Abstract
Background
Sepsis can cause immune dysregulation and multiple organ failure in patients and eventually lead to death. The gut microbiota has demonstrated its precise therapeutic potential in the treatment of various diseases. This study aimed to discuss the structural changes of the gut microbiota in patients with sepsis and to analyze the differences in the gut microbiota of patients with different prognoses.
Methods
We conducted a multicenter study in which rectal swab specimens were collected on the first and third days of sepsis diagnosis. A total of 70 specimens were collected, and gut microbiota information was obtained by 16S rRNA analysis.
Results
The relative abundance of Enterococcus decreased in rectal swab specimens during the first three days of diagnosis in patients with sepsis, while the relative abundance of inflammation-associated Bacillus species such as Escherichia coli, Enterobacteriaceae, and Bacteroidetes increased. By comparing the differences in the flora of the survival group and the death group, we found that the abundance of Veillonella and Ruminococcus in the death group showed an increasing trend (p < 0.05), while the abundance of Prevotella_6 and Prevotella_sp_S4_BM14 was increased in surviving patients (p < 0.05).
Conclusions
The Firmicutes/Bacteroidetes ratio, reflecting overall gut microbial composition, was significantly lower on day three of sepsis diagnosis. Changes in the abundance of specific gut microbiota may serve as prognostic markers in patients with sepsis.
Similar content being viewed by others
Introduction
There are 100 trillion parasitic microorganisms in the human gastrointestinal (GI) tract [1], including bacteria, viruses, fungi, archaea, and protozoa, which are collectively termed the gut microbiota [2]. Research has long focused on the pathogenicity of gut microbiota, but new studies suggest that the gut microbiota is a complex ecosystem that plays an important role in many aspects of host metabolism, immunity and health [3, 4]. Perturbations of the gut microbiota even during infancy can affect growth, development and health [5].
Sepsis is a serious global health problem and the most common cause of death in hospitals [6]; it can lead to immune dysregulation and multiple organ failure, causing 11 million deaths worldwide every year [7,8,9]. Over the past few decades, in critical illness, the gut has been considered the driver of associated infectious complications [10]. However,current studies have found that the gastrointestinal tract (GIT) is the largest immune organ and maintains systemic immune homeostasis [11]. Patients with sepsis suffer from both sustained excessive inflammation and immune suppression [12], and the gut microbiota and its associated metabolites play an essential role in regulating the host immune response to infection [13].
The gut microbiota has enormous genetic and metabolic diversity, and it has fully demonstrated promise in precision medicine and personalized therapy in recent years [14]. The relationship between intestinal gut microbiota and sepsis has become a hot field [15]. Increasing evidence shows that gastrointestinal dysfunction is associated with high mortality in patients with sepsis [16, 17]. Necessary medical treatment of sepsis patients leads to the collapse of gut microbiota diversity [18], and the examination and management of the gut microbiota in patients with sepsis has become an important part of clinical treatment [19], Therefore, understanding the changes of gut microbiota in sepsis patients has become an urgent problem to be solved.
In this study, we continuously collected rectal swabs from sepsis patients on the first and third day after diagnosis, and tried to describe the characteristics of gut microbiota in sepsis patients by dynamic observation and combining with the prognosis and clinical factors of patients, so as to determine the relationship between gut microbiota and the survival rate of patients, and the relationship between the changes of gut microbiota and the development of sepsis. We attempted to elucidate the correlation of gut microbiota with age, lactate, procalcitonin, mean arterial pressure (MAP) and other factors.
Methods
Subjects and inclusion criteria
This was a multicenter study with patients from the ICU wards of the First Affiliated Clinical Medical College of Harbin Medical University and the Second Affiliated Clinical Medical College of Harbin Medical University. The inclusion criteria are in line with the definition of sepsis and septic shock in the third international consensus [20]. Patients younger than 18 years old, pregnant, with IBD or terminal diseases, and patients undergoing colostomy or ileostomy were excluded. In the presence of clinicians, patients’ families were informed of the study and signed informed consent forms. In this study,all research was conducted in accordance with the Declaration of Helsinki,all patients’ privacy rights are respected. A total of 70 clinical specimens from 41 sepsis patients were collected during a six-month period from March to September 2021.
