences before CBPT-based correction for multiple comparisons, i.e., indicating statistical trends
Discussion
The current study explored the impact of different anesthetics and pharmacological tool compounds in wild-type and Nrxn1α KO Sprague Dawley rats and shows for the first time that rat ABRs are unaffected by diverse pharmacological modulators.
First, using three of the most widely used anesthetics for rodents, we confirmed that ABRs without additional pharmacological intervention are similar between adult Nrxn1α KO Sprague Dawley rats and their wild-type littermates. Our results align with our previous studies that probed ABRs in adult wild-type and Nrxn1α KO Sprague Dawley rats under isoflurane anesthesia only [33]. Our current study expands this finding by demonstrating the lack of genotypic differences also under ketamine/xylazine and medetomidine anesthesia. This finding is important, since previous studies showed that the choice of anesthesia (e.g., isoflurane vs. ketamine/xylazine) significantly affected ABR characteristics [41], raising the possibility that genotypic differences may be missed with just using one type of anesthesia with a specific mode of action. Isoflurane and ketamine/xylazine (the two most widely-used anesthetics for rodents ABRs [1]) share many molecular targets, including glycine receptors [42], GABAA [42,43,44,45] and GABAB receptors [46, 47], glutamate receptors [48,49,50] (including NMDA receptors [51,52,53]), and nACh receptors [54, 55]. All these receptors are widely expressed in the brainstem and along the auditory pathway [12, 13]. Any changes in these neurotransmitter systems may affect the transmission of auditory information from the cochlea to higher brain areas [54]. Indeed, Santarelli et al. showed that the latencies of ABR waves are significantly increased during isoflurane anesthesia compared to awake ABRs in Sprague Dawley rats [56]. These differences could be due to isoflurane reducing the glutamatergic neurotransmission at pre- and postsynaptic sites of inner hair cells [56] or by augmenting GABAergic inhibition within the auditory brainstem circuits. While similar circuit engagement can be expected with ketamine/xylazine, Ruebhausen et al. showed that isoflurane elevates hearing thresholds by around 30 dB more than ketamine/xylazine-based anesthesia [41]. This could be due to an additional effect of isoflurane by increasing blood flow to the brainstem and tissue perfusion [41, 57], in addition to a decrease in synaptic glutamate release [58], potentially reducing stimulus-driven activity [41]. As an alternative to isoflurane or ketamine/xylazine, we used medetomidine, an α2-adrenoceptor agonist, which is a common choice for fMRI studies as it preserved the dynamics of the brain better than α-chloralose or isoflurane [59, 60]. Indeed, previous studies show that medetomidine administration only marginally influences auditory-evoked potentials, picked up in the midbrain [61] and the cortex [62]. Other studies show that dexmedetomidine, a medetomidine isomer, demonstrated a minimal effect on ABRs in children [63] and it could be a better alternative for the commonly used oral chloral hydrate sedation [64].
A key point of the current study is that testing a diverse set of pharmacological modulators showed either none or only marginal effects on ABRs. This is surprising since the tool compounds, and doses used, engage receptors that are involved in signal transmission within auditory brainstem circuits. Only nicotine and retigabine treatment led to significant, but minor effects in the ABR. The effects of nicotine were confined to the very late phase of the ABR, resembling the activation of higher-order brain regions, and only at 90 dB stimulus intensity. While the major targets of nicotine (nACh receptors) are expressed at outer hair cells to regulate their sensitivity [65], no effects on the very early components of the ABR were evident. Therefore, our data imply for the action of nicotine on higher-order brain circuits to alter auditory processing [66]. For retigabine, we observed slightly reduced amplitudes of late components of the ABR at 80 dB, but not at 90 dB or at 70 dB. The volume-specific effect challenges the robustness and interpretability of the finding. More importantly, the fact that retigabine enhances voltage-gated potassium channels (such as Kv7.4) expressed in the auditory brainstem [67], but does not clearly affect the ABR, highlights yet again the resistance of ABRs to pharmacological modulation. Our findings are in line with previous studies, showing a lack of ABRs and hearing threshold modulation with retigabine [39]. Beyond our findings with other compounds (such as diazepam, baclofen, or biopterin), the notion of a more general issue with pharmacological modulation of ABRs, is further supported by other rodent studies, demonstrating limited modulations of ABRs (slight increase in wave 1 amplitude, but no effects on latency) even with a high dose of opioids [68]. This is different from earlier studies demonstrating that theophylline [69] or cocaine [70] change ABR characteristics likely due to ototoxic rather than neuromodulatory effects.
