Abstract
Background
Previous studies have indicated that selenium supplementation may be beneficial in neuroprotection against glutamate-induced cell damage, in which mitochondrial dysfunction is considered a major pathogenic feature. However, the exact mechanisms by which selenium protects against glutamate-provoked mitochondrial perturbation remain ambiguous. In this study glutamate exposed murine hippocampal neuronal HT22 cell was used as a model to investigate the underlying mechanisms of selenium-dependent protection against mitochondria damage.
Results
We find that glutamate-induced cytotoxicity was associated with enhancement of superoxide production, activation of caspase-9 and -3, increases of mitochondrial fission marker and mitochondrial morphological changes. Selenium significantly resolved the glutamate-induced mitochondria structural damage, alleviated oxidative stress, decreased Apaf-1, caspases-9 and -3 contents, and altered the autophagy process as observed by a decline in the ratio of the autophagy markers LC3-I and LC3-II.
Conclusion
These findings suggest that the protection of selenium against glutamate stimulated cell damage of HT22 cells is associated with amelioration of mitochondrial dynamic imbalance.
Similar content being viewed by others
Background
l-Glutamate, the most abundant excitatory neurotransmitter in the nervous system is involved in a wide variety of brain functions and plays a key role in the pathogenesis of many neurological disorders. It is a potent neurotoxin capable of neuronal destruction when present in high concentration. Glutamate-evoked excitotoxicity has been implicated in the etiology of many neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), and ischemic stroke [1]. Glutamate-induced cell death is mediated in part by overstimulation of the postsynaptic glutamate receptor system [2] and non-receptor mediated oxidative toxicity [3]. Prolonged exposure to high concentrations of extracellular glutamate promotes oxidative toxicity by activation of mechanisms that negatively impact cysteine uptake into cells via the cystine/glutamate antiporter leading to depletion of glutathione (GSH) [3]. Depletion of GSH causes a downregulation of the cystine-dependent antioxidant system leading to excessive accumulation of reactive oxygen species (ROS) accompanied by oxidative stress. Oxidative stress perturbs mechanisms that regulate Ca2 + homeostasis in the mitochondria and activates pathways that lead to collapse of the mitochondrial membrane polarity and opening of the mitochondrial permeability transition pore (MPTP).
Mitochondria are autonomous double membrane-enclosed organelles present in most mammalian cells, and mainly involved in aerobic respiration. They are dynamic organelles that continuously undergo remodeling by fusion and fission in response to the cellular and environmental cues. Mitochondrial fusion and fission play critical roles in the maintenance of mitochondrial function and regulation of bioenergetic state of the cell. Three dynamin-related GTPases, Mitofusins 1 (Mfn1), Mitofusin 2 (Mfn2) and optic atrophy 1 (Opa1) are required for fusion of the mitochondrial outer and inner membranes in mammalian cells [4–6]; while mitochondrial fission is mediated by the dynamin-like protein-1 (Drp1) and the mitochondrial fission 1 protein (Fis1) [7]. Mitochondrial fusion results in enlargement of the mitochondria through merging of two separate units. In addition, mitochondrial fusion presumably regulates electron transport, mitochondrial metabolism and calcium homeostasis [8]. In contrast, mitochondrial fission is essential to establish new mitochondria and to eliminate defective mitochondria by mitochondrial autophagy [9]. Both processes are tightly controlled and uniformly balanced under physiological conditions.
Autophagy is a vital intracellular catabolic process that causes cellular protein and organelle turnover, through sequestering and priming proteins for lysosomal degradation [10]. Autophagy can be stimulated by a variety of stress-inducing conditions including nutrient depletion, reactive oxygen species, and hypoxia [11]. Activation of this pathway may not necessarily lead to cell death [12]. Defects in the autophagy-regulation and signaling have been associated with many human diseases, including neurodegenerative disorders [13]. Three types of autophagy (macroautophagy, microautophagy, and chaperone-mediated autophagy) have been identified of which, microautophagy has been studies extensively [10]. A number of key signaling pathways ties autophagy to stress responses. Activation of these pathways is orchestrated by a sequence of core autophagy-related (ATG) genes that are evolutionarily conserved. One of the proteins critical to this process is Beclin-1, the mammalian orthologue of the yeast autophagy protein Atg6. Beclin-1 induces autophagy by interacting with several cofactors and Vps-34, a class III phosphatidylinositol-3-phosphate kinase (PI3kIII/Vps34), to form a complex that promotes autophagosome formation in the early stages of autophagy [14]. Unlike Beclin-1, another member of the ATG protein family, microtubule-associated protein 1 light chain 3 (LC3) localize to different autophagic membranes and is essential for final autophagosome formation. LC3, synthesized as a pro-protein is rapidly converted to LC3-I by Atg4 and further transformed by a series of conjugating and activating Atg proteins to LC3-II, the autophagosomal membrane bound form considered as a marker of autophagy activation.
Selenium is central component at the catalytic sites of various selenium-dependent enzymes including Glutathione peroxidase (GPx). Selenium has been demonstrated to reduce ROS production [15, 16], protect cells against glutamate toxicity [17], oxidative stress [18] and inflammatory cytokines [19, 20]. Animal experiments have showed that selenium supplementation reduces ROS production, ameliorates cisplatin induced neurotoxicity and ischemia-induced brain damage [21, 22]. However, the mechanisms by which selenium exerts its protective effect against glutamate insult in neuronal cells remains ambiguous. In this study, glutamate exposed HT22 cells were used as an in vitro model to examine signaling pathway through which, sodium selenite could potentially moderate glutamate-induced toxicity in neuronal cells. Cellular viability, ultrastructural changes, ROS production, and biomarkers for autophagy and mitochondrial fission were measured respectively. Our results showed that sodium selenite blocked glutamate provoked neuronal death by restoring mitochondrial function, reducing ROS production, inhibiting caspase activation, and impairment in autophagy.