Specimen collection and preservation
For patients who met the inclusion criteria, specimens were collected on the first and third days of sepsis diagnosis using sealed RNase- and DNase-free cotton swabs, which were then placed in protective tubes made of polystyrene. With the patient in the left lateral decubitus position, a rectal swab was inserted 2-3 cm into the rectal sphincter, rotated 360°, removed and checked for successful stool collection. Immediately after successful collection, the specimens were transferred and placed in a − 80 °C freezer until DNA extraction.
Data records
We collected clinical data through electronic medical records, including sex, age,PH,heart rate(HR),sequential organ failure assessment (SOFA) score, physiology and chronic health (APACHE) II score, blood lactate content(LAC), procalcitonin(PCT) level, ICU length of stay, systolic and diastolic blood pressure(SBP,DBP), and we calculated the mean arterial pressure(MAP).After one month, follow-up was performed to record the mortality of patients on the 7th and 28th days after diagnosis to complete the evaluation.
16SrRNA
Extract the DNA in the specimen and use NanoDropOne to detect the concentration and purity, and use the primers with barcode for PCR amplification. Amplicons were sequenced on the MiSeq platform.The sequence clustering algorithm was used to cluster sequences with a similarity of more than 97% to generate an OTU table. The specimens were analyzed for alpha and beta diversity based on the OTU table.
Statistical analysis
The demographic characteristics of patients were assessed using descriptive statistics. Using the grouped case–control method, 70 specimens were grouped and compared according to the time of specimen collection and the survival time of patients. The t test was used for those that conformed to the normal distribution, the rank-sum test was used for those that did not conform to the normal distribution, and the diversity of different groups was analyzed. The z value was obtained by normalizing the relative abundance of the species (the difference between the relative abundance of this sample in this taxonomy and the average relative abundance of all specimens in this taxonomy divided by the standard deviation of all specimens in this taxonomy). The obtained values are displayed in the Species Abundance Analysis Graph. The relative abundance of all OTUs in different specimens was counted, the dissimilarity coefficient matrix between different specimens was calculated using the selected distance formula, and the matrix was hierarchically clustered to obtain the correlation map between gut microbiota and specific factors (SOFA score, ICU length of stay, pH, age, heart rate, procalcitonin, systolic blood pressure, diastolic blood pressure, mean arterial pressure and blood lactate). In the LEfSe analysis, the nonparametric Kruskal–Wallis rank-sum was first used to detect the abundance differences between different groups, and then the Wilcoxon rank-sum was used to test the difference consistency of the different substances in different subgroups between groups. Finally, linear discreminant analysis (LDA) was used to estimate the size of the effect of each microbiota on the difference. All tests used p = 0.05 as the threshold for significance.
Results
Study cohort
We obtained 70 specimens from 41 sepsis patients. Patient demographics and clinical characteristics are reported in Table 1.
Analysis of differences in the gut micro supplementarybiota between the first and third days of sepsis
First, according to the time of specimen collection, we divided 70 specimens into the SepsisD1 group (35 specimens collected on the first day of diagnosis of sepsis) and SepsisD3 group (35 specimens collected on the third day after the diagnosis of sepsis). The core group of gut microbiota, microbial diversity and community structure of the specimens were preliminarily assessed.
Core group of gut microbiota
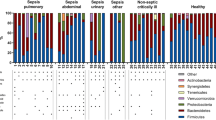
Calculate the relative abundance of bacteria in each sample; OTUs with relative abundance over 1% were selected to draw the relative abundance distribution map of top 15 intestinal microflora in SepsisD1 group and SepsisD3 group.(Fig. 1a). We can see that the core genus of sepsis patients is Enterococcus (OTU1), Escherichia-Shigella (OTU2), Enterobacteriaceae (OTU5), Bacteroides (OTU9, OTU13), Corynebacterium (OTU3), Porphyromonas (OTU18), Anaerococcus (OTU23), Acinetobacter (OTU4), Staphylococcus (OTU17), Campylobacter (OTU6), etc. Compared with the sepsisD1 group, we found that the relative abundance of Enterococcus was decreased in the sepsisD3 group, and the relative abundance of Escherichia-Shigella, Enterobacteriaceae and Bacteroides was increased. The Firmicutes/Bacteroidetes ratio, reflecting overall gut microbial composition, was significantly lower on day three of sepsis diagnosis(Fig. 1e).