An intuitive explanation for the lack of pharmacological modulation of ABRs in rodents is the “masking” effects of anesthesia, which may either block the target receptors and/or reduce neuronal dynamics to the extent that does not allow for further pharmacological modulation. We mitigated this caveat by using diverse anesthetic protocols, including medetomidine, which largely preserves network dynamics. Further support for the resistance of ABRs to pharmacological modulation comes from human and non-human primate studies which allow awake ABR experiments. In this context, Samra et al. showed in awake rhesus monkeys that neither Scopolamine nor Morphine intravenous injection could modulate the ABR waves [71]. In addition, studies in humans report no effects of anesthetic agents, or drugs such as benzodiazepines, propofol, and ketamine on ABRs [2, 72].Nonetheless, in our rodent study, a technical detail worth mentioning is the placement of the ground electrode under the contralateral ear in an open sound field configuration, there is a possibility that activation of the contralateral pathways interferes with the signals measured between the ipsilateral ear and the vertex. Given that key ABR features (e.g. 4–5 waves at defined latencies, and dependency of ABR amplitudes on stimulus intensity) are intact in our measurements and because the contralateral grounding introduces a systematic difference, we do not expect that a potential impact of genotype or pharmacological modulation on auditory brainstem processing would remain unnoticed in our measurements. Also, it is worth mentioning that our study was restricted to measuring ABRs with click sound stimulation protocols. Future studies could investigate whether tone ABRs at specific frequencies might be more sensitive to pharmacological modulation than click ABRs. Also, while dose selection rigorously followed the literature, higher doses could be explored in future work.
However, a more general concern with ABR measurements is that it primarily detects the neural response to sound onset and therefore might limit the identification of pharmacological effects, e.g. on later components of auditory signal processing. Therefore, complementary methods such as surface EEG recordings represent useful tools for studying the physiology of auditory signal processing.
Independent of these considerations, our study suggests that rodent ABR measurements are unsuited for testing auditory circuit modulation by diverse pharmacology. This conclusion is critical for drug development programs that aim to tackle auditory processing deficits, such as in psychiatric and neurodevelopmental disorders, where sensory abnormalities might stem from early-life disruption of auditory brainstem circuits [3].
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ABRs:
-
Auditory brainstem responses
- Nrxn1α:
-
Neurexin1α
- AN:
-
Auditory nerve
- CN:
-
Cochlear nucleus
- SOC:
-
Superior olivary complex
- IC:
-
Inferior colliculus
- AC:
-
Auditory cortex
- LSO:
-
Lateral superior olive
- MNTB:
-
Medial nucleus of the trapezoid body
- CBPT:
-
Cluster-based permutation tests
References
Domarecka E, et al. Reporting data on auditory brainstem responses (ABR) in rats: recommendations based on review of experimental protocols and literature. Brain Sci. 2021;11:1596.
Nunes RR, Bersot CDA, Garritano JG. Intraoperative neurophysiological monitoring in neuroanesthesia. Curr Opin Anaesthesiol. 2018;31:532–8.
Seif A, Shea C, Schmid S, Stevenson RA. A systematic review of brainstem contributions to autism spectrum disorder. Front Integr Neurosci. 2021;15: 760116.
Abernathy MM, Gauvin DV, Tapp RL, Yoder JD, Baird TJ. Utility of the auditory brainstem response evaluation in non-clinical drug safety evaluations. J Pharmacol Toxicol. 2015;75:111–7.
