Abstract
Background
Antimicrobial resistance (AMR) is a critical global issue that poses significant threats to human health, animal welfare, and the environment. With the increasing emergence of resistant microorganisms, the effectiveness of current antimicrobial medicines against common infections is diminishing. This study aims to conduct a competitive meta-analysis of surveillance data on resistant microorganisms and their antimicrobial resistance patterns in two countries, Egypt and the United Kingdom (UK).
Methods
Data for this study were obtained from published reports spanning the period from 2013 to 2022. In Egypt and the UK, a total of 9,751 and 10,602 food samples were analyzed, respectively. Among these samples, 3,205 (32.87%) in Egypt and 4,447 (41.94%) in the UK were found to contain AMR bacteria.
Results
In Egypt, the predominant resistance was observed against β-lactam and aminoglycosides, while in the United Kingdom, most isolates exhibited resistance to tetracycline and β-lactam. The findings from the analysis underscore the increasing prevalence of AMR in certain microorganisms, raising concerns about the development of multidrug resistance.
Conclusion
This meta-analysis sheds light on the escalating AMR problem associated with certain microorganisms that pose a higher risk of multidrug resistance development. The significance of implementing One Health AMR surveillance is emphasized to bridge knowledge gaps and facilitate accurate AMR risk assessments, ensuring consumer safety. Urgent actions are needed on a global scale to combat AMR and preserve the effectiveness of antimicrobial treatments for the well-being of all living beings.
Highlights
1. Egypt and the United Kingdom (UK) have the highest prevalence rates of E. coli.
2. Chicken has the highest prevalence of microbial resistance strains.
3. Egypt has the highest rate of β-lactam and aminoglycoside resistance.
4. UK has the highest rate of tetracycline and β-lactam resistance.
5. Resistance to multiple drugs has been identified in both nations.
Similar content being viewed by others
Introduction
Antibiotic resistance (AMR) has been identified as one of the biggest global health concerns to people and animals, affecting not only developed and developing nations but throughout the world. It is spreading across the globe due to pathogenic bacteria. AMR is a concern, regardless of geographical boundaries, healthcare knowledge levels, or national economic status [1]. According to the United Kingdom (UK) Government-commissioned Review on AMR, Current estimates put the annual global mortality rate from AMR at 700,000. By 2050, this figure could potentially reach a staggering 10 million annual fatalities [2,3,4]. Estimates suggest that AMR is responsible for 25,000 annual deaths in the EU alone [5]. According to a recent article, in 2019, bacterial AMR directly caused 1.27 million deaths and was additionally associated with over twice that number of fatalities. In 2019, antibiotic-resistant Escherichia coli alone resulted in the deaths of nearly 200,000 people [6]. Most of these fatalities are expected to occur in underdeveloped nations.AMR will also have a negative impact on the world economy and hinder development efforts. It is estimated that failing to address AMR until 2050 will cost $100 trillion in total losses. The global GDP may decline by as much as 3.5%. Most of the developing world's poorest nations will experience significant economic losses [7]. The latest data published by the UK Health Security Agency (UKHSA) [8] reveals that the estimated total number of serious antibiotic resistant infections in England rose by 2.2% in 2021 compared to 2020. While in Egypt, a total of 4.95 million individuals died in 2019 were afflicted with drug-resistant infections. Among these cases, 1.27 million deaths were directly attributed to AMR [9].
Beside humans, billions of pets, livestock, and fish rely on these drugs, as curative or preventive medicines, or as questionable growth boosters. However, every time we use antibiotics, we put bacteria under selection pressure to modify or transfer pieces of DNA, potentially leading to drug resistance [10]. As a result, the misuse and overuse of antibiotics are significant contributors to the resistance phenomena, along with other local and global variables that promote the spread of resistant bacteria and their genes [11]. AMR is characterized by complex interactions involving many microbial populations. With this complexity and ecological nature in perspective, it becomes important to address the resistance issue via a coordinated, multi-sectorial strategy, such as One Health [12,13,14], as depicted in Fig. 1.
Interconnections of complex AMR amongst several health sectors. A schematic diagram for the complex transmission pathways of AMR genes and drug-resistant bacteria between human, livestock and environmental reservoirs. The dashed lines represent potential transmission pathways [15]. (Reference?)
One Health is defined as "the collaborative approach of numerous health science professionals, as well as their allied disciplines and institutions, working locally, nationally, and internationally to achieve optimal health for humans, livestock, biodiversity, flora, and our ecosystem." by the World health organization (WHO) [16] and the Food and Agriculture Organization of the United Nations (FAO) [17, 18]. This comprehensive viewpoint makes it possible to comprehend how human interactions with the environment influence the spread and occurrence of diseases as well as how society may be prone to the potential burden of these diseases [19]. Several International organizations, such as the WHO, FAO and World Organization for Animal Health (OIE), have a significant impact on monitoring antibiotic consumption providing the necessary information to combat AMR. The WHO has unveiled several international measures to address AMR at the global level such as “Global Action Plan (GAP)”, “Global Antimicrobial Resistance and Use Surveillance System (GLASS) [20,21,22] and locally “Egypt’s National Action Plan on Antibiotic Resistance”, “Central Asian and European surveillance of AMR (CAESAR)”, “UK’s National Action Plan”, “European Surveillance of Antimicrobial Consumption Network”, “European Antimicrobial Resistance Surveillance Network (EARS-Net)” and “Joint Programming Initiative on AMR (JPIAMR)”. These programmes focus on the One Health concept, which links human and animal health to their respective ecosystems (Table 3).
The major carriers of AMR in the UK are raw and undercooked poultry [23]. From farm to product, there are numerous processes involved in producing pork, meat, and poultry, including breeding and finishing, animal transportation, slaughter, cutting, processing, and packing. Each of these processes might be a source of bacterial contamination [24, 25]. While meat is the primary source of protein in Egypt, raw milk is also believed to contribute significantly to the development of various health issues [26, 27]. Additionally, due to the poor quality, Egyptian street food, particularly meat products, may pose a risk. The following factors, including raw material use, inadequate worker cleanliness, and holding for a long time cause food to become contaminated with pathogenic bacteria [28].
