Abstract
Background
Escherichia coli, Enterobacter spp., Klebsiella pneumoniae and Enterococcus spp., common gut bacteria in giant pandas, include opportunistic pathogens. The giant panda is an endangered species, classified as vulnerable by the World Wildlife Foundation. Continuous monitoring for the emergence of antimicrobial resistance (AMR) among bacterial isolates from giant pandas is vital not only for their protection but also for public health.
Results
A total of 166 E. coli, 68 Enterobacter spp., 116 K. pneumoniae and 117 Enterococcus spp. isolates were collected from fecal samples of 166 giant pandas. In the antimicrobial susceptibility tests, 144 E. coli isolates, 66 Enterobacter spp. isolates, 110 K. pneumoniae isolates and 43 Enterococcus spp. isolates were resistant to at least one antimicrobial. The resistant isolates carried antimicrobial resistance genes (ARGs), including sul3, blaTEM, blaSHV and tetA. The differences in the prevalence of the bla types implied that the genetic basis for β-lactam resistance among the E. coli, Enterobacter spp. and K. pneumoniae isolates was different. The strain K. pneumoniae K85 that was resistant to sixteen antimicrobials was selected for whole genome sequencing. The genome contained Col440I, IncFIBK and IncFIIK plasmids and altogether 258 ARGs were predicted in the genome; 179 of the predicted ARGs were efflux pump genes. The genetic environment of the β-lactamase genes blaCTX-M-3 and blaTEM-1 in the K. pneumoniae K85 genome was relatively similar to those in other sequenced K. pneumoniae genomes. In comparing the giant panda age groups, the differences in the resistance rates among E. coli, K. pneumoniae and Enterobacter spp. isolates suggested that the infections in giant pandas of different age should be treated differently.
Conclusions
Antimicrobial resistance was prevalent in the bacterial isolates from the giant pandas, implying that the gut bacteria may pose serious health risks for captive giant pandas. The resistance genes in the genome of K. pneumoniae K85 were associated with insertion sequences and integron-integrase genes, implying a potential for the further spread of the antimicrobial resistance.
Similar content being viewed by others
Background
The giant panda, Ailuropoda melanoleuca, is a mammal species endemic to China, where the sparse giant panda population is limited to Sichuan, Shanxi and Gansu provinces [1]. The captive panda population was approximately 600 by the end of 2019. Although the number of both wild and captive pandas has increased, the giant pandas are still endangered due to several threats. Intestinal tract diseases caused by pathogenic bacteria has become a considerable threat to the health of giant pandas [2]. Escherichia coli, Enterobacter spp., Klebsiella and Enterococcus spp. are common gut bacteria in humans and other animals, including giant pandas [3,4,5]. These species play important commensal roles in gut; however, they are also opportunistic pathogens, and can cause various diseases [6,7,8]. For example, some E. coli strains cause hemorrhagic colitis, and these enterohaemorrhagic E. coli have been isolated from giant pandas [9]. Enterobacter spp., K. pneumoniae and Enterococcus faecium have been associated with hospital-acquired infections and outbreaks [10,11,12,13,14,15]. Clinical infections caused by Enterococcus spp. have been increasing in recent years [16], and Klebsiella and Enterobacter spp. can cause a wide range of infections [12, 17,18,19].
Antimicrobials have been widely used to prevent and cure infectious diseases in captive giant pandas in recent decades [20,21,22]. However, with the widespread use of antimicrobials, the number of drug-resistant strains has increased and the development and spread of multidrug-resistant (MDR) bacteria in humans and the environment has accelerated [23]. In China, more antimicrobial agents are consumed than in most other countries. According to a 2007 survey, almost half of the 210,000 tons of antimicrobials produced in China were used in livestock as therapeutic drugs and feed additives [24]. In addition, antimicrobials like ceftriaxone sodium are used not only in humans but also in giant pandas [25]. Thus, antimicrobial resistant strains may develop in giant pandas and spread to humans and other animals.
Antimicrobial resistance has caused serious problems in clinical practice [26]. Infections by Klebsiella spp., especially K. pneumonia, are frequently caused by MDR strains that produce extended-spectrum β-lactamases (ESBLs; mainly including blaTEM, blaCTX-M, blaSHV and blaGES types) [19, 26]. K. pneumonia may be also naturally resistant to certain antimicrobials, including ampicillin, amoxicillin, carbenicillin and ticarcillin [27, 28]. Likewise, Enterobacter spp., especially Enterobacter cloacae, may be naturally resistant to, for example, ampicillin, kanamycin and tetracycline [7]. Generally, Enterococcus spp. are intrinsically resistant to many antimicrobials and can easily acquire resistance to other agents [29]. Acquired high-level aminoglycoside or penicillin resistance, as well as erythromycin or tetracycline resistance, have increased among Enterococcus spp. [16, 30, 31].
Several investigations have been carried out to monitor the distribution of antimicrobials and disinfectant resistance genes in E. coli and K. pneumoniae isolates from the giant pandas [2, 20, 22, 32]. In giant pandas, E. coli infections were frequently caused by MDR strains [2, 20, 32]. To our knowledge, detailed gene and genome level information on the antimicrobial resistant bacteria, especially on Enterobacter and Enterococcus spp., from giant pandas is still lacking. Therefore, comprehensive investigation at molecular level to monitor the distribution of antimicrobial resistant, opportunistic pathogens from giant pandas was needed. We isolated E. coli, Enterobacter spp., K. pneumoniae and Enterococcus spp. from giant panda feces and assessed their antimicrobial resistance and related genetic properties, with the aims to 1) characterize the antimicrobial resistance phenotypes and genotypes, 2) compare the antimicrobial resistance between the four taxa, and to 3) further understand the resistance based on whole-genome sequencing of a MDR K. pneumoniae isolate.
Results
Antimicrobial susceptibility of all isolates
A total of 166 E. coli, 68 Enterobacter spp., 116 K. pneumoniae and 117 Enterococcus spp. isolates were purified from fecal samples of 166 giant pandas. Only one isolate per genus per giant panda was kept for further analyses. In the antimicrobial susceptibility tests, 87% (n = 144) E. coli isolates, 97% (n = 68) Enterobacter spp. isolates, 95% (n = 110) K. pneumoniae isolates and 37% (n = 37) Enterococcus spp. isolates were resistant to at least one antimicrobial (Fig. 1, Supplementary Table S1).
