Abstract
Background
The emergence of different viral infections calls for the development of new, effective, and safe antiviral drugs. Glycyrrhiza glabra is a well-known herbal remedy possessing antiviral properties.
Objective
The objective of our research was to evaluate the effectiveness of a newly developed combination of the probiotics Lactobacillus acidophilus and G. glabra root extract against two viral models, namely the DNA virus Herpes simplex virus-1 (HSV-1) and the RNA virus Vesicular Stomatitis Virus (VSV), with regards to their antiviral properties.
Methodology
To examine the antiviral impacts of various treatments, we employed the MTT assay and real-time PCR methodology.
Results
The findings of our study indicate that the co-administration of L. acidophilus and G. glabra resulted in a significant improvement in the survival rate of Vero cells, while also leading to a reduction in the titers of Herpes Simplex Virus Type 1 (HSV-1) and Vesicular Stomatitis Virus (VSV) in comparison to cells that were not treated. Additionally, an investigation was conducted on glycyrrhizin, the primary constituent of G. glabra extract, utilizing molecular docking techniques. The results indicated that glycyrrhizin exhibited a greater binding energy score for HSV-1 polymerase (− 22.45 kcal/mol) and VSV nucleocapsid (− 19.77 kcal/mol) in comparison to the cocrystallized ligand (− 13.31 and − 11.44 kcal/mol, respectively).
Conclusions
The combination of L. acidophilus and G. glabra extract can be used to develop a new, natural antiviral agent that is safe and effective.
Similar content being viewed by others
Introduction
Herpes simplex virus (HSV) is a member of the Herpesviridae family, which consists of a wide variety of enveloped DNA viruses that pose significant global health risks. HSV is a common sexually transmitted infection (STI) that can cause lifelong infections with intermittent recurrence, resulting in painful symptoms and distress for those affected. Moreover, HSV infections can have severe consequences for newborns and immunocompromised individuals, highlighting the need for effective antiviral treatments [1]. According to the World Health Organisation, oral HSV-1 infections affect several billion people, while genital HSV-1 infections affect over 500 million people. This virus places numerous people at risk of various diseases and can cause clinical complications in both adults and neonates [2,3,4]. The treatment of HSV infection typically involves the use of acyclovir, foscarnet, vidarabine, and other synthetic nucleoside analogues [5, 6], the unintended outcomes of this have led to the emergence of resistant strains of the drug [7, 8]. In addition, the prevention of HSV infection has not been achieved through vaccines. Hence, the focus has shifted towards developing new antiviral drugs and innovative prophylactics in recent decades.
Antiviral properties can be attributed to probiotic bacteria, which generate bacteriocins and lactic acid to inhibit the proliferation of enteric pathogens. Moreover, the interaction of probiotic bacteria with epithelial cell surface receptors can stimulate the production of cytokines, affecting the performance of mucosal lymphocytes [9, 10]. Bioactive secondary metabolites can be sourced from plants and used to develop new natural products with properties such as antiviral, antioxidant, anti-inflammatory, and cytotoxic effects, making them valuable resources in the treatment of various diseases [11,12,13,14].
The plant G. glabra, also known as licorice from the Fabaceae family, is renowned for its traditional use as an herbal supplement with anti-cancer and anti-viral properties. The roots of this plant are used to treat chronic inflammatory conditions [15, 16].
Secondary metabolites, including terpenoids, saponins, flavonoids, polyamines, and polysaccharides, were found to be abundant in various species of G. glabra during chemical analysis [17, 18]. The main active component in G. glabra is glycyrrhizin, which belongs to the oleanane category of pentacyclic triterpenoid saponins (as shown in Fig. 1) [17, 19]. The antiviral, anti-inflammatory, antiarrhythmic, antibacterial, antioxidant, and expectorant properties of licorice roots are attributable to glycyrrhizic and aglycone glycyrrhetinic acids present in them [17, 20, 21]. G. glabra and its active component, Glycyrrhizin, have been reported to possess antiviral activity against various viruses, including the hepatitis C virus (HCV).
Several studies have demonstrated the potential of glycyrrhizin to inhibit HCV replication in vitro and in vivo. For example, Ikeda et al. (2006) reported that glycyrrhizin inhibited the entry of HCV into hepatocytes by blocking virus binding to cell surface receptors [22]. Similarly, Matsumoto et al. (2013) and Calland et al. (2012) found that glycyrrhizin reduced HCV replication in a dose-dependent manner by inhibiting the expression of HCV RNA-dependent RNA polymerase [23, 24].
In addition to HCV, glycyrrhizin has also been shown to possess antiviral activity against other viruses. For instance, studies have demonstrated the ability of glycyrrhizin to inhibit the replication of influenza viruses [25, 26], herpes simplex viruses [27], and human immunodeficiency viruses [28].
The aim of this study is to assess the potential antiviral effects of combining Lactobacillus acidophilus, a probiotic bacterium, with G. glabra against herpes simplex virus type 1 (HSV-1) and vesicular stomatitis virus (VSV), two enveloped viruses that can cause mild to severe illnesses. Previous research has indicated that probiotics possess antiviral properties, while G. glabra has been found to have multiple biological activities, including antiviral effects. Thus, this research aims to determine whether the combination of L. acidophilus and G. glabra can enhance antiviral activity against HSV-1 and VSV.
Results
Cytotoxicity
Once the concentrations of probiotics and prebiotics were determined, the Vero cell line underwent a cytotoxicity test, and the microplate reader was used to analyze the results. The the minimum non-toxic concentration (MNTC) for each sample was determined by observing the alterations in cellular morphology.