a Histogram of gut microbiota composition. b-c The alpha diversity of sepsisD1 and sepsisD3 groups was analyzed, and the b Chao1 and c Simpson index of the samples were evaluated.d The NMDS analysis chart shows the β diversity analysis of sepsisd 1 and sepsisD3. e Comparison of the Firmicutes/Bacteroidetes ratio
Alpha diversity analysis
The alpha diversity index was analyzed for the SepsisD1 group and SepsisD3 group of specimens (Fig. 1b,c), and the Chao-1 index and the Simpson index were used for quantification. There was no significant difference in the diversity index between the sepsisD1 and sepsisD3 groups (p = 0.83).
Beta diversity analysis
Afterward, beta diversity analysis was performed on the SepsisD1 group and SepsisD3 group of specimens, and the spatial location map of the specimens was obtained by NMDS (Fitness Multi-Microscale Method) (Fig. 1d). The close spatial distance between the sepsisD1 and sepsisD3 specimens indicated that the gut microbiota did not undergo drastic changes in species composition during the first three days of sepsis diagnosis.
Gut microbiota and factors
We selected 10 factors, including the SOFA score, ICU length of stay, PH, age, HR, PCT, SBP,DBP, MAP and LAC. The distance between specimens was used to obtain the beta diversity matrix, which is displayed by a heatmap (Fig. 2).
Through the heatmap, we found that Ezakiella, Proteus, Pyramidobacter, Gardnerella, Ureaplasma, Pseudomonas and Citrobacter microorganisms were proportional to the blood lactate value and inversely proportional to the pH value (p < 0.05). Among them, Proteus, Pyramidobacter, Gardnerella and blood lactate were more significantly related (p < 0.01). Aggregatibacter, Mycoplasma, CAG-873, and Akkermansia were inversely proportional to patients’ systolic blood pressure, diastolic blood pressure and mean arterial pressure, and diastolic blood pressure was significantly affected (p < 0.01). Veillonella was proportional to PCT (p < 0.01) and inversely proportional to the average length of stay in the ICU of patients. Mycoplasma was the only factor closely related to age (p < 0.01) and was inversely proportional to diastolic blood pressure, mean arterial pressure and pH.
Analysis of differences in gut microbiota between survivors and deceased patients
To study the relationship between the prognosis of patients with sepsis and the gut microbiota, the 7-day and 28-day survival rates of the patients were calculated, and the patient specimens were divided into the following groups according to the survival rate: Live7D1 (specimens from 7-day survivors on the first day of diagnosis), Death7D1 (specimens from 7-day deceased patients on the first day of diagnosis), Live7D3 (specimens from 7-day survivors on the third day after the diagnosis), Death7D3 (specimens from 7-day deceased patients on the third day after the diagnosis), Live28D1 (specimens from 28-day survivors on the first day of diagnosis), Death28D1 (specimens from 28-day deceased patients on the first day of diagnosis), Live28D3 (specimens from 28-day survivors on the third day after the diagnosis), and Death28D3 (specimens from 28-day deceased patients on the third day after the diagnosis). Furthermore, the community structure and species differences of the specimens were analyzed to determine the differential microbiota related to the patient prognosis.
Species community structure analysis
First, we counted the occurrence of OTUs in the grouping, and by plotting the Venn diagram to show the number of common and unique OTUs, we found that the patients in the death group had fewer types of OTUs and fewer unique OTUs (Fig. 3).
Core group composition analysis
The top 15 species in the SepsisD1 group and SepsisD3 group of specimens were selected, the relative abundance was compared, and the differences in dominant species between the two groups were analyzed.