The rising prevalence of bacteria in various food sources contributes to an escalation in drug resistance and affects the susceptibility of infections to antibiotics. It is important to determine the burden based on both of these counterfactual scenarios because we do not know the amount to which drug-resistant infections would be replaced by susceptible infections or by no infections in a scenario in which all drug resistance was removed. In this study, a systematic research and meta-analysis on AMR present in food samples were conducted in an African country Egypt (developing) and a European country UK (highly developed), using data published on Medline search engine between 2013–2022.
Materials and methods
Search methodology
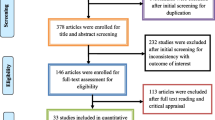
Data from different Medline search engines, including PubMed (https://pubmed.ncbi.nlm.nih.gov/), Google Scholar (https://scholar.google.com/), and Science Direct (https://www.sciencedirect.com/), were examine to find relevant publications that had been published between January 2013 and December 2022. The papers that are suggested for reporting systematic reviews and meta-analyses. Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline (http://www.prisma-statement.org/) was followed to obtain the data, and the pertinent medical subject heading (MeSH) word was also used to retrieve the data provided below. For e.g., “Pathogen transfer from different food sources along with antimicrobial resistance”, “AMR Spreading from Different pathogens along with One Health”, “Multi drug resistance”, “Drug susceptibility tests of different pathogens isolated from different food sources”, “AMR assessment method”, “MDR”, “Egypt”, “United Kingdom” “UK” “One Health” “surveillance” are the keywords as well as the MeSH terms that were used. The search queries were applied using the Boolean operators "AND" and "OR." Fig. 2 depicts the search strategy for the PubMed/MEDLINE database in accordance with PRISMA guidelines.
Selection criteria
A total of 49 papers were selected for retrieval of information and for the inclusion or omission of data. The following was the basis for the studies that mainly composed the meta-analysis: (i) accessibility of the article's full text and abstract; (ii) studies with author names, publication year, region, overall number of isolates, total samples collected, and circumstances; (iii) observations of food pathogens and AMR; (iv) mention of the pathogen analysis method; (v) mention of sample sources (such as animal and vegetable food origins, dairy products, environmental samples, food handlers, slaughterhouses, etc.); and (vi) an AMR evaluation approach that takes into account several molecular methodologies.
Exclusion criteria
Studies were disqualified if they (a) were not review articles (b) proper screening techniques were not mentioned (c) did not have any book chapters included, (d) essential statistics were missing (e) AMR was not analyzed, and (f) were not conducted in Egypt and UK (g) did not have any publications published between 2013 and 2022.
Data extraction and quality assessment
To establish a baseline, full versions of allegedly relevant articles were obtained. The author names, publication year, geography, the total number of isolates, and total samples from each article were collected independently and recorded on a spreadsheet (Microsoft Excel® 2013) for pre-testing prior to full extraction. Text, tables, and figures were used to extract the data. The results were examined, and a pie chart was used to show the AMR data and the cited articles using Mendeley (version 1.19.8).
Food category
The food categories considered were beef, chicken, retail chicken, raw meat, ready-to-eat (RTE) meat, fish, milk, other dairy product (cheese, ice cream, yoghurt, cream), water, vegetables, animals (pig, sheep, dog, badger, fox), and environmental samples (water, drainages) shown in Tables 1 and 2.
Result
Identification of studies
The initial search strategy approach yielded 506 citations from electronic databases. After 225 duplicates were deleted, 281 records were evaluated for title and abstract. Out of these, 281 full-text papers were assessed, and in the initial systematic search, 133 records were excluded; 79 due to improper screening of data, 15 for no relevant interventions in animals to reduce antibiotics use, 37 were due to being review articles on MDR and 2 due to full access which could not be achieved. Of these 148 full-text articles, 98 were again excluded, of which 54 were excluded because they did not address AMR in the results, 32 were excluded due to irreverent outcomes, and 12 were excluded due to missing essential statistics. This systematic review and meta-analysis included 50 articles in total. The entire set of criteria for inclusion and exclusion are shown in Fig. 2.
One health and AMR training programs in Egypt
Several international organizations such as the World Organization for Animal Health (OIE), World Health Organization (WHO) and Food and Agriculture Organization of the United Nations (FAO), have taken significant steps to address antibiotic resistance. These measures encompass the adoption of various plans, such as the National Action Plan on Antibiotic Resistance in 2018–2024 and [29, 30] the Global Action Plan on Antimicrobial Resistance-WHO [31]. These initiatives focus on infection prevention and control, AMR surveillance and management supported by the antimicrobial stewardship program, increasing public awareness, and investing in novel medications. Measures to build national capacity were identified, along with a variety of other interventions [32]. It will make it possible to examine the relationship between AMR and antimicrobial use in many contexts (including those involving animals, people, and the environment) and to evaluate the impact of interventions within and across sectors [1, 33, 34]. An additional operational initiative is the Global Antimicrobial Resistance and Use Surveillance System (GLASS) [21], It promotes nations to adopt surveillance methods based on systems that include epidemiological, clinical, and population-level data rather than only laboratory data and fosters the development of the AMR evidence base (Table 3).
