Abstract
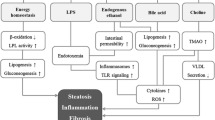
Liver steatosis is the most frequent liver disorder and its advanced stage, non-alcoholic steatohepatitis (NASH), will soon become the main reason for liver fibrosis and cirrhosis. The “multiple hits hypothesis” suggests that progression from simple steatosis to NASH is triggered by multiple factors including the gut microbiota composition. The Epstein Barr virus induced gene 2 (EBI2) is a receptor for the oxysterol 7a, 25-dihydroxycholesterol synthesized by the enzymes CH25H and CYP7B1. EBI2 and its ligand control activation of immune cells in secondary lymphoid organs and the gut. Here we show a concurrent study of the microbial dysregulation and perturbation of the EBI2 axis in a mice model of NASH.
We used mice with wildtype, or littermates with CH25H−/−, EBI2−/−, or CYP7B1−/− genotypes fed with a high-fat diet (HFD) containing high amounts of fat, cholesterol, and fructose for 20 weeks to induce liver steatosis and NASH. Fecal and small intestinal microbiota samples were collected, and microbiota signatures were compared according to genotype and NASH disease state.
We found pronounced differences in microbiota composition of mice with HFD developing NASH compared to mice did not developing NASH. In mice with NASH, we identified significantly increased 33 taxa mainly belonging to the Clostridiales order and/ or the family, and significantly decreased 17 taxa. Using an Elastic Net algorithm, we suggest a microbiota signature that predicts NASH in animals with a HFD from the microbiota composition with moderate accuracy (area under the receiver operator characteristics curve = 0.64). In contrast, no microbiota differences regarding the studied genotypes (wildtype vs knock-out CH25H−/−, EBI2−/−, or CYP7B1−/−) were observed.
In conclusion, our data confirm previous studies identifying the intestinal microbiota composition as a relevant marker for NASH pathogenesis. Further, no link of the EBI2 – oxysterol axis to the intestinal microbiota was detectable in the current study.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) has a global prevalence of 32% [1, 2], and currently constitutes the most frequent liver disorder. With global trends towards adaptation of a Western lifestyle worldwide, the number of people affected by NAFLD will rise even further. NAFLD comprises benign liver steatosis; however, it may progress to non-alcoholic steatohepatitis (NASH) [3, 4] which can lead to liver fibrosis and liver cirrhosis with related complications including hepatocellular carcinoma. Since viral hepatitis has been substantially declining in the last decade due to the availability of effective antiviral treatments, NAFLD/NASH will soon become the main reason for liver fibrosis and cirrhosis [5]. Subsequently, NAFLD/NASH will pose a significant clinical and economic burden on the healthcare systems [6, 7].
The pathogenesis of NAFLD and NASH is critically linked to a metabolic state with overabundance of macronutrients including free fatty acids (FFA). NAFLD is characterized by synthesis and accumulation of triglycerides in the liver. Importantly, NAFLD is associated with other metabolic diseases such as obesity, type 2 diabetes mellitus and dyslipidemia, a constellation of conditions termed “metabolic syndrome” [8, 9]. NASH is characterized by a hepatic inflammatory reaction [10]. However, only a subset of patients exhibiting similar metabolic comorbidities and dietary risk eventually develops NASH. The key steps mediating the progression of benign steatosis to NASH remain incompletely understood. A multiple hits hypothesis has been suggested in which additional adverse effects mediate hepatic inflammation [11]. Main inflammatory triggers include the alteration of lipid metabolism, with changes in the generation of adipokines and cytokines and increased oxidative stress inside the liver. Further the gut microbiota has been suggested as one key factor for NASH pathogenesis [10, 12, 13].
A possible causal role of the gut microbiota in the generation of hepatic steatohepatitis has been suggested, as in mice NASH can be induced by transplantation of dysbiotic gut microbiota, whereas the transplantation of complete healthy gut microbiota can alleviate NASH susceptibility [14,15,16,17]. The exact role of the gut microbiota in NASH pathogenesis is still unknown. Gut endotoxins, mainly bacterial lipopolysaccharides (LPS), have been shown to promote hepatic inflammation [18]. Further possible hypotheses involve induction of a microbiota-induced gut barrier dysfunction by increasing epithelial permeability, shifts in bile acid composition influencing microbiota community structure, and bacterial metabolites, like ethanol, influencing hepatic metabolism [19]. Increased permeability of the intestinal epithelium facilitates the transfer of bacterial lipopolysaccharides to the systemic circulation which can further promote proinflammatory effects [20].
Oxysterols are cholesterol metabolites, generated by enzymatic and non-enzymatic cholesterol oxidation [21]. They constitute precursors for bile acid synthesis but have also been implicated in sterol homeostasis and immune regulation [22]. One example of immune regulation via oxysterols is the EBI2-oxysterol axis. The Epstein Barr virus induced protein 2 (EBI2 – also known as GPR183) belongs to the family of G protein-coupled receptors [23]. Two landmark papers have identified EBI2 as a high-affinity receptor for the oxysterol 7a, 25-dihydroxycholesterol (7a, 25-diHC), generated by the enzymes cytochrome P450 7B1 (CYP7B1) and cholesterol 25-hydroxylase (CH25H) [24, 25]. EBI2 and its ligand 7a, 25-diHC have been implicated in the positioning and the activation of B cells, T cells, and dendritic cells and the generation of an efficient antibody response [26,27,28]. Further, the 7a, 25-diHC synthesizing enzyme CH25H has additional roles in inflammation [29] and has been involved in intestinal fibrosis [30].
Some evidence suggests a role of oxysterols in liver inflammation including NASH [22, 31,32,33,34,35]. However, the role of oxysterols in NASH pathogenesis is not yet fully understood. In a detailed previous study from our group, levels of 24- and 7-hydroxylated oxysterols were significantly increased in human NASH. However, murine knockouts of CH25H, EBI2 and CYP7B1 neither effected liver function tests, nor liver inflammatory markers, histological NAFLD activity or liver fibrosis in a long-term feeding model of NASH [31].
A connection between the microbiota and the EBI2-oxysterol axis, as well as the oxysterol precursor cholesterol has been suggested [36,37,38]. The bacterial metabolite lauroyl tryptamine can act as an antagonist of EBI2 in vitro. Intestinal lauroyl tryptamine production was attributed to gene clusters found in diverse Clostridia and has been demonstrated in vitro by Eubacterium rectale [39]. Other bacterial metabolites such as short chain fatty acids (SCFA) can act as GPR41 and GPR43 ligands and in turn down-regulate cholesterol transport and synthesis [36, 40]. Finally, the intestinal microbiota also has general effects on cholesterol levels. For example, bile salt hydrolases from Lactobacillus strains can deconjugate bile acids, leading to reduced reabsorption and lower circulating cholesterol levels [36]. Further, multiple Eubacterium and Lactobacillus strains can biotransform cholesterol into coprostanol, facilitating elimination with feces [37, 41].