Results
Glutamate decreased and selenite increased cell viability
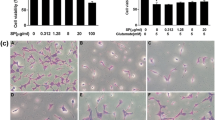
The cell viabilities in vehicle and various concentrations of glutamate treated cells were assessed using MTT assay kit after 24 h of incubation (Fig. 1a). The results showed that incubation with 2 mM of glutamate reduced the cell viability from 98.8 ± 1.2% in control to 94.2 ± 0.6% (p > 0.05), while 4 mM of glutamate reduced it to 85.2 ± 1.4% (p < 0.01 compared with control). The cell viability was further decreased with the increased concentrations of glutamate. Therefore, the cell viabilities were 72.0 ± 1.6% at 6 mM, 54.2 ± 1.6% at 8 mM, and 42.5 ± 2.4% at 10 mM of glutamate concentrations (p < 0.01 with all three concentrations compared with control). In next experiments, we incubated the cell with 6 mM glutamate and 100 nM of sodium selenite concurrently and measured cell viability after 6, 10 and 24 h (Fig. 1b). The results showed that the cell viabilities were not significantly altered in selenite, glutamate and glutamate plus selenite groups compared with vehicle control after 6 h of incubation. The cell viabilities were significantly decreased after 10 h of glutamate incubation to 85.8 ± 2.5% comparing to control of 100 ± 0.9% (p < 0.01). The viability was further decreased to 68.9 ± 3.9% after 24 h of glutamate incubation compared with control (p < 0.01). Compared with glutamate incubation alone, the HT22 cells co-treated with glutamate and selenite had a mild improved cell viability after 10 h (92.2 ± 1.3%, p > 0.05). However, the viability was significantly improved by selenite co-treatment at 24 h comparing to glutamate alone (83.6 ± 1.5% vs. 68.9 ± 3.9%, p < 0.01).
Cell viabilities in three experimental groups assessed by MTT assay. a cell viability assays were performed at 24 h of incubation with various concentrations of glutamate. The viabilities of the cells were reduced to 94.2 ± 0.6, 85.2 ± 1.4, 72.0 ± 1.6, 54.2 ± 1.6, and 42.5 ± 2.4% with the glutamate at 2, 4, 6, 8, and 10 mM concentrations. N = 10 in each group. **p < 0.01 versus control by Student’s t test. b Cell viability after 6, 10 and 24 h of glutamate (6 mM) incubation with or without concurrent addition of selenium (100 nM). After 10 h of incubation, cell viabilities were reduced to 85.8 ± 2.5% by glutamate and the reduction was moderately improved by selenite (92.2 ± 1.3%). F(3, 23) = 13.6 and p < 0.001 by ANOVA. After 24 h of culture, glutamate further reduced the cell viability to 68.9 ± 3.9%, while selenite rescued the viability to 83.6 ± 1.5%. F(3, 23) = 18.9. *p < 0.05, **p < 0.01 versus control and ## p < 0.01 versus glutamate by ANOVA with post hoc Tukey’s test. Glu glutamate, Se sodium selenite, Glu + Se = glutamate + sodium selenite. N = 6 in each group
Light and electron microscopic findings
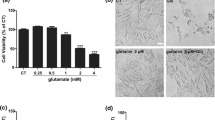
Consistent to the results obtained from viability assay, cell images captured by inverted light microscope demonstrated normal cell morphology in non-glutamate treated control HT22 cells. Glutamate exposure resulted in cell shrinkage and nuclear condensation, suggesting cell death (Fig. 2a). Selenite concurrent treatment ameliorated glutamate-caused cellular morphological changes. Electron microscopic study showed that abundant endoplasmic reticulum (yellow arrow) and mitochondria (red arrow), mostly elongated, in the cytosol of the control cells. Comparing to the control, mitochondria in glutamate-treated cells became lucent and swelling (red arrow). In addition, distended rough endoplasmic reticulum, abundant presence of lysosomes (blue arrow), autophagosomes, and nuclear chromatin margination (green arrow) were observed. Selenite concurrent treatment ameliorated glutamate caused alterations in the mitochondrion, endoplasmic reticulum and lysosome (Fig. 2b).
Cell morphology observed by light microscope (a) and electron microscope (b). a Glutamate exposure causes cell shrinkage and nuclear condensation. Selenite concurrent treatment reduced glutamate caused cell death. b Glutamate exposure 24 h caused mitochondrial lucency and swelling (red arrow), nuclear chromatin margination (green arrow), decreased presence of subcellular organelles, distention of rough endoplasmic reticulum (yellow arrow), and increased presence of lysosome (blue arrow). Selenite concurrent treatment reduced glutamate-caused alterations in the mitochdrion, endoplasmic reticulum and lysosome
Selenium decreased glutamate-induced superoxide production
Superoxide productions were measured by DHE fluorescent probe following glutamate and/or selenite incubation. As shown in Fig. 3, faint DHE immunofluorescence was detected in normal HT22 control cells, representing the basal amount of superoxide (value of 6.0 ± 0.8) produced during normal cellular metabolic activity. Glutamate exposure resulted in a surge of DHE signal (value of 81.0 ± 2.1), suggesting significantly increased superoxide production (p < 0.01). Treatment with selenium in glutamate-incubated cells reduced the DHE fluorescent intensity to 47.0 ± 1.2, a 42% reduction comparing to that of non-selenite treated, glutamate exposed cultures (p < 0.01).
