Abstract
Background
Pu-erh tea is a traditional Chinese tea and produced by natural solid-state fermentation. Several studies show that the natural microbiota influence caffeine level in pu-erh tea. Our previous research also found that the caffeine declined significantly (p < 0.05) in the fermentation, which suggested that the caffeine level could be influenced by specific strains. The purpose of this study was to isolate and identify microorganisms for caffeine degradation, and this research explored the degradation products from caffeine and optimal condition for caffeine degradation.
Results
11 Fungi were isolated from pu-erh tea fermentation and 7 strains could survive in caffeine solid medium. Two superior strains were identified as Aspergillus niger NCBT110A and Aspergillus sydowii NRRL250 by molecular identification. In the substrate tests with caffeine, A. niger NCBT110A could use caffeine as a potential carbon source while glucose is absent, A. sydowii NRRL250 could degrade 600 mg/L caffeine completely in a liquid medium. During the degradation product analysis of A. sydowii NRRL250, theophylline and 3-methlxanthine were detected, and the level of theophylline and 3-methlxanthine increased significantly (p < 0.05) with the degradation of caffeine. The single factor analysis showed that the optimum conditions of caffeine degradation were 1) substrate concentration of 1200 mg/L, 2) reaction temperature at 30 °C, and 3) pH of 6. In the submerged fermentation of tea infusion by A. sydowii NRRL250, 985.1 mg/L of caffeine was degraded, and 501.2 mg/L of theophylline was produced.
Conclusions
Results from this research indicate that Aspergillus sydowii NRRL250 was an effective strain to degrade caffeine. And theophylline and 3-methlxanthine were the main caffeine degradation products.
Similar content being viewed by others
Background
Caffeine (1, 3, 7-trimethylxanthine or 3, 7-dihydro-1, 3, 7-trimethyl-1H-2, 6dione) belongs to a group of compounds known as purine alkaloids. Caffeine is a key flavor substance in many popular drinks, especially in tea and coffee. Although caffeine has lots of benefits, such as regulating the central nervous system, excess caffeine intake could develop hypertension and induce osteoporosis. Based on the recent researches, caffeine content only changes in different physiological metabolism of tea tree (Camellia sinesis (L.) O. Kuntze) [1], and caffeine content is influenced by tea tree varieties [2], but caffeine level remains stable among different kind of teas, which showed that tea processing cannot impact caffeine content [3, 4].
Pu-erh tea (pu-erh shucha) (PET) is produced though a natural solid-state fermentation (SSF) process with sun-dried green tea leaves (Camellia sinensis var. assamica (JW Masters) Kitamura) as the raw material [5,6,7]. PET has been produced and drank by some minorities of the Yunnan people in China for centuries [8, 9]. Microorganisms, involved in pu-erh tea solid-state fermentation (PETSSF), have been mainly studied using culture-based approaches and culture-independent approaches [6,7,8,9,10,11,12]. Many fungi and yeast have been isolated from PET, especially Aspergillus niger, A. tubingensis, A. fumigatus, A. acidus, A. awamori, Penicllium sp., Rhizomucor pusillus, Rhizomucor tauricus, Blastobotrys adeninivorans, Arxula adeninivorans, Pichia farinose and Candida tropicalis, which have been reported widely [8,9,10,11,12,13,14,15,16].
Due to the participation of microorganisms, caffeine content is changeable during PETSSF [17,18,19,20]. Cephalosporium acremonium dramatically increases 60–70% caffeine content during PETSSF, whereas Saccharomycetes sp. and A. niger could potentially reduce caffeine content [19,20,21]. In addition, the level of caffeine content is relatively stable with the effect of A. fumigatu and Lactobacillus sp. [14]. Therefore, microorganisms have a certain effect on caffeine and other purine alkaloids [22].
In this paper, samples from PETSSF were used to select target strains with the capability of caffeine degradation. This report found that Aspergillus sydowii NRRL250 leads caffeine biodegradation. In addition, this paper investigated the effect of Aspergillus niger NCBT110A on caffeine degradation.
Methods
Ethics statement
No specific permits were required for the described study. No specific permissions were required for these locations/activities, because specimens used in this study were manufactured in the laboratory.
Material and reagents
Assam sun-dried green tea leaves (C. sinensis var. assamica (JW Masters) Kitamura) with moisture content 6.25% by weight were obtained from Yunnan province, China. Caffeine (purity about 95%), used in culture medium, was purchased from Tianjin Guangfu fine chemical industry institute, China. Caffeine (≥99%), theophylline (≥99%) and 3-methylxanthine (≥99%) standards were purchased form Sigma Company, USA. SP fungal DNA kit, DNA marker, polymerase chain reaction (PCR) spread reagent, internal transcribed spacers (ITS): ITS1 (5`-TCCGTAGGTGAACCTGCGG-3`) and ITS4 (5`-TCCTCCGCTTATTGATAGC-3`) were purchased from TaKaRa Company, Japan. High performance liquid chromatography (HPLC) grade of acetonitrile was purchased from Beijing Mreda Biotechnology Company, China. Other reagents were of analytical grade.
