Abstract
Objective
Cerebellar injury can not only cause gait and postural instability, nystagmus, and vertigo but also affect the vestibular system. However, changes in connectivity regarding the vestibular projection pathway after cerebellar injury have not yet been reported. Therefore, in the current study, we investigated differences in the connectivity of the vestibular projection pathway after cerebellar injury using diffusion tensor imaging (DTI) tractography.
Methods
We recruited four stroke patients with cerebellar injury. Neural connectivity in the vestibular nucleus (VN) of the pons and medulla oblongata in patients with cerebellar injury was measured using DTI. Connectivity was defined as the incidence of connection between the VN on the pons and medulla oblongata and target brain regions such as the cerebellum, thalamus, parieto-insular vestibular cortex (PIVC), and parietal lobe.
Results
At thresholds of 10 and 30, there was lower connectivity in the ipsilateral hemisphere between the VN at the medullar level and thalamus in the patients than in healthy adults. At a threshold of 1 and 10, the patient group showed lower VN connectivity with the PIVC than healthy adults. At a threshold of 1, VN connectivity with the parietal lobe in the contralateral hemisphere was lower in the patients than in healthy adults. Additionally, at a threshold of 30, VN connectivity at the pons level with the cerebellum was lower in healthy adults than in the patients.
Conclusion
Cerebellar injury seems to be associated with decreased vestibular projection pathway connectivity, especially in the ipsilateral thalamus, PIVC, and contralateral parietal lobe.
Similar content being viewed by others
Introduction
Balance is a key component that maintains the center of mass within the base of support for ambulation and reduces fall risk [1]. It requires complex integration of the visual, vestibular, and somatosensory systems [2]. In particular, the vestibular system, which is composed of the peripheral vestibular organs in the inner ear, ocular system, and projections of the central nervous system, has relatively low importance for balance in static environments such as horizontal and stable surfaces; however, it is crucial for balance in dynamic environments, where the surface is unstable from tilting and oscillating [3,4,5,6,7].
Vestibular function is controlled by interactions between various brain areas and neuropathways; it affects the balance and vertical position of the head and the body [8]. Studies have reported that vestibular projection pathways were mainly connected with the vestibular nuclei (VN), parieto-insular vestibular cortex (PIVC), cerebellum, and cerebral cortex [9, 10]. The VN, which is located in the pons and medulla oblongata, receives sensory information from eye and head movements as well as body orientation in space to control the movements [11]. The PIVC, which is a core region of vestibular input, contributes to the processing of bodily self-consciousness, estimation of verticality, and integration of visual motion [12]. The cerebellum, which receives vestibular information and projects vestibular information through projection pathways to the VN, contributes to equilibrium [9, 13]. The cerebral cortex contributes to the conscious perception of movement and spatial orientation [11].
Because the vestibular projection pathway is connected to various brain areas, injury to the vestibular system can be accompanied by problems related to balance, spatial orientation, vertigo, and dizziness [14,15,16,17,18,19]. Moreover, the vestibular projection pathway is connected to the cerebellum [20]. Cerebellar injury can cause not only gait and postural instability, nystagmus, and vertigo but also vestibular symptoms; this is due to the fact that the nodulus of the cerebellum has reciprocal connections with numerous structures in the peripheral and central vestibular networks [13, 14, 21,22,23]. However, changes in connectivity regarding the vestibular projection pathway after cerebellar injury have not yet been reported.
Recently developed diffusion tensor tractography (DTT), which is derived from diffusion tensor imaging (DTI), has enabled three-dimensional reconstruction and estimation of the microstructural integrity of neural tracts [24,25,26]. Additionally, DTI enables the projection and reconstruction of functional connectivity and anatomical structures by visualizing water diffusion patterns [25]. Thus, DTI is a useful tool to provide images of the diffusion properties of white matter by quantifying multidirectional connectivity [25]. Studies have reconstructed human neural connectivity in the VN and other brain areas in three dimensions [9, 10, 25]. Therefore, in the current study, we investigated the differences in the connectivity of the vestibular projection pathway after cerebellar injury using DTI tractography.
Materials and methods
Subjects
In this study, four stroke patients (three males, one female; mean age 70.75 ± 7.76 years) with cerebellar injury on magnetic resonance imaging (MRI) and 6 control subjects (four males, two females, mean age 30.00 ± 5.66 years) with no history of a neurological or psychiatric disease were recruited for this study at the University Hospital. The inclusion criteria were as follows: (1) first-ever stroke, (2) no traumatic brain injury, and (3) cerebellar injury due to infarction or hemorrhage. All subjects provided informed consent before undergoing DTI and functional evaluations. The study was approved by the Institutional Review Board of Dankook University.