Comparing the Live7D1 and Death7D1 groups (Fig. 4a), the relative abundance of OTU_2 (g_Escherichia-Shigella), OTU_60 (g_Aggregatibacter), OTU_69 (g_Fusobacterium), and OTU_81 (g_Veillonella) was higher in deceased patient specimens.
Comparing the Live7D3 and Death7D3 groups (Fig. 4b), the relative abundance of Escherichia-Shigella (OTU2) remained higher in the death group, and the relative abundance of OTU14 and OTU15 was also higher in the death group. It is worth noting that these OTUs come from the same genus, Ruminococcus, and we found no significant difference in OTU15 between surviving and deceased patients on the first day of diagnosis.
Comparing the Live28D1 and Death28D1 groups, Porphyromonas (OTU18), Aggregatibacter (OTU60), Fusobacterium (OTU69), Corynebacterium (OTU52), and Veillonella (OTU81) were more abundant in the specimens of deceased patients (Fig. 4c).
Comparing the Live28D3 and Death28D3 groups, Escherichia-Shigella (OTU2), Bacteroides (OTU9), Acinetobacter (OTU4), Pyramidobacter (OTU59), and Ruminococcus (OTU15) were more abundant in the specimens of deceased patients (Fig. 4d).
Species difference analysis
Linear discriminant analysis effect size (LEfSe) was used to estimate the effect size of each microbiota on the difference in patient survival/death. Comparing the Live7D1 and Death7D1 groups (Fig. 5a), 12 bacterial groups were found to be associated with patient death, of which Veillonella was among the top 15 most abundant. Comparing the Live7D3 and Death7D3 groups (Fig. 5b), it was found that 9 bacterial groups were associated with death, and Prevotella_sp_S4_BM14 and Prevotella_6 were associated with survival. we also found that, whether comparing the specimens from the first day of sepsis diagnosis or the third day of the diagnosis of sepsis, Ruminococcaceae was associated with patient death.
The LDA graph represents species with significantly different abundances in different groups when the LDA value was greater than the set value. a-b According to 7-day survival/death, LDA value of gut microbes in a SepsisD1 and b SepsisD3 groups. c-d According to 28-day survival/death, LDA value of gut microbes in c SepsisD1 and d SepsisD3 groups
Comparing the Live28D1 and Death28D1 groups (Fig. 5c), we found that 5 bacterial groups were associated with patient death. Comparing the Live28D3 and Death28D3 groups (Fig. 5d), we found that 3 bacterial groups were associated with survival, and 1 bacterial group was associated with death. Here we also found that Prevotella_6 is associated with patient survival.
Changes in gut microbiota in patients with different prognoses
To observe the relationship between the changes in gut microbiota and the prognosis of patients, the top 30 genera of relative abundance were selected for cluster analysis, and the different changing trends of the gut microbiota of the patients who survived or died were observed.
Grouped according to the 7-day survival rate (Fig. 6a), compared with the Live7D1 group, the abundance of Porphyromonas (OTU18), Corynebacterium-1(OTU12), Gardnerella(OTU8), Anaerobicococcus(OTU23), Finegoldia(OTU7), Peptoniphilus(OTU46), Parabacteroides(OTU66), Staphylococcus(OTU17) and Bacteroides(OTU42) was lower in the Live7D3 group, and Acinetobacter(OTU4), Lactobacillus(OTU29), Akkermansia(OTU10), Corynebacterium (OTU21), Ezakiella(OTU11), Campylobacter(OTU6) and Prevotella(OTU24) were more abundant. Compared with the Death7D1 group, Anaerococcus(OTU30), Enterobacteriaceae(OTU5) and Citrobacter(OTU40) were less abundant in the Death7D3 group, and Escherichia-Shigella(OTU2),Faecalibacterium(OTU25), Ruminococcus(OTU15) torques, Ruminococcus gnavus(OTU14) and Porphyromonas(OTU57) were more abundant. Faecalibacterium, Ruminococcus torques and Ruminococcus gnavus decreased in abundance in the survival group and increased in abundance in the death group.