Taken together, EBI2 and CH25H have profound effects on the intestinal immune system; however, the role of the EBI2-oxysterol axis on the intestinal microbiota in health and disease (e.g., NASH) is unknown, justifying a detailed investigation. The high degree of interconnection between metabolic state, oxysterol regulation, and microbiota, creates a challenge for disentangling individual effects. We used samples from a previous study [31] to assess any concurrent relationship between players of the EBI2-oxysterol axis and microbiota composition in a murine-feeding model of NASH [31].
Methods
Murine feeding model
Approval by the local animal welfare authority (Tierschutzkommision Zürich, Zurich, Switzerland; registration number ZH 50/2013) was granted for the experimental protocol for animal testing. All animals used had a C57BL/6 background. Knockout animals for CH25H (Ch25h−/−), EBI2 (Ebi2−/−), and CYP7B1 (Cyp7b1−/−) were generated as previously described [31]. The initial knockout animals were provided by Novartis Institutes (Ch25h−/− and Ebi2−/−) or purchased from Jackson Laboratories (Cyp7b1−/−). Heterozygous mice were subsequently crossed with each other to obtain wild-type (Ch25h+/+, Ebi2+/+, or Cyp7b1+/+) and knockout (Ch25h−/−, Ebi2−/−, or Cyp7b1−/−) littermates. The mice used were eight-week-old littermates, housed under specific pathogen-free conditions in individually ventilated cages.
We induced NAFLD/NASH by a murine feeding model in C57BL/6 mice by supplying the animals for 20 weeks with a high-fat, high-cholesterol diet (HFD; ssniff Spezialdiäten GmbH) and a high-fructose corn syrup equivalent (55% fructose and 45% glucose, at a concentration of 42 g/l) in the drinking water as described previously [31]. Control mice (standard diet, STD) were fed standard chow (Provimi Kliba) and water ad libitum. Wildtype and knockout animals were sacrificed and tested regarding clinical and histological hallmarks of NASH after 20 weeks [31]. Further, microbiota samples from the small intestine and feces were collected. Liver samples were assessed by histology to score for the presence of steatosis, the occurrence of cellular hypertrophy or ballooning, and the presence of necroinflammation to diagnose NASH.
In total, 68 mice were investigated under HFD in the study and 42 fecal samples could be analyzed. From control animals under STD 54 fecal samples were included in the analysis (Table 1).
16S rRNA amplicon sequencing
DNA was extracted using DNEasy PowerSoil kits (Qiagen, Hilden, Germany) as per the manufacturer’s instructions. Targeted amplification of the 16S rRNA V4 region (primer sequences F515 5’-GTGCCAGCMGCCGCGGTAA-3’ and R806 5’-GGACTACHVGGGTWTCTAAT-3’ [42], was performed in a two-step barcoded PCR protocol using the FailSafe PCR PreMix (Lucigen, WI, USA) according to the manufacturer’s instructions. PCR products were pooled, purified using size-selective SPRIselect magnetic beads (0.8 left-sized), and then sequenced at 2 × 250bp on an Illumina MiSeq (Illumina, San Diego, CA, USA) at the Genomics Core Facility, European Molecular Biology Laboratory, Heidelberg.
Raw 16S rRNA reads were trimmed, denoised, and filtered to remove chimeric PCR artefacts using DADA2 [43]. The resulting Amplicon Sequence Variants (ASVs) were then clustered into Operational Taxonomic Units (OTUs) at 98% sequence similarity using an open-reference approach: reads were first mapped to the pre-clustered reference set of full-length 16S rRNA sequences at 98% similarity included with MAPseq v1.2.6 [44]. Reads that did not confidently map were aligned to bacterial and archaeal secondary structure-aware SSU rRNA models using Infernal [45] and clustered into OTUs with 98% average linkage using hpc-clust [46], as described previously [47]. The resulting OTU count tables were noise filtered by asserting that samples retained at least 400 reads and taxa were prevalent in at least 1% of samples; these filters removed 45% of OTUs as spurious, corresponding to 0.16% of total reads.
Data analysis
Local sample diversities were calculated as OTU richness, exponential Shannon entropy and inverse Simpson index (corresponding to Hill diversities of order 0, 1, and 2 [48]) as average values of 100 rarefaction iterations to 400 reads per sample. Between-sample community diversity was calculated as Bray–Curtis dissimilarity [49]. Trends in community composition were quantified using ordination methods (Principal Coordinate Analysis, distance-based Redundancy Analysis) and tested using permutational multivariate analysis of variance (PERMANOVA [50]) or ANOVA, as implemented in the R package vegan [51].
Machine learning models were built by randomly splitting data into test and training sets in 10 times repeated tenfold cross-validation. For each fold, models were trained using the Elastic Net algorithm as implemented in the R package siamcat [52]. Models were evaluated based on the average Area Under the Receiver Operating Characteristic curve (AUROC), averaged across validation folds. All codes used are deposited in the git repository at https://git.embl.de/tschmidt/ch25h-microbiome.
Results
Impact of a high-fat diet on the intestinal microbiota
We used a high-fat diet (HFD) containing high amounts of fat and cholesterol with high-fructose corn syrup equivalent in the drinking water (42g/l) to induce NASH in mice. Animals were sacrificed at 20 weeks, and the degree of steatohepatitis was assessed in liver histology. As previously described, the HFD induced liver steatosis in most animals and NASH in approximately 50% of animals at 20 weeks, while the liver histology in controls remained normal, as described in detail before [31].
We analyzed the microbiota in fecal samples at 20 weeks by 16S sequencing. The HFD resulted in significant microbial changes in the mice. Taxa richness in stool samples of mice fed HFD was significantly lower than that of mice fed STD (Fig. 1A). This pattern persisted when richness was assessed by the exponential Shannon and the inversed Simpson diversity indices (Fig. S1). Diet further induced gross changes in the microbiota community structure. The ordination plot showed a clear distinction of the samples clustered together according to diet type (PERMANOVA R2 = 0.079, p ≤ 10^-4; Fig. 1B). Overall, these results suggest that HFD diet impacts on the microbiota composition and leads to a reduction of alpha diversity. Taken together, reduced alpha diversity and beta diversity shifts, hint towards microbial adaptation with a reduced number of taxa in HFD.
Comparison of species richness and beta-diversity of mice under standard diet and high-fat diet. A Individual measurements of OTU richness after rarefaction to the lowest sequencing depth are depicted. Each dot represents a sample from a total of n = 43 mice. B PCoA depiction of beta-diversity measured by Bray–Curtis dissimilarity with colors indicating samples belonging to the standard diet and the high-fat diet group
Microbiota profile in stool samples and the small intestine
To analyze the small intestinal microbiota, we isolated samples from the small intestine in mice with the HFD and STD controls. Species richness was higher in fecal samples than from samples collected from the small intestine; however, no difference in diversity was observed between the proximal, middle, and distal small intestines (Fig. 2A, Fig. S2). In the ordination analysis, fecal samples and small intestinal samples were significantly separated, indicating a different microbiota composition (PERMANOVA R2 = 0.112, p ≤ 10^-4). Further, samples from different sites of the small intestine clustered together, and a gradual shift from the proximal to the distal intestine was detected with samples from the distal small intestine being closest to fecal samples (Fig. 2B).
