Abstract
Background
Only a few studies dealt with the occurrence of endospore-forming clostridia in the microbiota of infants without obvious health complications.
Methods
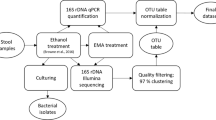
A methodology pipeline was developed to determine the occurrence of endospore formers in infant feces. Twenty-four fecal samples (FS) were collected from one infant in monthly intervals and were subjected to variable chemical and heat treatment in combination with culture-dependent analysis. Isolates were identified by MALDI-TOF mass spectrometry, 16S rRNA gene sequencing, and characterized with biochemical assays.
Results
More than 800 isolates were obtained, and a total of 21 Eubacteriales taxa belonging to the Clostridiaceae, Lachnospiraceae, Oscillospiraceae, and Peptostreptococcaceae families were detected. Clostridium perfringens, C. paraputrificum, C. tertium, C. symbiosum, C. butyricum, and C. ramosum were the most frequently identified species compared to the rarely detected Enterocloster bolteae, C. baratii, and C. jeddahense. Furthermore, the methodology enabled the subsequent cultivation of less frequently detectable gut taxa such as Flavonifractor plautii, Intestinibacter bartlettii, Eisenbergiella tayi, and Eubacterium tenue. The isolates showed phenotypic variability regarding enzymatic activity, fermentation profiles, and butyrate production.
Conclusions
Taken together, this approach suggests and challenges a cultivation-based pipeline that allows the investigation of the population of endospore formers in complex ecosystems such as the human gastrointestinal tract.
Similar content being viewed by others
Background
One of the most complex and densely inhabited ecosystems is found inside the human gut [1, 2]. Obligate anaerobes make up the majority of bacteria that colonize the gut, with lower proportions of facultative anaerobes, yeast, and archaea [3, 4]. In the human gut microbiota, different genera of obligate anaerobes are found including Bacteroides, Bifidobacterium, Clostridium, Eubacterium, and Ruminococcus [5, 6]. A functional subpopulation of the intestinal microbiota is able to form endospores [7, 8] including Lachnospiraceae, Peptostreptococcaceae, and Clostridiaceae families belonging to Eubacteriales order. The endospore cohort of a microbial community is referred to as sporobiota [9].
The majority of spore-forming species are considered commensals while some species or even strains have been associated with disease (e.g., strains of Clostridioides difficile and Clostridium perfringens) [10]. Moreover, endospore formers can be important butyrate producers [11, 12], and Clostridiaceae and Peptostreptococcaceae have been associated with intestinal butyrate formation capacity of infants during the first year of life [10].
Despite considerable progress in identifying and categorizing the intestinal microbial community during the last decades, the crucial biological role of many microbes remains still unknown [2, 13]. The cultivation of prokaryotes to obtain pure isolates remains essential to fully characterize the microorganisms residing in the gut [14], and expanding the spectrum of culturable gut microorganisms by including spore formers would be of great interest [15].
The number of studies focused on the cultivable endospores in infant gut microbiota is limited due to difficulties in culturing under anaerobic conditions and numerous unknown germination factors [3, 10]. Moreover, isolations and characterizations of these unknown common endospore formers would be desirable for future studying of their properties in connection with elucidating their functional role in infant’s gut microbiota. Therefore, we aimed to develop a culture-dependent screening approach that allowed isolating and characterizing variable taxa of clostridial endospore formers using Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS) or 16S rRNA gene sequencing. The second goal of this work was to evaluate the influence of age and other factors such as diet changes on the presence of these endospore-forming clostridia in the infant microbiota. Long-term screening of one infant was chosen to achieve the selected goals.
Materials and methods
Sampling of fecal samples
Twenty-four FS of one vaginally born infant were selected for this study. The infant was partially breastfed for up to one year, obtained complementary infant formula (BEBA COMFORT HM-O, Nestlé, Switzerland) for two years, and was not treated with antibiotics. Solid food was introduced starting from month five. The infant received oral probiotic drops BioLac Baby (Generica, Czechia; Bifidobacterium breve BR03 and Lactobacillus plantarum LP01) when it was one month old. A diagram showing the infant’s age, sampling, diet, and probiotic intervention is displayed in Supplementary Fig. 1. FS were collected to detect and characterize the occurring spore-forming anaerobic bacteria via culture-dependent methods. Fresh FS were collected directly from the infant’s diapers with the sterile spoons and were transferred to sterile tubes containing dilution medium (5 g L-1 tryptone, 5 g L-1 Nutrient broth No. 2, 2.5 g L-1 yeast extract (all Oxoid, UK), 0.5 g L-1 L-cysteine, 1 mL L-1 Tween 80 (both Sigma-Aldrich, USA), 30% glycerol (VWR, Radnor, Pennsylvania, USA), and glass pearls for homogenization). Tubes with dilution medium were prepared in an oxygen-free carbon dioxide environment [16] and then sterilized. Each FS was directly frozen, transported on ice, and then kept at − 20 °C.