A solid-state fermentation
In this study, PTSSF was manufactured in Tea Processing Laboratory, College of Long Run Pu-erh Tea, Yunnan Agricultural University, Kunming of Yunnan province to simulate pu-erh tea industrial production. Sun-dried green leaves (400 g) were moistened with distilled water (220 mL) to achieve a final moisture content of 35% (w/w) [23]. SSF was carried out with the natural microbiota exist on the leaves [21]. The whole fermentation process continued for 35 d in a nature condition. The leaves were turned over with sprayed by moderate sterile water for every 5 days to ensure a homogeneous fermentation. Samples were collected periodically from fermentation for chemical and microbial analyses [23]. In addition, parallel tests were carried out to ensure the data reliability.
Isolation and identification of target strains
Fermented sample would be used to isolate the fungi and they were counted by dilution plating method [23]. The colony forming units (CFU) was calculated by per gram dry weight of tea leaves after 2 days of cultivation at 30 °C. The caffeine content of related samples was determined by HPLC [24].
The target strains were inoculated and cultivated in the potato dextrose agar (PDA) and Czapek-Dox mediums at 30 °C, respectively. The colony morphological characteristics and conidia structure were observed after cultivation for 5 d. The target strains grew aerobically as pure cultures in 20 mL of Czapek-Dox liquid medium in 125 mL shake flasks at 30 °C, 250 rpm, for 2 d. The fresh cells were obtained by centrifugation at 1700 g for 5 min and freeze-dried at − 80 °C [10, 23]. DNA was extracted by using SP fungal DNA kit. The extracted DNA was subject to amplify the ITS region, the universal fungal primers ITS1 and ITS4 were used in the PCR [11, 12, 23]. The final volume of 50 μL, 1.0 μL of containing template DNA, 5 μL of 10 x buffer, 5 μL of dNTPs (2.5 mM), 0.5 μL of Taq polymerase, 1.0 μL (10 μM) of each primer, and 36.5 μL of sterile distilled water were used to implement amplifications [12, 23]. The PCR reaction procedure was as follows. Pre-degeneration at 95 °C for 5 min, degeneration at 94 °C for 1 min, annealing at 54 °C for 1 min, extension at 72 °C for 1.5 min, with 35 cycles, extension at 72 °C for 10 min [15]. It was stored at 10 °C in the end of the reaction process.
The PCR mixtures were analyzed by using ABI3730 automatic DNA sequencer (Applied Biosystems, USA). The received sequence was sent to Genbank of NCBI to seek semi-root sequence. ITS1–5.8S rRNA-ITS2 gene sequence of related strains were transferred and compared with ClustalX 1.8. The evolution distance was calculated through a Kimura2-parameter of the MEGA 4 Soft. Neighbor-Joining method was used to establish phylogenetic trees. 1000 random samples were taken to calculate Bootstrap for evaluation of the phylogenetic confidence level.
Growth of tea-derived fungi in a solid medium
Qualitative screenings were carried out on Petri dishes containing a solid culture medium with glucose (2% w/v) (control culture) and a selection medium with caffeine instead of glucose in three different concentrations: 600, 1200 and 1800 mg/L per plate, respectively [25]. Fungal mycelia from recent cultures were transferred to the surface of the agar plates with an inoculating loop. Strains were incubated at 30 °C for 5 d. Compared with the control culture, the strains utilized caffeine was estimated by the size of the colony grown on the plates (Table 1).
Growth of tea-derived fungi in a liquid medium
Spore solutions of target strains were prepared by growing the fungi for 5 d at 30 °C in dishes containing solid culture medium with glucose [25]. Two loopfuls of target strains were transferred aseptically from a dish slant into 25 mL of a sterile liquid medium (per liter: potato starch 4 g, dextrose 20 g, chloramphenicol 0.1 g) with 600 mg/L of caffeine in a 125 mL Erlenmeyer flask. The flask was incubated aerobically on an incubator shaker (250 rpm) at 30 °C for 48 h. The volume of the seed was 10% (v/v) of total initial volume [23]. The flask was incubated in an orbital shaker during 3, 6, 9, 12 and 15 d (130 rpm, 30 °C). The mycelium was collected after the culture was filtered in a Buchner funnel, and rinsed in 20 mL of water: ethyl acetate (1:1) [25]. The mycelial mass was determined as fungal dry mass after drying at 35 °C for 24 h [25]. Biodegraded products of caffeine were analyzed by HPLC [24]. The results were summarized in Table 2.