Superoxide accumulation measured by dihydroethidium (DHE) fluorescence. Accumulation of superoxide in the cells was measures by incubation in DHE (red color) after 24 h following glutamate and/or selenium treatments. Cells were counterstained with DAPI to label the nuclei (blue color). Relative immunointensity increased from 6.0 ± 0.8 in control to 81.0 ± 2.1 after glutamate exposure. Concurrent treatment of the cells with glutamate and selenite reduce the DHE immunointensity to 47.0 ± 1.2. F(2, 23) = 633.5. **p < 0.01 versus control and ## p < 0.01 versus glutamate by ANOVA with post hoc Tukey’s test. N = 10 in each group
Glutamate activated and selenium suppressed mitochondria-initiated cell death pathway
Since Apaf-1, caspase-9, and caspase-3 play key roles in mediating mitochondria-initiated cell death pathway, we performed immunocytochemistry using antibodies against Apaf-1, cleaved caspase-9, and cleaved caspase-3. The results were further verified with Western blots using antibodies against Apaf-1, total and cleaved caspase-9, and total and cleaved caspase-3. The results showed that there were very few faintly stained Apaf-1 positive neurons in the normal control cells. Glutamate exposure for 24 h significantly increased the immunoreactivity of Apaf-1 in the cytoplasm as shown in Fig. 4. Concurrent treatment with selenite reduced the immunoreactivity of Apaf-1 in glutamate-exposed neurons. Western blot using cytosolic fraction further support the findings showing that Apaf-1 protein band intensity in the cytosolic fraction were significantly increased from 25.3 ± 1.4% in control to 51.8 ± 1.4 in glutamate treated cells (p < 0.01). Selenite treatment significantly reduced the Apaf-1 protein band intensity to 32.7 ± 1.4 (p < 0.05 vs. glutamate treatment).
Immunocytochemistry and protein blotting of Apaf-1. Apaf-1 (green color) was detected using antibody after 24 h of glutamate or glutamate plus selenite treatments. DAPI (blue color) was used to label the nuclei. Western blot was performed using cytosolic fraction. Relative band intensity was measured and presented in the bar graph. Β-tubulin served as internal protein loading control. Glutamate incubation increased the Apaf-1 protein band intensity to 51.8 ± 1.4 and concurrent treatment with selenite reduced the value to 32.7 ± 1.4. F(2, 29) = 97.1. *p < 0.05 and **p < 0.01 versus control; # p < 0.05 versus Glutamate by ANOVA with post hoc Tukey’s test. N = 10 in each group
Similarly, there were very few faintly stained caspase-9 positive cells in control cultures. After glutamate treatment for 24 h, the number of caspase-9 positively stained neurons increased and concurrent treated of selenium decreased immunointensity of caspase-9 (Fig. 5). The results were further confirmed by Western blot. Consistently, glutamate caused significant elevation of cleaved caspase-9 (38.4 ± 1.7) compared to control (21.8 ± 1.8). Treatment with selenite significantly ablated the glutamate-induced increase (27.1 ± 1.1).
Immunocytochemistry and protein blotting of caspase-9. Immunocytochemistry was performed 24 h after treatments using antibody against cleaved caspase-9 (green color) and counterstained with DAPI (blue color). Western blots were performed using antibodies against total and cleaved caspase-9 in the cytosolic fraction. Beta-tubulin served as an internal control. Relative band intensities were measured and presented in the bar graph. Cleaved caspase-9 protein band intensity increased from 21.8 ± 1.8 in control to 38.4 ± 1.7 after glutamate incubation and reduced to 27.1 ± 1.1 by selenite treatment. F(2, 29) = 102.4. *p < 0.05, **p < 0.01 versus control; # p < 0.05 versus Glutamate by ANOVA with post hoc Tukey’s test. N = 10 in each group
There was faint punctuated caspase-3 positive staining localized in the cytoplasm of non-glutamate-incubated control cells. After glutamate incubation for 24 h, caspase-3 immunoreactivity significantly enhanced in the cytosol and nuclear translocation was noted. Concurrent treatment with selenite prevented caspase-3 nuclear translocation but did not seem to reduce the immunoreactivity in the cytosol and prevented caspase-3 nuclear translocation (Fig. 6). Western blot using nuclear fraction revealed that cleaved caspase-3 increased significantly in glutamate-incubated cells (25.5 ± 1.5) compared with control (13.6 ± 0.8). Such increases were ameliorated by selenite treatment (18.7 ± 1.2).
Immunocytochemistry and protein blotting of caspase-3. Immunocytochemistry was performed 24 h after treatments using antibody against cleaved caspase-3 (green color) and counterstained with DAPI (blue color). Western blots were performed using antibodies against cleaved caspase-3 in the nuclear fraction. Beta-tubulin served as an internal control. Relative band intensities were measured and presented in the bar graph. Cleaved caspase-3 protein band intensity increased from 13.6 ± 0.8 in control to 25.5 ± 1.5 after glutamate incubation and concurrent treatment of selenite reduced it to 18.7 ± 1.2. F(2, 29) = 67.5. *p < 0.05 and **p < 0.01 versus control; # p < 0.05 versus Glutamate by ANOVA with post hoc Tukey’s test. N = 10 in each group
Selenium reduces glutamate-induced mitochondrial fission
Mitochondrial fission markers Fis1, Drp1, and p-Drp1 were detected in the mitochondrial fraction of the cell lysate using Western blotting. As shown in Fig. 7, Protein band intensity of Fis1 increased significantly in glutamate treated cells (51.1 ± 1.4) comparing to control cells (31.4 ± 1.3). Concurrent treatment of glutamate incubated cells with selenite significantly reduced the level of Fis1 to 40.5 ± 1.3 (p < 0.01). Similarly, glutamate increased the p-Drp1 protein relative band intensity from 12.3 ± 1.1 in control to 32.3 ± 1.4 in glutamate incubated cells. Treatment with selenite reduced the p-Drp1 intensity to 16.6 ± 1.0 (p < 0.01). This is further supported by the following imaging study.