Comparison of species richness and beta-diversity of intestinal samples from different body sites. A Individual measurements of OTU richness after rarefaction to the lowest sequencing depth are depicted. Each dot is representing a sample from a total of n = 77 mice. Plots are split according to sampling location. B PCoA depiction of beta diversity measured by Bray–Curtis dissimilarity with colors indicating sampling location
Similar to fecal samples, small intestine samples also tended to cluster according to feeding type and disease status in all small intestinal sampling locations (Fig. 3A-C). However, the number of samples with sufficient recovered DNA from the HFD group was very low (n = 27), thus limiting detailed comparisons. These findings indicate a systematic shift in the microbiota composition in regard to feeding type and the NASH status on top of an underlying gradient of the microbiota composition along the longitudinal axis of the intestinal tract.
Comparison of beta-diversity according to diet type between intestinal samples stratified by genotypes. PCoA depiction of beta diversity measured by Bray–Curtis dissimilarity of genotypes and diet type. Samples are color-coded as indicated by the figure legend. Genotypes: wildtype (wt) and knockout. Diet: standard diet (STD) and high-fat diet (HFD). A Distal small intestine. B Mid small intestine. C Proximal small intestine. The plot represents 14 HFD and 38 STD samples in the distal, 9 HFD and 26 STD samples in the mid, and 4 HFD and 42 STD samples in the proximal small intestine. Missing samples originate from technical inabilities to obtain successful sequencing results
Microbiota profile according to NASH disease state
Upon 20 weeks of HFD, approximately 50% of animals developed NASH. Interestingly, NASH was associated with a higher (although only borderline significant) microbiota diversity compared to animals with HFD without NASH (Fig. 4A), indicating an association of the intestinal microbiota with liver inflammation beyond the HFD. The community analysis also indicated differences in microbiota community structures according to liver histology (PERMANOVA R2 = 0.108, p ≤ 10^-4) even though we observed a pronounced overlap in the multidimensional scaling map (Fig. 4B). Thus, the change in alpha and beta diversity appears to be linked to NASH progression. This suggests that even under the same feeding regimen (all mice with HFD) the microbiota differed between individual animals. Limited associations could be detected between individual microbiota profiles and the occurrence of NASH, either as a triggering factor or a consequence of the liver disease.
Comparison of species richness and beta-diversity of fecal samples according to NASH status. A Individual measurements of OTU richness after rarefaction to the lowest sequencing depth are depicted. Each dot is representing a sample from a total of n = 42 mice. Plots are split according to disease status. B PCoA depiction of beta-diversity measured by Bray–Curtis dissimilarity with colors indicating NASH status of mice
Microbiota profile according to genotypes
Animals in our HFD trial comprised wildtype animals but also littermates with knockouts in the oxysterol receptor EBI2 or one of the 7a, 25-diHC synthesizing enzymes CH25H or CYP7B1. When the fecal samples were stratified by diet, NASH status and genotypes, microbiota clustered distinctly according to diet and NASH (Fig. 5A-C) without apparent effects of the investigated genotypes wildtype, CH25H, EBI2, or CYP7B1. Similarly, samples from the distal, mid and proximal small intestine also showed no or only minimal deviation according to genotype (Supplementary Fig. 3C-F). This indicates that the known modulating effect of the EBI2-axis on gut lymphoid structures was not relevant for gut microbiota alterations related to NASH pathogenesis.
Distance-based redundancy analysis of fecal microbiota samples according to genotypes with diet, genotype, and NASH status as explanatory variables. Samples are colored according to feeding type (blue: standard diet, green: high-fat diet). A EBI2−/− (dark/light) versus wildtype. B CH25H−/− (dark/light) versus wildtype. C CYP7B1−/− (dark/light) versus wildtype. In these plots global centroids for genotype (`KO` vs `WT`) and diet (`STD` vs `HFD`) are shown; a larger distance of the centroids from `[0, 0]` indicate a stronger effect on community composition. The (constrained) vectors of continuous variable effects are shown (for NAS, q.0, q.1, q.2); the direction of the vectors relative to each other indicates if effects are correlated (same direction) or anti-correlated (different direction). q.0, q.1, and q.2 are Hill alpha-diversities; q.0 is (rarefied) taxa richness, q.1 and q.2 are effective taxa numbers weighted by taxa abundances
Differential abundance analysis
We observed a significant increase of 33 OTUs and a decrease of 17 OTUs in animals with NASH compared to animals without NASH (Wilcoxon test, FDR-corrected p < 0.05). Among the enriched OTUs, 17 belong to either the Clostridiales order and/ or the Clostridiaceae family, 3 were belonging to the Bacteroidales order, 2 belonging to the Rikenellaceae family, one was identified as Akkermansia muciniphila, 2 were belonging to Ruminococcaceae family, two were Lactobacillus, one belonged to the Prevotellaceae family, one was an Eubacterium, another belonged to the Eubacteriaceae, two to the Oscillospiraceae and one to the Eggerthellaceae family (Fig. 6).
Identification of microbiota signatures according to NASH status after classification by SIMCAT. The importance of specific microbial taxa for the classification according to NASH status is shown. The weight of individual taxa used for classification is indicated together with individual effect size and robustness of classification. OTUs are named by their lowest taxonomic resolution and are colored according to their classification status (brown: NASH, green: no-NASH). The heatmap indicates taxa as features with sample abundance colored by the corresponding Z-scores. The panel at the bottom of the figures indicates the metadata available to the model (Classification: Result of Enet classification. Gene (cohabitation): Mice raised in the same cage. Genotype: wildtype vs knock-out CH25H−/−, EBI2−/−, or CYP7B1.−/−. Fibrosis: Histology scoring of liver. NASH: Outcome measure of occurrence of NASH. q0: (rarefied) taxa richness).The panel with the title ENET model represents the weight statistic of the model (number of top features and their weight in the complete model)
Among the 17 OTUs depleted in NASH, 4 belong to Bacteroidales, one to Bacteroides, 2 to Lactobacillus, 3 to Clostridiales, 1 to Clostridium symbiosum, 1 to Prevotellaceae, one to Delftia, one belonging to Firmicutes, one to Porphyromonadaceae, one to Lachnospiraceae and one Oscillospiraceae (Fig. 6).