Cultivation analyses
Each frozen FS corresponding to 1 g of stool was homogenized and analyzed according to the protocols described below to kill vegetative cells and stimulate spore former sporulation. Diluted FS from the second dilution (10− 2) representing 100 mg FS/10 mL were further analyzed using three independent methods: heat-treatment at 80 °C according to Fakhry et al. [17], with modification of the total treatment time to 20 min; heat-treatment at 60 °C for 60 min [18]; and chemical treatment with 70% (v/v) ethanol for 4 h at room temperature following the instructions of Browne et al. [7]. Heat treatment was done directly with tubes containing the diluted samples. For the ethanol treatment, the tube containing the 100-fold dilution was centrifugated at maximum speed (12’000 RPM) for 5 min at 20 °C, and the pellet was resuspended in 10 mL 70% (v/v) ethanol for 4 h at room temperature. Centrifugation was repeated and the biomass was washed and resuspended again in dilution buffer. After treatment, all the samples were serially diluted and plated using two different media. Wilkins-Chalgren Anaerobe Agar was supplemented with 5 g L-1 GMO-Free Soya Peptone (both Oxoid), 0.5 g L-1 L-cysteine, and 1 mL L-1 Tween 80 (WSP) and Brain Heart Infusion Broth (BHI, GranuCult, Germany) supplemented with 1.2 g L-1 Agar Technical (Agar n°2) (Oxoid). Plates were incubated under anaerobic conditions with GENbag Anaer (bioMérieux, Craponne, France) and plates were cultivated at 37 °C for 2 days.
Strains isolation and identification
The selection of colonies was performed based on morphology and aimed at obtaining as many variable isolates as possible while isolating at least five colonies per treatment from each medium type. Bacterial colonies from agar plates were cultured in Wilkins-Chalgren broth (Oxoid) supplemented with 5 g L− 1 GMO-Free Soya Peptone (both Oxoid), 0.5 g L− 1 L-cysteine, and 1 mL L− 1 Tween 80 using the roll-tube approach [16] in a carbon dioxide atmosphere at 37 °C for 24 h. The cell morphology and culture purity were determined by phase-contrast microscopy (Nikon Eclipse E200, Japan). Obtained isolates were identified using MALDI-TOF MS with ethanol-formic acid extraction procedure with HCCA matrix to the species level using Biotyper software (server distribution version 4.1.100 (PYTH), build 174; server module version 4.3.18, build 330) according to the manufacturer’s instructions (Bruker Daltonik GmbH, Bremen, Germany) and Modrackova et al. [19]. A Bruker criterium score in a range of 2.00–3.00 was followed for an assignment to the species level with a high degree of certainty, while 1.70–1.99 for identification with a low degree of certainty, and 0.00–1.69 for not reliable identification.
Based on morphology and MALDI-TOF MS identification, DNA from representative strains (n = 90) was isolated using PrepMan Ultra™ (Applied Biosystems, USA) according to the manufacturer’s instructions and stored at − 20 °C. Bacterial culture stocks were prepared in 30% glycerol and/or in Cooked Meat Medium (CMM, ThermoFisher Scientific, UK) and stored at − 20 °C and room temperature, respectively.
Primers fd1/rp2 [20], or 27F/1492R [21] were used to amplify the 16S rRNA gene through PCR reaction as described in Supplementary Table 1. PCR reaction mixtures (25 µL) contained 12.5 µL of DreamTaq Green PCR Master Mix (2X) (Thermo Fisher Scientific), 0.2 mM primers (Eurofins Genomics, Ebersberg, Germany), and 1 µL of template DNA. PCR products were purified with E.Z.N.A. Cycle Pure Kit (Omega Bio-Tek, Norcross, Georgia, USA) and were Sanger sequenced by Eurofins Genomics. The obtained sequences were processed in Chromas Lite 2.5.1 (Technelysium Pty Ltd., Australia) and BioEdit [22] using the ClustalW algorithm [23]. Bacteria were classified based on comparative using the EzBioCloud database [24].
Phenotypic characterization of selected strains
Selected isolates (n = 29) representing detected species belonging to the Eubacteriales taxa that were capable of repeated cultivation under in vitro conditions were characterized for specific enzymatic and fermentation activity. Strains were inoculated from the stock frozen or CMM copy and cultivated under anaerobic conditions at 37 °C for 48 h on WSP agar. Selected colonies were again inoculated and cultivated for 24 h in WSP broth to obtain working cultures. All experimental work was performed in at least independent duplicates.
Fermentation profiles of tested strains were analyzed using ANAEROTEST 23 (Erba Lachema, Czech Republic) according to the manufacturer’s instructions. The incubation occurred in anaerobic atmosphere at 37 °C for 24/48 hours. The reading of the results based on color changes was carried along with the instructions reported by the manufacturer.
Hemolytic activity was tested on Columbia Blood Agar Base (Oxoid) supplemented with 5% of Horse Blood Defibrinated (Thermo Scientific), when 10 µL of bacterial cultures was inoculated by a sterile loop with the four-quadrant streak method, and anaerobically cultivated overnight at 37 °C for 48 h. Based on visual examination, incomplete hemolysis or α-hemolysis was assigned if the color of the medium below the colony had changed slightly while complete hemolysis or β-hemolysis occurred when the red cells around the colony had completely lysed. Strains without visible hemolytic activity were considered non-hemolytic (γ-hemolysis) (Supplementary Fig. 2).
Lecithinase activity of the tested isolates was confirmed on Egg Yolk Agar Base (HiMedia, India) supplemented with Egg Yolk Emulsion (VHR, UK) according to manufacturer instructions. The isolate was inoculated onto the plate using a sterilized loop and incubated anaerobically for 72 h at 37 °C. The presence of opalescence around a colony was taken to indicate lecithinase activity.