Western blotting of mitochondrial fission proteins. Fission proteins Fis-1, total Drp-1 and phospho-Drp1 (p-Drp1) were semi-quantified in the mitochondrial fractions using specific antibodies. COX IV served as an internal control. Band relative densities were measured and presented in the bar graphs. Fis-1 protein band intensity increased from 31.4 ± 1.3 in control to 51.1 ± 1.4 after glutamate incubation and reduced back to 40.5 ± 1.3 by selenite. F(2, 29) = 24.2. *p < 0.05 and **p < 0.01 versus control; # p < 0.05 and ## p < 0.01 versus Glutamate by ANOVA host hoc Tukey’s test. Similarly, p-Drp-1 ban intensity increased from 12.3 ± 1.1 in control to 32.3 ± 1.4 in glutamate and declined to 16.6 ± 1.0 in selenite and glutamate co-treated cells. F(2, 29) = 54.7. *p < 0.05 and **p < 0.01 versus control; # p < 0.05 and ## p < 0.01 versus Glutamate by ANOVA with post hoc Tukey’s test. N = 10 in each group
Selenium suppressed glutamate-activated autophagy
The last step of autophagy is the fusion of autophagosome with lysosome. Therefore, colocalization of mitochondrial marker MitoTracker Green and lysosomal marker LysoTracker Red could be used as an indication of mitochondrial autophagy. As shown in Fig. 8, in control cells, punctate green staining was observed, representing normal mitochondria detected by the probe. There were almost no lysosomes were detected. After glutamate treatment for 24 h, both MitoTracker Green and LysoTracker Red staining increased significantly in the cytosol, suggesting increased numbers of mitochondria and lysosomes. Treatment with selenite reduced the intensity of LysoTracker labeling.
Co-localization of MitoTracker Green and LysoTracker Red. The cells were incubated for 30 min with MitoTracker Green (green color) and LysoTracker Red (red color) to visualize the colocalization of mitochondria and lysosomes. Colocalization of the two dyes turns the color into orange. Western blot detected LC3-I and II in the mitochondrial fraction. Relative LC3-II band intensities were measured and presented in the bar graph. LC3-II protein band intensity increased from 46.8 ± 1.7 in control to 105.4 ± 4.2 in glutamate and reduced to 57.4 ± 1.8 in selenite treated groups. F(2, 29) = 124.9. *p < 0.05 and **p < 0.01 versus control; ## p < 0.01 versus glutamate by ANOVA with post hoc Tukey’s test. N = 10 in each group
Another marker of autophagy is the conversion of LC3-I to LC3-II. LC-3 levels were measure in the cytosolic fraction of the cells. It was shown that the levels of LC3-II band intensities significantly increased from 46.8 ± 1.7 in control to 105.4 ± 4.2 in glutamate treated cells (p < 0.01) and selenite reverted the glutamate-induced elevation back to 57.4 ± 1.8 (p < 0.01).
Discussion
Neuronal cell injury and death are major pathological features of many CNS disorders such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and stroke. These disorders provoke alterations in cell function and survival in distinct regions of the brain. For example, stroke triggers a significant neuronal death and several theories, including excitotoxicity, production of oxygen free radicals, nitric oxide level, and mitochondrial injury have been proposed to explain the cause of damage to neurons during stroke [23, 24]. Ischemic stroke occurs as a result of focal cerebral ischemia associated with permanent brain infarction. Ischemic stroke often leads to excessive glutamate release, acute excitotoxicity and nerve cell damage. In this study, HT22 cells exposed to glutamate was used as in vitro model of stroke to explore the effects of selenium on glutamate excitotoxicity and to assess the underlying mechanisms that lead to attenuation of glutamate-induced toxicity in the cell. Our findings showed that selenium could attenuate cell apoptosis, diminish superoxide production, and decrease expression of Apaf-1, caspase 9, and caspase 3. In addition, selenium decreased the level of the LC-3II, a proxy for autophagosome and protected against mitochondrial impairment induced by glutamate. Our results further indicate that glutamate-induced cell death appears to be a results caused by a mechanism that involves structural changes typical of apoptosis, including swelling of the mitochondria, chromatin condensation, and appearance of lysosomes.
Selenium has traditionally been used as a supplement for several decades in the prevention and treatment of human diseases including Keshan cardiomyopathy [25], Kashin-Beck osteoarthropathy [26, 27], osteopenia [28] and certain forms of cancers [29–31], and has been shown to modulate the functions of a variety of intracellular proteins [32–34]. Recent research has focused on the neuroprotective potential of selenium as a candidate for therapeutic intervention in hypoxic injuries and ischemia-induced neuronal damage [35–39]. The neuroprotective effect of selenium may be dependent on its antioxidant properties. Selenium in the form of selenoprotein is present in the active site of glutathione peroxidase and regulates the activity of the enzyme. Glutathione peroxidase participates in the complex antioxidant defense signaling network that is essential for the detoxification of cellular stresses induced by free radicals. ROS are chemically reactive molecules that contain oxygen such as superoxide, singlet oxygen, and hydrogen peroxide (H2O2). They are produced as natural byproducts of normal metabolism and play beneficial roles in cell signaling [40]. However, excessive ROS can be detrimental to cells as a result of induction of oxidative stress and cellular damage [41]. Earlier studies describing the chronic effect of glutamate exposure in cellular toxicity point to the crucial role of oxidative stress-induced ROS as a major stimulus of glutamate-induced cell death [17, 42–45]. It has been reported that excessive concentrations of extracellular glutamate antagonize the glutamate/cystine-antiporter and inhibit cystine uptake. This inhibitory effect leads to accumulation of ROS, oxidative stress, and mitochondrial hyperactivation [46]. Previous studies have shown that selenium attenuates the level of ROS following various in vitro injury models [16, 47–49]. Our results are in agreement with these studies. We observed high production of cellular superoxide radical with glutamate treatment that was considerably reduced upon co-treatment with selenium. This suggest that selenium exerts its influence partly by enhancing the activity of antioxidant systems in cells under stress.