To test how these univariate associations could be harnessed to predict NASH status, we next trained predictive models using the Elastic Net (ENET) algorithm using 10 times repeated tenfold cross-validation (see Methods). The resulting models were moderately predictive of NASH at an AUROC of 0.64; interestingly, the algorithm exclusively picked microbiome features, as other features were homogenously distributed among the populations with and without NASH.
Discussion
We provide evidence that fecal and intestinal microbiota composition in a murine feeding model of NASH differ between mice developing NASH and the ones that do not, according to liver histology upon feeding with a high fat diet. In mice with NASH, 33 OTUs were significantly enriched, mainly stemming from the Clostridiales order or the Clostridiaceae family, while 17 OTUs were significantly depleted (Fig. 7). In contrast, the microbiota composition did not reveal strong differences between wildtype, CH25H−/−, EBI2−/−, or CYP7B1−/− animals. The most striking difference between the enriched OTUs comparing NASH and non-NASH mice in our study was the generally higher abundance of members of the Clostridiales order or the Clostridiaceae family in the NASH mice, while some members were also decreased. This might reflect a high baseline abundance of these taxa in the murine intestinal microbiota under a high-fat diet [53]. It has to be noted that several members of this clade are metabolically active and might therefore benefit from the high influx of easily accessible metabolites [54, 55]. Many species of these anaerobic bacteria ferment indigestible polysaccharides and are one of the main producers of SCFAs. SCFA have anti-inflammatory effects and are generally associated with positive health outcomes in many settings. However, NASH-associated taxa from our study with documented SCFA production are not limited to Clostridiales and Clostridiaceae. The family Oscillospiraceae belongs to the Clostridia class as well and includes SCFA producing bacteria like Faecalibacterium prausnitzii [56]. Other NASH-associated taxa in our study, involved in SCFA production are Prevotellaceae and Lachnospiraceae [57,58,59,60,61].
Summary of the evidence and model of the interplay between gut microbiota, oxysterol synthesizing enzymes and NASH: Levels of 24- and 7 hydroxylated oxysterol in liver biopsies are elevated in human NASH (upper left [31]). In a murine feeding model of NASH, relative abundance of some bacterial taxa of the intestinal differed between animals with and without NASH. Because of limits in the taxonomic resolution some OTUs with identical assignment were found in both conditions. Mechanistic effects of the intestinal microbiota and NASH have not been addressed by the current study but microbial metabolites absorbed from the intestine might mediate the induction of liver inflammation
On the other hand, effects of Clostridiales and Clostridiaceae might also be mediated by other mechanisms. Some Clostridiaceae can degrade polyphenols, thereby mitigating potential anti-oxidative effects of this class of molecules [62]. Furthermore, Eubacteriaceae of the Clostridia class also contribute to enzymatic bile acid and cholesterol transformations [63]. Among NASH-associated non-Firmicutes, Eggerthellaceae of the phylum Actinomycetota are not saccharolytic and perform unusual chemical transformations involving amino-acids and polyphenols [64, 65].
Lactobacillus are characterized by their fermentation of carbohydrate to lactic acid which is widely used in the food industry. Apart from this they are able to produce a wide range of other metabolites like SCFAs, amines, bacteriocins and vitamins [57]. They are commonly used as probiotics for intestinal and metabolic health [58]. Some Lactobacillus taxa were positively, others negatively associated with NASH in our study (Fig. 6); however, the reasons for the respective effects are unknown.
Surprisingly, also Akkermansia muciniphila was associated with NASH in our analysis. Generally, Akkermansia is considered to be a probiotic bacterium with positive influence against metabolic diseases, including NASH and obesity [66,67,68]. It is using mucin as a carbon and energy source producing SCFAs and oligosaccharides as main metabolites [69]. Porphyromonadaceae were negatively associated with NASH in our study; in agreement with past human observations related to increased human life span and reduced visceral fat [70, 71].
Previous microbiota signatures found in mice with NASH induced by several feeding models proved inconsistent so far. Similar to our results, a differential expression of some taxa with a high abundance of Lactobacillus and Akkermansia has been described, [72, 73] while other groups reported differing findings [72,73,74,75,76,77].
The microbiota composition between animals fed with the HFD compared to the STD was markedly different. The lower species richness of the intestinal microbiota under a HFD is lower in accordance with previous studies [59]. On a taxonomic level, microbiota signatures in mice provided with an HFD are consistently characterized by an increase of Firmicutes and a decrease of Bacteroidetes [78]. On lower taxonomic levels, findings are more complex; however, in one study a swift and distinct increase of Clostroidales, Verrucomicrobiales and Erysipelotrichiales upon a high-fat diet challenge was a reproducible finding across multiple wildtype strains of mice [79]. Animal studies have thus provided important insights into microbiota changes associated with NASH. The use of animal models permits standardization of the investigated subjects regarding sex, feeding patterns and in regard to metabolic comorbidities (especially type 2 diabetes) that are often difficult to control in human studies [80].
Our findings are in line with differences in human microbiota profiles in NAFLD and NASH compared to the composition in controls. Signatures differ from the ones observed in mice models. In human NASH, an enrichment of Proteobacteria has been consistently described [81,82,83,84,85,86]. On the family level an increase of Enterobacteriaceae [81, 84] and a decrease of Rikenellaceae [87] and Ruminococcaceae [81, 82, 88,89,90] has been observed. Several genera are consistently found to be increased (e.g. Dorea [82, 87, 91]) or decreased (Faecalibacterium [84], Coprococcus [84, 87, 90, 92, 93], Anaerosporobacter [92]). Furthermore, a differential abundance in Lachnospiraceae [81, 82, 87, 92] has been commonly but less consistent described. To note, NASH shows some shared dysbiotic patterns similar to other metabolic diseases like obesity or type 2 diabetes mellitus [94] with decreased Clostridium levels [83, 85, 87, 88, 95] and increases in Lactobacillaceae including Lactobacillus [82, 85, 87, 88, 90,91,92,93, 95,96,97] which in turn are shared with the profile detected in the current study.
The comparison of wildtype mice with CH25H−/−, EBI2−/−, or CYP7B1−/− animals did not reveal relevant differences in their microbiota composition. Therefore, effects of the EBI2-oxysterol axis on the formation of intestinal lymphoid structures, positioning of B cells in the colon and intestinal IgA levels did not notably affect bacterial populations [24,25,26]. Notably, the design of our study with litter mates of wildtype and knockout animals with the same diet but differing only by the genotype would enable detection also of subtle microbiota shifts with high sensitivity. While this argues against a relevant impact of the EBI2-oxysterol axis on the microbiota composition in NASH, other oxysterols might still be relevant, especially since bile acids and other products of liver metabolism can shape the gut microbiota [98]. Bile acids are modified by the intestinal microbiota and are reabsorbed as secondary bile acids which have important immune regulatory functions [99,100,101]. The composition and amount of bile acid secreted has been shown to be influenced by dysbiosis [102], and concurrent changes of bile acid composition and microbiota aberrations have been found in human NASH patients with hepatocellular carcinoma [88]. The upregulation of bile acids can thus have a direct effect on microbiota composition, favoring strains with better adaption to higher bile acid concentrations [103]. Therefore, complex indirect effects of changes in sterol synthesis affecting the gut microbiota have to be considered in studies addressing the intestinal microbiota and hepatic sterol metabolism.