Catalase activity was tested using isolates smeared on microscope slides. A drop of 3% hydrogen peroxide (Coopharma, Czech Republic) was added, and the production of copious bubbles was taken to indicate that the bacteria were positive for catalase.
Butyrate detection by ion chromatography with suppressed conductivity detection
Butyrate was measured in culture supernatants by capillary high-pressure ion-exchange chromatography with suppressed conductivity detection (IC-SC). The supernatants of freshly grown cultures in WSP broth after 24 h cultivation at 37 °C as is described above for phenotypic characterization. Supernatants were diluted (500×) and filtered through a 0.45 μm nylon membrane and analyzed using a Dionex ICS 4000 system equipped with IonPac AS11-HC 4 μm guard and analytical columns (Thermo Scientific, USA). Eluent composition was as follows: 0–10 min isocratic: 1 mM KOH; 10–20 min linear gradient: 1–60 mM KOH; and 20–25 min again isocratic: 60 mM KOH. The flow rate was set to 0.012 mL min− 1. An ACES 300 suppressor (Thermo Scientific, USA) was used to suppress eluent conductivity, while a carbonate Removal Device 200 (Thermo Scientific) was implemented to suppress carbon dioxide baseline shift. Chromatograms were processed with Chromeleon 7.20 (Thermo Fisher). Standards were prepared from 1 g L− 1 stock solutions (Analytika, Czechia; Inorganic Ventures, USA) and diluted to range from 0.1 to 40 mg L− 1. Deionized water (conductivity < 0.055 µS cm− 1) was used for eluent and standard preparation. All measurements were performed in triplicates.
Statistical analyses
Total counts of bacterial colonies expressed as log CFU g− 1 within the three different treatments and the two different agars were evaluated for statistical differences. The normality of the data was evaluated by Shapiro–Wilk W test (α = 0.05). Differences in bacterial counts were assessed using a Mann–Whitney U Test (α = 0.05) within the agars, and a Kruskal-Wallis test within the treatments (α = 0.05) using StatSoft, Inc. (2013) (STATISTICA – data analysis software system, version 12, www.statsoft.com) and MS Excel (Redmond, WA, USA).
Results
Counts of heat/ethanol resistant bacterial cells including endospores in feces after cultivation
A total of 24 infant FS was examined, and the number of anaerobic heat/ethanol resistant bacterial cells including germinated endospores (log colony forming units (CFU) g− 1 of the fecal sample) are presented in Fig. 1.
Detected counts of anaerobic heat/ethanol resistant bacterial cells including germinated endospores. Detected counts of viable bacterial cell presented as log CFU g− 1 of fecal sample after heat (60 or 80 °C) or chemical treatment (EtOH 70% v/v) in Wilkins-Chalgren agar supplemented with soya peptone (WSP) or Brain Heart Infusion agar (BHI)
The total counts detected on BHI agar (5.67 ± 0.84 log CFU g− 1) was similar as on WSP agar (5.61 ± 0.84 log CFU g− 1). The method of treatment influenced the detected numbers of bacteria. Cell counts were significantly higher (p = 0.0178) after EtOH (5.85 ± 0.61 log CFU g− 1) compared to the 80 °C treatment (5.27 ± 1.14 log CFU g− 1) but not statistically significant within the comparison with 60 °C (5.80 ± 0.71 log CFU g− 1). All three treatments allowed the detection of endospore-forming bacteria. For 3 FS, no germinating cultures were recovered after treatment at 80 °C with a detection limit of < 2 log CFU g− 1.
During the first 6 months, the counts of cultivable germinating endospore formers ranged from 5.59 to 6.91 log CFU g− 1. From month 7 until the end of the study, the range was more variable (3.66–6.91 log CFU g− 1).
Detected endospore-forming taxa
In total, 809 colonies were collected from all cultivation and treatment variants. Microscopical identification based on cell morphology led to the exclusion of co-cultures (a mixture of two or more cultures; n = 152), which were not subjected to further analysis.
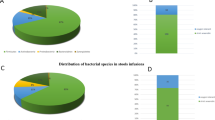
A total of 657 isolates were chosen and subjected to MALDI-TOF MS analysis, as shown in Table 1. In total, 21 distinct taxa belonging to the Clostridiaceae, Oscillospiraceae, Lachnospiraceae, and Peptostreptococcaceae families were identified. C. perfringens (n = 113), C. paraputrificum (n = 66), C. tertium (n = 52), and Eubacterium tenue (n = 47) were the most represented species. Besides the isolates belonging to the Eubacteriales order, other species (n = 56) from Bacillales, Lactobacillales, and Erysipelotrichales orders were identified (Table 1). Treatment-resistant, non-sporulating bacteria accounted for 3.6% of all isolates. A proportion of isolates (n = 160) could not be classified using MALDI-TOF MS.