Apoptosis, also known as programmed cell death, is a tightly regulated cell suicide process necessary for growth, development, and aging in multicellular organisms. Two well defined apoptotic pathways, intrinsic and extrinsic pathways, have been identified. Both pathways can be are activated by a variety of physiological and pathological conditions or stimuli and converge on a common execution point driven by caspases. Caspases are a family of cysteine-aspartic proteases endoproteases. Caspases are generally divided into two subtypes, initiator and effector caspases. Initiator caspases (caspases-8, -9 and -10) contain large prodomains and act as regulators of the caspase proteolytic cascade. Effector caspases (caspases-3, -6 and -7) are activated by initiator caspases, through proteolytic cleavage, and function in disassembly of cells into apoptotic bodies [50]. Glutamate induces cell death through processes that involve both necrosis and apoptosis [44, 51] although, apoptosis appears to be the major driver of cell fate at late time points. Apoptotic process culminate in alterations in the integrity of the inner mitochondrial membrane that results in the formation of the MPTP [52], failure of the mitochondrial membrane potential and release of pro-apoptogenic proteins into the cytosol. Among the proteins released from the mitochondria is Cytochrome c (Cyt c), a major component of the electron transport chain. Cyt c binds and activates Apaf-1, recruits procaspase-9 and dATP, and thus facilitates assembly of the apoptosome. Formation of the multi-protein apoptosome complex results in the activation of effector caspases 3, 6, and 7, which in turn translocates to the nucleus, and initiates cleavage of DNA and subsequent degradation of nucleosomal proteins [53]. Our findings show that the magnitude of Apaf-1, increased significantly in HT-22 cells under glutamate tension, while selenium antagonized the glutamate-induced increase in Apaf-1. Furthermore, glutamate-dependent increase in Apaf-1 was accompanied by concomitant increases in cleaved caspase 9 and cleaved caspase 3. Our data indicate that selenium also reduced the stimulation of caspase 9 and caspase-3-like protease activity that occurs in glutamate exposed cells. These selenium-dependent influences on effector caspase have been demonstrated previously in other cellular and in vivo animal models [15, 16, 54]. Apaf-1 is one of the well-studied physiological regulator of events of mitochondria-dependent apoptosis in most death pathways. Typically, it exists in an auto-inhibited form in the cytosol until it binds to Cyt c and promotes assembly of the apoptosome [55]. Our present findings suggest that inhibition of Apaf-1 activity may play a central role as a potential mechanism by which selenium protects HT22 cells from glutamate excitotoxicity.
The structure of mitochondria is mainly regulated by cycles of fusion and fission. During apoptosis, mitochondria undergo fission, chromatin condensation, and becomes highly fragmented [56]. The mammalian Drp1 is a dynamin-related cytoplasmic GTPase that mediates mitochondrial fission. Drp1 is a highly regulated protein and undergoes a number of steps including translocation from cytoplasm to the mitochondrial outer membrane for fission to occur [57, 58]. Our results showed that p-Drp1, as well as Fis-1, increased significantly after glutamate exposure, suggesting that glutamate triggered mitochondrial fission. Selenium treatment hampered the increases of p-Drp1 and Fis-1, indicating its ability of preventing mitochondrial fission. It is widely accepted that fragmentation associated with mitochondrial fission is a key activator of mitochondrial autophagy. During autophagy, components of damaged mitochondria are encapsulated in autophagosomes. Progression of autophagy is dependent on the recruitment of LC3-II, a c-terminal lipidated form of cytosolic LC3-I as a structural component of the autophagosomal membrane. Thereafter, LC3-II tagged autophagosomes are delivered to the lysosome/vacuole for degradation by lysosomal hydrolases. Our results revealed that glutamate increased the presence of lysosome in the cytoplasm and colocalization with the mitochondrial marker, supporting the prediction that glutamate induces mitochondrial autophagy. The presence of autophagy was supported by quantifying the relationship between LC3-I and LC3-II. Glutamate exposure led to an increase in autophagy as reflected in the conversion of LC3-I to LC3-II using Western blotting. Selenium on the other hand, significantly reduced the intensity of LysoTracker labeling and blocked the alteration of LC3-I to LC3-II, and thus, protected against glutamate-dependent cell death. Increased levels of Beclin 1 and upregulation of LC3-II relative to LC3-I as markers of autophagy in cells exposed to glutamate have been reported in our earlier studies [17]. Although the mechanisms by which selenium altered the ratios of LC3-I and LC3-II in the presence of glutamate is uncertain, we posit that selenium could modulate this process potentially by blocking the upstream conjugation of phosphatidylethanolamine to LC3-I, or enable interconversion of LC3-II back to LC3-I. The actual mechanism(s) remains to be elucidated.
Conclusions
Our results indicate that glutamate exposure causes increase of superoxide radical, activation of mitochondria-initiated cell death pathway, tilting mitochondrial dynamic balance towards fission and triggers mitochondrial autophagy. Concurrent treatment with sodium selenite rescues glutamate-induced cell death by reducing superoxide production, inhibiting intrinsic cell death pathway, blocking mitochondrial fission and suppressing mitochondrial autophagy. It is concluded that the protective effects of selenium against glutamate cytotoxicity is associated with preserving mitochondrial integrity and dynamic balance.