The cell monolayers were treated with varying amounts of the test substances and left to incubate for 48 hours at 37°C and 5% CO2. The determination of non-toxic concentrations was based on a comparison of the treated cell's morphological changes with those of the untreated cells. The MNTC was determined as the maximum concentration that did not cause any damage to the cells.
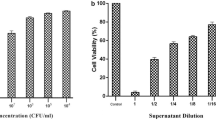
Once incubated, concentrations of the drug that did not harm live cells were assessed and compared to normal cells to confirm their non-toxicity. Any concentration that led to damaged cell rows was considered hazardous. Additionally, the maximum drug concentrations that had no impact on cells were identified as non-toxic concentrations. By comparing treated and untreated cultures, the minimum non-toxic concentrations (MNTCs) were determined. Table 1 and Figure 2 display the results.
HSV-1 log reduction titre
After establishing the safe concentration of the prebiotic, including L. acidophilus suspension and supernatant, and their combination, the cells were exposed to these concentrations. Following this, the cells were inoculated with HSV-1 using varying concentrations obtained through serial dilution of the virus.
The Reed and Munch programs were used to determine the log reduction of the virus by comparing its concentration to the initial titer of HSV-1. Table 2 displays the TCID50 values for each sample, indicating the log reduction of HSV-1 inhibition, starting from an initial concentration of 1.00E+07. The findings are presented in Table 2 and Figure 3.
VSV log reduction titer
The Vero cells were exposed to a non-toxic concentration of the prebiotic, comprising of L. acidophilus suspension and supernatant, and their combination. Subsequently, VSV was introduced to the cells at different concentrations, achieved through serial dilution of the virus.
The log reduction of the virus was computed by comparing it to the initial VSV titer using the Reed and Munch programs. The VSV's initial concentration was 1.00E+05 TCID50, and the resulting virus titer after treatment is presented in Table 2. Each sample was found to inhibit VSV by a certain log reduction, with the results presented in Table 2 and Figure 2.
Real time PCR
Real-time PCR results indicated that the CT ratio of L. acidophilus suspension was 37.1 upon exposure to the VSV virus, whereas the combination of L. acidophilus and G. glabra had a lower CT ratio of 25.2. The control had a CT value of 0. These outcomes imply that the combination of L. acidophilus and G. glabra could potentially be more efficacious in hindering the VSV virus than L. acidophilus suspension alone, as presented in Table 3 and Figure 4.
In silico studies
Molecular docking investigations were conducted to investigate and predict the possible biological targets of glycyrrhizin, including HSV-1 and VSV. The binding energy scores of glycyrrhizin were compared to those of the co-crystallized ligands, and the results are presented in Table 4.
In Table 4, it is evident that glycyrrhizin exhibits a remarkable energy binding score (-22.45 kcal/mol) against the HSV-1 polymerase enzyme, which is notably higher than the energy score of the co-crystallized ligand (-13.31 kcal/mol). The interaction of glycyrrhizin with crucial amino acids in the enzyme is facilitated through conventional hydrogen bonds, which involves its oxygen atom with Asp717 and Lys928, its carboxylic OH group with Val812, Gln617, Ser816, and Gly819, as well as the guanine base of DG-10, as illustrated in Figures 5 and 6 [29]. These findings indicate a higher likelihood of glycyrrhizin serving as an inhibitor of HSV-1 polymerase. The results imply that glycyrrhizin is more likely to act as a HSV-1 polymerase inhibitor because of its high energy binding score and the precise amino acid interactions it establishes with the enzyme.
The binding affinity between glycyrrhizin and VSV nucleocapsid was found to be significant, with a score of -19.77 kcal/mol, surpassing that of the co-crystallized ligand, which had a score of -11.44 kcal/mol. Glycyrrhizin binds to uridine (U25) via its OH group and to uridine (U26) via two other hydroxyl groups, as shown in Figures 7 and 8. Furthermore, the carboxylic acid's carbonyl oxygen of glycyrrhizin interacts with uridine (U27), and the Lys410 amino acid interacts with the pyran oxygen [30]. Based on the significant interactions and high binding score, it can be inferred that glycyrrhizin has the ability to hinder the activity of VSV nucleocapsid.
Discussion
Phenolic compounds and triterpene saponins are a diverse collection of natural compounds found in plants that possess a range of biological activities, such as antiviral effects. Phenolic compounds have been found to exhibit antiviral properties against various viruses such as herpes simplex virus, influenza virus, and human immunodeficiency virus, among others, according to multiple studies. Phenolic compounds have the ability to hinder viral attachment and entry into host cells. Caffeic acid, which is a common phenolic acid, inhibits the entry of herpes simplex virus type 1 (HSV-1) into Vero cells by possibly interacting with the viral envelope glycoproteins [29]. The inhibition of hepatitis C virus (HCV) entry into Huh-7.5 cells can be attributed to quercetin, a flavonoid present in various fruits and vegetables. This is believed to occur through the obstruction of the interaction between the virus and its receptor on the surface of the cell [30]. Studies have demonstrated that phenolic compounds can hinder viral replication and assembly. Resveratrol, a type of polyphenolic compound present in red wine and grapes, has been found to impede the replication of three strains of influenza virus both in vitro and in vivo, by potentially disrupting the activity of the viral polymerase [31]. Furthermore, the assembly and release of HCV virions from infected cells were hindered by gallic acid, which is another prevalent phenolic acid [32,33,34]. In addition, it has been reported that phenolic compounds can alter the immune response of the host to viral infections. One example is curcumin, a phenolic compound present in turmeric, which has been demonstrated to boost the antiviral effects of interferon-alpha (IFN-α) against dengue virus in vitro by increasing the expression of IFN-stimulated genes [35]. In the same way, it was discovered that epigallocatechin gallate (EGCG), a main catechin present in green tea, boosted the effectiveness of IFN-α against hepatitis B virus (HBV) by increasing the expression of IFN-stimulated genes in human hepatoma cells [35]. Phenolic and triterpene compounds are a promising class of natural compounds that have antiviral activity against a wide range of viruses. Their mechanism of action involves inhibiting viral attachment and entry, replication and assembly, and modulation of the host immune response.