Grouped according to 28-day survival(Fig. 6b), compared with the Live28D1 group, Enterococcus(OTU1), Akkermansia(OTU10), Parabacteroides(OTU66), Gardnerella(OTU8), Staphylococcus(OTU17), Bacteroides(OTU42) and Fusobacterium(OTU31) were less abundant in the Live28D3 group, and Lactobacillus(OTU29), Porphyromonas(OTU57), Corynebacterium (OTU21),Campylobacter(OTU6), Prevotella(OTU24), Ezakiella(OTU11), Enterobacteriaceae(OTU5) and Citrobacter(OTU409) were more abundant.Compared with the Death28D1 group, Anaerococcus, Finegoldia, Peptoniphilus and Porphyromonas were less abundant in the Death28D3 group, and Escherichia, Acinetobacter, Faecalibacterium, and Ruminococcus were more abundant. The abundance of Campylobacter, Prevotella and Ezakiella increased gradually in the survival group and decreased in the death group.
Discussion
Dysregulation of gut microbiota is associated with a variety of digestive diseases, such as irritable bowel syndrome (IBS), inflammatory bowel disease, colorectal cancer (CRC), liver disease, and pancreatic disease [21,22,23,24]. At present, the help of the gut microbiota in the diagnosis and treatment of diseases is not limited to intestinal diseases. In diseases such as type 2 diabetes, Alzheimer’s disease, and cardiovascular and cerebrovascular diseases, the gut microbiota also shows great prospects for biological treatment [22,23,24,25,26,27]. However, there are still few relevant studies on the gut microbiota in patients with sepsis. By recording the dynamic changes of the gut microbiota in patients with sepsis, we correlated the changes in the gut microbiota in patients with sepsis with patient prognosis. Through differential analysis, three bacteria groups related to the prognosis of sepsis were summarized, and there are few studies on the relationship between these bacteria groups and sepsis at present.
Veillonella
In the heatmap of environmental factors, we found that PCT was proportional to the Veillonella microbiota (OTU81 Veillonella) (p < 0.01). Comparing the top 15 microbiota between 7-day surviving and deceased patients, we also found the content of Veillonella was higher in the specimens of the deceased patients. The LEfSe analysis predicted that patient mortality was associated with Veillonellaceae (p < 0.05), a lactic acid-fermenting bacterium that colonizes the oral, genitourinary, respiratory, and gut microbiomes found in healthy humans. Veillonella is usually found in the mouth [28]. Loomba R et al. found that Veillonella is associated with bile acid metabolism and has the potential to be a marker for predicting efficacy in the treatment of primary sclerosing cholangitis, nonalcoholic fatty liver disease and other diseases [29]. At the same time, some studies have found that the dominant bacteria in the upper gastrointestinal tract, Veillonella, is almost absent in the lower gastrointestinal tract, and that rectal swabs of deceased patients have higher levels of Veillonella. The abnormal migration of microbiota is likely to predict relatively poor clinical outcomes.
Ruminococcus
When comparing the differences among the top 15 microbiota in the specimens of 7-day surviving/deceased patients on the third day after the diagnosis, we found that OTU14 and OTU15 were higher in the specimens of the deceased patients. Interestingly, OTU14 and OTU15 both belong to Ruminococcus. In the LEfSe analysis, we found that Ruminococcaceae had a worse effect on the prognosis of patients by comparing the specimens of patients with different prognoses at 7 days (p < 0.05). Ruminococcus is a Gram-positive anaerobic coccus [30]. Enrique et al. found a transient, dramatic increase in Ruminococcus abundance that corresponds to increased disease activity in patients with inflammatory bowel disease [31]. There are also multiple reports linking Ruminococcus with activity in Crohn’s disease and other immune diseases [32,33,34,35]. Matthew et al. found that Ruminococcus synthesizes and secretes glucorhamnan, which can effectively induce dendritic cells to secrete inflammatory cytokines (TNFα) [36]. In the early stage of sepsis, overactivated Toll-like receptor (TLR) activates intracellular transcription factors, such as NF-κB, and induces the generation and release of proinflammatory cytokines(e.g., interferon-α [IFN-α], interleukin-6 [IL-6], interleukin-8 [IL-8], and tumor necrosis factor-α [TNF-α]), thus triggering a cytokine storm and leading to the first death peak of sepsis patients [37]. We do not yet know whether Ruminococcus affects immune dysregulation in sepsis patients and thus affects prognosis.