Methods
Cell culture and materials
Murine hippocampal neuronal HT22 cells (originally purchased from ATCC) were cultured in Dulbecco’s Modified Eagle Medium (DMEM)/F12 (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum (FBS, HyClone), 100 nM penicillin/streptomycin, at 37 °C in 5% CO2 and 90% relative humidity. The culture medium was renewed every 2 days. Sodium glutamate, and sodium selenite (Sigma-Aldrich, St Lois, MO), were reconstituted in water and diluted to appropriate concentrations in cell culture medium. Antibodies directed against caspase-3, caspase-9, apoptosis protease-activating factor-1 (Apaf-1), cleaved caspase-3, cleaved caspase-9 were purchased from Cell Signaling Technology, Danvers, MA. Anti LC3A/B, Fis1, Drp1, p-Drp1, and β-actin were purchased from Abcam, Cambridge, MA.
Cell viability assay
Cell viability was assessed with MTT cell proliferation assay kit (Trevigen Inc., Gaithersburg, MD) according to manufacturer’s instructions. In brief, HT22 cells were plated into 96-well plates (5000/well, Corning Inc., New York, NY). After seeding 24 h the cultures exposed to various concentrations of glutamate (2, 4, 6, 8, and 10 mM). After 24 h of incubation, the results were read in the SpectraMax microplate reader (Molecular Devices, Sunnyvale, CA). Glutamate of 6 mM was selected for the subsequent experiments.
Selenium treatment
Sodium selenite (100 nM) was added to the media concurrently with the glutamate. The selection of selenium concentration was based on results of previous publications [17, 23], which showed that 100–200 nM selenium provided the adequate neuroprotection in vitro, while 500 nM was toxic to the cells.
Electron microscopy
Log-phase cultures were collected, centrifuged and the pellets were fixed with 2% glutaraldehyde solution for 2 h at 4 °C. The samples were incubated in osmic acid for 2 h and 0.1 M dimethyl sodium arsenate buffer for 15 min for 2 times at 4 °C. Thereafter, the pellets were dehydrated in ascending ethanol, rinsed in propylene oxide, and incubated in propylene oxide and resin before embedding resin. The pellets were sliced and imaged on a transmission electron microscope.
Determination of superoxide radical
Superoxide anion production was measured by dihydroethidine (DHE) in glutamate only or selenium-co-treated HT22 cells exposed to glutamate (6 mM). Briefly, cells (5000 per well) were seeded on glass slides. After 24 h treatment period, the slides were incubated with the DHE (50 μM) for 45 min at 37 °C, washed with PBS, and fixed in 4% paraformaldehyde. Images were captured under 400× magnification using a fluorescent microscope (Olympus CHC-212) and fluorescent intensity was measured by IPP6.0 software.
Western blotting
For immunoblotting, treated cells were lysed in RIPA buffer containing protease (Roche Diagnostics Corp., Indianapolis, IN) and phosphatase (ThermoFisher Scientific, Waltham, MA) inhibitor cocktails.
The cells were lysed and subcellular fractions of the cytosol, mitochondrion, and nucleus were isolated through various peed of centrifugations [17]. Twenty micrograms of proteins in the total cell lysates were separated on 4–12% NuPAGE SDS-PAGE gels (Invitrogen), transferred to nitrocellulose membrane, and probed with the following antibodies:anti-Apaf-1 (1:500) and anti-caspase-9 (1:1000) for the cytosolic fraction; anti-caspase-3 (1:500) for the nuclear fraction; and anti-LC3A/B (1:1000), Fis 1 (1:2000), Drp1 (1:2000), p-Drp1 (1:1000) for the mitochondrial fraction. Anti β-actin (1:1000) was used as internal control for protein loading. After washing, the membranes were incubated in HRP-conjugated secondary anti IgG (1:5000) for 3 h at room temperature. Antibody binding was detected by enhanced chemiluminescence using Gel imaging analyzer (BIO-Rad Inc., Hercules, CA) and band intensity was measured using Image-J2x software.
Immunofluorescence
Cells were seeded for 24 h on glass slides and treated with glutamate or glutamate and selenium for additional 24 h in a humidified incubator at 37 °C and 5% CO2. The slides were washed with PBS, fixed with 4% paraformaldehyde for 15 min and permeabilized with 1% TritonX-100 for 30 min. The nonspecific binding sites were blocked with 10% goat serum and incubated overnight at 4 °C with antibodies against Apaf-1 (1:100), cleaved caspase-3 (1:100), and cleaved caspase-9 (1:100). After washing 3 times in PBS the slides were incubated secondary antibody (1:200) for 1 h at 4 °C and DAPI for 5 min in a dark environment. Images were captured in each well at a magnification of 400× and fluorescence intensities were measured as describe previously.
Detection of autophagy
Autophagy was detected by immunofluorescent double labeling with LysoTracker Red and MitoTracker Green. Cells were seeded in 12-well plate and treated with glutamate and selenium as previously described. Following treatment, LysoTracker Red (75 nM), was added to the culture medium and the cells were returned to the incubator for 45 min. MitoTracker Green (100 nM) was then added into culture medium an incubation continued for additional 45 min in a humidified incubator at 37 °C and 5% CO2. The slides were washed with PBS and fixed with 4% paraformaldehyde. Images were captured under the magnification of 400× using a fluorescent microscope (Olympus CHC-212) and fluorescent intensity were measured by IPP6.0 software.
Statistics
All experiments were repeated at least 2 times and carried out in triplicate. Statistical analysis was performed with IBM SPSS Statistics 19.0. All values were expressed as mean ± SEM. Data in Fig. 1a were analyzed by Student’s t test. Data in Figs. 1b and 3, 4, 5, 6, 7 and 8 were analyzed by one-way ANOVA and followed by Tukey’s test to detect differences between groups. Statistical significance was set at p < 0.05.