Glycyrrhizin, extracted from the roots of the licorice plant, is often misconceived as a phenolic compound, but in reality, it is a triterpene saponin. Research has been conducted on its antiviral and anti-inflammatory properties, specifically its ability to hinder the replication of several viruses including hepatitis C virus, HIV-1, and SARS-associated coronavirus (SARS-CoV) [36,37,38,39,40,41,42,43].
The aim of the study was to analyze the separate impact of L. acidophilus as a probiotic and G. glabra extract as a prebiotic, as well as their combined effect as a symbiotic, on the Vero cell line that was infected with HSV-1 and VSV viruses. Although L. acidophilus and G. glabra were given to the infected Vero cells, the exact mechanism through which L. acidophilus fights viral infections L. acidophilus fights viral infections by blocking the pathogen's interaction with DC-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN), a trans-membrane protein, through the surface layer protein (S-layer) present on the bacterial cell-envelope of L. acidophilus. By doing so, it inhibits viral and bacterial infections [44]. The S-layer protein of L. acidophilus is also shown to have antiviral activity by impacting virus infections and virus-induced apoptosis [45]. Additionally, healthy bacteria like L. acidophilus can boost the immune system and help reduce the risk of viral infections [46, 47], the byproducts of metabolic activity by L. acidophilus, such as bacteriocins, lactic acid, and H2O2, could potentially aid in preventing virus proliferation and improving the innate immune response [48, 49], this is supported by previous research indicating that the metabolites generated by L. acidophilus can promote the production of cytokines, which are crucial in the fight against viral infections such as COVID-19 [50,51,52,53,54]. Additionally, bacteriocins produced by L. acidophilus have been shown to hinder the replication of certain viruses, such as herpes simplex virus and human papillomavirus [55].
The introduction of L. acidophilus and G. glabra resulted in a noteworthy decrease in viral multiplication, as demonstrated by the MTT assay conducted on a microplate reader and real-time PCR. These tests indicated a reduction in the expression rate on the MX gene. Earlier studies have confirmed that G. glabra exhibits anti-HSV1 activity, with an IC50 value of 225 ± 24.1 µM [56]. The immunoregulatory and anti-inflammatory properties of G. glabra can impede the entry of HSV-1 virus into cell [29, 57].
The study demonstrated that the combination of L. acidophilus and G. glabra is a powerful antiviral agent, showing a greater reduction in viral titer than either treatment alone. Additionally, the presence of L. acidophilus and G. glabra significantly increased the viability of the Vero cell line both before and after infection with HSV-1. These results suggest that the combination of these probiotic and prebiotic agents can improve Vero cell viability against viral infections and decrease viral titers. The study also indicates that the combined treatment is more effective than either one used alone.
Healthy women's vaginal discharge was the source of the samples, as reported by Goudarzi and Fazeli (2015) [58].
The present strains of Lactobacilli were differentiated using biochemical and molecular analysis, and the antiviral efficacy of the culture supernatant (CS) of L. acidophilus species LA-5 was tested against the standard strain of HSV-1. Recent research has indicated that the L. acidophilus culture supernatant has a noteworthy capability to decrease the formation of HSV-1 and HIV plaques on cells [59].
Notably, the inhibitory effect is observed in both the standard strain and the wild L. acidophilus, without the need for an acidic environment or the production of H2O2 or H+ ions, suggesting that active metabolites may be responsible. With an estimated 50 million people in the US infected with genital herpes through direct contact and up to 60% of sexually active adults carrying the virus, the use of L. acidophilus for controlling HSV-1 is a significant possibility. It is important to note that the virus can spread through asymptomatic carriers, leading to high transmission rates [60].
Our study demonstrated a significant decrease in HSV-1 replication using a supernatant culture of L. acidophilus. This finding suggests that lactobacilli possess a potent antiviral capacity, potentially through the production of bacteriocins or other fragments that can neutralize the virus. This mode of antiviral activity differs from previously reported mechanisms that involve the secretion of H2O2 and H+ ions [55]. The culture supernatant (CS) utilized in our study had a neutral pH and did not exhibit any toxic effects on the target cells. This may be attributed to the release of active molecules in the CS of the L. acidophilus strain, which exerted a significant inhibitory effect on the replication of the virus [55, 61, 62].
In our study, the antiviral defense mechanism displayed by L. acidophilus did not involve the generation of H2O2 or H+ ions. Rather, it is plausible that the byproducts produced by the lactobacilli impeded the attachment of HSV virions to host cells or counteracted the viral particles. This mechanism is a recent proposal and contradicts the previous notion that lactobacilli deactivate viral particles by reducing pH levels or discharging disinfectant agents [63].