Church MW, Kaltenbach JA. The Hamster’s auditory brain stem response as a function of stimulus intensity, tone burst frequency, and hearing loss. Ear Hearing. 1993;14:249–57.
Popelar J, Grecova J, Rybalko N, Syka J. Comparison of noise-induced changes of auditory brainstem and middle latency response amplitudes in rats. Hearing Res. 2008;245:82–91.
Alvarado JC, Fuentes-Santamaría V, Jareño-Flores T, Blanco JL, Juiz JM. Normal variations in the morphology of auditory brainstem response (ABR) waveforms: a study in wistar rats. Neurosci Res. 2012;73:302–11.
Eggermont JJ. Chapter 30 Auditory brainstem response. Handb Clin Neurol. 2019;160:451–64.
Picton TW, Stapells DR, Campbell KB. Auditory evoked potentials from the human cochlea and brainstem. J Otolaryngology Suppl. 1981;9:1–41.
Melcher JR, Guinan JJ, Knudson IM, Kiang NYS. Generators of the brainstem auditory evoked potential in cat. II. Correlating lesion sites with waveform changes. Hearing Res. 1996;93:28–51.
Møller AR, Jannetta PJ, Jho HD. Click-evoked responses from the cochlear nucleus: a study in human. Electroencephalogr Clin Neurophysiol Evoked Potentials Sect. 1994;92:215–24.
Pollak GD, Burger RM, Klug A. Dissecting the circuitry of the auditory system. Trends Neurosci. 2003;26:33–9.
Petralia, R. S. & Wenthold, R. J. Encyclopedia of Neuroscience. 2009; 2847–2853. https://doi.org/10.1007/978-3-540-29678-2_3957
Knipper M, Dijk PV, Nunes I, Rüttiger L, Zimmermann U. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol. 2013;111:17–33.
Winer JA, Chernock ML, Larue DT, Cheung SW. Descending projections to the inferior colliculus from the posterior thalamus and the auditory cortex in rat, cat, and monkey. Hearing Res. 2002;168:181–95.
Mellott JG, Bickford ME, Schofield BR. Descending projections from auditory cortex to excitatory and inhibitory cells in the nucleus of the brachium of the inferior colliculus. Frontiers Syst Neurosci. 2014;8:188.
Jewett DL, Romano MN, Williston JS. Human auditory evoked potentials: possible brain stem components detected on the scalp. Science. 1970;167:1517–8.
Castro AC, Monteiro P. Auditory dysfunction in animal models of autism spectrum disorder. Front Mol Neurosci. 2022;15: 845155.
Fujihira H, Itoi C, Furukawa S, Kato N, Kashino M. Auditory brainstem responses in adults with autism spectrum disorder. Clin Neurophysiol Pract. 2021;6:179–84.
Jamal L, Khan AN, Butt S, Patel CR, Zhang H. The level and distribution of the GABABR1 and GABABR2 receptor subunits in the rat’s inferior colliculus. Front Neural Circuit. 2012;6:92.
Cai S-P, Fang Z-Y, Yang S-M, Doi T. The effects of GABAergic neurotransmitters and GABAA receptors on the auditory afferent pathway in the brainstem analyzed by optical recording. Zhongguo Ying Yong Sheng Li Xue Za Zhi Zhongguo Yingyong Shenglixue Zazhi Chin J Appl Physiol. 2008;24:42–5.
Magnusson AK, Park TJ, Pecka M, Grothe B, Koch U. Retrograde GABA signaling adjusts sound localization by balancing excitation and inhibition in the brainstem. Neuron. 2008;59:125–37.
Campos ML, de Cabo C, Wisden W, Juiz JM, Merlo D. Expression of GABAA receptor subunits in rat brainstem auditory pathways: cochlear nuclei, superior olivary complex and nucleus of the lateral lemniscus. Neuroscience. 2001;102:625–38.