One health and AMR training programs in United Kingdom (UK)
Several international organizations such as the WHO, OIE and FAO adopt several plans such as UK’s National Action Plan 2019–2024, which is a national action plan to combat AMR both within and beyond our borders. Developed in collaboration with a diverse variety of partners from various sectors [22], another is WHO’s Global Antimicrobial Resistance Surveillance System (GLASS) which is an international cooperative effort to standardize AMR surveillance that was started to advance knowledge through monitoring and investigation [35]. European Surveillance of Antimicrobial Consumption Network, European Antimicrobial Resistance Surveillance Network (EARS-Net) evaluates the overall comparability of routinely collected test results and assess the accuracy of quantitative antimicrobial susceptibility test results [34]. Furthermore, the Central Asian and European Surveillance of Antimicrobial Resistance network (CAESAR) serves as a network of encompassing national antimicrobial resistance (AMR) surveillance systems that includes all WHO European Region countries that are not members of the European Antimicrobial Resistance Surveillance Network (EARS-Net), which is coordinated by the European Union's European Centre for Disease Prevention and Control [36]. Additionally, the EU established the Joint Programming Initiative on AMR (JPIAMR), aims to better coordinate global AMR research efforts (Table 3).
Comparative meta-analysis
Out of 506 eligible papers, as indicated in Fig. 2, 50 publications (34 from Egypt and 16 from the UK) were included in the meta-analysis and systematic study. A total of 7,652 AMR tests (3,205 from Egypt and 4447 samples from the UK) were obtained for various bacteria found in various food items, animals, and environments. A total of 12 different antimicrobial agents/drugs and 13 different bacteria showedresistance. The sample sources, species of bacteria, and their proportions are represented in Tables 1 and 2.
Comparative meta-analysis in Egypt
Out of 220 eligible studies related to pathogens carrying AMR in Egypt from 2013 to 2022, 34 full-text articles were included for further examination [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70]. Out of 9,751 samples, 3205 (32.87%) found positive prevalence of which samples included beef 204 (28.73%), chicken 1315 (41.42%), raw meat 239 (24.26%), ready to eat food 239 (41.57%), fish 137 (24.46%), milk 484 (30.42%), other dairy products 608 (42.13%), vegetables 51 (9.46%), and water 44 (25.43%) showed positive prevalence for various pathogens including E. coli 1725 (17.69%), Staphylococcus 643 (6.59%), Salmonella spp. 187 (1.92%), Lactobacillus 144 (1.48%), L. monocytogenes 138 (1.42%), Aeromonas spp. 115 (1.18%), Streptococcus 72 (0.74%), P. aeruginosa 34 (0.35%), Lactococcus 29 (0.30%), K. pneumonia 26 (0.27%), Enterococcus spp. 13 (0.13%), Citrobacter spp. 2 (0.02%) and other 77 (0.79%) (Table 1). It can be assumed that E. coli and Staphylococcus are the most prevalent in Egypt. Approximately 34% E. coli isolates in Egypt originated from chicken and 25% from water. Furthermore, it was found that L. monocytogenes is the only species that was found in vegetables only. Listeria monocytogenes is the causative agent of listeriosis and a serious threat to the health of certain populations, including the elderly, immunocompromised people, and pregnant women. It is an uncommon foodborne disease with a 20%-30% death rate. L. monocytogenes is also common in the environment and can infect food-processing settings, posing a threat to the food chain [71, 72].
Comparative meta-analysis in UK
In a comprehensive analysis of 286 research papers pertaining to foodborne pathogens in the UK between 2013 and 2022, only 16 full-text articles were selected for meta-analysis, as referenced in sources [15, 21, 71,72,73,74,75,76,77,78,79,80,81,82,83]. Within a dataset comprising 10,602 samples, it was observed that 41.94% of these samples exhibited a positive prevalence of pathogens. Notably, chicken 824 (89.66%), retail chicken 1023 (66.86%), and dairy products 271 (90.33%) displayed particularly high positive prevalence rates, while other sources such as pigs 696 (62.37%), raw meat 285 (7.20%), animals 1046 (59.06%), and the environment 418 (41.51%) also demonstrated varying degrees of pathogen prevalence. Key pathogens identified included E. coli. 2037 (19.21%), Campylobacter 1603 (15.12%), Salmonella 676 (6.38%), and Staphylococci 131 (1.24%) (Table 2). It can be assumed that E. coli and Campylobacter are the most prevalent in the UK. About 53% of E. coli isolates in the UK originated from pigs. Additionally, it was found that Campylobacter is the only pathogen i.e., prevalent in retail chicken and raw meat only. It is one of the most prevalent causes of bacterial diarrheal sickness globally, including acute enteritis, extra intestinal infections (for example, bacteremia, abscess, and meningitis), and post infectious complications. In most cases, Campylobacter causes a self-limiting clinical illness that lasts 5 to 7 days; the infection resolves without antimicrobial therapy in the vast majority of cases, although 5% to 10% of individuals experience a recurrence after their initial illness [73].
Comparison of AMR isolates in Egypt and UK and their antimicrobial resistance
A comparison of their AMR isolates in Egypt and UK is provided in Fig. 3, indicating a similar incidence of E. coli in both nations, representing about 54% in Egypt and 46% in the UK. Similarly, Campylobacter is the second most common pathogen in the UK, accounting for 36%, however in the Egypt Staphylococcus is the second most prevalent pathogen with 19%. Staphylococcus abundance in Egypt which is mostly associated with high intake of raw and RTE meat, milk, and other dairy items, but Campylobacter prevalence in the UK is solely related to vegetable consumption.
Diagram showing the flow of the study selection process according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [84]
The analyses of antimicrobial agents found and antibiotic resistances observed for each pathogen isolate in Egypt and the UK are illustrated in Figs. 4 and 5. E. coli with multiple drug resistance was observed in both Egypt and the UK, as well as MDR strains of Staphylococcus spp., Salmonella, and K. pneumonia in Egypt and Staphylococcus spp., and Campylobacter spp. in the UK. Figure 4 shows that the majority of the bacteria found in Egypt exhibited microbial resistance to β-lactams and aminoglycosides, followed by fluoroquinolones and tetracyclines, whereas the majority of the bacteria found in the UK showed microbial resistance to tetracyclines and β-lactams, followed by aminoglycosides and sulfonamide (Fig. 5). It is concerning because the majority of the microorganisms reported were multidrug resistant.