A total of 90 isolates, 57 that had been identified to the species level by MALDI-TOF MS and 33 that could not be reliably classified, were chosen for additional identification based on 16 S rRNA gene sequencing. For 49 of isolates, which had been identified by MALDI-TOF MS, identity was confirmed based on 16S rRNA gene sequencing as: C. perfringens (n = 8), C. paraputrificum (n = 7), Cl. difficile (n = 5), C. tertium (n = 5), C. symbiosum (n = 3), Flavonifractor plautii (n = 3), Intestinibacter bartlettii (n = 3), Ruminococcus gnavus (n = 3), C. baratii (n = 2), C. jeddahense (n = 2), C. ramosum (n = 2), E. tenue (n = 2), Paeniclostridium sordellii (n = 2), Eisenbergiella tayi (n = 1), and Paraclostridium bifermentans (n = 1). With the used primers, it was not possible to amplify DNA from 8 strains that were identified by MALDI-TOF MS as Enterocloster clostridioformis, En. bolteae, C. butyricum, and En. aldenensis with a score > 2.00 (high confidence identification). All 33 isolates that were not reliably classified by MALD-TOF-MS were successfully identified by 16S rRNA sequencing as Blautia wexlerae (n = 18), I. bartlettii (n = 6), C. disporicum (n = 4), C. ramosum (n = 1), R. albus (n = 1), R. gnavus (n = 1), C. perfringens (n = 1), and F. plautii (n = 1).
Treatment dependent species variability of isolates
We recovered most bacterial species (n = 17) from the ethanol-treated samples, including En. aldenensis, En. bolteae, and Eis. tayi which were not identified after the heat treatments (Table 2). Ethanol treatment proved to be selective for the otherwise heat-resistant non-sporulating species such as Limosilactobacillus reuteri, Enterococcus faecium, and Escherichia coli. The highest frequency of species not belonging to Eubacteriales taxa and co-cultures was recorded at 60 °C treatment. E. tenue and R. gnavus were more often recovered after thermal than chemical treatment.
Occurrence of endospore-forming anaerobes depending on the infant age
Species including C. ramosum, C. tertium, and C. paraputrificum were more often detected during the first year of the infant life, while C. butyricum predominately occurred during the first 6 months. C. perfringens isolates were reliably recovered over the course of two years of life, while F. plautii and Cl. difficile were present sporadically throughout the first few months of infant’s life. C. baratii, C. jeddahense, En. bolteae, P. bifermentans, and C. disporicum were detected only once or twice during the two-year sampling period. C. symbiosum and E. tenue were repeatedly detected in consecutive samples, from the 15th month onwards and between the 17th and 24th months, respectively (Table 1).
Phenotypic characterization and fermentation profiling
We next characterized representative strains of the identified endospore-forming taxa combining biochemical and fermentation-based assays. Fifteen of the 21 identified taxa were examined, because 6 species (I. bartlettii, P. bifermentans, Eis. tayi, En. aldenensis, R. albus, and R. gnavus) did not grow/did not grow in a pure culture after re-inoculation of the stock cultures at the described cultivation conditions. Up to three distinct strains of the same species were characterized. Fermentation profiles and characteristics such as hemolysis, lecithinase, and catalase activity are presented in Table 3. Based on the fermentation profiles, all tested strains were capable of utilizing glucose, mannose, and sucrose. For the other substrates, there were differences at the level of family, genus, species, and strain. C. jeddahense fermented only glucose, mannose, and sucrose. B. wexlerae the same and in addition maltose. En. aldenensis was able to utilize rhamnose, arabinose, and xylose. Arabinose and xylose were also used by E. tenue, C. butyricum, and partially C. tertium. C. baratii, C. butyricum, C. paraputrificum, C. perfringens, C. ramosum, C. tertium, Cl. difficile, and En. aldenensis used both lactose and galactose. C. baratii, C. butyricum, C. jeddahense, C. paraputrificum, C. perfringens (strain-specific), C. tertium, Cl. difficile, P. sordellii, B. wexlerae, and F. plautii were able to produce butyrate after cultivation in WSP broth. Butyrate was not detected in supernatants of C. ramosum, En. bolteae, C. symbiosum, En. aldenensis, and E. tenue. Only strains of C. perfringens and P. sordellii displayed a β-hemolysis, and the same strains and C. baratii strains were lecithinase positive. While the tested strains of C. tertium, B. wexlerae, F. plautii, Cl. difficile, and E. tenue displayed a ɣ-hemolysis. An α-hemolysis was detected in strains belonging to C. baratii, C. butyricum, C. paraputrificum, C. ramosum, C. symbiosum, En. aldenensis, and En. bolteae. None of the tested isolates had a positive result for catalase activity. The accession numbers of the partial 16 S rRNA gene sequences of selected characterized isolates are listed in Table 4.
Discussions
Treatment and culture conditions impact the recovery of Eubacteriales endospore formers
Whole-genome and metagenomic sequencing, combined with computational and phenotypic analysis, suggest that at least 50–60% of the bacterial genera from the intestinal microbiota of an individual without obvious health problems produce resilient spores specialized for host-to-host transmission [7]. Our approach comparing chemical and heat treatment, and cultivation to determine the abundance and composition of chemical/heat resistant cells within the infant fecal microbiota, which encompassed the endospore community, yielded 21 species of endospore-forming bacteria belonging to five families within the Eubacteriales order from the feces of one infant during monthly sampling over 2 years. Based on the species identity of the obtained isolates in comparison with the used methods of FS treatment, it can be stated that the method of treatment at 60 °C for one hour was redundant. Using only the 80 °C and 70% ethanol methods, we would have obtained the same number of bacterial species, with the possibility of isolating more isolates per method and medium, which could bring higher species variability. Another factor could be the agar used when greater species variability of the obtained isolates was recorded on BHI agar.