Hassfurth B, Grothe B, Koch U. The mammalian interaural time difference detection circuit is differentially controlled by GABAB receptors during development. J Neurosci. 2010;30:9715–27.
Szczepaniak WS, Møller AR. Effects of (−)-baclofen, clonazepam, and diazepam on tone exposure-induced hyperexcitability of the inferior colliculus in the rat: possible therapeutic implications for pharmacological management of tinnitus and hyperacusis. Hearing Res. 1996;97:46–53.
Martina M, et al. Glycine transporter type 1 blockade changes NMDA receptor-mediated responses and LTP in hippocampal CA1 pyramidal cells by altering extracellular glycine levels. J Physiology. 2004;557:489–500.
Cubelos B, Giménez C, Zafra F. Localization of the GLYT1 glycine transporter at glutamatergic synapses in the rat brain. Cereb Cortex. 2005;15:448–59.
Brill SE, et al. Glycinergic transmission in the presence and absence of functional GlyT2: lessons from the auditory brainstem. Frontiers Synaptic Neurosci. 2021;12: 560008.
Korsgaard MPG, et al. Anxiolytic effects of maxipost (BMS-204352) and retigabine via activation of neuronal Kv7 channels. J Pharmacol Exp Ther. 2005;314:282–92.
Kharkovets T, et al. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc National Acad Sci. 2000;97:4333–8.
Taranda J, et al. A point mutation in the hair cell nicotinic cholinergic receptor prolongs cochlear inhibition and enhances noise protection. Plos Biol. 2009;7: e1000018.
Viñas-Jornet M, et al. A common cognitive, psychiatric, and dysmorphic phenotype in carriers of NRXN1 deletion. Mol Genet Genom Med. 2014;2:512–21.
Janz P, et al. Neurexin1α knockout rats display oscillatory abnormalities and sensory processing deficits back-translating key endophenotypes of psychiatric disorders. Transl Psychiat. 2022;12:455.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the arrive guidelines for reporting animal research. Plos Biol. 2010;8: e1000412.
Yohn SE, Alberati D, Correa M, Salamone JD. Assessment of a glycine uptake inhibitor in animal models of effort-related choice behavior: implications for motivational dysfunctions. Psychopharmacology. 2017;234:1525–34.
Sun W, et al. Neonatal nicotine exposure impairs development of auditory temporal processing. Hearing Res. 2008;245:58–64.
Janz P, Nicolas MJ, Redondo RL, Valencia M. GABABR activation partially normalizes acute NMDAR hypofunction oscillatory abnormalities but fails to rescue sensory processing deficits. J Neurochem. 2022;161:417–34.
Szczepaniak WS, Møller AR. Effects of (-)-baclofen, clonazepam, and diazepam on tone exposure-induced hyperexcitability of the inferior colliculus in the rat: possible therapeutic implications for pharmacological management of tinnitus and hyperacusis. Hearing Res. 1996;97:46–53.
Li S, Choi V, Tzounopoulos T. Pathogenic plasticity of Kv7.2/3 channel activity is essential for the induction of tinnitus. Proc National Acad Sci. 2013;110:9980–5.
Scott KE, et al. Altered auditory processing, filtering, and reactivity in the cntnap2 knock-out rat model for neurodevelopmental disorders. J Neurosci. 2018;38:8588–604.
Ruebhausen MR, Brozoski TJ, Bauer CA. A comparison of the effects of isoflurane and ketamine anesthesia on auditory brainstem response (ABR) thresholds in rats. Hearing Res. 2012;287:25–9.
Grasshoff C, Antkowiak B. Effects of isoflurane and enflurane on GABAA and glycine receptors contribute equally to depressant actions on spinal ventral horn neurones in rats. Bja Br J Anaesth. 2006;97:687–94.
Wang D-S, Penna A, Orser BA. Ketamine increases the function of &ggr;-aminobutyric acid type a receptors in hippocampal and cortical neurons. Anesthesiology. 2017;126:666–77.
Topf N, Jenkins A, Baron N, Harrison NL. Effects of isoflurane on γ-aminobutyric acid type a receptors activated by full and partial agonists. Anesthesiology. 2003;98:306–11.