Therapeutic efforts in liver steatosis and NASH are currently centered around lifestyle modification. While these are effective in reversing early disease stages, patient compliance is often lacking, limiting therapeutic success [104]. Additional medical therapies are currently under investigation [11, 83, 105], potentially preventing and reversing NAFLD; however, no effective treatment for fibrosis is available yet. In end-stage liver disease, liver transplantation ultimately remains the only treatment option. Thus, understanding relevant factors of the gut microbiota influencing the progression of liver steatosis to NASH can potentially indicate new therapeutic targets. Notably, gut microbiota directed therapies have shown clinically relevant results. Modulation of gut microbiota in by symbiotic therapy was able to reduce liver steatosis [76, 106,107,108,109].
Our study has several strengths and limitations. Strengths include the use of a relevant animal feeding model mimicking human NASH pathogenesis. Although generally NASH mice models do not show the full phenotype of human steatosis (especially hepatocyte ballooning) they are useful to investigate hepatic metabolic pathways [110]. Notably, blinded histological analysis of mice livers in the current study confirmed histological hallmarks of NASH in our feeding model [31]. Our study used littermates as controls, further reducing possible confounding factors.
An obvious limitation is the limited possibility of a direct translation of mice microbiota results to the human physiology. Further, human and murine oxysterol chemistry is complex, and our results only apply to EBI2 and its ligands and cannot be generalized to other oxysterols. Moreover, from many animals receiving a HFD, no sequencing results could be obtained in small intestinal samples, most likely for technical reasons. More generally, 16S amplicon sequencing based microbiome profiling provides a limited taxonomic resolution, although our analysis of both OTUs and ASVs exploited the available taxonomic profile information as far as currently possible. A notable further limitation is that Enterobacteriaceae which were reported to be related to NASH status in previous studies are only present in low abundance in our specific pathogen free mice population, which has likely precluded their detection as discriminatory taxa in our analysis.
In conclusion, differential expression of several intestinal microbiota taxa was detectable according to NASH disease state, confirming and expanding previous results. However, no impact of all tested genotypes (EBI2, CH25H, CYP7B1) on NASH could be detected, arguing against a relevant interaction of gut microbiota dysbiosis and the EBI – oxysterol axis in our murine NASH model.
Availability of data and materials
"The codes uses for analysis presented in this article are available in the githup repository https://git.embl.de/tschmidt/ch25h-microbiome. The dataset supporting the conclusions of this article is available in the European Nucleotide Archive (ENA) database under the accession number PRJEB67581.
Abbreviations
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- FFA:
-
Free fatty acids
- OTU:
-
Operational taxonomic unit
- AUROC:
-
Area under the receiver operating characteristic curve
- EBI2:
-
Epstein Barr virus-induced gene 2 - Also known as GPR183
- CH25H:
-
Cholesterol 25-hydroxylase
- CYP7B1:
-
Cytochrome P450 7B1
- 7a, 25-diHC:
-
7α,25-dihydroxycholesterol
- 25-OHC:
-
25-hydroxycholesterol
- 27-OHC:
-
27-hydroxycholesterol
- 24(S)-OHC:
-
24-S-hydroxycholesterol
- 7-OHC:
-
7-hydroxycholesterol
- HFD:
-
High fat diet
- STD:
-
Standard diet
- SCFA:
-
Short chain fatty acids
References
Teng ML, Ng CH, Huang DQ, Chan KE, Tan DJ, Lim WH, et al. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2023;29(Suppl):S32-42.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatol Baltim Md Juli. 2016;64(1):73–84.
Wong VWS, Wong GLH, Choi PCL, Chan AWH, Li MKP, Chan HY, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59(7):969–74.
Nasr P, Ignatova S, Kechagias S, Ekstedt M. Natural history of nonalcoholic fatty liver disease: A prospective follow-up study with serial biopsies. Hepatol Commun. 2018;2(2):199–210.
Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70(3):531–44.
Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatol Baltim Md. 2016;64(5):1577–86.
Morgan A, Hartmanis S, Tsochatzis E, Newsome PN, Ryder SD, Elliott R, et al. Disease burden and economic impact of diagnosed non-alcoholic steatohepatitis (NASH) in the United Kingdom (UK) in 2018. Eur J Health Econ HEPAC Health Econ Prev Care. 2021;22(4):505–18.
Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37(S1):81–4.
Alberti KGM, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–62.
Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15(6):349–64.
Machado MV, Diehl AM. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology. 2016;150(8):1769–77.
Noureddin M, Sanyal AJ. Pathogenesis of NASH: The Impact of Multiple Pathways. Curr Hepatol Rep. 2018;17(4):350–60.
Yang M, Qi X, Li N, Kaifi JT, Chen S, Wheeler AA, et al. Western diet contributes to the pathogenesis of non-alcoholic steatohepatitis in male mice via remodeling gut microbiota and increasing production of 2-oleoylglycerol. Nat Commun. 2023;14(1):228.
Duarte SMB, Stefano JT, Oliveira CP. Microbiota and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH). Ann Hepatol. 2019;18(3):416–21.
Roy TL, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62(12):1787–94.
Zhou D, Pan Q, Shen F, Cao H xia, Ding W jin, Chen Y wen, et al. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7(1):1529.
Koning M, Herrema H, Nieuwdorp M, Meijnikman AS. Targeting nonalcoholic fatty liver disease via gut microbiome-centered therapies. Gut Microbes. 2023;15(1):2226922.
de Faria Ghetti F, Oliveira DG, de Oliveira JM, de Castro Ferreira LEVV, Cesar DE, Moreira APB. Influence of gut microbiota on the development and progression of nonalcoholic steatohepatitis. Eur J Nutr. 2018;57(3):861–76.
Brandl K, Schnabl B. The intestinal microbiota and NASH. Curr Opin Gastroenterol. 2017;33(3):128–33.
Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm. 2010;7(1):15.
van Reyk DM, Brown AJ, Hult’en LM, Dean RT, Jessup W. Oxysterols in biological systems: sources, metabolism and pathophysiological relevance. Redox Rep Commun Free Radic Res. 2006;11(6):255–62.
Lefort C, Cani PD. The Liver under the Spotlight: Bile Acids and Oxysterols as Pivotal Actors Controlling Metabolism. Cells. 2021;10(2):400.
Misselwitz B, Wyss A, Raselli T, Cerovic V, Sailer AW, Krupka N, et al. The oxysterol receptor GPR183 in inflammatory bowel diseases. Br J Pharmacol. 2021;178(16):3140–56.