Several research studies have explored the antiviral mechanism of different treatments for viruses like HSV-1, VSV, and MERS-CoV, and their correlation with the Mx-A gene's expression profiles. In one such study, it was observed that the MxA protein obstructed the transcription of VSV, which aligns with the inhibitory properties of these treatments against VSV [64].
In addition, it has been observed that the MxA protein produced in Escherichia coli can hinder the RNA synthesis of VSV and influenza A virus in vitro [65]. Other investigations have also highlighted the capability of MxA protein to impede thogoto virus [64], bunya, phlebo, and hanta viruses [66], as well as La-Crosse virus [67], and puumala and tula hantaviruses [65].
The inhibitory effect of MxA protein on DUGV replication was observed to be due to its ability to decrease the expression levels of DUGV antigen, as per the findings of [68]. However, the antiviral activity of MxA protein is dependent on the type of virus and host cell. For instance, MxA protein demonstrated inhibitory effects on measles virus in human mononuclear and glioblastoma cell lines but not in Vero or Hep-2 cells [69]. In U87 or Vero cells, MxA protein did not inhibit respiratory syncytial virus (RSV) [55]. Nevertheless, transgenic mouse cells that expressed bovine Mx protein exhibited complete abolition of murine pneumovirus infectivity [70].
Conclusions
The study findings indicate that probiotics and prebiotics have antiviral effects against HSV-1 and VSV, as shown by MTT assay and real-time PCR. The combination of L. acidophilus and G. glabra was observed to enhance cell survival against viral infection, possibly by hindering intracellular viral reproduction or interfering with early infection stages. The study also revealed that glycyrrhizin, the primary component in G. glabra extract, exhibits high binding energy scores against HSV-1 polymerase enzyme and favorable interactions with VSV nucleocapsid. Combining L. acidophilus and G. glabra extract could lead to the development of a natural antiviral agent that is both safe and effective. The synergistic effect of L. acidophilus and glycyrrhizin makes them a suitable combination for viral therapy. Further research is needed to investigate the potential of the major bioactive ingredient and its antiviral efficacy in developing a viable natural antiviral medication against HSV-1 and VSV.
Methods
Preparation of G. glabra plant extract
Licorice roots harvested from Afghanistan were procured from an Egyptian market and ground to a fine powder in a Waring blender. Water extraction of the root samples was conducted for 20 hours to prevent microbial growth that could result from prolonged soaking in cold water. The extraction process was performed only once, and the resultant extract was filtered using linen filter cloth and concentrated via a rotary evaporator. The concentrated residue was stored in dark, sealed glass vials at a temperature of -20°C for future analysis.
Bacterial strain
The lyophilized strain of the probiotic L. acidophilus was procured from the American Type Culture Collection (43121) and grown in Man, Rogosa, and Sharpe (MRS) media (Acumedia, United States) at 37°C with 180 rpm shaking overnight. The L. acidophilus was isolated from a fresh MRS culture by centrifugation at 3000 rpm, and the pellet was washed twice with phosphate-buffered saline (PBS) to remove any residual MRS. The supernatant was filtered through a 0.2 m membrane. The bacterial suspension from an overnight culture was sonicated at 60% intensity using bacterial suspension aliquots (Fisher Scientific Co., NJ, USA) with the help of the Sonic 300 Dismembrator's intermediate sonication horn. The horn was inserted into a 15-ml polypropylene tube with 5–6 ml of cell solution, measuring approximately 9.5 mm in diameter, which was placed in an ice water bath to cool while sonicating the samples.
In vitro antiviral study
Test viruses
The DNA virus model HSV-1 and the RNA virus model VSV Indiana strain-156 were generously provided by the International Center for Training and Advanced Research in Egypt.
Cell line and growth conditions
The Vero cell line (ATCC no. CCL-81), derived from African green monkeys, was provided by VACSERA Egypt for this study. The cells were cultured in Dulbecco's Modified Eagle Medium (Sigma) supplemented with 5% fetal bovine serum (FBS, Sigma) and incubated in a humidified atmosphere with 5% CO2 at 37°C (Jouan-France) for 48 hours in 96-well plates to attain a confluent monolayer. The culture medium used for the Vero cells contained 10% heat inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM/ml glutamine, all procured from Sigma Aldrich in the United States.
Cytotoxicity assay
The evaluation of cytotoxicity was carried out by observing changes in the cellular morphology, as previously explained [71]. The samples were distributed into four Falcon tubes: the first tube was given 300 μl of the prebiotic (G. glabra extract), the second was given 300 μl of the probiotic L. acidophilus (cell sonicate), the third was given 300 μl of the probiotic L. acidophilus (cell supernatant), and the fourth tube was given a mixture of the prebiotic and probiotic (100 μl of G. glabra extract + 100 μl of L. acidophilus cell supernatant + 100 μl of cell precipitate). Vero cells were seeded onto a 96-well ELISA plate, followed by incubation at 37°C in a 5% CO2 atmosphere for 24 hours. Another sterile 96-well plate was labeled and divided into three columns for each sample. Samples were pipetted into the first row of the plate (200 μl each) and then subjected to twofold dilution. Subsequently, 100 μL of Vero cells were added to each well, and the plate was incubated at 37°C for 24 hours. After incubation, the medium was discarded, and the monolayer was washed with PBS (pH 7.4).