Irifune M, et al. Evidence for GABAA receptor agonistic properties of ketamine: convulsive and anesthetic behavioral models in mice. Anesthesia Analgesia. 2000;91:230–6.
Hung W-C, et al. GABAB receptor-mediated tonic inhibition of locus coeruleus neurons plays a role in deep anesthesia induced by isoflurane. NeuroReport. 2020;31:557–64.
Rosa PB, Neis VB, Ribeiro CM, Moretti M, Rodrigues ALS. Antidepressant-like effects of ascorbic acid and ketamine involve modulation of GABAA and GABAB receptors. Pharmacol Rep. 2016;68:996–1001.
Niciu MJ, Henter ID, Luckenbaugh DA, Charney DS. Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annu Rev Pharmacol. 2014;54:119–39.
Zuo Z. Isoflurane enhances glutamate uptake via glutamate transporters in rat glial cells. NeuroReport. 2001;12:1077–80.
Lazarevic V, Yang Y, Flais I, Svenningsson P. Ketamine decreases neuronally released glutamate via retrograde stimulation of presynaptic adenosine A1 receptors. Mol Psychiatr. 2021;26:7425–35.
Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86.
Dong Y, et al. Isoflurane facilitates synaptic NMDA receptor endocytosis in mice primary neurons. Curr Mol Med. 2012;13:488–98.
Zorumski CF, Izumi Y, Mennerick S. Ketamine: NMDA receptors and beyond. J Neurosci Off J Soc Neurosci. 2016;36:11158–64.
Cederholm JME, et al. Differential actions of isoflurane and ketamine-based anaesthetics on cochlear function in the mouse. Hearing Res. 2012;292:71–9.
Piao M-H, Liu Y, Wang Y-S, Qiu J-P, Feng C-S. Volatile anesthetic isoflurane inhibits LTP induction of hippocampal CA1 neurons through α4β2 nAChR subtype-mediated mechanisms. Ann Françaises D’anesthésie Réanimation. 2013;32:e135–41.
Santarelli R, et al. Effects of isoflurane on the auditory brainstem responses and middle latency responses of rats. Acta Oto-laryngol. 2003;123:176–81.
Boarini DJ, Kassell NF, Coester HC, Butler M, Sokoll MD. Comparison of systemic and cerebrovascular effects of isoflurane and halothane. Neurosurgery. 1984;15:400.
Larsen M, Valø E, Berg-Johnsen J, Langmoen I. Isoflurane reduces synaptic glutamate release without changing cytosolic free calcium in isolated nerve terminals. Eur J Anaesthesiol. 1998;15:224–9.
Sirmpilatze N, Baudewig J, Boretius S. Temporal stability of fMRI in medetomidine-anesthetized rats. Sci Rep-uk. 2019;9:16673.
Petrinovic MM, et al. A novel anesthesia regime enables neurofunctional studies and imaging genetics across mouse strains. Sci Rep-uk. 2016;6:24523.
Ter-Mikaelian M, Sanes DH, Semple MN. Transformation of temporal properties between auditory midbrain and cortex in the awake Mongolian gerbil. J Neurosci. 2007;27:6091–102.
Thornton C, Lucas MA, Newton DE, Doré CJ, Jones RM. Effects of dexmedetomidine on isoflurane requirements in healthy volunteers. 2: auditory and somatosensory evoked responses. Brit J Anaesth. 1999;83:381–6.
Godbehere J, et al. Auditory brainstem response testing using intranasal dexmedetomidine sedation in children: a pilot study. Int J Audiol. 2021;60:549–54.
Reynolds J, Rogers A, Medellin E, Guzman JA, Watcha MF. A prospective, randomized, double-blind trial of intranasal dexmedetomidine and oral chloral hydrate for sedated auditory brainstem response (ABR) testing. Pediatr Anesth. 2016;26:286–93.