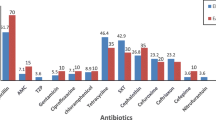
Antimicrobial resistance of E. coli, Enterobacter spp., K. pneumoniae and Enterococcus spp. isolates against 18 antimicrobials. The indicator on the right denotes the relationship between the antimicrobial resistance and color range. KAN, kanamycin; GEN, gentamicin; AZM, azithromycin; ERY, erythromycin; NOR, norfloxacin; OFX, ofloxacin; CIP, ciprofloxacin; LOM, lomefloxacin; LEV, levofloxacin; SD, sulfadiazine; TMP, trimethoprim; CRO, ceftriaxone; CFX, cefixime; AMP, ampicillin; AML, amoxicillin; ATM, aztreonam; IPM, imipenem; TET, tetracycline
Many of the isolates were resistant to at least three different antimicrobial classes and were considered MDR strains. Out of the E. coli isolates, 72% were resistant to sulfadiazine (SD), 38% to tetracycline (TET), and approximately 23% to amoxicillin (AML) and ampicillin (AMP) (Fig. 2); 18% (n = 29) were resistant to three or more tested antimicrobials (Supplementary Table S1). Out of the Enterobacter spp. isolates, 88% were resistant to AML, 84% to AMP, and 7% (n = 5) were MDR strains. Out of the K. pneumoniae isolates, 85% were resistant to AML, 73% to AMP, 47% to SD, and 15% (n = 17) were MDR strains. Out of the Enterococcus spp. isolates, 35% were resistant to TET, 29% to erythromycin (ERY), and 3% (n = 15) were MDR strains.
Antimicrobial resistance of E. coli, Enterobacter spp., K. pneumoniae and Enterococcus spp. isolates against 18 antimicrobial agents. KAN, kanamycin; GEN, gentamicin; AZM, azithromycin; ERY, erythromycin; NOR, norfloxacin; OFX, ofloxacin; CIP, ciprofloxacin; LOM, lomefloxacin; LEV, levofloxacin; SD, sulfadiazine; TMP, trimethoprim; CRO, ceftriaxone; CFX, cefixime; AMP, ampicillin; AML, amoxicillin; ATM, aztreonam; IPM, imipenem; TET, tetracycline. Different letters above columns indicate statistically significant differences at P < 0.05
The prevalence of gentamicin (GEN) resistance was highest among the Enterobacter spp. isolates, and that of SD resistance was highest among the E. coli isolates and second highest among the K. pneumoniae isolates. The prevalence of AMP and AML resistances were highest and that of TET lowest among the Enterobacter spp. and K. pneumoniae isolates.
Antimicrobial resistant strains by giant panda sex and age
Antimicrobial resistant isolates were detected in 161 of the 166 giant pandas (Fig. 1). The difference in the proportion of antimicrobial resistant isolates from female and male giant pandas was limited to Enterococcus isolates: 26.9 and 46.0% of the isolates from females and males, respectively, were resistant to tetracycline (P < 0.05) (Fig. S1).
Among the E. coli isolates, the prevalence of resistance to six antimicrobials was highest in isolates from pandas in their infancy (P < 0.05) (Fig. 3a). All the E. coli isolates from infant and old pandas were resistant to SD, and the prevalence of SD resistance was lowest among isolates from adolescent pandas (P < 0.05). The prevalence of AMP resistance was higher among Enterobacter spp. isolates from adult pandas than among those from infant and adolescent pandas (P < 0.05) (Fig. 3b). For the K. pneumoniae isolates, the prevalence of resistance to four antimicrobials was highest in isolates from old pandas (P < 0.05), and the prevalence of SD resistance was highest in isolates from adult pandas (P < 0.05) (Fig. 3c). For Enterococcus spp. isolates, there was almost no significant difference in resistance to tested antimicrobials among giant pandas of different ages (Fig. 3d).
The proportion of antimicrobial resistant isolates from (a) infant, (b) adolescent, (c) adult and (d) old giant pandas. KAN, kanamycin; GEN, gentamicin; AZM, azithromycin; ERY, erythromycin; NOR, norfloxacin; OFX, ofloxacin; CIP, ciprofloxacin; LOM, lomefloxacin; LEV, levofloxacin; SD, sulfadiazine; TMP, trimethoprim; CRO, ceftriaxone; CFX, cefixime; AMP, ampicillin; AML, amoxicillin; ATM, aztreonam; IPM, imipenem; TET, tetracycline
Prevalence of ARGs
The genotypes of antimicrobial resistant E. coli, Enterobacter spp., K. pneumoniae and Enterococcus spp. isolates were characterized by analyzing the ARGs in the isolates with different antimicrobial resistance phenotypes.
Among the E. coli isolates, tetA was detected in 67% (42/63) of the tetracycline-resistant isolates, blaTEM and blaCTX were detected in 36% (16/45) and 18% (8/45) of the β-lactam-resistant isolates, respectively, sul2 and sul3 were detected in 7 and 9% of the sulfonamide-resistant isolates, respectively, qnrB was detected in one of the eight fluoroquinolone-resistant isolates, and both acc (3)-IIa and ant (3″)-Ia were detected in one of the five aminoglycoside-resistant isolates (Table 1).
The gene tetA was detected in 77% (10/13) of the tetracycline-resistant Enterobacter spp. isolates, blaTEM, blaSHV and blaCTX were detected in 11% or less of the β-lactam-resistant isolates, sul1 was detected in two of the eight sulfonamide-resistant isolates, and ant (3″)-Ia was detected in one of the nine aminoglycoside-resistant isolates (Table 1).
ARGs for β-lactam-resistance were detected in all the resistant K. pneumoniae isolates, with blaSHV in 83% (82/99) of them, tetA was detected in 76% (10/13) of the tetracycline-resistant isolates, and sul1, sul2 and sul3 were detected in 13% or less of the sulfonamide-resistant isolates. The only K. pneumoniae aminoglycoside-resistant isolate carried acc (6′)-Ib gene.