Prevotella
Comparing the changes in the microbiota of the specimens from the first day and the third day of the surviving patients, it was found that the abundance of Prevotella increased in the specimens of the surviving patients on the third day. Prevotella is a diverse genus of gram-negative anaerobic bacteria [38]. Fifty-seven species of Prevotella have been identified [39]. They colonize many parts of the human body and are also the main genus of the three reported human enterotypes. Their relative abundances were negatively correlated with the relative abundance of Bacteroides [40]. The higher the diversity of Prevotella, the more fermentative the microbiome for the benefit of the human gut. In our results, it was found that Prevotella_6 and Prevotella_sp_S4_BM14 were significantly different in the microbiota specimens of the survival group on the third day. It may be that the abundance of Prevotella_6 and Prevotella_sp_S4_BM14 in sepsis patients increased on the third day after the diagnosis, indicating a good prognosis.
Unlike the previous study by Gloria M et al. [10], that showed a significant reduction in gut microbial diversity during the ICU stay, We think this may be due to the fact that the second sample in Gloria M et al’s study was collected on the fifth to seventh day after admission to ICU. We can collect samples from sepsis patients admitted to ICU on the 1st, 3rd, 5th and 7th day after diagnosis in the follow-up experiments to verify the changes of intestinal microflora. The imbalance of immune homeostasis is the pathological mechanism of sepsis death. As a broad-spectrum immunomodulator, gut microbiota can regulate the functions of various immune cells in the host, and understand the changes of gut microbiota and the abundance of specific microbiota in sepsis patients, which may provide new ideas for individualized treatment of sepsis patients.
Conclusion
Compared with the samples on the first day after diagnosis of sepsis, the relative abundance of Enterococcus in the samples on the third day after diagnosis decreased, while the relative abundance of Bacillus species associated with inflammation, such as Escherichia-Shigella, Enterobacteriaceae and Bacteroides, increased. There was no significant difference in microbial community diversity in the first three days after the diagnosis of sepsis, and the ratio of Firmicutes/Bacteroides reflecting the overall intestinal microbial composition decreased.
Species community structure analysis showed that the patients in the death group had fewer bacterial species, and through the analysis of species composition and species differences, it was found that Ruminococcus and Veillonella were relatively abundant in the deceased patients and may have a certain impact on poor prognosis (p < 0.05), while Prevotella_6 and Prevotella_sp_S4_BM14 may predict a good prognosis (p < 0.05).
Limitations
Small sample size of patients in the death group; Some patients failed to have specimens collected on the third day of sepsis due to gastrointestinal dysfunction and total parenteral nutrition support.
Availability of data and materials
The datasets generated or analyzed during this study areavailable from the corresponding author on reasonable request.
Abbreviations
- GI:
-
gastrointestinal
- GIT:
-
Gastrointestinal tract
- ICU:
-
Intensive Care Unit
- SOFA:
-
Sequential Organ Failure Assessment Score
- APACHE II:
-
Physiology And Chronic Health Score
- LAC:
-
Blood Lactate Content
- PCT:
-
Procalcitonin
- SBP:
-
Systolic Blood Pressure
- DBP:
-
Diastolic Blood Pressure
- MAP:
-
Mean Arterial Pressure
- PCR:
-
Polymerase Chain Reaction
- OTU:
-
Operational Taxonomic Unit
- LEfSe:
-
Linear Discriminant Analysis Effect Size
References
Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–70.
Sender R, Fuchs S, Milo R. Revised estimates for the number of human and Bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533.
Sanna S, Kurilshikov A, van der Graaf A, Fu J, Zhernakova A. Challenges and future directions for studying effects of host genetics on the gut microbiome. Nat Genet. 2022;54(2):100–6.