The anaerobic taxa we isolated agreed with previously published works that treated FS with heat or ethanol before cultivation for the detection of endospore formers. Clostridiaceae, Lachnospiraceae, Oscillospiraceae, and Peptostreptococcaceae endospore-forming families were detected using anaerobic cultivation in our study. The study by Appert et al. [10] used heat treatment and a combination of most probable number analysis and 16 S rRNA gene sequencing successfully enriched members of Clostridiaceae, Peptostreptococcaceae, Lachnospiraceae, Ruminococcaceae, and Erysipelotrichaceae families. Ethanol treatment followed by cultivation was used in the study of Avershina et al. [3] and led to the detection of F. plautii, I. bartlettii, Cl. difficile, P. sordellii, C. perfringens, R. gnavus, C. ramosum, En. bolteae, and C. symbiosum in the infant fecal samples. Other studies employed ethanol resistance or a series of enzymatic and chemical lysis treatments as selection criteria to enrich stress-resistant cells including endospores from adult feces [7, 25]. Several different culture-dependent approaches enable cultivating more species from an identical fecal sample. In addition, the number of selected isolates for identification is always limiting for determining microbial occurrence by culture-dependent methods [26]. Despite a variety of approaches that were used, there is a relatively consistent core of endospore-forming species isolated from infant feces.
Taxonomic and functional diversity of endospore formers in feces of one infant
Vertical transmission of commensal intestinal bacteria is initiated during birth and usually remains selective and persistent during the first year of infant’s life. Microorganisms are also taken up from the environment [27] including the hospital environment, which has been discussed as a source of clostridia [28]. Our results showed relatively high and stable prevalence of Eubacteriales endospore formers during the first 6 months of life with higher variability of endospore counts from months 7 to 24, which may be related to a change in the newborn’s diet, such as the introduction of solid food [29].
Representatives of the family Clostridiaceae were dominantly detected during the first year of life. Other studies observed a reliable presence of C. tertium [30], C. butyricum [31], and C. ramosum [32] during the early stages of life. The second major endospore-forming family during the first months was Peptostreptococcaceae, when Cl. difficile in healthy infants is associated with multiple indicators of healthy gut microbiome maturation [33]. In addition, Clostridiaceae and Peptostreptococcaceae taxa are found as earliest butyrate producers [10]. Most tested taxa of the Clostridiaceae and Peptostreptococcaceae fermented lactose and galactose and possessed N-acetylglucosamine activity, which can be beneficial for harvesting breast milk nutrients during breastfeeding [34]. In addition, some of the analyzed strains across taxa found showed urease activity. Human milk nitrogen sources such as urea can also contribute to the composition of this early life microbiome [35].
In the second year of the infant life, our results indicate repeated occurrence and numerous representations of E. tenue together with C. symbiosum, suggesting their commensal character. The colonization of E. tenue in human feces has been previously reported [4]. Still, this species was only later isolated from human feces using culturomics [36]. Interestingly, Ludwig et al. [37] affiliated this species among Clostridiaceae and as a true member of the genus Paeniclostridium and according to Bartlett et al. [38] E. tenue is an established human pathogen. Conversely, C. symbiosum seems to be common infant bacteria [3], as well as I. bartlettii, P. sordellii, and P. bifermentans, which were detected in our study, as well, were previously detected in young human gut microbiota [39].
Future prospects of Eubacteriales endospore formers
The endospore-forming Eubacteriales population is taxonomically and functionally diverse [40]. Endospore formers can be important butyrate producers [12], as well as some are pathobionts with transmission potential [9]. Conversely, non-toxigenic strains are validated in clinical practice for their probiotic properties [11, 40, 41]. Atarashi et al. [42] have identified Clostridium species from the human microbiota as potent inducers of colonic Tregs capable of reducing inflammatory and allergic disorders. Their cellular constituents and metabolites, such as propionate and butyrate, secondary bile acids, and indole-based metabolites, could play a postbiotic or paraprobiotic role by energizing intestinal epithelial cells, enhancing the intestinal barrier, and interacting with the immune system [11]. In addition, Ogita et al. [43] results suggest that F. plautii might be employed as a probiotic in allergy treatment.
The application of endospores may pave the way for their application as promising possible probiotic supplementations that are secure, efficient, and simple to use due to their stable and resistant nature and ability to germinate and develop in a gut environment [44]; however, there are still some nonnegligible risks and challenges in approaching their application [11] and further analyzes are necessary.
Conclusions
We show that a cultivation dependent approach combining endospore germination and MALDI-TOF MS analyses can yield an extensive strain catalogue of endospore-forming taxa. The chemical and heat treatments with the following cultivation promoted the isolation of a diverse collection of endospore-forming bacteria, including species only recently identified as endospore formers. In general, heat treatment enabled the detection and isolation of most detected clostridial taxa instant of species belonging to Lachnospiraceae, for which the ethanol treatment is more suitable. To exclude the presence of resistant non-spore-forming bacteria, a higher temperature seems to be more suitable.
Our results of one infant study show that the number of culturable spore-forming anaerobes belonging to the Eubacteriales family is relatively stable during the first two years, especially in the first six months. Even so, an infant’s microbiota contains many species of culturable endospores that were not detailly described till today. Moreover, the species diversity during the first two years of this infant indicates sporobiota development reflecting age, diet, and other factors.