The gene ermE was detected in 24% of the macrolide resistant Enterococcus spp. isolates, and 35% of the tetracycline resistant isolates carried tetM or tetL genes.
Antibiotic resistance features in the K. pneumoniae K85 genome
The strain K. pneumoniae K85 that was resistant to sixteen antimicrobials was selected for whole genome sequencing. The 1454 reads (Clean Data) were assembled into 91 contigs with a combined length of 5,514,535 bp. The longest contig was 368,946 bp. A total of 5349 ORFs were detected in the K. pneumoniae K85 genome with an average gene length of 897 bp. K. pneumoniae K85 contained Col440I, IncFIBK and IncFIIK plasmids. Altogether 258 ARGs were predicted in the K. pneumoniae K85 genome (Table 2). Altogether 179 of the predicted ARGs were efflux pump genes, and the rest were related to enzymatic inactivation of antimicrobials, alteration, protection and replacement of the antimicrobial target, and reduced permeability to antimicrobials. The predicted aminoglycoside-modifying enzyme genes included aadA16, aph (3′)-Ia, and acc (6′)-Ib-cr that can simultaneously confer fluoroquinolone resistance. The predicted blaCTX-M-3, blaSHV-93 and blaTEM-1 confer resistance to β-lactams. In addition, genes encoding general mechanisms that mediate antibiotic resistance to fluoroquinolone (qnrB2 and qnrS1), sulfonamide (sul1 and sul3) and tetracycline (tet34 and tetT) were also predicted.
The genetic environment of the β-lactamase genes blaCTX-M-3 and blaTEM-1 in the K. pneumoniae K85 genome was relatively similar to those in other sequenced K. pneumoniae genomes (Fig. 4). The gene blaTEM-1 was adjacent to blaCTX-M-3, and this resistance region also included another two ARGs (floR and tetA) conferring resistance to tetracycline and florfenicol. More importantly, these antimicrobial resistance regions were flanked by various IS elements. The gene blaTEM-1 was adjacent to blaCTX-M-3, and this region also included floR and tetA that confer resistance to florfenicol and tetracycline, respectively. The isolate K. pneumoniae K85 harbored a class 1 integron gene cassette with resistance genes aac (6′)-Ib-cr, arr-3, dfrA5 and aadA16 (Fig. 5).
Discussion
We studied the distribution of antimicrobial resistant, opportunistic pathogens in giant panda guts by isolating E. coli, Enterobacter spp., K. pneumoniae and Enterococcus spp. from giant panda feces. The results showed that antimicrobial resistance was common among the isolates, ranging from 95% or more among the Enterobacter spp. and K. pneumoniae isolates to 37% among the Enterococcus spp. isolates.
Our results showed that five E. coli isolates were resistant to ten or more antimicrobials, implying that MDR E. coli may pose serious health risks for captive giant pandas. Compared to the 88 E. coli strains from giant pandas in Bifengxia, China [20], in our study the antimicrobial resistance range of the isolates was wider and the prevalence of resistance to amoxicillin was higher. However, the prevalence of resistances to six antimicrobials were lower than an earlier study on giant pandas from Wolong and Dujiangyan, the China Conservation and Research Center for Giant Panda [22], possibly partly due to the controlled use of antimicrobials [32]. In addition, the variation in antimicrobial resistance profiles at different times and sites may result from giant pandas obtaining antimicrobial-resistant bacteria via contacts with feeders, feeding environment or tourists that violate the feeding regulations of the zoos [32,33,34], thus increasing the risks of cross-infection and exposure to pathogens and ARGs through the digestive tract.
Enterobacter spp. that are opportunistic pathogens in humans, fish and other animals [35,36,37,38] have been found in the intestines of giant pandas [8, 39]. However, to our knowledge their resistance to antimicrobials has not been investigated. Compared to our E. coli isolates, the rates of resistance to ampicillin and amoxicillin were higher among the Enterobacter spp. isolates. Enterobacter spp. carry resistance genes that promote the MDR phenotype [40,41,42,43], it could be due to their ability to acquire numerous genetic mobile elements containing resistance genes [44], making them a potential problem for giant pandas. Unlike the Enterobacter spp. strains from humans and companion animals [45, 46], the giant panda Enterobacter spp. isolates were not resistant to ciprofloxacin, indicating that quinolone antimicrobials may remain effective in treating Enterobacter infections [47].
Over 70% of the K. pneumoniae isolates were resistant to ampicillin and amoxicillin. The prevalence of ESBL-producing K. pneumoniae in many areas of the world has reached 50%, indicating that its antimicrobial resistance is ubiquitous [48]. In Asia, the prevalence of resistance to most of the commonly used antimicrobials is high among K. pneumoniae [49]. In China, the probabilities of MDR K. pneumoniae infections are high, so management of antimicrobial resistance in MDR K. pneumoniae has been a major challenge for clinical veterinarians. K. pneumoniae may play a key role in disseminating ARGs from environmental microbes to clinically important pathogens because of its wider ecological distribution, greater ARG diversity or a higher mobile genetic element burden than other Gram-negative opportunists [50, 51].
Studies on the antimicrobial resistance of Enterococcus spp. derived from giant pandas are few. In our study, the Enterococcus isolates were mainly resistant to tetracycline, erythromycin and ampicillin. Compared with the giant pandas, the rate of tetracycline resistance among wild rabbit-derived Enterococcus spp. was higher [52], possibly due to the contamination of water or vegetation in the woodlands by fecal material from wild birds or even humans [53]. The intrinsic resistance of Enterococcus spp. to semisynthetic penicillin, aminoglycosides, vancomycin, polymyxins and streptogramins has compromised the choice of therapeutic options for the treatment of enterococcal infections [54]. It is suggested that when treating Enterococcus infections, antimicrobials should be selected according to the susceptibility and resistance among the isolates to reduce the generation of antimicrobial-resistant strains and the spread of antimicrobial-resistance genes.