MTT assay
To determine the cytotoxic effects of prebiotics, probiotics, and combination treatments, various concentrations were tested using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The initial concentration of treatment was 100 mg/ml, followed by two-fold serial dilutions in serum-free DMEM (100 L of each dilution/well). Treated Vero cells were incubated at 37°C for 24 hours, washed thrice with 250 μl of PBS, and 50 μl of MTT solution (0.5 mg/ml) was added to each well. After incubating the plates at 37°C for four hours, the formed, purple-colored formazan crystals were dissolved in 50 μl of dimethyl sulfoxide (Sigma Aldrich, USA) after washing the plates with PBS once more. The plates were incubated on a shaker at room temperature for 5 min, and the optical density (OD) was measured at 570 nm using an ELISA plate reader. The percentage of cellular viability was calculated using the given formula:
Preparation of viral assay
To assess the viral infectivity, Vero cells were exposed to 10-fold serial dilutions of HSV-1 and VSV, each added to eight wells. The plates were incubated at 37°C for seven days and examined daily for cytopathic effects using a microscope from Hund, Germany. The median tissue culture infectious dose (TCID50) was determined using the Reed and Muench method [60].
To evaluate the direct antiviral effects, Vero cells were infected with serially diluted HSV-1 or VSV for one hour at 37°C. After removing unabsorbed viruses, the cells were washed thrice with PBS. The infected cells were then treated with safe concentrations of the prebiotic, probiotic, or their combination and incubated for 24 hours at 37°C. The cells were examined microscopically for cytopathic effects [55].
Cells were treated with safe levels of the formulas for 24 hours at 37°C to evaluate their indirect antiviral activity. After removing the treatment medium, Vero cells were infected with a series of viruses diluted 10-fold [62].
Using established methods, the reduction in virus infectivity titer was measured as a percentage and compared between treated and untreated plates using both techniques.
Virus titration calculation of TCID50
Reed and Muench's method were used to calculate the inhibition of virus titer. The log reduction of the initial viral titer was then determined by subtracting the titer after treatment from the initial titer [72, 73].
RNA extraction from cell lines
Real-time PCR was used to evaluate the levels of MX gene expression. Total RNA was extracted from Vero cells that were either untreated or treated with prebiotic, probiotic, or their combination, using the GeneJET RNA Purification kit (Fermantus, UK) according to the manufacturer's protocol. The RNA's quality and quantity were assessed by measuring its absorbance at 260 and 280 nm. The Quantitect Reverse Transcription kit (Qiagen, Germany) was used to generate cDNA from 1 μg of the extracted RNA. The expression levels of the MxA gene were measured using the following primers:
In order to amplify the Mx-A gene, the primers F (5'-AAA TGG CTC AAG AGG TGGA-3') and R (5'-TAT CGC TGA CAG TTG GGTG-3') were utilized, and melting curves were created to confirm the successful amplification of the desired product. Additionally, a standard curve was generated to evaluate the amplification efficiency and relative alterations in expression levels [74].
In silico studies
The use of molecular docking in computer-assisted drug design has been widespread in recent times, as it aids in predicting binding affinities and analyzing the interactions between receptors and ligands [75]. For this study, the Molecular Operating Environment (MOE, version 2019.0102) software was employed [76] to execute all docking procedures. The 2D structure of glycyrrhizin was drawn using ChemDraw and transformed into a 3D structure using MOE. All ligands, including those that were co-crystallized, were subjected to energy minimization with the MMFF94x force field until a root mean square deviation gradient of 0.1 kcal mol-1Å-1 was achieved [77].
The X-ray crystallographic structures of the most important targets of HSV-1 were retrieved from Protein Data Bank with the following IDs: 6M5U (terminase), 2C53 (glycolase), 2KI5 (thymidine kinase), and 7LUF (polymerase). Nucleocapsid (6BJY) and L-protein (4UCZ) were chosen as possible targets for VSV.
To identify the binding site, the cocrystallized ligand present in each protein file was utilized. Docking was performed by employing Triangle Matcher as a placement technique and London dG as a scoring algorithm. The output of the docking was visualized using MOE software to create 2D and 3D images.
Availability of data and materials
The data generated during this study are available from the corresponding author upon reasonable request.
Abbreviations
- VSV:
-
Vesicular stomatitis virus
- HSV-1:
-
Herpes simplex virus-1
- MNTC:
-
Minimum non-toxic concentration
- CID50:
-
Tissue culture infectious dose 50
- CT:
-
Cycle threshold
References
James SH, Prichard MN. Current and future therapies for herpes simplex virus infections: Mechanism of action and drug resistance. Curr Opinion Virol. 2014;8:54–61.
Cole S. Herpes simplex virus: epidemiology, diagnosis, and treatment. Nurs Clin North Am. 2020;55:337–45.
James C, Harfouche M, Welton NJ, Turner KME, Abu-Raddad LJ, Gottlieb SL, et al. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020;98:315–29.
Valerio GS, Lin CC. Ocular manifestations of herpes simplex virus. Curr Opin Ophthalmol. 2019;30:525–31.
Das D, Hong J. Herpesvirus polymerase inhibitors. In: Viral Polymerases: Structures, Functions and Roles as Antiviral Drug Targets. Elsevier; 2018. p. 333–56.
Kharitonova MI, Konstantinova ID, Miroshnikov AI. Benzimidazole nucleosides: antiviral and antitumour activities and methods of synthesis. Russ Chem Rev. 2018;87:1111–38.
Gussone F, Cooperman Y. Acyclovir ointment: what you need to know. Herpes. 2021.
Zinser E, Krawczyk A, Mühl-Zürbes P, Aufderhorst U, Draßner C, Stich L, et al. A new promising candidate to overcome drug resistant herpes simplex virus infections. Antiviral Res. 2018;149:202–10.