Liu C, Yang XV, Wu J, Kuei C, Mani NS, Zhang L, et al. Oxysterols direct B-cell migration through EBI2. Nature. 2011;475(7357):519–23.
Hannedouche S, Zhang J, Yi T, Shen W, Nguyen D, Pereira JP, et al. Oxysterols direct immune cell migration through EBI2. Nature. 2011;475(7357):524–7.
Wyss A, Raselli T, Perkins N, Ruiz F, Schmelczer G, Klinke G, et al. The EBI2-oxysterol axis promotes the development of intestinal lymphoid structures and colitis. Mucosal Immunol. 2019;12(3):733–45.
Baptista AP, Gola A, Huang Y, Milanez-Almeida P, Torabi-Parizi P, Urban JF, et al. The Chemoattractant Receptor Ebi2 Drives Intranodal Naive CD4+ T Cell Peripheralization to Promote Effective Adaptive Immunity. Immunity. 2019;50(5):1188-1201.e6.
Chu C, Moriyama S, Li Z, Zhou L, Flamar AL, Klose CSN, et al. Anti-microbial Functions of Group 3 Innate Lymphoid Cells in Gut-Associated Lymphoid Tissues Are Regulated by G-Protein-Coupled Receptor 183. Cell Rep. 2018;23(13):3750–8.
Trindade BC, Ceglia S, Berthelette A, Raso F, Howley K, Muppidi JR, et al. The cholesterol metabolite 25-hydroxycholesterol restricts activation of the transcriptional regulator SREBP2 and limits intestinal IgA plasma cell differentiation. Immunity. 2021;54(10):2273-2287.e6.
Raselli T, Wyss A, Gonzalez Alvarado MN, Weder B, Mamie C, Spalinger MR, et al. The Oxysterol Synthesising Enzyme CH25H Contributes to the Development of Intestinal Fibrosis. J Crohns Colitis. 2019;13(9):1186–200.
Raselli T, Hearn T, Wyss A, Atrott K, Peter A, Frey-Wagner I, et al. Elevated oxysterol levels in human and mouse livers reflect nonalcoholic steatohepatitis. J Lipid Res. 2019;60(7):1270–83.
Serviddio G, Bellanti F, Villani R, Tamborra R, Zerbinati C, Blonda M, et al. Effects of dietary fatty acids and cholesterol excess on liver injury: A lipidomic approach. Redox Biol. 2016;9:296–305.
Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular Mechanisms of Lipotoxicity and Glucotoxicity in Nonalcoholic Fatty Liver Disease. Metabolism. 2016;65(8):1049–61.
Ikegami T, Hyogo H, Honda A, Miyazaki T, Tokushige K, Hashimoto E, et al. Increased serum liver X receptor ligand oxysterols in patients with non-alcoholic fatty liver disease. J Gastroenterol. 2012;47(11):1257–66.
Bieghs V, Hendrikx T, van Gorp PJ, Verheyen F, Guichot YD, Walenbergh SMA, et al. The Cholesterol Derivative 27-Hydroxycholesterol Reduces Steatohepatitis in Mice. Gastroenterology. 2013;144(1):167-178.e1.
Vourakis M, Mayer G, Rousseau G. The Role of Gut Microbiota on Cholesterol Metabolism in Atherosclerosis. Int J Mol Sci Januar. 2021;22(15):8074.
Kriaa A, Bourgin M, Potiron A, Mkaouar H, Jablaoui A, Gérard P, et al. Microbial impact on cholesterol and bile acid metabolism: current status and future prospects. J Lipid Res. 2019;60(2):323–32.
Le Roy T, Lécuyer E, Chassaing B, Rhimi M, Lhomme M, Boudebbouze S, et al. The intestinal microbiota regulates host cholesterol homeostasis. BMC Biol. 2019;17(1):94.
Chang FY, Siuti P, Laurent S, Williams T, Glassey E, Sailer AW, et al. Gut-inhabiting Clostridia build human GPCR ligands by conjugating neurotransmitters with diet- and human-derived fatty acids. Nat Microbiol. 2021;6(6):792–805.
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165(6):1332–45.
Juste C, Gérard P. Cholesterol-to-Coprostanol Conversion by the Gut Microbiota: What We Know, Suspect, and Ignore. Microorganisms. 2021;9(9):1881.
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4516–22.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–3.
Matias Rodrigues JF, Schmidt TSB, Tackmann J, von Mering C. MAPseq: highly efficient k-mer search with confidence estimates, for rRNA sequence analysis. Bioinformatics. 2017;33(23):3808–10.
Nawrocki EP, Eddy SR. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics. 2013;29(22):2933–5.
Matias Rodrigues JF, von Mering C. HPC-CLUST: distributed hierarchical clustering for large sets of nucleotide sequences. Bioinforma Oxf Engl. 2014;30(2):287–8.
Schmidt TSB, Matias Rodrigues JF, von Mering C. Limits to robustness and reproducibility in the demarcation of operational taxonomic units. Environ Microbiol. 2015;17(5):1689–706.
Hill MO. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology. 1973;54(2):427–32.
Schmidt TSB, Matias Rodrigues JF, von Mering C. A family of interaction-adjusted indices of community similarity. ISME J März. 2017;11(3):791–807.
Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26(1):32–46.
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens HH, Szoecs E, Wagner H. vegan: Community Ecology Package. R package version 2.5–7. 2020. https://CRAN.R-project.org/package=vegan.
Wirbel J, Zych K, Essex M, Karcher N, Kartal E, Salazar G, et al. Microbiome meta-analysis and cross-disease comparison enabled by the SIAMCAT machine learning toolbox. Genome Biol. 2021;22(1):93.
Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen Y, et al. High-Fat Diet Determines the Composition of the Murine Gut Microbiome Independently of Obesity. Gastroenterology. 2009;137(5):1716-1724.e2.
Guo CJ, Allen BM, Hiam KJ, Dodd D, Van Treuren W, Higginbottom S, et al. Depletion of microbiome-derived molecules in the host using Clostridium genetics. Science. 2019;366(6471):eaacv1282.
Tiffany CR, Lee JY, Rogers AWL, Olsan EE, Morales P, Faber F, et al. The metabolic footprint of Clostridia and Erysipelotrichia reveals their role in depleting sugar alcohols in the cecum. Microbiome. 2021;9(1):174.
Hu W, Gao W, Liu Z, Fang Z, Zhao J, Zhang H, et al. Biodiversity and Physiological Characteristics of Novel Faecalibacterium prausnitzii Strains Isolated from Human Feces. Microorganisms. 2022;10(2):297.
Wang Y, Wu J, Lv M, Shao Z, Hungwe M, Wang J, et al. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front Bioeng Biotechnol. 2021;9. Verfügbar unter: https://www.frontiersin.org/articles/10.3389/fbioe.2021.612285. Zitiert 8. Dezember 2023.