The only difference between the isolates from female and male giant pandas was the lower TET resistance rate in Enterococcus isolates from females. In comparing the giant panda age groups, the differences in the resistance rates among E. coli, K. pneumoniae and Enterobacter spp. isolates suggested that the infections in giant pandas of different age should be treated differently. Diet conversion from infancy to adolescence may induce higher prevalence of gastroenteritis that is treated with antimicrobials causing high antimicrobials-resistance rate [55]. In our study, the resistance prevalence to some antimicrobials were higher among the isolates from the infant giant pandas or the old giant pandas than in the other age groups. At the age of 7–18 months, the diet of the giant pandas changes gradually from breast milk or artificial milk to bamboo, which can lead to intestinal diseases and affect the health of the pandas [56]. The probability of intestinal infection is higher at old age because of weakened immunity, basic diseases and long-time application of wide-spectrum antimicrobials [20]. For the K. pneumoniae and E. coli isolates, the prevalence of resistance to sulfadiazine was highest and lowest, respectively, among isolates from adult pandas. The difference may be associated with differences in resistance mechanisms, spread of resistance genes or in inherent characteristics of the taxa, yet further research is needed to confirm the cause.
Enterobacter isolates are able to produce extended-spectrum β-lactamases of CTX-M, TEM and SHV types, and β-lactamases are the prominent reason for β-lactam resistance in most Enterobacter species [44]. The blaTEM, blaSHV and blaCTX-M genes that have been found in Enterobacter spp. isolates from other animals, including humans [57, 58], were detected in the isolates from the giant pandas as well. The differences in the prevalence of the bla types implied that the genetic basis for β-lactam resistance among the E. coli, Enterobacter spp. and K. pneumoniae isolates were different.
The genome of K. pneumoniae K85, an isolate resistant to sixteen antimicrobials, contained multiple ARGs. Efflux pump genes were the most numerous ARGs, indicating that the efflux pumps are the main determinants for the resistance. Efflux pumps are commonly found in bacteria and mediate resistance to antimicrobials, disinfectants, detergents and dyes [59]. Overexpression of the efflux pump genes can lead to multi-drug resistance: the efflux pump encoded by emrE can pump tetracycline, erythromycin, crystal violet and the stain ethidium bromide [60], and the pump encoded by mdfA can pump ciprofloxacin, kanamycin, neomycin, and quaternary ammonium disinfectants out of cells [61]. Even though K. pneumoniae K85 was resistant to all β-lactams except aztreonam, the genome of K. pneumoniae K85 contained the resistance gene blaCTX-M-3 that encodes an aztreonam hydrolyzing enzyme [62]. In addition, we detected mobile genetic elements including insertion sequences, transposons, integrons and plasmids that can mobilize antimicrobial resistance genes. The insertion sequence ISEcp1, adjacent to the bla genes in the K85 genome, is associated with the expression and mobilization of blaCTX-M genes [63, 64]. Thus, the location of insertion sequences and integron-integrase genes next to the resistance genes in the genome of K. pneumoniae K85 implied a potential for gene transfer between different plasmids.
Conclusions
In summary, the E. coli, Enterobacter spp., K. pneumoniae and Enterococcus spp. isolated from the feces of giant pandas showed resistance to various antimicrobials and carried several ARGs, implying that the gut bacteria may pose serious health risks for captive giant pandas. The resistance genes in the genome of K. pneumoniae K85 were associated with insertion sequences and integron-integrase genes, implying a potential for the further spread of the antimicrobial resistance.
Materials and methods
Bacterial isolation and identification
Fresh feces of 166 giant pandas were sampled in May to June 2018, including eight infant giant pandas (aged < 1.5 year), 51 adolescent giant pandas (aged 1.6 to 5 years), 98 adult giant pandas (aged 6 to 20 years) and nine old giant pandas (aged > 21 years) (Supplementary Table S2). Twenty-five-gram samples were taken aseptically, placed in sterile conical flasks with 225 mL of buffered peptone water (BPW; Huankai Microbial Technology Co., Ltd., Guangzhou, China) and incubated for 16–18 h at 200 rpm at room temperature. One loopful of overnight BPW culture was streaked onto MacConkey agar (MAC) and eosin methylene blue agar (EMB), Simmons Citrate Agar (SCA) and Pfizer Selective Enterococcous Agar (EA) (Huankai Microbial Technology Co., Ltd., Guangzhou, China), and incubated at 37 °C for 18–24 h. Typical E. coli colonies (large, blue-black and green metallic sheen) on EMB, K. pneumoniae colonies (the agar turns to blue) on SCA, Enterococcus spp. colonies (brown-black colony with brown-black halo) on EA and other colonies on EMB were streaked onto Soybean Casein Digest Agar (TSA, Huankai Microbial Technology Co., Ltd., Guangzhou, China). Isolates were purified using standard methods and grown in Tryptic Soy Polymyxin Broth Base (TSB; Huankai Microbial Technology Co., Ltd., Guangzhou, China) at 37 °C. After Gram-staining, Matrix-Assisted Laser Desorption/Ionisation Time of Flight Mass Spectrometry (MALDI-TOF-MS/ Autoflex speed TOF/TOF, Bruker, Germany) [65] was used for identification. The 166 E. coli, 68 Enterobacter spp., 116 K. pneumoniae and 117 Enterococcus spp. isolates were stored in TSB containing 25% glycerol at − 80 °C.
Antimicrobial susceptibility testing
Susceptibility to antimicrobials was determined in triplicate using the standard agar dilution method recommended by the Clinical and Laboratory Standards Institute (CLSI, 2020) [66]. The following eighteen antimicrobials were tested: kanamycin (KAN), gentamicin (GEN), erythromycin (ERY), azithromycin (AZM), norfloxacin (NOR), ofloxacin (OFX), ciprofloxacin (CIP), lomefloxacin (LOM), levofloxacin (LEV), sulfadiazine (SD), trimethoprim (TMP), ceftriaxone (CRO), cefixime (CFM), ampicillin (AMP), amoxicillin (AML), aztreonam (ATM), imipenem (IPM) and tetracycline (TET) (Meilun Biotechnology Co., LTD, Dalian, China). The isolates were grown on TSA plates, suspended in stroke-physiological saline solution to a turbidity equivalent to 0.5 McFarland Standard, and inoculated onto Mueller-Hinton agar plates using a multipoint inoculator (MIT-60P; Sakuma Seisakusyo, Tokyo, Japan). The final inoculum was approximately 104 CFU per spot. Plates were incubated at 37 °C for 16–18 h. The range of 2-fold concentrations used to determine the susceptibility was determined by CLSI criteria. In addition, the results were interpreted in accordance with CLSI criteria (Supplementary Table S3). E. coli ATCC25922 and E. faecalis ATCC29212 were used as quality control strains.