Discussion
This study provides a comprehensive assessment of the abundance of foodborne pathogens and the presence of AMR genes in Egypt and the UK. With the goal of establishing a link between the prevalence of AMR and various bacteria, data published over the last ten years (2013 to 2022) were examined. 56 papers were selected and met the inclusion criterion out of 190 papers. Beef, chicken, retail chicken, raw meat, ready to eat meat, fish, milk, other dairy products (cheese, ice cream, yoghurt, cream), water, vegetables, pigs, animals (sheep, dog, badger, fox), environmental samples (water, drainages) and other food sources were all subjected to a planned follow-up AMR risk group evaluation.
The meta-analysis suggested that foods were highly contaminated with E.coli, and Staphylococcus spp. in Egypt, while Campylobacter spp. was the prevalent bacterium detected in food in the UK. The obtained data reflected that contamination occurred in the food mostly eaten in each country. The isolated bacteria showed resistance mainly to β-Lactam, tetracyclines, fluoroquinolones, cephalosporin, quinolone, macrolides, aminoglycosides, lincosamide, amphenicols, Glycopeptides, Chloramphenicol, sulfonamide, carbapenem, and other. The wide range of resistance genes shows that strains derived from food may have an impact on the environment, animals, and humans.
In Egypt, out of a total of 20,353 isolates, 9,751 (48%) were found to be positive for AMR. Among these, 3,205 (16%) of the isolated bacteria exhibited resistance to β-lactams and aminoglycosides.The resistance to fluroquinolone, tetracycline, cephalosporin, macrolides and sulfonamide had middle prevalence whereas chloramphenicol, glycopeptides, quinolones, lincosamide and carbapenem showed low prevalence. Whereas in the UK, out of 20,353 isolates, 10,602 (52%) samples were positive for AMR by which 4447 (22%) bacteria were isolates. Tetracycline, and β-lactam showed the highest resistance occurrence, while aminoglycosides, sulfonamide and quinolones showed middle prevalence and chloramphenicol, cephalosporin, fluoroquinolones and macrolides showed low prevalence.
There was a noticeable difference in antimicrobial susceptibility patterns between the groups. This difference in AMR may be due to the antimicrobial drugs not only used to treat various infections in animals but to inhibit the growth of bacteria [74,75,76]. The Food Standards Agency (FSA) in the UK is responsible for ensuring food safety and hygiene in England, Wales, and Northern Ireland. It collaborates with local authorities to enforce food safety requirements, and its employees work in meat plants to ensure that criteria are met [77]. However, the Egyptian National Food Safety Authority’s (NFSA) goal is to protect Egyptian customers' health and safety by imposing minimum requirement standards for food exported to Egypt [78]. Among the different sources and transmission pathways examined, faecal fertilizers, irrigation, and surface water were discovered to contribute the most to AMR. Raw foods are regarded substantially risky to consumers because resistant microbes can thrive in untreated food [79, 80].
Today's health concerns are usually complex, transboundary, multifactorial, and cross-species, and it is unlikely that sustainable mitigation methods would be developed if handled just from a medical, veterinary, or ecological perspective [81, 82]. To better understand the complexity of the situation there is a need to implement One Health approach to a variety of sectors as well as the larger topic of antibiotic resistance at the animal-human–environment interface [83]. Therefor it is important to understand the top pathogen-drug combinations contributing to the burden of bacterial AMR trends worldwide, and the present severity of the problem. If AMR continues to progress without restraint, numerous bacterial pathogens could potentially become significantly more lethal in the future than they are at present.
Conclusion
This comprehensive systematic review and meta-analysis provided a summary of the current state of AMR in Egypt and the UK from the aspect of One Health. The levels of AMR reported in Egypt between 2013 and 2022 are of concern, especially regarding ancient antibacterial agents such as β-Lactam, 1st and 2nd generation cephalosporin, Aminoglycoside or tetracycline. The high levels of AMR and the identification of pertinent levels of other agent resistance Tetracycline, β-Lactam, Aminoglycosides, 3rd and 4th generation cephalosporin or fluoroquinolones, as well as the detected resistance to these drugs in both Egypt and the UK point to the necessity of enacting effective controls regarding access to antibacterial agents as well as the creation of educational campaigns to raise public awareness of the importance of prudent use of antibacterial agents.
The unusual number of isolates especially most dangerous pathogenic bacteria such as E. coli, Klebsiella spp., Streptococcus spp., Staphylococcus spp., Salmonella, Campylobacter spp. found on foodstuffs exhibiting intermediate levels of resistance to multiple antimicrobials highlights the necessity for a One Health approach to overcoming the impending pandemic. The success of the OH strategy is dependent not just on local initiatives, but also on socio-culture, socioeconomic, and institutional initiatives at an institutionalized and systemic level.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article.
References
Queenan K, Häsler B, Rushton J. A One Health approach to antimicrobial resistance surveillance: is there a business case for it? Int J Antimicrob Agents. 2016;48:422–7. https://doi.org/10.1016/J.IJANTIMICAG.2016.06.014.
Tackling drug-resistant infections globally: final report and recommendations the review on antimicrobial resistance chaired by jim o’neill. (2016) (Acessed on: 26 Jan 2023).
Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. (Acessed on: 26 Jan 2023).
Carlos Franco M, Prudencio CR, da CharlysCosta A, Leal E, Chang C-M, Pati Pandey R. Systematic Surveillance and Meta-Analysis of Antimicrobial Resistance and Food Sources from China and the USA. Antibiot. 2022;11:1471. https://doi.org/10.3390/ANTIBIOTICS11111471.
The bacterial challenge: time to react. https://doi.org/10.2900/2518
Thompson T. The staggering death toll of drug-resistant bacteria. Nature. 2022. https://doi.org/10.1038/D41586-022-00228-X.
MOHP, WHO: Egypt National Action Plan For Antimicrobial Resistance. Fed. Minist. Agric. Environ. Heal. 136, 66 (2022) (Acessed on: 26 Jan 2023).