Koduru L, Lakshmanan M, Lee YQ, Ho PL, Lim PY, Ler WX, et al. Systematic evaluation of genome-wide metabolic landscapes in lactic acid bacteria reveals diet- and strain-specific probiotic idiosyncrasies. Cell Rep. 2022;41(10):111735.
Abdugheni R, Wang WZ, Wang YJ, Du MX, Liu FL, Zhou N, et al. Metabolite profiling of human-originated Lachnospiraceae at the strain level. iMeta. 2022;1(4):e58.
Li Z, Zhou E, Liu C, Wicks H, Yildiz S, Razack F, et al. Dietary butyrate ameliorates metabolic health associated with selective proliferation of gut Lachnospiraceae bacterium 28–4. JCI Insight. 2023;8(4):e166655.
Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms. 2020;8(4):573.
Guo P, Zhang K, Ma X, He P. Clostridium species as probiotics: potentials and challenges. J Anim Sci Biotechnol. 2020;11(1):24.
Mukherjee A, Lordan C, Ross RP, Cotter PD. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes. 2020;12(1):1802866.
Noecker C, Sanchez J, Bisanz JE, Escalante V, Alexander M, Trepka K, et al. Systems biology elucidates the distinctive metabolic niche filled by the human gut microbe Eggerthella lenta. PLOS Biol. 2023;21(5):e3002125.
Soukup ST, Stoll DA, Danylec N, Schoepf A, Kulling SE, Huch M. Metabolism of Daidzein and Genistein by Gut Bacteria of the Class Coriobacteriia. Foods. 2021;10(11):2741.
Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–13.
Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–36.
Yan J, Sheng L, Li H. Akkermansia muciniphila: is it the Holy Grail for ameliorating metabolic diseases? Gut Microbes. 2021;13(1):1984104.
Li Z, Hu G, Zhu L, Sun Z, Jiang Y, Gao M jie, et al. Study of growth, metabolism, and morphology of Akkermansia muciniphila with an in vitro advanced bionic intestinal reactor. BMC Microbiol. 2021;21(1):61.
Ren M, Li H, Fu Z, Li Q. Succession Analysis of Gut Microbiota Structure of Participants from Long-Lived Families in Hechi, Guangxi, China. Microorganisms. 2021;9(12):2524.
Tavella T, Rampelli S, Guidarelli G, Bazzocchi A, Gasperini C, Pujos-Guillot E, et al. Elevated gut microbiome abundance of Christensenellaceae, Porphyromonadaceae and Rikenellaceae is associated with reduced visceral adipose tissue and healthier metabolic profile in Italian elderly. Gut Microbes. 2021;13(1):1–19.
Ahmad MI, Ijaz MU, Hussain M, Haq I ul, Zhao D, Li C. High-Fat Proteins Drive Dynamic Changes in Gut Microbiota, Hepatic Metabolome, and Endotoxemia-TLR-4-NFκB-Mediated Inflammation in Mice. J Agric Food Chem. 2020;68(42):11710–25.
Li C, Cui L, Wang X, Yan Z, Wang S, Zheng Y. Using intestinal flora to distinguish non-alcoholic steatohepatitis from non-alcoholic fatty liver. J Int Med Res. 2020;48(12):0300060520978122.
Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70(4):761–74.
Longo L, Tonin Ferrari J, Rampelotto PH, Hirata Dellavia G, Pasqualotto A, P Oliveira C, et al. Gut Dysbiosis and Increased Intestinal Permeability Drive microRNAs, NLRP-3 Inflammasome and Liver Fibrosis in a Nutritional Model of Non-Alcoholic Steatohepatitis in Adult Male Sprague Dawley Rats. Clin Exp Gastroenterol. 2020;13:351–68.
Schneider KM, Mohs A, Kilic K, Candels LS, Elfers C, Bennek E, et al. Intestinal Microbiota Protects against MCD Diet-Induced Steatohepatitis. Int J Mol Sci. 2019;20(2):308.
Yun Y, Kim HN, Lee E ju, Ryu S, Chang Y, Shin H, et al. Fecal and blood microbiota profiles and presence of nonalcoholic fatty liver disease in obese versus lean subjects. PLoS ONE. 2019;14(3):e0213692.
Bisanz JE, Upadhyay V, Turnbaugh JA, Ly K, Turnbaugh PJ. Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host Microbe. 2019;26(2):265-272.e4.
Carmody RN, Gerber GK, Luevano JM, Gatti DM, Somes L, Svenson KL, et al. Diet Dominates Host Genotype in Shaping the Murine Gut Microbiota. Cell Host Microbe. 2015;17(1):72–84.
Plaza-Díaz J, Solis-Urra P, Aragón-Vela J, Rodríguez-Rodríguez F, Olivares-Arancibia J, Álvarez-Mercado AI. Insights into the Impact of Microbiota in the Treatment of NAFLD/NASH and Its Potential as a Biomarker for Prognosis and Diagnosis. Biomedicines. 2021;9(2):145.
Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16(4):375–81.
Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, et al. Fecal Microbiome and Volatile Organic Compound Metabolome in Obese Humans With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2013;11(7):868-875.e3.
Loomba R, Noureddin M, Kowdley KV, Kohli A, Sheikh A, Neff G, et al. Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH. Hepatol Baltim Md. 2021;73(2):625–43.
Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–9.
Schwimmer JB, Johnson JS, Angeles JE, Behling C, Belt PH, Borecki I, et al. Microbiome Signatures Associated with Steatohepatitis and Moderate to Severe Fibrosis in Children With Nonalcoholic Fatty Liver Disease. Gastroenterology Oktober. 2019;157(4):1109–22.
Hoyles L, Fernández-Real JM, Federici M, Serino M, Abbott J, Charpentier J, et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med Juli. 2018;24(7):1070–80.
Del Chierico F, Nobili V, Vernocchi P, Russo A, De Stefanis C, Gnani D, et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. 2017;65(2):451–64.
Sydor S, Best J, Messerschmidt I, Manka P, Vilchez-Vargas R, Brodesser S, et al. Altered Microbiota Diversity and Bile Acid Signaling in Cirrhotic and Noncirrhotic NASH-HCC. Clin Transl Gastroenterol. 2020;11(3):e00131.
Tsai MC, Liu YY, Lin CC, Wang CC, Wu YJ, Yong CC, et al. Gut Microbiota Dysbiosis in Patients with Biopsy-Proven Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study in Taiwan. Nutrients. 2020;12(3):820.
Da Silva HE, Teterina A, Comelli EM, Taibi A, Arendt BM, Fischer SE, et al. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci Rep. 2018;8:1466.
Yang C, Xu J, Xu X, Xu W, Tong B, Wang S, et al. Characteristics of gut microbiota in patients with metabolic associated fatty liver disease. Sci Rep. 2023;13(1):9988.