Data Availability
The partial 16S rRNA gene sequences of selected isolates for future phenotypic and metabolite characterization were saved to NCBI database and are accessible at OP727730-OP727753. Their accession numbers are listed in Table 4.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BHI:
-
Brain Heart Infusion
- CFU:
-
Colony forming units
- CMM:
-
Cooked Meat Medium
- CZU:
-
Czech University of Life Sciences Prague
- FS:
-
Fecal sample
- IC-SC:
-
Capillary high-pressure ion-exchange chromatography with suppressed conductivity detection
- MALDI-TOF MS:
-
Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry
- WSP:
-
Wilkins-Chalgren Anaerobe Agar supplemented with 5 g L-1 GMO-Free Soya Peptone (both Oxoid), 0.5 g L-1 L-cysteine, and 1 mL L-1 Tween 80
References
Derrien M, Alvarez AS, de Vos WM. The gut microbiota in the First Decade of Life. Trends Microbiol. 2019;27(12):997–1010. https://doi.org/10.1016/j.tim.2019.08.001. Epub 2019 Aug 29. PMID: 31474424.
Dominguez-Bello MG, Godoy-Vitorino F, Knight R, Blaser MJ. Role of the microbiome in human development. Gut. 2019;68(6):1108–14. Epub 2019 Jan 22. PMID: 30670574; PMCID: PMC6580755. doi: 10.1136/gutjnl-2018-317503.
Avershina E, Larsen MG, Aspholm M, Lindback T, Storrø O, Øien T, Johnsen R, Rudi K. Culture dependent and Independent analyses suggest a low level of sharing of endospore-forming species between mothers and their children. Sci Rep. 2020;10(1):1832. https://doi.org/10.1038/s41598-020-58858-y. PMID: 32020012; PMCID: PMC7000398.
Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 2014;38(5):996–1047. https://doi.org/10.1111/1574-6976.12075. Epub 2014 Jun 27. PMID: 24861948; PMCID: PMC4262072.
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. https://doi.org/10.1038/nature11234. PMID: 22699609; PMCID: PMC3564958.
Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30(3):332–8. https://doi.org/10.1097/MOG.0000000000000057. PMID: 24625896; PMCID: PMC4215539.
Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, Goulding D, Lawley TD. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature. 2016;533(7604):543–6. https://doi.org/10.1038/nature17645. Epub 2016 May 4. PMID: 27144353; PMCID: PMC4890681.
Forster SC, Kumar N, Anonye BO, Almeida A, Viciani E, Stares MD, Dunn M, Mkandawire TT, Zhu A, Shao Y, Pike LJ, Louie T, Browne HP, Mitchell AL, Neville BA, Finn RD, Lawley TD. A human gut bacterial genome and culture collection for improved metagenomic analyses. Nat Biotechnol. 2019;37(2):186–92. https://doi.org/10.1038/s41587-018-0009-7. Epub 2019 Feb 4. PMID: 30718869; PMCID: PMC6785715.
Egan M, Dempsey E, Ryan CA, Ross RP, Stanton C. The Sporobiota of the Human Gut. Gut Microbes. 2021 Jan-Dec;13(1):1–17. doi: 10.1080/19490976.2020.1863134. PMID: 33406976; PMCID: PMC7801112.
Appert O, Garcia AR, Frei R, Roduit C, Constancias F, Neuzil-Bunesova V, Ferstl R, Zhang J, Akdis C, Lauener R, Lacroix C, Schwab C. Initial butyrate producers during infant gut microbiota development are endospore formers. Environ Microbiol. 2020;22(9):3909–21. https://doi.org/10.1111/1462-2920.15167. Epub 2020 Aug 20. PMID: 32686173.
Guo P, Zhang K, Ma X, He P. Clostridium species as probiotics: potentials and challenges. J Anim Sci Biotechnol. 2020;11:24. https://doi.org/10.1186/s40104-019-0402-1. PMID: 32099648; PMCID: PMC7031906.
Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1–8. https://doi.org/10.1111/j.1574-6968.2009.01514.x. Epub 2009 Feb 13. PMID: 19222573.
Senn V, Bassler D, Choudhury R, Scholkmann F, Righini-Grunder F, Vuille-Dit-Bile RN, Restin T. Microbial Colonization from the Fetus to Early Childhood-A Comprehensive Review. Front Cell Infect Microbiol. 2020;10:573735. https://doi.org/10.3389/fcimb.2020.573735. Erratum in: Front Cell Infect Microbiol. 2021;11:715671. PMID: 33194813; PMCID: PMC7661755.
Lagier JC, Hugon P, Khelaifia S, Fournier PE, La Scola B, Raoult D. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin Microbiol Rev. 2015;28(1):237–64. https://doi.org/10.1128/CMR.00014-14. PMID: 25567229; PMCID: PMC4284300.
Tanaka M, Onizuka S, Mishima R, Nakayama J. Cultural isolation of spore-forming bacteria in human feces using bile acids. Sci Rep. 2020;10(1):15041. https://doi.org/10.1038/s41598-020-71883-1. PMID: 32929101; PMCID: PMC7490687.
Hungate RE, Macy J. The roll-tube method for cultivation of strict anaerobes. Bulletins from the Ecological Research Committee, 1973; 123–6.
Fakhry S, Sorrentini I, Ricca E, De Felice M, Baccigalupi L. Characterization of spore forming Bacilli isolated from the human gastrointestinal tract. J Appl Microbiol. 2008;105(6):2178-86. https://doi.org/10.1111/j.1365-2672.2008.03934.x. PMID: 19120663.