Antimicrobial resistance - UK Health Security Agency, https://ukhsa.blog.gov.uk/category/priority3/antimicrobial-resistance/ (Acessed on: 27 Jan 2023.
The burden of antimicrobial resistance (AMR) in Egypt. (Acessed on: 27 Jan 2023
Harbarth S, Balkhy HH, Goossens H, Jarlier V, Kluytmans J, Laxminarayan R, Saam M, Van Belkum A, Pittet D. Antimicrobial resistance: One world, one fight! Antimicrob Resist Infect Control. 2015;4:1–15. https://doi.org/10.1186/S13756-015-0091-2/FIGURES/3.
Holmes AH, Moore LSP, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, Guerin PJ, Piddock LJV. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet (London, England). 2016;387:176–87. https://doi.org/10.1016/S0140-6736(15)00473-0.
Collignon PJ, McEwen SA. One Health—Its Importance in Helping to Better Control Antimicrobial Resistance. Trop Med Infect Dis. 2019;4:22. https://doi.org/10.3390/TROPICALMED4010022.
So AD, Shah TA, Roach S, Ling Chee Y, Nachman KE. An Integrated Systems Approach is Needed to Ensure the Sustainability of Antibiotic Effectiveness for Both Humans and Animals. J Law Med Ethics. 2015;43(Suppl 3):38–45. https://doi.org/10.1111/JLME.12273.
Global action plan on antimicrobial resistance, https://apps.who.int/iris/handle/10665/193736 (Acessed on: 28 Jan 2023).
Vikesland PJ, Pruden A, Alvarez PJJ, Aga D, Bürgmann H, Li XD, Manaia CM, Nambi I, Wigginton K, Zhang T, Zhu YG. Toward a Comprehensive Strategy to Mitigate Dissemination of Environmental Sources of Antibiotic Resistance. Environ Sci Technol. 2017;51:13061–9. https://doi.org/10.1021/ACS.EST.7B03623/ASSET/IMAGES/LARGE/ES-2017-036232_0003.JPEG.
One health, https://www.who.int/health-topics/one-health#tab=tab_1 (Acessed on: 28 Jan 2023)
One Health Joint Plan of Action, 2022–2026. One Heal. Jt. Plan Action, 2022–2026. (2022). https://doi.org/10.4060/CC2289EN
One Health Basics | One Health | CDC, https://www.cdc.gov/onehealth/basics/index.html (Acessed on: 28 Jan 2023).
Kienberger S, Hagenlocher M. Spatial-explicit modeling of social vulnerability to malaria in East Africa. Int J Health Geogr. 2014;13:1–16. https://doi.org/10.1186/1476-072X-13-29/FIGURES/6.
Gunjan Vidic J, Manzano M, Raj VS, Pandey RP, Chang CM. Comparative meta-analysis of antimicrobial resistance from different food sources along with one health approach in Italy and Thailand. One Heal. 2023;16:100477. https://doi.org/10.1016/J.ONEHLT.2022.100477.
Tornimbene, B., Eremin, S., Abednego, R., Abualas, E.O., Boutiba, I., Egwuenu, A., Fuller, W., Gahimbare, L., Githii, S., Kasambara, W., Lukwesa-Musyani, C., Miamina, F.A., Mtapuri-Zinyowera, S., Najjuka, G., Perovic, O., Zayed, B., Ahmed, Y.A., Ismail, M.T., Pessoa Da Silva, C.L.: African Journal of Laboratory Medicine. 2225–2002. https://doi.org/10.4102/ajlm.v11i1.1594
Tackling antimicrobial resistance 2019 to 2024. (2019) (Acessed on: 28 Jan 2023).
Koluman A, Dikici A. Antimicrobial resistance of emerging foodborne pathogens: Status quo and global trends. Crit Rev Microbiol. 2013;39:57–69. https://doi.org/10.3109/1040841X.2012.691458.
Martins BTF, Botelho CV, Silva DAL, Lanna FGPA, Grossi JL, Campos-Galvão MEM, Yamatogi RS, Falcão JP, Bersot L, Dos S, Nero LA. Yersinia enterocolitica in a Brazilian pork production chain: Tracking of contamination routes, virulence and antimicrobial resistance. Int J Food Microbiol. 2018;276:5–9. https://doi.org/10.1016/J.IJFOODMICRO.2018.03.028.
Wheatley P, Giotis ES, McKevitt AI. Effects of slaughtering operations on carcass contamination in an Irish pork production plant. Ir Vet J. 2014;67:1–6. https://doi.org/10.1186/2046-0481-67-1/FIGURES/2.
Omar D, Al-Ashmawy M, Ramadan H, El-Sherbiny M. Occurrence and PCR identification of Salmonella spp. from milk and dairy products in Mansoura. Egypt Int Food Res J. 2018;25:446–52.
Al-Ashmawy MA, Sallam KI, Abd-Elghany SM, Elhadidy M, Tamura T. Prevalence, Molecular Characterization, and Antimicrobial Susceptibility of Methicillin-Resistant Staphylococcus aureus Isolated from Milk and Dairy Products. Foodborne Pathog Dis. 2016;13:156–62. https://doi.org/10.1089/FPD.2015.2038.
Shiga toxin-producing Escherichia coli (STEC) and food: attribution, characterization, and monitoring: report, https://apps.who.int/iris/handle/10665/272871 (Acessed on: 28 Jan 2023).
WHO country cooperation strategy at a glance: Egypt, https://www.who.int/publications/i/item/WHO-CCU-18.02-Egypt (Acessed on: 28 Jan 2023).
National Action Plan for Antimicrobial Resistance 2018–2022. | FAOLEX, https://www.fao.org/faolex/results/details/en/c/LEX-FAOC204655/ (Acessed on: 29 Jan 2023).
WHO: WHO Library Cataloguing-in-Publication Data Global Action Plan on Antimicrobial Resistance. Microbe Mag. 10, 354–355 (2015) (Acessed on: 29 Jan 2023).