Wang B, Jiang X, Cao M, Ge J, Bao Q, Tang L, et al. Altered Fecal Microbiota Correlates with Liver Biochemistry in Nonobese Patients with Non-alcoholic Fatty Liver Disease. Sci Rep. 2016;6(1):32002.
Li F, Sun G, Wang Z, Wu W, Guo H, Peng L, et al. Characteristics of fecal microbiota in non-alcoholic fatty liver disease patients. Sci China Life Sci. 2018;61(7):770–8.
Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17(5):279–97.
Peters BA, Shapiro JA, Church TR, Miller G, Trinh-Shevrin C, Yuen E, et al. A taxonomic signature of obesity in a large study of American adults. Sci Rep. 2018;8:9749.
Nobili V, Putignani L, Mosca A, Del Chierico F, Vernocchi P, Alisi A, et al. Bifidobacteria and lactobacilli in the gut microbiome of children with non-alcoholic fatty liver disease: which strains act as health players? Arch Med Sci AMS. 2018;14(1):81–7.
Demir M, Lang S, Martin A, Farowski F, Wisplinghoff H, Vehreschild MJGT, et al. Phenotyping non-alcoholic fatty liver disease by the gut microbiota: Ready for prime time? J Gastroenterol Hepatol. 2020;35(11):1969–77.
Masoodi M, Gastaldelli A, Hyötyläinen T, Arretxe E, Alonso C, Gaggini M, et al. Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat Rev Gastroenterol Hepatol. 2021;18(12):835–56.
Molinero N, Ruiz L, Sánchez B, Margolles A, Delgado S. Intestinal Bacteria Interplay With Bile and Cholesterol Metabolism: Implications on Host Physiology. Front Physiol. 2019;10. Verfügbar unter: https://www.frontiersin.org/article/10.3389/fphys.2019.00185. Zitiert 24. März 2022.
Hang S, Paik D, Yao L, Kim E, Jamma T, Lu J, et al. Bile acid metabolites control Th17 and Treg cell differentiation. Nature. 2019;576(7785):143–8.
Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016;45(4):802–16.
Wei M, Huang F, Zhao L, Zhang Y, Yang W, Wang S, et al. A dysregulated bile acid-gut microbiota axis contributes to obesity susceptibility. EBioMedicine. 2020;55:102766.
Foley MH, O’Flaherty S, Barrangou R, Theriot CM. Bile salt hydrolases: Gatekeepers of bile acid metabolism and host-microbiome crosstalk in the gastrointestinal tract. PLoS Pathog. 2019;15(3):e1007581.
Neuschwander-Tetri BA. Therapeutic Landscape for NAFLD in 2020. Gastroenterology. 2020;158(7):1984-1998.e3.
Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 2021;384(12):1113–24.
Tsay CJ, Lim JK. NASH and the Gut Microbiome: Implications for New Therapies. Clin Liver Dis. 2022;19(3):97–100.
Preethy S, Ikewaki N, Levy GA, Raghavan K, Dedeepiya VD, Yamamoto N, et al. Two unique biological response-modifier glucans beneficially regulating gut microbiota and faecal metabolome in a non-alcoholic steatohepatitis animal model, with potential applications in human health and disease. BMJ Open Gastroenterol. 2022;9(1):e000985.
Guohong-Liu Qingxi-Zhao, Hongyun-Wei,. Characteristics of intestinal bacteria with fatty liver diseases and cirrhosis. Ann Hepatol. 2019;18(6):796–803.
Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med Februar. 2019;11(2):e9302.
Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Animal Models of Fibrosis in Nonalcoholic Steatohepatitis: Do They Reflect Human Disease? Adv Nutr. 2020;11(6):1696–711.
Acknowledgements
The authors would like to thank all technicians and animal carers for their commitment.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by Swiss National Science Foundation Grant 32473B_156525 (B.M.), and a grant from the Hartmann-Müller Foundation (B.M.). The funding institutions had no role in the study design, analysis, interpretation of the data, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
J.W. was responsible for writing of the original manuscript and formal data analysis, T.R. established trial methodology and conducted laboratory investigation, A.W: conducted part of the laboratory investigation. A.T. prepared the data visualizations. T.SB.S. initialised bioinformatics pipeline, performed data curation, formal analysis and validation of results, B.M. was responsible for conceptualization, methodology and resources and administration of the project. T.SB.S. and B.M. both supervised the project. J.W., T.R., A.W., A.T., G.R., N.K., B.Y., B.M., and T.SB.S reviewed and edited the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval by the local animal welfare authority (Tierschutzkommision Zürich, Zurich, Switzerland; registration number ZH 50/2013.
Consent for publication
Not applicable.
Competing interests
G.R. was supported by grants from AbbVie, Ardeypharm, MSD, Falk, Flamentera, Novartis, Roche, Tillots, UCB, and Zeller. B.M. has served on an advisory board for Takeda and BMS and has received traveling fees or speaking fees from Abbvie, MSD, Falk, iQONE, Gilead, and Novartis and has received unrestricted research grants from MSD and BMS unrelated to the current work.
All other authors declare that they have no competing interests. No third party has influenced any aspect of this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Fig. S1. Comparison of samples between diet types according to alpha-diversity measures. Comparison of samples from the standard diet and the high-fat diet by diversity indices (species richness, exponential Shannon index, inverse Simpson index) measured by the effective number of species.
Additional file 2:
Fig. S2. Comparison of samples between body sites according to alpha-diversity measures. Comparison of samples from intestinal sampling locations and feces by diversity indices (species richness, exponential Shannon index, inverse Simpson index) is shown, assessed by the effective number of species.
Additional file 3:
Fig. S3. Distance-based redundancy analysis of small intestinal microbiota samples according to genotypes with diet, genotype, and NASH status as explanatory variables. Samples are colored according to feeding type (blue: standard diet, green: high-fat diet). A: Distal small intestine, Ch25h-/- (dark/light) versus wildtype (n=7 vs. 5). B: Distal small intestine, Cyp7b1-/- (dark/light) versus wildtype (n=18 vs. 11). C: Mid small intestine, Ch25h-/- (dark/light) versus wildtype (n=4 vs. 7). D: Mid small intestine, Cyp7b1-/- (dark/light) versus wildtype (n=12 vs. 7). E: Proximal small intestine. Ch25h-/- (dark/light) versus wildtype (n=7 vs. 5). F: Proximal small intestine, Cyp7b1-/- (dark/light) versus wildtype (n=10 vs. 10). No analysis of Ebi2-/- was feasible since only in a few small intestinal HFD samples from Ebi2-/- animals (9 out of 33 HFD Ebi2-/- animals) meaningful sequencing results were obtained.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wyss, J., Raselli, T., Wyss, A. et al. Development of non-alcoholic steatohepatitis is associated with gut microbiota but not with oxysterol enzymes CH25H, EBI2, or CYP7B1 in mice. BMC Microbiol 24, 69 (2024). https://doi.org/10.1186/s12866-024-03195-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-024-03195-7