Diffusion tensor image
DTI data were acquired using a 6-channel head coil on a 1.5 T Philips Gyro scan Intera (Philips, Best, The Netherlands) with single-shot echo-planar imaging. For each of the 32 non-collinear diffusion sensitizing gradients, 67 contiguous slices were collected parallel to the anterior commissure-posterior commissure line. The imaging parameters were as follows: acquisition matrix, 96 × 96; reconstructed matrix, 192 × 192; field of view, 240 × 240 mm2; TR, 10,726 ms; TE, 76 ms; parallel imaging reduction factor (SENSE factor) = 2; EPI factor = 49; b = 1000 s/mm2; NEX = 1; and a slice thickness of 2.5 mm with no gap (acquired voxel size 1.3 × 1.3 × 2.5 mm3) [27, 28].
Probabilistic fiber tracking
The DWI data were analyzed using the Oxford Center for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl). Affine multi-scale two-dimensional registration was used to correct the head motion effect and image distortion due to eddy currents. Fiber tracking uses a probabilistic image method based on a multifiber model; it was performed in this study by utilizing image routines implemented in FMRIB Diffusion (5000 streamline samples, 0.5 mm step lengths, curvature thresholds = 0.2) [29].
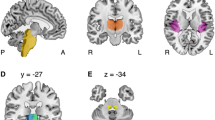
Both contra- and ipsilateral connectivity were defined as the incidence of connection between the VN (on pons and medulla oblongata) and the following target brain regions as well as were determined by whether the results passed through each target brain region: cerebellum, thalamus, PIVC, and parietal lobe. The incidence of connection was counted from the VN (on pons and medullar oblongata) to each brain region. Note that the seed region of interest (ROI) is located at the VN (on pons: Deitets’ and Schwalbe’s nuclei, on medullar oblongata). The fractional anisotropy (FA), mean diffusivity (MD), and tract volume (voxel number) of the projection pathway were also measured.
Statistical analysis
SPSS software (ver. 20.0; SPSS, Inc., Chicago, IL, USA) was used to analyze the results. The chi-square test was used to determine the significance of differences in the incidences of connectivity in the VN on pons and VN on medullar in patients with cerebellum. The level of statistical significance was accepted for p-values < 0.05.
Results
A summary of the demographic clinical characteristics of patients with cerebellar syndrome is presented in Table 1. All patients exhibited typical vestibular signs except diplopia: vertigo (n = 4, 100%), ataxia (n = 4, 100%), dysarthria (n = 1, 25%), dysphagia (n = 0, 0%), nystagmus (n = 1, 25%), diplopia (n = 0, 0%), and abnormal facial sensation (n = 1, 25%).
The reconstruction of VN on pons connectivity is shown in Table 2 and Fig. 1. The ipsilateral connectivity of VN on pons with the target brain regions (cerebellum, thalamus, and parietal lobe) was 100% in healthy adults, regardless of the threshold. In contrast, patients with cerebellar injury showed lower connectivity with the target brain area (cerebellum, thalamus, and parietal lobe). At thresholds of 1, 10, or 30, connectivity with the PIVC steadily decreased in patients (100.0%, 62.5%, and 37.5%, respectively) and in healthy adults (100.0%, 80.0%, and 75.0%, respectively). However, no significant difference was observed between the patients and healthy adults, regardless of the threshold (p > 0.05).
At thresholds of 1, 10, or 30, contralateral connectivity of the VN in the pons with the cerebellum steadily decreased in patients with cerebellar injury (100.0%, 87.5%, and 62.5%, respectively) and in healthy adults (100.0%, 50.0%, and 16.7%, respectively). Notably, at a threshold of 30, connectivity with the cerebellum was significantly lower in healthy adults (16.7%) than in patients (62.5%) (p < 0.05). Connectivity with PIVC and parietal lobe also showed decrements with increasing thresholds in patients and healthy adults. It should be noted that at a threshold of 30, connectivity with the parietal lobe was lower in healthy adults (58.3%) than in patients with cerebellar injury (62.5%). However, connectivity at each threshold was not significantly different between the two groups (p > 0.05). Connectivity with the thalamus was 75.0% in patients at thresholds of 1, 10, or 30. In contrast, healthy adults showed lower connectivity with the thalamus at thresholds of 1, 10, or 30 (100%, 58.3%, and 33.3%, respectively).