Lipovsek M, Marcovich I, Elgoyhen AB. The hair cell α9α10 nicotinic acetylcholine receptor: odd cousin in an old family. Front Cell Neurosci. 2021;15: 785265.
Metherate R, Intskirveli I, Kawai HD. Nicotinic filtering of sensory processing in auditory cortex. Front Behav Neurosci. 2012;6:44.
Caminos E, Garcia-Pino E, Martinez-Galan JR, Juiz JM. The potassium channel KCNQ5/Kv7.5 is localized in synaptic endings of auditory brainstem nuclei of the rat. J Comp Neurol. 2007;505:363–78.
Ramírez T, Soto E, Vega R. Opioid modulation of cochlear auditory responses in the rat inner ear. Synapse. 2020;74: e22128.
Church MW, Shucard DW. Theophylline-induced changes in the mouse brainstem auditory evoked potential. Neurotoxicol Teratol. 1987;9:59–66.
Gritzke R, Church MW. Effects of cocaine on the brain-stem auditory evoked potential in the Long-Evans rat. Electroencephalogr Clin Neurophysiol Evoked Potentials Sect. 1988;71:389–99.
Samra SK, Krutak-Krol H, Pohorecki R, Domino EF. Scopolamine, morphine, and brain-stem auditory evoked potentials in awake monkeys. Anesthesiology. 1985;62:437–41.
Banoub M, Tetzlaff JE, Schubert A. Pharmacologic and physiologic influences affecting sensory evoked potentials. Anesthesiology. 2003;99:716–37.
Acknowledgements
We would like to acknowledge Marie Bainier for the excellent technical assistance. Furthermore, we would like to thank the Roche Innovation Centre Graduate Students Internship Program for the funding of Samuel Marashli.
Funding
F. Hoffmann-La Roche (Roche).
Author information
Authors and Affiliations
Contributions
SM: Conceptualization, Methodology, Data acquisition, Formal analysis, Investigation, Software, Visualization, Writing-Original draft. PJ: Conceptualization, Methodology, Supervision, Methodology, Software, Validation, Writing—Review & Editing. RLR: Conceptualization, Supervision, Project administration, Funding acquisition, Writing—Review & Editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures were approved by the ethics committee of Federal Food Safety and Veterinary Office of Switzerland and conducted in adherence to the Swiss federal ordinance on animal protection and welfare.
Consent for publication
Not applicable.
Competing interests
SM received a graduate student internship from F. Hoffmann-La Roche (Roche). PJ and RLR were under employment by the company F. Hoffmann-La Roche (Roche). The funder provided support in the form of salaries for authors but did not have any additional role in the study design, data collection, analysis, decision to publish, or manuscript preparation. This does not alter the authors’ adherence to all the journal policies on sharing data and materials.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Comparison of auditory brainstem responses between Nrxn1α KO and wild-type littermates Sprague Dawley rats under isoflurane. Fig. S2. Comparison of auditory brainstem responses between Nrxn1α KO and wild-type littermates Sprague Dawley rats under ketamine/xylazine or medetomidine anesthesia. Fig. S3. Comparison of auditory brainstem responses between pharmacological modulations and vehicle in wild-type Sprague Dawley rats under isoflurane anesthesia. Fig. S4. Comparison of auditory brainstem responses between pharmacological modulations and vehicle in Nrxn1α KO Sprague Dawley rats under isoflurane anesthesia. Fig. S5. Comparison of auditory brainstem responses between pharmacological modulations and vehicle in wild-type Sprague Dawley rats under medetomidine anesthesia. Fig. S6. Comparison of auditory brainstem responses between pharmacological modulations and vehicle in Nrxn1α KO Sprague Dawley rats under medetomidine anesthesia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Marashli, S., Janz, P. & Redondo, R.L. Auditory brainstem responses are resistant to pharmacological modulation in Sprague Dawley wild-type and Neurexin1α knockout rats. BMC Neurosci 25, 18 (2024). https://doi.org/10.1186/s12868-024-00861-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12868-024-00861-4