Detection of antimicrobial resistance genes
DNA was extracted by suspending an overnight culture grown on TSA in 600 μl of reagent-grade water, incubating the suspension at 100 °C for 10 min, centrifuging at 1100 g for 5 min and collecting the supernatant. The concentration and purity of the extracted DNA was estimated with a NanoDROP ONE (Thermo Scientific, USA) and a Qubit3.0 system (Life Invitrogen, USA). DNA extracts were stored at − 20 °C. Antibiotic resistance genes were amplified using primers and amplification conditions as described previously [2, 20, 22, 67,68,69,70,71,72,73] (Supplementary Table S4). Amplification products were assessed using electrophoresis in 1.0% (w/v) agarose gel. All results were confirmed by at least two independent experiments. Confirming that the amplification products were the target resistance genes was done using Sanger sequencing.
Whole-genome sequencing of K. pneumoniae
Genomic DNA of K. pneumoniae K85 was extracted using an UltraClean1 Microbial DNA Isolation Kit (MoBio Laboratories, Inc., Carlsbad, CA, USA). The concentration and purity of the extracted DNA was estimated as described above. The genome of K. pneumoniae K85 was sequenced using Illumina NovaSeq PE150 at the Beijing Novogene Bioinformatics Technology Co., Ltd. (Beijing, China). The Raw data was filtered to obtain valid data (Clean Data). The sequences were assembled using SOAPdenovo (version 2.04) [74, 75], SPAdes [75] and ABySS [76], the assemblies were integrated with CISA [77] with default parameters. Then filling the gaps of preliminary assembly results, fragments below 500 bp were filtered out and the final result was counted for gene prediction. Antimicrobial resistance genes were predicted using the Comprehensive Antibiotic Research Database (CARD, https://card.mcmaster.ca) with default BLAST expectation value ≤ e− 30 and annotated with the highest score (default identity ≥40%, coverage ≥40%). The sequences were compared using BLASTN (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and EasyFig 2.2.7 with default parameters [78]. Plasmid type analysis was done using database Enterobacteriales in Plasmid Finder v.2.0 with 100% identity and 60% coverage (https://cge.cbs.dtu.dk/services/PlasmidFinder/) [79].
Data analysis
Statistical testing of the differences was tested using χ2 test of independence or Fisher’s exact test in IBM SPSS Statistics 26 software with default parameters [20]. A P-value < 0.05 was considered statistically significant. Other statistical analyses were done using Microsoft Excel (Microsoft, Inc., Washington DC, USA).
Availability of data and materials
All data generated and analyzed in this study are included in this published article and the supplementary materials. The assembly genome sequencing data for K. pneumoniae strain K88 were deposited at CNGB Sequence Archive (CNSA) of China National GeneBank DataBase (CNGBdb) with accession number: CNP0002458 (https://db.cngb.org/cnsa/project/page/sub026616).
Abbreviations
- GPs:
-
Giant pandas
- AMR:
-
Antimicrobial resistance
- ARGs:
-
Antibiotic resistance genes
- MDR:
-
Multidrug-resistant
- ESBLs:
-
Extended-spectrum β-lactamases
- BPW:
-
Buffered peptone water
- MAC:
-
MacConkey agar
- EMB:
-
Eosin methylene blue agar
- SCA:
-
Simmons Citrate Agar
- EA:
-
Pfizer Selective Enterococcus Agar
- TSA:
-
Soybean Casein Digest Agar
- TSB:
-
Tryptic Soy Polymyxin Broth Base
- MALDI-TOF-MS:
-
Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry
- KAN:
-
Kanamycin
- GEN:
-
Gentamicin
- ERY:
-
Erythromycin
- AZM:
-
Azithromycin
- NOR:
-
Norfloxacin
- OFX:
-
Ofloxacin
- CIP:
-
Ciprofloxacin
- LOM:
-
Lomefloxacin
- LEV:
-
Levofloxacin
- SD:
-
Sulfadiazine
- TMP:
-
Trimethoprim
- CRO:
-
Ceftriaxone
- CFX:
-
Cefixime
- AMP:
-
Ampicillin
- AML:
-
Amoxicillin
- ATM:
-
Aztreonam
- IPM:
-
Imipenem
- TET:
-
Tetracycline
- CLSI:
-
The Clinical and Laboratory Standards Institute
- CARD:
-
The Comprehensive Antibiotic Research Database
References
Qiu-Hong W, Hua W, Sheng-Guo F. A new subspecies of giant panda (Ailuropoda melanoleuca) from Shaanxi, China. J Mammal. 2005;86(2):397–402.
Zhang AY, Wang HN, Tian GB, Zhang Y, Yang X, Xia QQ, et al. Phenotypic and genotypic characterisation of antimicrobial resistance in faecal bacteria from 30 Giant pandas. Int J Antimicrob Agents. 2009;33(5):456–60.
Vitetta L, Vitetta G, Hall S. Immunological tolerance and function: associations between intestinal bacteria, probiotics, prebiotics, and phages. Front Immunol. 2018;9:2240.
Rojo D, Méndez-García C, Raczkowska BA, Bargiela R, Moya A, Ferrer M, et al. Exploring the human microbiome from multiple perspectives: factors altering its composition and function. FEMS Microbiol Rev. 2017;41(4):453–78.
Zhu L, Wu Q, Dai J, Zhang S, Wei F. Evidence of cellulose metabolism by the giant panda gut microbiome. Proc Natl Acad Sci USA. 2011;108(43):17714–9.