Ducarmon QR, Zwittink RD, Hornung BVH, van Schaik W, Young VB, Kuijper EJ. Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol Mol Biol Rev. 2019;83(3):e00007–19.
Healy DB, Ryan CA, Ross RP, Stanton C, Dempsey EM. Clinical implications of preterm infant gut microbiome development. Nat Microbiol. 2022;7(1):22–33.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–11.
Mira JC, Gentile LF, Mathias BJ, Efron PA, Brakenridge SC, Mohr AM, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med. 2017;45(2):253–62.
Dickson RP. The microbiome and critical illness. Lancet Respir Med. 2016;4(1):59–72.
Tang D, Wang H, Billiar TR, Kroemer G, Kang R. Emerging mechanisms of immunocoagulation in sepsis and septic shock. Trends Immunol. 2021;42(6):508–22.
Agudelo-Ochoa GM, Valdés-Duque BE, Giraldo-Giraldo NA, Jaillier-Ramírez AM, Giraldo-Villa A, Acevedo-Castaño I, et al. Gut microbiota profiles in critically ill patients, potential biomarkers and risk variables for sepsis. Gut Microbes. 2020;12(1):1707610.
Zhou CB, Zhou YL, Fang JY. Gut microbiota in Cancer immune response and immunotherapy. Trends Cancer. 2021;7(7):647–60.
Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–74.
Cheng HY, Ning MX, Chen DK, Ma WT. Interactions between the gut microbiota and the host innate immune response against pathogens. Front Immunol. 2019;10:607.
Schupack DA, Mars RAT, Voelker DH, Abeykoon JP, Kashyap PC. The promise of the gut microbiome as part of individualized treatment strategies. Nat Rev Gastroenterol Hepatol. 2022;19(1):7–25.
Dickson RP, Singer BH, Newstead MW, Falkowski NR, Erb-Downward JR, Standiford TJ, et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol. 2016;1(10):16113.
Harris VC, Haak BW, Boele van Hensbroek M, Wiersinga WJ. The intestinal microbiome in infectious diseases: the clinical relevance of a rapidly emerging field. Open forum. Infect Dis. 2017;4(3):ofx144.
Haak BW, Prescott HC, Wiersinga WJ. Therapeutic potential of the gut microbiota in the prevention and treatment of Sepsis. Front Immunol. 2018;9:2042.
Keskey R, Cone JT, DeFazio JR, Alverdy JC. The use of fecal microbiota transplant in sepsis. Transl Res. 2020;226:12–25.
Haak BW, Levi M, Wiersinga WJ. Microbiota-targeted therapies on the intensive care unit. Curr Opin Crit Care. 2017;23(2):167–74.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Jacobs JP, Gupta A, Bhatt RR, Brawer J, Gao K, Tillisch K, et al. Cognitive behavioral therapy for irritable bowel syndrome induces bidirectional alterations in the brain-gut-microbiome axis associated with gastrointestinal symptom improvement. Microbiome. 2021;9(1):236.
Cai J, Sun L, Gonzalez FJ. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe. 2022;30(3):289–300.
Silveira MAD, Bilodeau S, Greten TF, Wang XW, Trinchieri G. The gut-liver axis: host microbiota interactions shape hepatocarcinogenesis. Trends Cancer. 2022;8(7):583–97.
Kartal E, Schmidt TSB, Molina-Montes E, Rodríguez-Perales S, Wirbel J, Maistrenko OM, et al. A faecal microbiota signature with high specificity for pancreatic cancer. Gut. 2022;71(7):1359–72.
Meijnikman AS, Gerdes VE, Nieuwdorp M, Herrema H. Evaluating causality of gut microbiota in obesity and diabetes in humans. Endocr Rev. 2018;39(2):133–53.
Bairamian D, Sha S, Rolhion N, Sokol H, Dorothée G, Lemere CA, et al. Microbiota in neuroinflammation and synaptic dysfunction: a focus on Alzheimer's disease. Mol Neurodegener. 2022;17(1):19.
Honarpisheh P, Bryan RM, McCullough LD. Aging microbiota-gut-brain Axis in stroke risk and outcome. Circ Res. 2022;130(8):1112–44.