The reconstruction of the VN on the medullary connectivity is shown in Table 3 and Fig. 2. At thresholds of 1, 10, or 30, the ipsilateral connectivity with the cerebellum, PIVC, and parietal lobe steadily decreased in both patients and healthy adults. Notably, at thresholds of 1 and 10, connectivity with the PIVC was significantly lower in patients than in healthy adults (p < 0.05). At thresholds of 1, 10, or 30, connectivity with the thalamus decreased in both patients (75.0%, 50.0%, and 50.0%, respectively) and healthy adults (100.0%, 100.0%, and 91.7%, respectively). At thresholds of 10 and 30, connectivity with the thalamus was significantly lower in patients than in healthy adults (p < 0.05).
At thresholds of 1, 10, or 30, contralateral connectivity of VN on the medulla with all target brain regions (cerebellum, thalamus, PIVC, and parietal lobe) steadily decreased in both patients and healthy adults. Notably, at a threshold of 1, connectivity with the parietal lobe was significantly lower in patients (62.5%) than in healthy adults (100%) (p < 0.05).
Discussion
In the current study, we investigated the differences in vestibular projection pathway connectivity after cerebellar injury using DTI tractography. We found that at thresholds of 10 and 30, there was lower connectivity in the ipsilateral hemisphere between the VN at the medullar level and thalamus in patients than in healthy adults. At thresholds of 1 and 10, the patient group showed lower VN connectivity with the PIVC compared to healthy adults. At a threshold of 1, VN connectivity with the parietal lobe in the contralateral hemisphere was lower in patients than in healthy adults. Additionally, at a threshold of 30, VN connectivity at the pons level with the cerebellum was lower in healthy adults than in patients. These results suggest that cerebellar injury due to hemorrhage might be associated with alterations in the connectivity of the vestibular projection pathway, especially the thalamus and PIVC in the ipsilateral hemisphere and parietal lobe in the contralateral hemisphere.
Studies have reported that vestibular projection pathways from VN at the level of the pons and medulla are typically connected to the thalamus, PIVC, VN, cerebral cortex, and cerebellum [9, 10, 30]. In 2004, Lee et al. showed that patients with cerebellar infarction presented with isolated vertigo, spontaneous ipsilesional nystagmus, and contralesional axial lateropulsion, without symptoms of cerebellar dysfunction [21]. In 2017, Kim et al. reported that isolated vestibular symptoms were associated with cerebellar injury due to infarctions without other neurologic deficits [13]. Specifically, cerebellar lesions involving the inferior cerebellar peduncle, which include the neural pathway that typically transfers vestibular information to the VN, can lead to isolated vertigo and postural imbalance without other neurological deficits [13, 31]. In 2018, Jang et al. suggested that the VN showed strong connectivity with the cerebellum, thalamus, and vestibular-related brain regions [9]. Our results are consistent with those of the previous studies. The cerebellum receives vestibular inputs and projects through the inferior cerebellar peduncle to the VN [13, 32]. Subsequently, the VN sends vestibular information to the PIVC, which is then processed and integrated with the thalamus [32,33,34,35]. When vestibular information is deficient due to cerebellar injury, VN may affect connectivity with the PIVC and thalamus [13, 21]. Hence, cerebellar injury might affect the connectivity of the vestibular projection pathway, especially in the thalamus and PIVC.
In the current study, the VN at the medullar level connectivity with the parietal lobe was lower in patients than in healthy adults, at a threshold of 1 in the contralateral hemisphere. In 1994, Akbarian et al. reported that connectivity was present between the VN and premotor and parietal cortices [36]. Recently, Jang et al. reported the VN connectivity in 37 healthy adults; it has also been reported that the VN showed connectivity with the primary motor cortex (95.9%, 83.8%, and 74.3% at thresholds of 1, 10, and 15, respectively), primary somatosensory cortex (90.5%, 68.9%, and 64.9%), and premotor cortex (87.8%, 52.7%, and 40.5% at thresholds of 1, 10, and 15 respectively) [9]. Our results are consistent with those of previous studies. Thus, cerebellar injury might affect VN connectivity with the parietal lobe.