WHO EMRO | Initiating Egypt’s antimicrobial resistance national action plan | Egypt-events | Egypt, https://www.emro.who.int/egy/egypt-events/antimicrobial-resistance-national-action-plan.html (Acessed on: 29 Jan 2023).
Integrated surveillance of antimicrobial resistance in foodborne bacteria: application of a one health approach: guidance from the WHO Advisory Group on Integrated Surveillanec of Antimicrobial Resistance (AGISAR), https://apps.who.int/iris/handle/10665/255747
Magouras I, Carmo LP, Stärk KDC, Schüpbach-Regula G. Antimicrobial usage and -resistance in livestock: Where should we focus? Front Vet Sci. 2017;4:148. https://doi.org/10.3389/FVETS.2017.00148/BIBTEX.
Global antimicrobial resistance and use surveillance system (GLASS) report: 2022, https://www.who.int/publications/i/item/9789240062702 (Acessed on: 30 Jan 2023).
Central Asian and European Surveillance of Antimicrobial Resistance (CAESAR), https://www.who.int/europe/groups/central-asian-and-european-surveillance-of-antimicrobial-resistance-(caesar) (Acessed on: 30 Jan 2023).
Zeinhom MMA, Abdel-Latef GK, Jordan K. The use of multiplex pcr to determine the prevalence of enterotoxigenic staphylococcus aureus isolated from raw milk, feta cheese, and hand swabs. J Food Sci. 2015;80:M2932–6. https://doi.org/10.1111/1750-3841.13147.
Enany, M.E., Algammal, A.M., Nasef, S.A., Abo-Eillil, S.A.M., Bin-Jumah, M., Taha, A.E., Allam, A.A.: The occurrence of the multidrug resistance (MDR) and the prevalence of virulence genes and QACs resistance genes in E. coli isolated from environmental and avian sources. AMB Express. 9, (2019). https://doi.org/10.1186/s13568-019-0920-4
Sahar MEA, Salwa FA, Samy AS, Mohamed HAA, Amira MZ, John DK. Prevalence and characterization of Shiga toxin O157 and non-O157 enterohemorrhagic Escherichia coli isolated from different sources in Ismailia. Egypt African J Microbiol Res. 2013;7:2637–45. https://doi.org/10.5897/ajmr2013.5417.
Abdeen EE, Mousa WS, Abdelsalam SY, Heikal HS, Shawish RR, Nooruzzaman M, Soliman MM, Batiha GE, Hamad A, Abdeen A. Prevalence and characterization of coagulase positive staphylococci from food products and human specimens in Egypt. Antibiotics. 2021;10:1–14. https://doi.org/10.3390/antibiotics10010075.
Elafify M, Khalifa HO, Al-Ashmawy M, Elsherbini M, El Latif AA, Okanda T, Matsumoto T, Koseki S, Abdelkhalek A. Prevalence and antimicrobial resistance of Shiga toxin-producing Escherichia coli in milk and dairy products in Egypt. J Environ Sci Heal - Part B Pestic Food Contam Agric Wastes. 2020;55:265–72. https://doi.org/10.1080/03601234.2019.1686312.
El Seedy FR, Samy AA, Salam HSH, Khairy EA, Koraney AA. Polymerase chain reaction detection of genes responsible for multiple antibiotic resistance Staphylococcus aureus isolated from food of animal origin in Egypt. Vet World. 2017;10:1205–11. https://doi.org/10.14202/vetworld.2017.1205-1211.
Bouymajane A, Rhazi Filali F, Oulghazi S, Lafkih N, Ed-Dra A, Aboulkacem A, El Allaoui A, Ouhmidou B, Moumni M. Occurrence, antimicrobial resistance, serotyping and virulence genes of Listeria monocytogenes isolated from foods. Heliyon. 2021;7:e06169. https://doi.org/10.1016/j.heliyon.2021.e06169.
Moawad AA, Hotzel H, Awad O, Tomaso H, Neubauer H, Hafez HM, El-Adawy H. Occurrence of Salmonella enterica and Escherichia coli in raw chicken and beef meat in northern Egypt and dissemination of their antibiotic resistance markers. Gut Pathog. 2017;9:1–13. https://doi.org/10.1186/s13099-017-0206-9.
Gharieb RM, Tartor YH, Khedr MHE. Non-Typhoidal Salmonella in poultry meat and diarrhoeic patients: Prevalence, antibiogram, virulotyping, molecular detection and sequencing of class I integrons in multidrug resistant strains. Gut Pathog. 2015;7:1–11. https://doi.org/10.1186/s13099-015-0081-1.
Mahros MA, Abd-Elghany SM, Sallam KI. Multidrug-, methicillin-, and vancomycin-resistant Staphylococcus aureus isolated from ready-to-eat meat sandwiches: An ongoing food and public health concern. Int J Food Microbiol. 2021;346:109165. https://doi.org/10.1016/j.ijfoodmicro.2021.109165.
Mahmoud M, Askora A, Barakat AB, Rabie OEF, Hassan SE. Isolation and characterization of polyvalent bacteriophages infecting multi drug resistant Salmonella serovars isolated from broilers in Egypt. Int J Food Microbiol. 2018;266:8–13. https://doi.org/10.1016/j.ijfoodmicro.2017.11.009.
Ammar AM, Abd El-Hamid MI, Eid SEA, El Oksh AS. Insights into antimicrobial resistance and virulence genes of emergent multidrug resistant avian pathogenic Escherichia coli in Egypt: How closely related are they? Rev Med Vet (Toulouse). 2015;166:304–14.