Tsai Y-L, Lin T-L, Chang C-J, Wu T-R, Lai W-F, Lu C-C, et al. Probiotics, prebiotics and amelioration of diseases. J Biomed Sci. 2019;26:1–8.
Dicks LMT, Grobbelaar MJ. Double-barrel shotgun: Probiotic lactic acid bacteria with antiviral properties modified to serve as vaccines. Microorganisms. 2021;9:1565.
Aly SH, Elissawy AM, Eldahshan OA, Elshanawany MA, Efferth T, Singab ANB. The pharmacology of the genus Sophora (Fabaceae): an updated review. Phytomedicine. 2019;64:153070.
El-Nashar HAS, Eldehna WM, Al-Rashood ST, Alharbi A, Eskandrani RO, Aly SH. Gc/ms analysis of essential oil and enzyme inhibitory activities of syzygium cumini (Pamposia) grown in egypt: chemical characterization and molecular docking studies. Molecules. 2021;26:6984.
Ads EN, Hassan SI, Rajendrasozhan S, Hetta MH, Aly SH, Ali MA. Isolation, structure elucidation and antimicrobial evaluation of natural pentacyclic triterpenoids and phytochemical investigation of different fractions of ziziphus spina-christi (L.) stem bark using LCHRMS analysis. Molecules. 2022;27:1805.
Aly SH, Elissawy AM, Fayez AM, Eldahshan OA, Elshanawany MA, Singab ANB. Neuroprotective effects of sophora secundiflora, sophora tomentosa leaves and formononetin on scopolamine-induced dementia. Nat Prod Res. 2020;35:1–5.
Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MBPP. Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Phytother Res. 2018;32:2323–39.
Batiha GES, Beshbishy AM, El-Mleeh A, Abdel-Daim MM, Devkota HP. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (fabaceae). Biomolecules. 2020;10:352.
Farag MA, Porzel A, Wessjohann LA. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC-MS, LC-MS and 1D NMR techniques. Phytochemistry. 2012;76:60–72.
Heidari S, Mehri S, Hosseinzadeh H. The genus Glycyrrhiza (Fabaceae family) and its active constituents as protective agents against natural or chemical toxicities. Phytother Res. 2021;35:6552–71.
Maatooq GT, Marzouk AM, Gray AI, Rosazza JP. Bioactive microbial metabolites from glycyrrhetinic acid. Phytochemistry. 2010;71:262–70.
Hazrati M, Mehrabani D, Japoni A, Montasery H, Azarpira N, Hamidian-Shirazi AR, et al. Effect of honey on healing of Pseudomonas aeruginosa infected burn wounds in rat. J Appl Anim Res. 2010;37:161–5.
Tanideh N, Rokhsari P, Mehrabani D, Samani SM, Sarvestani FS, Ashraf MJ, et al. The healing effect of licorice on Pseudomonas aeruginosa infected burn wounds in experimental rat model. World J Plast Surg. 2014;3:99.
Ikeda M, Yi M, Li K, Lemon SM. Selectable subgenomic and genome-length dicistronic RNAs derived from an infectious molecular clone of the HCV-N strain of hepatitis c virus replicate efficiently in cultured huh7 cells. J Virol. 2002;76:2997–3006.
Matsumoto Y, Matsuura T, Aoyagi H, Matsuda M, Hmwe SS, Date T, Watanabe N, Watashi K, Suzuki R, Ichinose S, Wake K, Suzuki T, Miyamura T, Wakita T, Aizaki H. Antiviral activity of glycyrrhizin against hepatitis C virus in vitro. PLoS ONE. 2013;8:e68992.
Calland N, Dubuisson J, Rouillé Y, Séron K. Hepatitis C virus and natural compounds: a new antiviral approach? Viruses. 2012;4:2197–217.
Michaelis M, Geiler J, Naczk P, Sithisarn P, Leutz A, Doerr HW, et al. Glycyrrhizin exerts antioxidative effects in H5N1 influenza A virus-infected cells and inhibits virus replication and pro-inflammatory gene expression. PLoS One. 2011;6:e19705.
Khuntia BK, Sharma V, Wadhawan M, Chhabra V, Kidambi B, Rathore S, et al. Antiviral Potential of Indian Medicinal Plants Against Influenza and SARS-CoV: A Systematic Review. Nat Prod Commun. 2022;17(3):1–10. https://doi.org/10.1177/1934578X221086988
Sato H, Goto W, Yamamura JI, Kurokawa M, Kageyama S, Takahara T, et al. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antiviral Res. 1996;30:171–7.
Fiore C, Eisenhut M, Ragazzi E, Zanchin G, Armanini D. A history of the therapeutic use of liquorice in Europe. J Ethnopharmacol. 2005;99:317–24.
Lin L-T, Chen T-Y, Chung C-Y, Noyce RS, Grindley TB, McCormick C, et al. Hydrolyzable tannins (Chebulagic Acid and Punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J Virol. 2011;85:4386–98.
Rojas N, Del Campo JA, Clement S, Lemasson M, García-Valdecasas M, Gil-Gómez A, et al. Effect of quercetin on hepatitis C virus life cycle: from viral to host targets. Sci Rep. 2016;6:31777.
Palamara AT, Nencioni L, Aquilano K, De Chiara G, Hernandez L, Cozzolino F, et al. Inhibition of influenza A virus replication by resveratrol. J Infect Dis. 2005;191:1719–29.