Clermont O, Olier M, Hoede C, Diancourt L, Brisse S, Keroudean M, et al. Animal and human pathogenic Escherichia coli strains share common genetic backgrounds. Infect Genet Evol. 2011;11(3):654–62.
Yan Y, Zhao CW, Zhang YZ, Zhang ZH, Pan GL, Liu WW, et al. Draft genome sequence of Enterobacter cloacae subsp. cloacae strain 08XA1, a fecal bacterium of giant pandas. J Bacteriol. 2012;194(24):6928–9.
Guo M, Chen J, Li Q, Fu Y, Fan G, Ma J, et al. Dynamics of gut microbiome in giant panda cubs reveal transitional microbes and pathways in early life. Front Microbiol. 2018;9:3138.
Lin CS, Xie YJ, Chen YC, Chen YS, Gao HY, Jiang GQ. Enterohaemorrhagic E. coli (EHEC) isolated from giant panda (in Chinese). Chin J Vet Med. 1992;18:7–9.
Akbari M, Bakhshi B, Najar PS. Particular distribution of Enterobacter cloacae strains isolated from urinary tract infection within clonal complexes. Iran Biomed J. 2016;20(1):49–55.
Bertrand X, Hocquet D, Boisson K, Siebor E, Plésiat P, Talon D. Molecular epidemiology of Enterobacteriaceae producing extended-spectrum beta-lactamase in a French university-affiliated hospital. Int J Antimicrob Agents. 2003;22(2):128–33.
Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115(8):585–90.
Paauw A, Caspers MPM, Leverstein-van Hall MA, Schuren FHJ, Montijn RC, Verhoef J, et al. Identification of resistance and virulence factors in an epidemic Enterobacter hormaechei outbreak strain. Microbiology (Reading, England). 2009;155(Pt 5):1478–88.
Morand PC, Billoet A, Rottman M, Sivadon-Tardy V, Eyrolle L, Jeanne L, et al. Specific distribution within the Enterobacter cloacae complex of strains isolated from infected orthopedic implants. J Clin Microbiol. 2009;47(8):2489–95.
Sanders WE Jr, Sanders CC. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin Microbiol Rev. 1997;10(2):220–41.
Murray BE. Diversity among multidrug-resistant enterococci. Emerg Infect Dis. 1998;4(1):37–47.
Davin-Regli A, Pagès JM. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front Microbiol. 2015;6:392.
Brisse S, Duijkeren E. Identification and antimicrobial susceptibility of 100 Klebsiella animal clinical isolates. Vet Microbiol. 2005;105(3–4):307–12.
de Oliveira GD, Doi Y, Szabo D, Adams-Haduch JM, Vaz TM, Leite D, et al. Multiclonal outbreak of Klebsiella pneumoniae producing extended-spectrum beta-lactamase CTX-M-2 and novel variant CTX-M-59 in a neonatal intensive care unit in Brazil. Antimicrob Agents Chemother. 2008;52(5):1790–3.
Guo L, Long M, Huang Y, Wu G, Deng W, Yang X, et al. Antimicrobial and disinfectant resistance of Escherichia coli isolated from giant pandas. J Appl Microbiol. 2015;119(1):55–64.
Yang X, Cheng G, Li C, Yang J, Li J, Chen D, et al. The normal vaginal and uterine bacterial microbiome in giant pandas (Ailuropoda melanoleuca). Microbiol Res. 2017;199:1–9.
Zou W, Li C, Yang X, Wang Y, Cheng G, Zeng J, et al. Frequency of antimicrobial resistance and integron gene cassettes in Escherichia coli isolated from giant pandas (Ailuropoda melanoleuca) in China. Microb Pathog. 2018;116:173–9.
Abd El-Aziz NK, Tartor YH, Gharieb RMA, Erfan AM, Khalifa E, Said MA, et al. Extensive Drug-Resistant Salmonella enterica Isolated From Poultry and Humans: Prevalence and Molecular Determinants Behind the Co-resistance to Ciprofloxacin and Tigecycline. Front Microbiol. 2021;12:738784.
Hvistendahl M. Public health. China takes aim at rampant antibiotic resistance. Science (New York, NY). 2012;336(6083):795.
Caiwu L, Min W, Senyan J, Yahui Z, Zhengquan H, Chunmao Q, et al. The Application of Rocephin in Gastrointestinal and Respiratory Infections in Giant Panda (in Chinese). Sichuan J Zool. 2017;36(6):669–73.
Zou LK, Wang HN, Zeng B, Zhang AY, Li JN, Li XT, et al. Phenotypic and genotypic characterization of β-lactam resistance in Klebsiella pneumoniae isolated from swine. Vet Microbiol. 2011;149(1–2):139–46.
Haeggman S, Löfdahl S, Paauw A, Verhoef J, Brisse S. Diversity and evolution of the class A chromosomal beta-lactamase gene in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2004;48(7):2400–8.
Mendonça N, Ferreira E, Caniça M. Genetic diversity of genes encoding OKP and LEN beta-lactamases produced by clinical Klebsiella pneumoniae strains in Portugal. Diagn Microbiol Infect Dis. 2009;63(3):334–8.
Poeta P, Costa D, Rodrigues J, Torres C. Antimicrobial resistance and the mechanisms implicated in faecal enterococci from healthy humans, poultry and pets in Portugal. Int J Antimicrob Agents. 2006;27(2):131–7.
Coque TM, Arduino RC, Murray BE. High-level resistance to aminoglycosides: comparison of community and nosocomial fecal isolates of enterococci. Clin Infect Dis. 1995;20(4):1048–51.
Schouten MA, Voss A, Hoogkamp-Korstanje JA. Antimicrobial susceptibility patterns of enterococci causing infections in Europe. The European VRE Study Group. Antimicrob Agents Chemother. 1999;43(10):2542–6.
Zhu Z, Pan S, Wei B, Liu H, Zhou Z, Huang X, et al. High prevalence of multi-drug resistances and diversity of mobile genetic elements in Escherichia coli isolates from captive giant pandas. Ecotoxicol Environ Saf. 2020;198:110681.
Dolejska M, Cizek A, Literak I. High prevalence of antimicrobial-resistant genes and integrons in Escherichia coli isolates from Black-headed Gulls in the Czech Republic. J Appl Microbiol. 2007;103(1):11–9.