In the current study, VN connectivity at the pons level with the cerebellum was higher in patients than in healthy adults, at a threshold of 30 in the contralateral hemisphere. Studies have reported that the unaffected hemisphere is associated with neuroplasticity in patients with brain injury [37,38,39]. In 2010, Kwak et al. demonstrated changes in the corticospinal tract in the unaffected hemisphere in stroke patients using DTI [37]. In 2013, Yeo et al. reported increased fiber volumes of the corticoreticular pathway in the unaffected hemisphere related to the recovery of motor function in stroke patients [38]. In 2016, Jang et al. demonstrated changes in the corticospinal tract in the unaffected hemisphere according to the severity of the corticospinal tract injury in stroke patients [39]. These studies suggest that the change in the neural pathway in the unaffected hemisphere can be regarded as neuroplasticity; therefore, the phenomenon of changes in the unaffected hemisphere can be regarded as a compensation for damage in the affected hemisphere [37,38,39]. The results of the current study are consistent with those of previous studies. Thus, greater connectivity with the cerebellum in patients than in healthy adults can be regarded as induced neuroplasticity.
The present study has a few limitations. First, it is limited by its small sample size. Second, we only investigated the vestibular projection pathway connectivity in patients with cerebellar injury without clinical evaluation. Third, because DTT cannot discern the direction, the afferent and efferent fibers could not be divided between the VN and target brain regions. Fourth, DTI analysis is operator-dependent; because of fiber complexity and the crossing fiber effect, it may underestimate the fiber tracts. Therefore, to overcome these limitations, in-depth studies as well as studies regarding the clinical application of our results in patients with cerebellar injury are encouraged.
Conclusion
We investigated the differences in the connectivity of the vestibular projection pathway after cerebellar injury using DTI tractography. We found that cerebellar injury seems to be associated with decreased vestibular projection pathway connectivity, especially in the ipsilateral thalamus, PIVC, and contralateral parietal lobe. Therefore, evaluating the vestibular pathway using DTT in patients with cerebellar injury might be useful for clinical evaluation.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are not publicly available due to the data containing information regarding individual diseases but are available from the corresponding author on reasonable request.
References
Dunsky A, Zeev A, Netz Y. Balance performance is task specific in older adults. Biomed Res Int. 2017. https://doi.org/10.1155/2017/6987017.
Cronin T, Arshad Q, Seemungal BM. Vestibular deficits in neurodegenerative disorders: balance, dizziness, and spatial disorientation. Front Neurol. 2017;8:538.
Allum J, Pfaltz C. Visual and vestibular contributions to pitch sway stabilization in the ankle muscles of normals and patients with bilateral peripheral vestibular deficits. Exp Brain Res. 1985;58(1):82–94.
Creath R, Kiemel T, Horak F, Jeka JJ. The role of vestibular and somatosensory systems in intersegmental control of upright stance. J Vestib Res. 2008;18(1):39–49.
Horak FB, Kluzik J, Hlavacka F. Velocity dependence of vestibular information for postural control on tilting surfaces. J Neurophysiol. 2016;116(3):1468–79.
Khan S, Chang R. Anatomy of the vestibular system: a review. NeuroRehabilitation. 2013;32(3):437–43.
Miles RD, Zapala DA. Vestibular function measurement devices. Semin Hear. 2015. https://doi.org/10.1055/s-0034-1396926.
Brandt T, Dieterich M, Strupp M. Vertigo and dizziness. Berlin: Springer; 2005.
Jang SH, Lee MY, Yeo SS, Kwon HG. Structural neural connectivity of the vestibular nuclei in the human brain: a diffusion tensor imaging study. Neural Regen Res. 2018;13(4):727.
Kirsch V, Keeser D, Hergenroeder T, Erat O, Ertl-Wagner B, Brandt T, Dieterich M. Structural and functional connectivity mapping of the vestibular circuitry from human brainstem to cortex. Brain Struct Funct. 2016;221(3):1291–308.
Barmack NH. Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull. 2003;60(5–6):511–41.
Pfeiffer C, Serino A, Blanke O. The vestibular system: a spatial reference for bodily self-consciousness. Front Integr Neurosci. 2014;8:31.
Kim S-H, Kim HJ, Kim J-S. Isolated vestibular syndromes due to brainstem and cerebellar lesions. J Neurol. 2017;264(1):63–9.
Dieterich M. Central vestibular disorders. J Neurol. 2007;254(5):559–68.
Kwon HG, Chang CH, Jang SH. Diagnosis of dizziness due to a core vestibular projection injury in a patient with intracerebral hemorrhage. Diagnostics. 2020;10(4):220.
Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9(1):20–6.
Brandt T. Management of vestibular disorders. J Neurol. 2000;247(7):491–9.