Calland N, Albecka A, Belouzard S, Wychowski C, Duverlie G, Descamps V, et al. (-)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology. 2012;55:720–9.
Calland N, Sahuc M-E, Belouzard S, Pène V, Bonnafous P, Mesalam AA, et al. Polyphenols inhibit hepatitis C Virus entry by a new mechanism of action. J Virol. 2015;89:10053–63.
Wang Y, Li J, Wang X, Penã JC, Li K, Zhang T, et al. (-)-Epigallocatechin-3-gallate enhances hepatitis C virus double-stranded RNA intermediates-triggered innate immune responses in hepatocytes. Sci Rep. 2016;6:21595.
Hong J, Smith TJ, Ho CT, August DA, Yang CS. Effects of purified green and black tea polyphenols on cyclooxygenase- and lipoxygenase-dependent metabolism of arachidonic acid in human colon mucosa and colon tumor tissues. Biochem Pharmacol. 2001;62:1175–83.
Sulutaniyah S, Darmawan E. Obat Herbal dari Akar Manis (Glycyrrhiza glabra L.) untuk Pencegahan dan Pengobatan Infeksi Virus H1N1, H5N1 dan COVID-19: Systematic Review. Jurnal Surya Medika. 2022;8.
Ahmad Bhat S, Islam Siddiqui Z, Ahmad Parray Z, Sultan A, Afroz M, Ali Azam S, et al. Naturally occurring inhibitor delineating the anti-hepatitis B virus mechanism of glycyrrhizin via in vitro and in silico studies. J Mol Liq. 2022;356:119029.
Khan T, Khan MA, Mashwani Z ur R, Ullah N, Nadhman A. Therapeutic potential of medicinal plants against COVID-19: The role of antiviral medicinal metabolites. Biocatalysis and Agricultural Biotechnology. 2021;31.
Al-Kamel H, Grundmann O. Glycyrrhizin as a potential treatment for the novel coronavirus (COVID-19). Mini-Reviews Med Chem. 2021;21:2204–8.
Zhang QH, Huang HZ, Qiu M, Wu ZF, Xin ZC, Cai XF, et al. Traditional uses, pharmacological effects, and molecular mechanisms of licorice in potential therapy of COVID-19. Front Pharmacol. 2021;12:3249.
Luo P, Liu D, Li J. Pharmacological perspective: glycyrrhizin may be an efficacious therapeutic agent for COVID-19. Int J Antimicrob Agents. 2020;55:105995.
Soleymani S, Naghizadeh A, Karimi M, Zarei A, Mardi R, Kordafshari G, et al. COVID-19: General strategies for herbal therapies. J Evid Based Integ Med. 2022;27:2515690X211053641.
Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–6.
Acosta MP, Geoghegan EM, Lepenies B, Ruzal S, Kielian M, Martinez MG. Surface (S) layer proteins of lactobacillus acidophilus block virus infection via DC-SIGN interaction. Front Microbiol. 2019;10:810.
Zhang X, Li P, Zheng Q, Hou J. Lactobacillus acidophilus S-layer protein-mediated inhibition of PEDV-induced apoptosis of Vero cells. Vet Microbiol. 2019;229:159–67.
Wang Y, Li X, Ge T, Xiao Y, Liao Y, Cui Y, et al. Probiotics for prevention and treatment of respiratory tract infections in children: A systematic review and meta-analysis of randomized controlled trials. Medicine (United States). 2016;95.
Shida K, Nanno M, Nagata S. Flexible cytokine production by macrophages and t cells in response to probiotic bacteria: A possible mechanism by which probiotics exert multifunctional immune regulatory activities. Gut Microbes. 2011;2:109–14.
Nader-Macías MEF, De Gregorio PR, Silva JA. Probiotic lactobacilli in formulas and hygiene products for the health of the urogenital tract. Pharmacol Res Perspect. 2021;9:e00787.
McGroarty JA. Probiotic use of lactobacilli in the human female urogenital tract. FEMS Immunol Med Microbiol. 1993;6:251–64.
Yuliana. The effects of probiotics in strengthening immunity against the COVID-19 infection. Indonesian Journal of Pharmacology and Therapy. 2022;3.
Azizi AFN, Uemura R, Omori M, Sueyoshi M, Yasuda M. Effects of Probiotics on Growth and Immunity of Piglets. Animals. 2022;12(14):1786.
Fikri B, Ridha NR, Putri SH, Salekede SB, Juliaty A, Tanjung C, Massi N. Effects of probiotics on immunity and iron homeostasis: A mini-review. Clinical Nutrition ESPEN. 2022;49:24-27.
Blázquez-Bondia C, Parera M, Català-Moll F, Casadellà M, Elizalde-Torrent A, Aguilo M, Espadaler-Mazo J, Ramon Santos J, Paredes R, Noguera-Julian M. Probiotic effects on immunity and microbiome in HIV-1 discordant patients. Front Immunol. 2022;13:1-16.
Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S. Probiotics: Effects on immunity. In: American Journal of Clinical Nutrition. 2001;73:444-50.
Conti C, Malacrino C, Mastromarino P. Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J Physiol Pharmacol. 2009;60(SUPPL.6):19–26.
Huang W, Chen X, Li Q, Li P, Zhao G, Xu M, et al. Inhibition of intercellular adhesion in herpex simplex virus infection by glycyrrhizin. Cell Biochem Biophys. 2012;62:137–40.
Choi HJ, Song JH, Park KS, Baek SH, Lee ES, Kwon DH. Antiviral activity of yogurt against enterovirus 71 in vero cells. Food Sci Biotechnol. 2010;19:289–95.