Abbreviations
- AD:
-
Alzheimer’s disease
- ANOVA:
-
analysis of variance
- Apaf-1:
-
apoptosis protease-activating factor-1
- Cyt c:
-
cytochrome c (Cyt c)
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DHE:
-
dihydroethidium
- Drp1:
-
dynamin-like protein 1
- FBS:
-
fetal bovine serum
- Fis1:
-
fission 1 protein
- GPx:
-
glutathione peroxidase
- GSH:
-
glutathione
- HRP:
-
horseradish peroxidase
- H2O2 :
-
hydrogen peroxide
- LC3:
-
microtubule-associated protein 1 light chain 3
- Mfn:
-
mitofusin
- MPTP:
-
mitochondrial permeability transition pore
- Opa1:
-
optic atrophy 1
- PD:
-
Parkinson’s disease
- RIPA buffer:
-
radioimmunoprecipitation assay buffer
- ROS:
-
reactive oxygen species
- SEM:
-
standard error of the mean
References
Blandini F, Greenamyre JT, Nappi G. The role of glutamate in the pathophysiology of Parkinson’s disease. Funct Neurol. 1996;11(1):3–15.
Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol. 2001;24(1–3):107–29.
Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989;2(6):1547–58.
Meeusen S, DeVay R, Block J, Cassidy-Stone A, Wayson S, McCaffery JM, Nunnari J. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006;127(2):383–95.
Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20(15):3525–32.
Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160(2):189–200.
Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8(11):870–9.
Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280(28):26185–92.
Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27(2):433–46.
Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–41.
Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–93.
Grander D, Kharaziha P, Laane E, Pokrovskaja K, Panaretakis T. Autophagy as the main means of cytotoxicity by glucocorticoids in hematological malignancies. Autophagy. 2009;5(8):1198–200.
Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13(7):805–11.
Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18(4):571–80.
Yeo JE, Kang SK. Selenium effectively inhibits ROS-mediated apoptotic neural precursor cell death in vitro and in vivo in traumatic brain injury. Biochim Biophys Acta. 2007;1772(11–12):1199–210.
Zhou YJ, Zhang SP, Liu CW, Cai YQ. The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK(1) cells. Toxicol In Vitro. 2009;23(2):288–94.
Kumari S, Mehta SL, Li PA. Glutamate induces mitochondrial dynamic imbalance and autophagy activation: preventive effects of selenium. PLoS ONE. 2012;7(6):e39382.
Touat-Hamici Z, Legrain Y, Bulteau AL, Chavatte L. Selective up-regulation of human selenoproteins in response to oxidative stress. J Biol Chem. 2014;289(21):14750–61.
Verma S, Hoffmann FW, Kumar M, Huang Z, Roe K, Nguyen-Wu E, Hashimoto AS, Hoffmann PR. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J Immunol. 2011;186(4):2127–37.
Tsuji PA, Carlson BA, Anderson CB, Seifried HE, Hatfield DL, Howard MT. Dietary selenium levels affect selenoprotein expression and support the interferon-gamma and IL-6 immune response pathways in mice. Nutrients. 2015;7(8):6529–49.
Mehta SL, Kumari S, Mendelev N, Li PA. Selenium preserves mitochondrial function, stimulates mitochondrial biogenesis, and reduces infarct volume after focal cerebral ischemia. BMC Neurosci. 2012;13:79.
Karavelioglu E, Boyaci MG, Simsek N, Sonmez MA, Koc R, Karademir M, Guven M, Eser O. Selenium protects cerebral cells by cisplatin induced neurotoxicity. Acta Cir Bras. 2015;30(6):394–400.
Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol. 2014;115:157–88.
Prentice H, Modi JP, Wu JY. Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxid Med Cell Longev. 2015;2015:964518.
Burke MP, Opeskin K. Fulminant heart failure due to selenium deficiency cardiomyopathy (Keshan disease). Med Sci Law. 2002;42(1):10–3.
Zou K, Liu G, Wu T, Du L. Selenium for preventing Kashin–Beck osteoarthropathy in children: a meta-analysis. Osteoarthr Cartil. 2009;17(2):144–51.
Peng A, Yang CL. Examination of the roles of selenium in the Kaschin–Beck disease. Cartilage cell test and model studies. Biol Trace Elem Res. 1991;28(1):1–9.
Moreno-Reyes R, Egrise D, Neve J, Pasteels JL, Schoutens A. Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J Bone Miner Res. 2001;16(8):1556–63.
Clark LC, Dalkin B, Krongrad A, Combs GF Jr, Turnbull BW, Slate EH, Witherington R, Herlong JH, Janosko E, Carpenter D, et al. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998;81(5):730–4.
Amaral AF, Cantor KP, Silverman DT, Malats N. Selenium and bladder cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev. 2010;19(9):2407–15.
Wei WQ, Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Sun XD, Fan JH, Gunter EW, Taylor PR, Mark SD. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutr. 2004;79(1):80–5.
Sarker KP, Biswas KK, Rosales JL, Yamaji K, Hashiguchi T, Lee KY, Maruyama I. Ebselen inhibits NO-induced apoptosis of differentiated PC12 cells via inhibition of ASK1-p38 MAPK-p53 and JNK signaling and activation of p44/42 MAPK and Bcl-2. J Neurochem. 2003;87(6):1345–53.
Kim IY, Stadtman TC. Inhibition of NF-kappaB DNA binding and nitric oxide induction in human T cells and lung adenocarcinoma cells by selenite treatment. Proc Natl Acad Sci USA. 1997;94(24):12904–7.