Franke LM, Walker WC, Cifu DX, Ochs AL, Lew HL. Sensorintegrative dysfunction underlying vestibular disorders after traumatic brain injury: a review. J Rehab Res Dev. 2012. https://doi.org/10.1682/jrrd.2011.12.0250.
Jang SH, Kwon HG. Injury of the ipsilateral vestibulothalamic tract in a patient with pontine hemorrhage. Acta Neurol Belg. 2020;120(4):951–4.
Jang SH, Kim JH, Kim DH, Kwon HG. The vestibulocerebellar tract in the human brain: a diffusion tensor tractography study. Curr Med Imag Rev. 2018;14(4):617–20.
Lee H, Cho Y-W. A case of isolated nodulus infarction presenting as a vestibular neuritis. J Neurol Sci. 2004;221(1–2):117–9.
Dichgans J. Clinical symptoms of cerebellar dysfunction and their topodiagnostical significance. Hum Neurobiol. 1984;2(4):269–79.
Amarenco P. The spectrum of cerebellar infarctions. Neurology. 1991;41(7):973–973.
Mori S, Crain BJ, Chacko VP, Van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–9.
Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34(1):51–61.
Mori S, Van Zijl PC. Fiber tracking: principles and strategies—a technical review. NMR Biomed. 2002;15(7–8):468–80.
Yeo SS, Jang SH, Kwon JW. Central vestibular disorder due to ischemic injury on the parieto-insular vestibular cortex in patients with middle cerebral artery territory infarction: observational study. Medicine. 2017;96(51):0000000000009349.
Jang SH, Lim HW, Yeo SS. The neural connectivity of the intralaminar thalamic nuclei in the human brain: a diffusion tensor tractography study. Neurosci Lett. 2014;579:140–4.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(1):051.
Henkel C, Martin G. The vestibular complex of the American opossum Didelphis virginiana. II. Afferent and efferent connections. JCN. 1977;172(2):321–48.
Choi JH, Seo JD, Choi Y, Kim MJ, Kim HJ, Kim J, Choi K. Inferior cerebellar peduncular lesion causes a distinct vestibular syndrome. Eur J Neurol. 2015;22(7):1062–7.
Yeo SS, Jang SH, Kwon JW. Lateral medullary syndrome following injury of the vestibular pathway to the core vestibular cortex: diffusion tensor imaging study. Neurosci Lett. 2018;665:147–51.
Bronstein A, Yardley L, Moore A, Cleeves LJN. Visually and posturally mediated tilt illusion in Parkinson’s disease and in labyrinthine defective subjects. Neurology. 1996;47(3):651–6.
Wirth AM, Frank SM, Greenlee MW, Beer AL. White matter connectivity of the visual–vestibular cortex examined by diffusion-weighted imaging. Brain Connect. 2018;8(4):235–44.
Dieterich M, Brandt T. Perception of verticality and vestibular disorders of balance and falls. Front Neurol. 2019;10:172.
Akbarian S, Grüsser OJ, Guldin WO. Corticofugal connections between the cerebral cortex and brainstem vestibular nuclei in the macaque monkey. J Comp Neurol. 1994;339(3):421–37.
Kwak SY, Yeo SS, Choi BY, Chang CH. Corticospinal tract change in the unaffected hemisphere at the early stage of intracerebral hemorrhage: a diffusion tensor tractography study. Eur Neurol. 2010;63(3):149–53.
Jang SH, Chang CH, Lee J, Kim CS, Seo JP, Yeo SSJS. Functional role of the corticoreticular pathway in chronic stroke patients. Stroke. 2013;44(4):1099–104.
Jang SH, Yi JH, Choi BY, Chang CH, Jung YJ, Lee HD, Yeo SS. Changes of the corticospinal tract in the unaffected hemisphere in stroke patients: a diffusion tensor imaging study. Somatosens Mot Res. 2016;33(1):1–7.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2021R1A2C1095047).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
BUG: investigation, writing—original draft. IHC: conceptualization, methodology, investigation, data curation. SSY: conceptualization, investigation, writing—review and editing, supervision. JWK: conceptualization, methodology, investigation. SHJ: methodology, investigation. SO: investigation, data curation, writing—original draft, writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The studies involving human participants were reviewed and approved by the institutional review board of Dankook University (DKU 2020-07-009) and was performed in accordance with the relevant guidelines and regulations. The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gam, B.U., Cho, I.H., Yeo, S.S. et al. Comparative study of vestibular projection pathway connectivity in cerebellar injury patients and healthy adults. BMC Neurosci 23, 17 (2022). https://doi.org/10.1186/s12868-022-00702-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12868-022-00702-2