Goudarzi MM, Fazeli MR. Isolation of Lactobacillus acidophilus and assessment for its antiviral effect against herpes simplex virus type II. Mol Genet Microbiol Virol. 2015;30:237–41.
Zabihollahi R, Motevaseli E, Sadat SM, Azizi-Saraji AR, Asaadi-Dalaie S, Modarressi MH. Inhibition of HIV and HSV infection by vaginal lactobacilli in vitro and in vivo. DARU J Pharmaceut Sci. 2012;20:1–7.
Nagot N, Ouedraogo A, Defer MC, Vallo R, Mayaud P, Van De Perre P. Association between bacterial vaginosis and Herpes simplex virus type-2 infection: implications for HIV acquisition studies. Sex Transm Infect. 2007;83:365–8.
Kaur S, Sharma P, Kalia N, Singh J, Kaur S. Anti-biofilm properties of the fecal probiotic lactobacilli against Vibrio spp. Front Cell Infect Microbiol. 2018;8:120.
Song J, Abraham SN. Innate and adaptive immune responses in the urinary tract. European Journal of Clinical Investigation. 2008;38 SUPPL.2.
Ranjbar R, Moazzami Goudarzi M, Jounaidi N. Lactobacillus acidophilus and assessment for its antiviral effect against herpes simplex virus type I. Biosci Biotechnol Res Asia. 2015;12:1351–6.
Hoe NL, Tuke PW, Tedder RS, Aysha BK, Eglin RP, Atkinson CE, et al. The prevalence of chromosomally integrated human herpesvirus 6 genomes in the blood of UK blood donors. J Med Virol. 2007;79:45–51.
Frese M, Kochs G, Feldmann H, Hertkorn C, Haller O. Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein. J Virol. 1996;70:915–23.
Frese M, Kochs G, Meier-Dieter U, Siebler J, Haller O. Human MxA protein inhibits tick-borne Thogoto virus but not Dhori virus. J Virol. 1995;69:3904–9.
Kanerva M, Melén K, Vaheri A, Julkunen I. Inhibition of Puumala and Tula hantaviruses in vero cells by MxA protein. Virology. 1996;224:55–62.
Reichelt M, Stertz S, Krijnse-Locker J, Haller O, Kochs G. Missorting of LaCrosse virus nucleocapsid protein by the interferon-induced MxA GTPase involves smooth ER membranes. Traffic. 2004;5:772–84.
Bridgen A, Dalrymple DA, Weber F, Elliott RM. Inhibition of dugbe nairovirus replication by human MxA protein. Virus Res. 2004;99:47–50.
Atreya PL, Kulkarni S. Respiratory syncytial virus strain A2 is resistant to the antiviral effects of type I interferons and human MxA. Virology. 1999;261:227–41.
Özçelik B, Orhan I, Toker G. Antiviral and antimicrobial assessment of some selected flavonoids zeitschrift fur naturforschung - section C. J Biosci. 2006;61:632–8.
Lei C, Yang J, Hu J, Sun X. On the calculation of TCID50 for quantitation of virus infectivity. Virol Sin. 2021;36:141–4.
LaBarre DD, Lowy RJ. Improvements in methods for calculating virus titer estimates from TCID50 and plaque assays. J Virol Methods. 2001;96:107–26.
Burgos KL, Javaherian A, Bomprezzi R, Ghaffari L, Rhodes S, Courtright A, et al. Identification of extracellular miRNA in human cerebrospinal fluid by next-generation sequencing. RNA. 2013;19:712–22.
Fan J, Fu A, Zhang L. Progress in molecular docking. Quanti Biol. 2019;7:83–9.
Vilar S, Cozza G, Moro S. Medicinal chemistry and the Molecular Operating Environment (MOE): application of qsar and molecular docking to drug discovery. Curr Top Med Chem. 2008;8:1555–72.
Wintachai P, Kaur P, Lee RCH, Ramphan S, Kuadkitkan A, Wikan N, et al. Activity of andrographolide against chikungunya virus infection. Sci Rep. 2015;5:14179.
Acknowledgements
Not available.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research was conducted without financial support.
Author information
Authors and Affiliations
Contributions
Dalia Elebeedy; Aml Ghanem; Shaza H. Aly; Mohamed A. Ali; Ahmed H. I. Faraag, Mohamed K. Alashry; Aya M. salem; Mahmoud A. El Hassab; Ahmed I. Abd El Maksoud have prepared a study, written a manuscript, designed and conducted experiments, analyzed, reviewed and interpreted data, and supervised research. Mohamed A. Ali; Ahmed H. I. Faraag also read, revised, and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This project has received ethical approval from BUC University's Ethics Committee, and ethical considerations and responsible practices will be observed during experimental research and field studies on cultivated plants. Our commitment to ethical principles includes respect for the intrinsic value of plants and their ecosystems, adherence to relevant laws and ethical guidelines, and maximizing benefits while minimizing harm to plants. By upholding these ethical principles, we aim to advance our understanding of plant biology and ecology while respecting the value of plant life and their supporting ecosystems.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elebeedy, D., Ghanem, A., Aly, S.H. et al. Synergistic antiviral activity of Lactobacillus acidophilus and Glycyrrhiza glabra against Herpes Simplex-1 Virus (HSV-1) and Vesicular Stomatitis Virus (VSV): experimental and In Silico insights. BMC Microbiol 23, 173 (2023). https://doi.org/10.1186/s12866-023-02911-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-023-02911-z