Setlow P, Wang S, Li YQ. Germination of spores of the orders Bacillales and Clostridiales. Annu Rev Microbiol. 2017;71:459–77. https://doi.org/10.1146/annurev-micro-090816-093558. Epub 2017 Jul 11. PMID: 28697670.
Modrackova N, Stovicek A, Burtscher J, Bolechova P, Killer J, Domig KJ, Neuzil-Bunesova V. The bifidobacterial distribution in the microbiome of captive primates reflects parvorder and feed specialization of the host. Sci Rep. 2021;11(1):15273. https://doi.org/10.1038/s41598-021-94824-y. PMID: 34315970; PMCID: PMC8316555.
Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173(2):697–703. https://doi.org/10.1128/jb.173.2.697-703.1991. PMID: 1987160; PMCID: PMC207061.
Miller CS, Handley KM, Wrighton KC, Frischkorn KR, Thomas BC, Banfield JF. Short-read assembly of full-length 16S amplicons reveals bacterial diversity in subsurface sediments. PLoS ONE. 2013;8(2):e56018. https://doi.org/10.1371/journal.pone.0056018. Epub 2013 Feb 6. PMID: 23405248; PMCID: PMC3566076.
Hall T, Biosciences I, Carlsbad C. BioEdit: an important Software for Molecular Biology. GERF Bull Biosci. 2011;2:60–1.
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of Progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–80. https://doi.org/10.1093/nar/22.22.4673. PMID: 7984417; PMCID: PMC308517.
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol. 2017;67(5):1613–7. https://doi.org/10.1099/ijsem.0.001755. Epub 2017 May 30. PMID: 28005526; PMCID: PMC5563544.
Kearney SM, Gibbons SM, Poyet M, Gurry T, Bullock K, Allegretti JR, Clish CB, Alm EJ. Endospores and other lysis-resistant bacteria comprise a widely shared core community within the human microbiota. ISME J. 2018;12(10):2403–16. https://doi.org/10.1038/s41396-018-0192-z. Epub 2018 Jun 13. PMID: 29899513; PMCID: PMC6157131.
Ito T, Sekizuka T, Kishi N, Yamashita A, Kuroda M. Conventional culture methods with commercially available media unveil the presence of novel culturable bacteria. Gut Microbes. 2019;10(1):77–91. https://doi.org/10.1080/19490976.2018.1491265. Epub 2018 Aug 17. PMID: 30118379; PMCID: PMC6363062.
Korpela K, Costea P, Coelho LP, Kandels-Lewis S, Willemsen G, Boomsma DI, Segata N, Bork P. Selective maternal seeding and environment shape the human gut microbiome. Genome Res. 2018;28(4):561–8. https://doi.org/10.1101/gr.233940.117. Epub 2018 Mar 1. PMID: 29496731; PMCID: PMC5880245.
Ferraris L, Butel MJ, Campeotto F, Vodovar M, Rozé JC, Aires J. Clostridia in premature neonates’ gut: incidence, antibiotic susceptibility, and perinatal determinants influencing colonization. PLoS ONE. 2012;7(1):e30594. https://doi.org/10.1371/journal.pone.0030594. Epub 2012 Jan 26. PMID: 22291996; PMCID: PMC3266918.
Fallani M, Amarri S, Uusijarvi A, Adam R, Khanna S, Aguilera M, Gil A, Vieites JM, Norin E, Young D, Scott JA, Doré J, Edwards CA, The Infabio Team. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiol (Reading). 2011;157(Pt 5):1385–92. https://doi.org/10.1099/mic.0.042143-0. Epub 2011 Feb 17. PMID: 21330436.
Kiu R, Caim S, Alcon-Giner C, Belteki G, Clarke P, Pickard D, Dougan G, Hall LJ. Preterm Infant-Associated Clostridium tertium, Clostridium cadaveris, and Clostridium paraputrificum strains: genomic and evolutionary insights. Genome Biol Evol. 2017;9(10):2707–14. https://doi.org/10.1093/gbe/evx210. PMID: 29044436; PMCID: PMC5647805.
Cassir N, Benamar S, Khalil JB, Croce O, Saint-Faust M, Jacquot A, Million M, Azza S, Armstrong N, Henry M, Jardot P, Robert C, Gire C, Lagier JC, Chabrière E, Ghigo E, Marchandin H, Sartor C, Boutte P, Cambonie G, Simeoni U, Raoult D. La Scola B. Clostridium butyricum strains and Dysbiosis Linked to Necrotizing enterocolitis in Preterm neonates. Clin Infect Dis. 2015;61(7):1107–15. https://doi.org/10.1093/cid/civ468. Epub 2015 Jun 17. PMID: 26084844.
Brook I, Long SS. Anaerobic bacteria: classification, normal flora, and clinical concepts. In: Long SS, Fischer M, Prober GC, editors. Principles and practice of pediatric infectious Diseases. Elsevier; 2018. pp. 987–95.
Ferretti P, Wirbel J, Maistrenko OM, Van Rossum T, Alves R, Fullam A, Akanni W, Schudoma C, Schwarz A, Thielemann R, Thomas L, Kandels S, Hercog R, Telzerow A, Letunic I, Kuhn M, Zeller G, Thomas SB, Schmidt. TSB, C. Difficile may be overdiagnosed in adults and is a prevalent commensal in infants. Peer Bork bioRxiv. 2022. https://doi.org/10.1101/2022.02.16.480740.