Wojewoda M, Duszynski J, Szczepanowska J. NARP mutation and mtDNA depletion trigger mitochondrial biogenesis which can be modulated by selenite supplementation. Int J Biochem Cell Biol. 2011;43(8):1178–86.
Yousuf S, Atif F, Ahmad M, Hoda MN, Khan MB, Ishrat T, Islam F. Selenium plays a modulatory role against cerebral ischemia-induced neuronal damage in rat hippocampus. Brain Res. 2007;1147:218–25.
Ahmad A, Khan MM, Ishrat T, Khan MB, Khuwaja G, Raza SS, Shrivastava P, Islam F. Synergistic effect of selenium and melatonin on neuroprotection in cerebral ischemia in rats. Biol Trace Elem Res. 2011;139(1):81–96.
Ansari MA, Ahmad AS, Ahmad M, Salim S, Yousuf S, Ishrat T, Islam F. Selenium protects cerebral ischemia in rat brain mitochondria. Biol Trace Elem Res. 2004;101(1):73–86.
Bordoni A, Biagi PL, Angeloni C, Leoncini E, Danesi F, Hrelia S. Susceptibility to hypoxia/reoxygenation of aged rat cardiomyocytes and its modulation by selenium supplementation. J Agric Food Chem. 2005;53(2):490–4.
Wang Y, Ji HX, Zheng JN, Pei DS, Hu SQ, Qiu SL. Protective effect of selenite on renal ischemia/reperfusion injury through inhibiting ASK1-MKK3-p38 signal pathway. Redox Rep. 2009;14(6):243–50.
Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;2012:936486.
Wittmann C, Chockley P, Singh SK, Pase L, Lieschke GJ, Grabher C. Hydrogen peroxide in inflammation: messenger, guide, and assassin. Adv Hematol. 2012;2012:541471.
Yang EJ, Kim GS, Jun M, Song KS. Kaempferol attenuates the glutamate-induced oxidative stress in mouse-derived hippocampal neuronal HT22 cells. Food Funct. 2014;5(7):1395–402.
Gliyazova NS, Huh EY, Ibeanu GC. A novel phenoxy thiophene sulphonamide molecule protects against glutamate evoked oxidative injury in a neuronal cell model. BMC Neurosci. 2013;14:93.
Fukui M, Song JH, Choi J, Choi HJ, Zhu BT. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur J Pharmacol. 2009;617(1–3):1–11.
Tan S, Schubert D, Maher P. Oxytosis: a novel form of programmed cell death. Curr Top Med Chem. 2001;1(6):497–506.
Kang Y, Tiziani S, Park G, Kaul M, Paternostro G. Cellular protection using Flt3 and PI3Kalpha inhibitors demonstrates multiple mechanisms of oxidative glutamate toxicity. Nat Commun. 2014;5:3672.
Yoon SO, Kim MM, Park SJ, Kim D, Chung J, Chung AS. Selenite suppresses hydrogen peroxide-induced cell apoptosis through inhibition of ASK1/JNK and activation of PI3-K/Akt pathways. FASEB J. 2002;16(1):111–3.
Panee J, Liu W, Nakamura K, Berry MJ. The responses of HT22 cells to the blockade of mitochondrial complexes and potential protective effect of selenium supplementation. Int J Biol Sci. 2007;3(5):335–41.
Sarada SK, Himadri P, Ruma D, Sharma SK, Pauline T. Mrinalini: selenium protects the hypoxia induced apoptosis in neuroblastoma cells through upregulation of Bcl-2. Brain Res. 2008;1209:29–39.
Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J. 2004;384(Pt 2):201–32.
Xu X, Chua CC, Zhang M, Geng D, Liu CF, Hamdy RC, Chua BH. The role of PARP activation in glutamate-induced necroptosis in HT-22 cells. Brain Res. 2010;1343:206–12.
Korde AS, Pettigrew LC, Craddock SD, Pocernich CB, Waldmeier PC, Maragos WF. Protective effects of NIM811 in transient focal cerebral ischemia suggest involvement of the mitochondrial permeability transition. J Neurotrauma. 2007;24(5):895–908.
Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15(6):725–31.
Ma W, Jing L, Valladares A, Mehta SL, Wang Z, Li PA, Bang JJ. Silver nanoparticle exposure induced mitochondrial stress, caspase-3 activation and cell death: amelioration by sodium selenite. Int J Biol Sci. 2015;11(8):860–7.
Hill MM, Adrain C, Duriez PJ, Creagh EM, Martin SJ. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. EMBO J. 2004;23(10):2134–45.
Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci. 2003;23(21):7881–8.
Otera H, Ishihara N, Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta. 2013;1833(5):1256–68.
Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann NY Acad Sci. 2010;1201:34–9.
Authors’ contributions
YMM carried out cell culture, detected autophagy, carried out mitochondrial fission, performed data analyses and drafted the manuscript. LYW carried out in vitro, measured superoxide, carried out Electron Microscopy and MTT. LYW performed cell cultures and participated in Immunofluorescence. PAL and LJ conceived, developed and oversaw the study. JJZ, YC, JDD performed data interpretation and analyses. YMM, JL, GI, PAL wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors appreciate Dr. Robert Onyenwoke for proofread the manuscript. BRITE is partially supported by Golden Leaf Foundation. This work was supported by funds from Natural Science Foundation of China (81560208, 81360184). The funding agency did not play a role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Materials and data can be requested from Dr. Li Jing, 1203220205@qq.com.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ma, YM., Ibeanu, G., Wang, LY. et al. Selenium suppresses glutamate-induced cell death and prevents mitochondrial morphological dynamic alterations in hippocampal HT22 neuronal cells. BMC Neurosci 18, 15 (2017). https://doi.org/10.1186/s12868-017-0337-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12868-017-0337-4