Abed, A.H., Menshawy, A.M.S., Zeinhom, M.M.A., Hossain, D., Khalifa, E., Wareth, G., Awad, M.F.: Subclinical mastitis in selected bovine dairy herds in north upper egypt: Assessment of prevalence, causative bacterial pathogens, antimicrobial resistance and virulence-associated genes. Microorganisms. 9, (2021). https://doi.org/10.3390/microorganisms9061175
Awad A, Arafat N, Elhadidy M. Genetic elements associated with antimicrobial resistance among avian pathogenic Escherichia coli. Ann Clin Microbiol Antimicrob. 2016;15:1–8. https://doi.org/10.1186/s12941-016-0174-9.
Bendary MM, Solyman SM, Azab MM, Mahmoud NF, Hanora AM. Genetic diversity of multidrug resistant Staphylococcus aureus isolated from clinical and non clinical samples in Egypt. Cell Mol Biol. 2016;62:55–61. https://doi.org/10.14715/cmb/2016.62.10.9.
Ammar AM, Attia AM, Abd El-Hamid MI, El-Shorbagy IM, Abd El-Kader SA. Genetic basis of resistance waves among methicillin resistant Staphylococcus aureus isolates recovered from milk and meat products in Egypt. Cell Mol Biol. 2016;62:7–15. https://doi.org/10.14715/cmb/2016.62.10.2.
Algammal AM, El-Kholy AW, Riad EM, Mohamed HE, Elhaig MM, Al Yousef SA, Hozzein WN, Ghobashy MOI. Genes encoding the virulence and the antimicrobial resistance in enterotoxigenic and shiga-toxigenic E. coli isolated from diarrheic calves. Toxins (Basel). 2020;12:1–13. https://doi.org/10.3390/toxins12060383.
Ramadan H, Ibrahim N, Samir M, Abd El-Moaty A, Gad T. Aeromonas hydrophila from marketed mullet (Mugil cephalus) in Egypt: PCR characterization of β-lactam resistance and virulence genes. J Appl Microbiol. 2018;124:1629–37. https://doi.org/10.1111/jam.13734.
Gad GFM, Abdel-Hamid AM, Farag ZSH. Antibiotic resistance in lactic acid bacteria isolated from some pharmaceutical and dairy products. Brazilian J Microbiol. 2014;45:25–33. https://doi.org/10.1590/S1517-83822014000100005.
Amer MM, Mekky HM, Amer AM, Fedawy HS. Antimicrobial resistance genes in pathogenic Escherichia coli isolated from diseased broiler chickens in Egypt and their relationship with the phenotypic resistance characteristics. Vet World. 2018;11:1082–8. https://doi.org/10.14202/vetworld.2018.1082-1088.
Moawad AA, Hotzel H, Neubauer H, Ehricht R, Monecke S, Tomaso H, Hafez HM, Roesler U, El-Adawy H. Antimicrobial resistance in Enterobacteriaceae from healthy broilers in Egypt: Emergence of colistin-resistant and extended-spectrum β-lactamase-producing Escherichia coli 06 Biological Sciences 0604 Genetics 11 Medical and Health Sciences 1108 Medical Mi. Gut Pathog. 2018;10:1–12. https://doi.org/10.1186/s13099-018-0266-5.
Younis R, Nasef S, Salem W. Detection of Multi-Drug Resistant Food-borne Bacteria in Ready-to-Eat Meat Products in Luxor City. Egypt SVU-International J Vet Sci. 2019;2:20–35. https://doi.org/10.21608/svu.2019.23168.
Osman KM, Al-Maary KS, Mubarak AS, Dawoud TM, Moussa IMI, Ibrahim MDS, Hessain AM, Orabi A, Fawzy NM. Characterization and susceptibility of streptococci and enterococci isolated from Nile tilapia (Oreochromis niloticus) showing septicaemia in aquaculture and wild sites in Egypt. BMC Vet Res. 2017;13:1–10. https://doi.org/10.1186/s12917-017-1289-8.
Hassan A-RHA, Salam HSH, Abdel-Latef GK. Serological identification and antimicrobial resistance of Salmonella isolates from broiler carcasses and human stools in Beni-Suef Egypt. Beni-Suef Univ J Basic Appl Sci. 2016;5:202–7. https://doi.org/10.1016/j.bjbas.2016.04.002.
Bendary MM, Solyman SM, Azab MM, Mahmoud NF, Hanora AM. Characterization of methicillin resistant staphylococcus aureus isolated from human and animal samples in Egypt. Cell Mol Biol. 2016;62:94–100. https://doi.org/10.14715/cmb/2016.62.2.16.
Samy AA, Mansour AS, Khalaf DD, Khairy EA. Development of multidrug-resistant Escherichia coli in some Egyptian veterinary farms. Vet World. 2022;15:488–95. https://doi.org/10.14202/vetworld.2022.488-495.
El-Gohary FA, Abdel-Hafez LJM, Zakaria AI, Shata RR, Tahoun A, El-Mleeh A, Abo Elfadl EA, Elmahallawy EK. Enhanced antibacterial activity of silver nanoparticles combined with hydrogen peroxide against multidrug-resistant pathogens isolated from dairy farms and beef slaughterhouses in egypt. Infect Drug Resist. 2020;13:3485–99. https://doi.org/10.2147/IDR.S271261.
Rasha IM, Mohamed AA, Heba MA. Virulence and antimicrobial susceptibility profile of Listeria monocytogenes isolated from frozen vegetables available in the Egyptian market. African J Microbiol Res. 2018;12:218–24. https://doi.org/10.5897/ajmr2018.8794.
Elrais, A.M., Arab, W.S., Sallam, K.I., Elmegid, W.A., Elgendy, F., Elmonir, W., Imre, K., Morar, A., Herman, V., Elaadli, H.: Prevalence, Virulence Genes, Phylogenetic Analysis, and Antimicrobial Resistance Profile of Helicobacter Species in Chicken Meat and Their Associated Environment at Retail Shops in Egypt. Foods. 11, (2022). 10.3390/foods11131890.
Sallam KI, Mohammed MA, Hassan MA, Tamura T. Prevalence, molecular identification and antimicrobial resistance profile of salmonella serovars isolated from retail beef products in mansoura, egypt. Food Control. 2014;38:209–14. https://doi.org/10.1016/j.foodcont.2013.10.027.