Garrido D, Ruiz-Moyano S, Mills DA. Release and utilization of N-acetyl-D-glucosamine from human milk oligosaccharides by Bifidobacterium longum subsp. infantis. Anaerobe. 2012;18(4):430–5. https://doi.org/10.1016/j.anaerobe.2012.04.012. Epub 2012 May 9. PMID: 22579845; PMCID: PMC7568402.
Schimmel P, Kleinjans L, Bongers RS, Knol J, Belzer C. Breast milk urea as a nitrogen source for urease positive Bifidobacterium infantis. FEMS Microbiol Ecol. 2021;97(3):fiab019. https://doi.org/10.1093/femsec/fiab019. PMID: 33538807; PMCID: PMC7947585.
Lagier JC, Khelaifia S, Alou MT, Ndongo S, Dione N, Hugon P, Caputo A, Cadoret F, Traore SI, Seck EH, Dubourg G, Durand G, Mourembou G, Guilhot E, Togo A, Bellali S, Bachar D, Cassir N, Bittar F, Delerce J, Mailhe M, Ricaboni D, Bilen M, Dangui Nieko NP, Dia Badiane NM, Valles C, Mouelhi D, Diop K, Million M, Musso D, Abrahão J, Azhar EI, Bibi F, Yasir M, Diallo A, Sokhna C, Djossou F, Vitton V, Robert C, Rolain JM, La Scola B, Fournier PE, Levasseur A, Raoult D. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol. 2016;1:16203. https://doi.org/10.1038/nmicrobiol.2016.203. PMID: 27819657.
Ludwig W, Viver T, Westram R, Francisco Gago J, Bustos-Caparros E, Knittel K, Amann R, Rossello-Mora R. Release LTP_12_2020, featuring a new ARB alignment and improved 16S rRNA tree for prokaryotic type strains. Syst Appl Microbiol. 2021;44(4):126218. Epub 2021 May 24. PMID: 34111737.
Bartlett A, Padfield D, Lear L, Bendall R, Vos M. A comprehensive list of bacterial pathogens infecting humans. Microbiology (Reading). 2022;168(12). https://doi.org/10.1099/mic.0.001269. PMID: 36748702.
Ohashi Y, Fujisawa T. Analysis of Clostridiumcluster XI bacteria in human feces. Biosci Microbiota Food Health. 2019;38(2):65–8. https://doi.org/10.12938/bmfh.18-023. Epub 2018 Dec 7. PMID: 31106109; PMCID: PMC6502712.
Saggese A, Baccigalupi L, Ricca E. Spore formers as beneficial microbes for humans and animals. Appl Microbiol. 2021;3498–509. https://doi.org/10.3390/applmicrobiol1030032.
Cassir N, Benamar S, La Scola B. Clostridium butyricum: from beneficial to a new emerging pathogen. Clin Microbiol Infect. 2016;22(1):37–45. https://doi.org/10.1016/j.cmi.2015.10.014. Epub 2015 Oct 20. PMID: 26493849.
Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–6. https://doi.org/10.1038/nature12331. Epub 2013 Jul 10. PMID: 23842501.
Ogita T, Yamamoto Y, Mikami A, Shigemori S, Sato T, Shimosato T. Oral administration of Flavonifractor plautii strongly suppresses Th2 Immune responses in mice. Front Immunol. 2020;11:379. https://doi.org/10.3389/fimmu.2020.00379. PMID: 32184789; PMCID: PMC7058663.
Koopman N, Remijas L, Seppen J, Setlow P, Brul S. Mechanisms and applications of bacterial sporulation and germination in the intestine. Int J Mol Sci. 2022;23(6):3405. https://doi.org/10.3390/ijms23063405. PMID: 35328823; PMCID: PMC8953710.
Acknowledgements
The authors thank Blanka Krausova for her valuable help during the cultivation analysis and bacterial identifications.
Funding
The research was funded by European Regional Development Fund-Project, “Centre for the investigation of synthesis and transformation of nutritional substances in the food chain in interaction with potentially harmful substances of anthropogenic origin: comprehensive assessment of soil contamination risks for the quality of agricultural products” [No: CZ.02.1.01/0.0/0.0/16_019/0000845] and by METROFOOD‐CZ research infrastructure project [MEYS Grant No: LM2023064 including access to its facilities.
Author information
Authors and Affiliations
Contributions
VN-B and CS participated in the study conception and design. VNB supervised the research work. EI performed the laboratory work and drafted the manuscript. VN-B, NM, JK, VT did data analysis and data curation. VN-B, NM, and CS critically reviewed the manuscript. VN-B and NM revised the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study belongs to the file with reference number 06/2022 which was approved by the Ethics Committee of the Czech University of Life Sciences Prague (CZU). The Ethics Committee declared that the research complied with the rules of ethics in the research work with the code of Ethics of CZU and did not infringe upon the dignity of the participants in the research. Parents gave informed consent for FS collection and analyses.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ingribelli, E., Modrackova, N., Tejnecky, V. et al. Culture-dependent screening of endospore-forming clostridia in infant feces. BMC Microbiol 23, 347 (2023). https://doi.org/10.1186/s12866-023-03104-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-023-03104-4