Aujoulat F, Bouvet P, Jumas-Bilak E, Jean-Pierre H, Marchandin H. Veillonella seminalis sp. nov., a novel anaerobic gram-stain-negative coccus from human clinical samples, and emended description of the genus Veillonella. Int J Syst Evol Microbiol. 2014;64(Pt 10):3526–31.
Loomba R, Ling L, Dinh DM, DePaoli AM, Lieu HD, et al. The commensal microbe Veillonella as a marker for response to an FGF19 analog in NASH. Hepatology. 2021;73(1):126–43.
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65.
Zozaya-Valdés E, Wong SQ, Raleigh J, Hatzimihalis A, Ftouni S, Papenfuss AT, et al. Detection of cell-free microbial DNA using a contaminant-controlled analysis framework. Genome Biol. 2021;22(1):187.
Breban M, Tap J, Leboime A, Said-Nahal R, Langella P, Chiocchia G, et al. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis. 2017;76(9):1614–22.
Chua HH, Chou HC, Tung YL, Chiang BL, Liao CC, Liu HH, et al. Intestinal Dysbiosis featuring abundance of Ruminococcus gnavus associates with allergic diseases in infants. Gastroenterology. 2018;154(1):154–67.
Nishino K, Nishida A, Inoue R, Kawada Y, Ohno M, Sakai S, et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J Gastroenterol. 2018;53(1):95–106.
Azzouz D, Omarbekova A, Heguy A, Schwudke D, Gisch N, Rovin BH, et al. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann Rheum Dis. 2019;78(7):947–56.
Henke MT, Kenny DJ, Cassilly CD, Vlamakis H, Xavier RJ, Clardy J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn's disease, produces an inflammatory polysaccharide. Proc Natl Acad Sci U S A. 2019;116(26):12672–7.
Li Y, Zhang H, Chen C, Qiao K, Li Z, Han J, et al. Biomimetic immunosuppressive exosomes that inhibit cytokine storms contribute to the alleviation of Sepsis. Adv Mater. 2022;34(19):e2108476.
Christensen L, Roager HM, Astrup A, Hjorth MF. Microbial enterotypes in personalized nutrition and obesity management. Am J Clin Nutr. 2018;108(4):645–51.
Tett A, Pasolli E, Masetti G, Ercolini D, Segata N. Prevotella diversity, niches and interactions with the human host. Nat Rev Microbiol. 2021;19(9):585–99.
Rolhion N, Chassaing B, Nahori MA, de Bodt J, Moura A, Lecuit M, et al. A listeria monocytogenes Bacteriocin can target the commensal Prevotella copri and modulate intestinal infection. Cell Host Microbe. 2019;26(5):691–701.e5.
Acknowledgements
We shall thank all the doctors, nurses and clinical scientists who worked in the hospital during the period of patient recruitment as well as the patients who were involved in this study.
Funding
This project was funded by the National Natural Science Foundation of China-Regional Innovation and Development Joint Fund (U20A20366).
Author information
Authors and Affiliations
Contributions
All authors participated in the design, KY, MZ, CW, FL, YZ, XM and YL searched for the articles, screened titles and abstracts and extracted data. FL,YZ,XM and MM performed statistical analysis and interpretation of data. YL, YP, XJ, NL, XW, QZ and CW drafted the manuscript, and all authors revised it for important intellectual content. Final approval of the version submitted for publication was obtained for all authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the study was approved by the Ethics Committee of the first affiliated hospital of Harbin Medical University (IRB-AF/SC-04/02.0), Harbin, China And this study was conducted adhering to a protocol that was approved by the Medical Ethics Research Committee of our hospital. In the presence of clinicians, patients’ families were informed of the study and signed informed consent forms. In this study,all research was conducted in accordance with the Declaration of Helsinki,all patients’ privacy rights are respected.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Luan, F., Zhou, Y., Ma, X. et al. Gut microbiota composition and changes in patients with sepsis: potential markers for predicting survival. BMC Microbiol 24, 45 (2024). https://doi.org/10.1186/s12866-024-03188-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-024-03188-6