Kruse H, kirkemo AM, Handeland K. Wildlife as source of zoonotic infections. Emerg Infect Dis. 2004;10(12):2067–72.
Sekar VT, Santiago TC, Vijayan KK, Alavandi SV, Raj VS, Rajan JJ, et al. Involvement of Enterobacter cloacae in the mortality of the fish, Mugil cephalus. Lett Appl Microbiol. 2008;46(6):667–72.
Kanemitsu K, Endo S, Oda K, Saito K, Kunishima H, Hatta M, et al. An increased incidence of Enterobacter cloacae in a cardiovascular ward. J Hosp Infect. 2007;66(2):130–4.
Saini AG, Rathore V, Ahuja CK, Chhabra R, Vaidya PC, Singhi P. Multiple brain abscesses due to Enterobacter cloacae in an immune-competent child. J Infect Public Health. 2017;10(5):674–7.
Mezzatesta ML, Gona F, Stefani S. Enterobacter cloacae complex: clinical impact and emerging antibiotic resistance. Futur Microbiol. 2012;7(7):887–902.
Zhao S, Li C, Li G, Yang S, Zhou Y, He Y, et al. Comparative analysis of gut microbiota among the male, female and pregnant giant pandas (Ailuropoda Melanoleuca). Open Life Sci. 2019;14:288–98.
Diene SM, Merhej V, Henry M, El Filali A, Roux V, Robert C, et al. The rhizome of the multidrug-resistant Enterobacter aerogenes genome reveals how new “killer bugs” are created because of a sympatric lifestyle. Mol Biol Evol. 2013;30(2):369–83.
Humann JL, Wildung M, Cheng CH, Lee T, Stewart JE, Drew JC, et al. Complete genome of the onion pathogen Enterobacter cloacae EcWSU1. Stand Genomic Sci. 2011;5(3):279–86.
Philippe N, Maigre L, Santini S, Pinet E, Claverie JM, Davin-Régli AV, et al. In Vivo Evolution of Bacterial Resistance in Two Cases of Enterobacter aerogenes Infections during Treatment with Imipenem. PloS One. 2015;10(9):e0138828.
Ren Y, Ren Y, Zhou Z, Guo X, Li Y, Feng L, et al. Complete genome sequence of Enterobacter cloacae subsp. cloacae type strain ATCC 13047. J Bacteriol. 2010;192(9):2463–4.
Davin-Regli A, Lavigne JP, Pagès JM. Enterobacter spp.: Update on Taxonomy, Clinical Aspects, and Emerging Antimicrobial Resistance. Clin Microbiol Rev. 2019;32(4):e00002–19.
Harada K, Shimizu T, Mukai Y, Kuwajima K, Sato T, Kajino A, et al. Phenotypic and molecular characterization of antimicrobial resistance in Enterobacter spp. isolates from companion animals in Japan. PloS One. 2017;12(3):e0174178.
Yamaguchi K, Ohno A, Ishii Y, Tateda K, Iwata M. In vitro susceptibilities to levofloxacin and various antibacterial agents of 12,866 clinical isolates obtained from 72 centers in 2010. Jpn J Antibiot. 2012;65(3):181–206.
Tartor YH, Gharieb RMA, Abd El-Aziz NK, El Damaty HM, Enany S, Khalifa E, et al. Virulence determinants and plasmid-mediated colistin resistance mcr genes in gram-negative bacteria isolated from bovine milk. Front Cell Infect Microbiol. 2021;11:761417.
Wang G, Zhao G, Chao X, Xie L, Wang H. The characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. Int J Environ Res Public Health. 2020;17(17):6278.
Effah CY, Sun T, Liu S, Wu Y. Klebsiella pneumoniae: an increasing threat to public health. Ann Clin Microbiol Antimicrob. 2020;19(1):1.
Wyres KL, Holt KE. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr Opin Microbiol. 2018;45:131–9.
Tartor YH, Abd El-Aziz NK, Gharieb RMA, El Damaty HM, Enany S, Soliman EA, et al. Whole-genome sequencing of gram-negative bacteria isolated from bovine mastitis and raw milk: the first emergence of colistin mcr-10 and fosfomycin fosA5 resistance genes in Klebsiella pneumoniae in middle east. Front Microbiol. 2021;12:770813.
Ben Said L, Jouini A, Fliss I, Torres C, Klibi N. Antimicrobial resistance genes and virulence gene encoding intimin in Escherichia coli and Enterococcus isolated from wild rabbits (Oryctolagus cuniculus) in Tunisia. Acta Vet Hung. 2019;67(4):477–88.
Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J. Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol. 2010;8(4):251–9.
Lebreton F, Willems RJL, Gilmore MS. Enterococcus Diversity, Origins in Nature, and Gut Colonization. In: Gilmore MS, Clewell DB, Ike Y, Shankar N, editors. Enterococci: from commensals to leading causes of drug resistant infection. Boston: Massachusetts Eye and Ear Infirmary; 2014.
Swami SK, Banerjee R. Comparison of hospital-wide and age and location - stratified antibiograms of S. aureus, E. coli, and S. pneumoniae: age- and location-stratified antibiograms. SpringerPlus. 2013;2(1):63.
Zhou S, Luo B, Song S, Huang J, Li W, Zhou J, et al. Analysis of factors influencing the viability of captive-bred pandas: based on the data of 2019 international studbook for giant panda (in Chinese). Sichuan J Zool. 2021;40(03):275–84.
Ho PL, Shek RHL, Chow KH, Duan RS, Mak GC, Lai EL, et al. Detection and characterization of extended-spectrum β-lactamases among bloodstream isolates of Enterobacter spp. in Hong Kong, 2000–2002. J Antimicrob Chemother. 2005;55(3):326–32.
Paterson DL, Hujer KM, Hujer AM, Yeiser B, Bonomo MD, Rice LB, et al. Extended-spectrum beta-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: dominance and widespread prevalence of SHV- and CTX-M-type beta-lactamases. Antimicrob Agents Chemother. 2003;47(11):3554–60.