Elmonir W, Shalaan S, Tahoun A, Mahmoud SF, Remela EMA, Eissa R, El-Sharkawy H, Shukry M, Zahran RN. Prevalence, antimicrobial resistance, and genotyping of Shiga toxin-producing Escherichia coli in foods of cattle origin, diarrheic cattle, and diarrheic humans in Egypt. Gut Pathog. 2021;13:1–11. https://doi.org/10.1186/s13099-021-00402-y.
Abdeen EE, Mousa WS, Harb OH, Fath-Elbab GA, Nooruzzaman M, Gaber A, Alsanie WF, Abdeen A. Prevalence, antibiogram and genetic characterization of listeria monocytogenes from food products in Egypt. Foods. 2021;10:1–13. https://doi.org/10.3390/foods10061381.
Pasricha S. Research Article Research Article. Arch Anesthesiol Crit Care. 2020;4:527–34.
Ameen F, Reda SA, El-Shatoury SA, Riad EM, Enany ME, Alarfaj AA. Prevalence of antibiotic resistant mastitis pathogens in dairy cows in Egypt and potential biological control agents produced from plant endophytic actinobacteria. Saudi J Biol Sci. 2019;26:1492–8. https://doi.org/10.1016/j.sjbs.2019.09.008.
Jordan K, McAuliffe O. Listeria monocytogenes in Foods. Adv Food Nutr Res. 2018;86:181–213. https://doi.org/10.1016/BS.AFNR.2018.02.006.
Lomonaco S, Nucera D, Filipello V. The evolution and epidemiology of Listeria monocytogenes in Europe and the United States. Infect Genet Evol. 2015;35:172–83. https://doi.org/10.1016/J.MEEGID.2015.08.008.
Fitzgerald C. Campylobacter. Clin Lab Med. 2015;35:289–98. https://doi.org/10.1016/J.CLL.2015.03.001.
Chokshi A, Sifri Z, Cennimo D, Horng H. Global Contributors to Antibiotic Resistance. J Glob Infect Dis. 2019;11:36. https://doi.org/10.4103/JGID.JGID_110_18.
ANTIBACTERIAL AGENTS IN CLINICAL DEVELOPMENT. (Acessed on: 30 Jan 2023).
Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev. 2011;24:718–33. https://doi.org/10.1128/CMR.00002-11.
Food Standards Agency - GOV.UK, https://www.gov.uk/government/organisations/food-standards-agency (Acessed on: 30 Jan 2023).
About NFSA, https://www.exports-to-egypt.com/en/about-nfsa (Acessed on: 30 Jan 2023).
Ahmed F, Cappai MG, Morrone S, Cavallo L, Berlinguer F, Dessì G, Tamponi C, Scala A, Varcasia A. Raw meat based diet (RMBD) for household pets as potential door opener to parasitic load of domestic and urban environment. Revival of understated zoonotic hazards? A review. One Heal. 2021;13:100327. https://doi.org/10.1016/J.ONEHLT.2021.100327.
Manyi-Loh, C., Mamphweli, S., Meyer, E., Okoh, A.: molecules Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. (2018). https://doi.org/10.3390/molecules23040795
Mackenzie, J.S., Jeggo, M.: The One Health Approach—Why Is It So Important? Trop. Med. Infect. Dis. 4, (2019). https://doi.org/10.3390/TROPICALMED4020088
Destoumieux-Garzón D, Mavingui P, Boetsch G, Boissier J, Darriet F, Duboz P, Fritsch C, Giraudoux P, Roux FL, Morand S, Paillard C, Pontier D, Sueur C, Voituron Y. The one health concept: 10 years old and a long road ahead. Front Vet Sci. 2018;5:14. https://doi.org/10.3389/FVETS.2018.00014/BIBTEX.
Allal L, Mahrous H, Saad A, Refaei S, Attia M, Mahrous I, Fahim M, Elfadaly S, Abdelnabi A. From Four-Way Linking to a One Health Platform in Egypt: institutionalisation of a multidisciplinary and multisectoral One Health system. Rev Sci Tech Off Int Epiz. 2019;38:261–70. https://doi.org/10.20506/rst.38.1.2958.
Page, M.J., McKenzie, J.E., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., Shamseer, L., Tetzlaff, J.M., Akl, E.A., Brennan, S.E., Chou, R., Glanville, J., Grimshaw, J.M., Hróbjartsson, A., Lalu, M.M., Li, T., Loder, E.W., Mayo-Wilson, E., McDonald, S., McGuinness, L.A., Stewart, L.A., Thomas, J., Tricco, A.C., Welch, V.A., Whiting, P., Moher, D.: The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 372, (2021). https://doi.org/10.1136/BMJ.N71
Acknowledgements
This work was supported through industry-academia collaboration projects, VtR Inc-CGU, R.O.C., project grant (SCRPD1L0221), DOXABIO-CGU, R.O.C., project grant (SCRPD1K0131), and also the CGU project grant (UZRPD1L0011).
Funding
Project grants (SCRPD1L0221); DOXABIO-CGU, R.O.C., (SCRPD1K0131), and the CGU project grant (UZRPD1L0011).
Author information
Authors and Affiliations
Contributions
G., H., R.P.P., and C.M.C. designed this study. G., H., R.P.P., C.M.C., V.S.R., E.L., contributed in methodology and data analysis. G., H., R.P.P., C.M.C. wrote the original manuscript text; R.M., J.V., M.M., E.L., V.S.R., reviewed and edited the writing; C.M.C. supplied the funding. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gunjan, Himanshu, Mukherjee, R. et al. Comparative meta-analysis of antimicrobial resistance from different food sources along with one health approach in the Egypt and UK. BMC Microbiol 23, 291 (2023). https://doi.org/10.1186/s12866-023-03030-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-023-03030-5