Poole K. Efflux pumps as antimicrobial resistance mechanisms. Ann Med. 2007;39(3):162–76.
Yerushalmi H, Lebendiker M, Schuldiner S. EmrE, an Escherichia coli 12-kDa multidrug transporter, exchanges toxic cations and H+ and is soluble in organic solvents. J Biol Chem. 1995;270(12):6856–63.
Edgar R, Bibi E. MdfA, an Escherichia coli multidrug resistance protein with an extraordinarily broad spectrum of drug recognition. J Bacteriol. 1997;179(7):2274–80.
Maryam L, Khan AU. Combination of aztreonam and cefotaxime against CTX-M-15 type β-lactamases: A mechanism based effective therapeutic approach. Int J Biol Macromol. 2018;116:1186–95.
Poirel L, Decousser JW, Nordmann P. Insertion sequence ISEcp1B is involved in expression and mobilization of a blaCTX-M beta-lactamase gene. Antimicrob Agents Chemother. 2003;47(9):2938–45.
Dhanji H, Doumith M, Hope R, Livermore DM, Woodford N. ISEcp1-mediated transposition of linked blaCTX-M-3 and blaTEM-1b from the IncI1 plasmid pEK204 found in clinical isolates of Escherichia coli from Belfast, UK. J Antimicrob Chemother. 2011;66(10):2263–5.
Singh NK, Bezdan D, Checinska Sielaff A, Wheeler K, Mason CE, Venkateswaran K. Multi-drug resistant Enterobacter bugandensis species isolated from the International Space Station and comparative genomic analyses with human pathogenic strains. BMC Microbiol. 2018;18(1):175.
CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 31st ed; 2020.
Edelstein M, Pimkin M, Palagin I, Edelstein I, Stratchounski L. Prevalence and molecular epidemiology of CTX-M extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrob Agents Chemother. 2003;47(12):3724–32.
Essack SY, Hall LM, Pillay DG, McFadyen ML, Livermore DM. Complexity and diversity of Klebsiella pneumoniae strains with extended-spectrum beta-lactamases isolated in 1994 and 1996 at a teaching hospital in Durban, South Africa. Antimicrob Agents Chemother. 2001;45(1):88–95.
Kim J, Kwon Y, Pai H, Kim JW, Cho DT. Survey of Klebsiella pneumoniae strains producing extended-spectrum beta-lactamases: prevalence of SHV-12 and SHV-2a in Korea. J Clin Microbiol. 1998;36(5):1446–9.
Senda K, Arakawa Y, Ichiyama S, Nakashima K, Ito H, Ohsuka S, et al. PCR detection of metallo-beta-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum beta-lactams. J Clin Microbiol. 1996;34(12):2909–13.
Tsakris A, Pournaras S, Woodford N, Palepou MF, Babini GS, Douboyas J, et al. Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece. J Clin Microbiol. 2000;38(3):1290–2.
Ng LK, Martin I, Alfa M, Mulvey M. multiplex PCR for the detection of tetracycline resistant genes. Mol Cell Probes. 2001;15(4):209–15.
Gu X, Shang J, Zhang W, Jiang Q. Analysis of genotype and antimicrobial resistance of Enterococcus faecalis isolates from pigs (in Chinese). Chin J Antibiot. 2017;42(03):225–9.
Li R, Zhu H, Ruan J, Qian W, Fang X, Shi Z, et al. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010;20(2):265–72.
Li R, Li Y, Kristiansen K, Wang J. SOAP: short oligonucleotide alignment program. Bioinformatics (Oxford, England). 2008;24(5):713–4.
Simpson JT, Wong K, Jackman SD, Schein JE, Jones SJ, Birol I. ABySS: a parallel assembler for short read sequence data. Genome Res. 2009;19(6):1117–23.
Lin SH, Liao YC. CISA: contig integrator for sequence assembly of bacterial genomes. PloS One. 2013;8(3):e60843.
Sullivan MJ, Petty NK, Beatson SA. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27(7):1009–10.
Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58(7):3895–903.
Acknowledgements
We thank China Conservation and Research Center for Giant Panda for providing the giant pandas’ feces for isolation of bacteria.
Funding
This work was supported by the Department of Science and Technology of Sichuan Province (2020YJ0338), and Open Project of Key Laboratory of State Forestry and Grassland Administration on Conservation Biology of Rare Animals in the Giant Panda National Park, the China Conservation and Research Center for the Giant Panda (KLSFGAGP2020.003).
Author information
Authors and Affiliations
Contributions
Xin Wang, Yi Zhang and Caiwu Li contributed equally to this work. All authors approved the manuscript’s final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was carried out with approval of China Conservation and Research Center for Giant Panda and all sample collection protocols in this study were approved by the CCRCGP. All experiments were performed in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
The proportion of antimicrobial resistant isolates from female and male giant pandas. KAN, kanamycin; GEN, gentamicin; AZM, azithromycin; ERY, erythromycin; NOR, norfloxacin; OFX, ofloxacin; CIP, ciprofloxacin; LOM, lomefloxacin; LEV, levofloxacin; SD, sulfadiazine; TMP, trimethoprim; CRO, ceftriaxone; CFX, cefixime; AMP, ampicillin; AML, amoxicillin; ATM, aztreonam; IPM, imipenem; TET, tetracycline.
Additional file 2: Table S1.
Antimicrobial resistance profiles of the 144 E. coli, 66 Enterobacter spp., 110 K. pneumoniae and 43 Enterococcus spp. isolates from the feces of giant pandas in China. Table S2 The location, sex and age of the sampled giant pandas. Table S3 MIC breakpoints for Enterobacterales and Enterococcus spp. according to the Clinical and Laboratory Standards Institute (CLSI) guidelines. Table S4 Primers, expected product sizes and annealing temperatures in the amplification of antimicrobial resistance genes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, X., Zhang, Y., Li, C. et al. Antimicrobial resistance of Escherichia coli, Enterobacter spp., Klebsiella pneumoniae and Enterococcus spp. isolated from the feces of giant panda. BMC Microbiol 22, 102 (2022). https://doi.org/10.1186/s12866-022-02514-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-022-02514-0