Biodegradation in a liquid medium
Studies were performed during 15 d in the liquid medium to evaluate the kinetic parameters for biodegradation reactions of caffeine. Effects of substrate concentration, reaction temperature and pH on the kinetic parameters were investigated [25].
-
1)
Substrate concentration. The seed was inoculated into the sterile liquid medium by 10% (v/v) of above noted inoculum with different initial caffeine concentration (600, 1200 and 1800 mg/L, respectively). The flask was incubated in an orbital shaker during 5, 10 and 15 d (130 rpm, 30 °C).
-
2)
Reaction temperature. The seed was inoculated into the sterile liquid medium by 10% (v/v) of above noted inoculum with the initial caffeine concentration of 1200 mg/L. The flask was incubated in an orbital shaker with different reaction temperature (25, 30, 35 °C, respectively) during 5, 10 and 15 d.
-
3)
pH. The seed and liquid medium were adjusted for different pH (5.0, 6.0,7.0, respectively) by phosphate buffer. The seed was inoculated into the sterile liquid medium by 10% (v/v) of above noted inoculum with the initial caffeine concentration of 1200 mg/L. The flask was incubated in an orbital shaker during 5, 10 and 15 d (130 rpm, 30 °C).
Fungal dry mass was determined. The final caffeine and biodegraded products viz. theophylline and 3-methylxanthine were determined by HPLC [24].
A submerged fermentation (SMF) of tea infusion
Sun-dried green tea leaves (1.0 g) were infused for 15 min in boiling distilled water (30 mL) and the tea infusion was made up to 30 mL with distilled water after filtration [23]. Caffeine and other functional ingredients (tea polyphenols and theabrownins) are relatively stable in high temperature condition. Based on the investigation of several thermal treatment methods (Additional file 1: Table S1), including the control (no further treatment), high temperature sterilization at 121 °C for 5 min, pasteurization at various conditions (65 °C, 30 min; 75 °C, 30 min and 80 °C, 30 min) and microwave heating (640 W, 2 min) [23], sterilization can kill viable microorganisms with minimal damage in main functional components. Therefore, sterilization was selected as the reasonable treatment for SMF.
Two loopfuls of target strains were transferred aseptically from a dish slant into 25 mL of sterile tea infusion in a 125 mL Erlenmeyer flask [23]. The flask was incubated aerobically at 30 °C for 48 h on an incubator shaker (250 rpm). The volume of the seed was 10% (v/v) of total initial volume of the inoculation [23]. Non-inoculation (control) and non-sterilization (natural treatment) were carried out. The flask was incubated in an orbital shaker for 3, 6, 9, 12 and 15 d (130 rpm, 30 °C), respectively. Fungal dry mass, caffeine and theophylline contents were determined.
Results
Caffeine content and fungi count during PETSSF
The Fig. 1 shows that the fugal colony count dramatically increased from 0 to 10 days. Then, it increased slowly from 4.8 × 105 CFU/g dry weight of tea leaves to 1.2 × 106 CFU/g on day 20. Due to nutrient limitation in the tea leaves, the colony count decreased after day 20. With changing of fungi count, caffeine content (Fig. 1) declined significantly (p < 0.05) from 3.685 ± 0.1006% (w/w) to 2.612 ± 0.1398% (w/w) during the fermentation. According to the analysis above, it suggested that the fungal colonies cause the decrease of caffeine content. Through separation and purification, 11 fungi were isolated from PETSSF.
Screening result of tea-derived fungi in a solid medium
The screening was carried out in an agar solid medium for selecting the tea-derived fungi able to utilize caffeine. In order to evaluate the biocatalytic potential for degradation of caffeine, all investigated strains were inoculated into an agar solid medium with presence of glucose and they were also inoculated into a set of agar solid medium with presence of different concentration caffeine. The sizes of the colonies were measured and showed in Table 1.
Among 11 tea-derived fungi, 7 strains could survive in the agar solid medium (2% w/v) with caffeine alone. Strains No. 1, No. 3 and No. 5 showed the best growth in all concentrations evaluated. And, stains No. 4 and No. 7 had no growth in low caffeine concentration, which showed that the utilization ratio of caffeine was restricted. If fungi had a higher growth in a low caffeine concentration, it may indicate that fungi could use caffeine as a carbon source directly or indirectly. Because strains No. 5 and No. 1 had a high growth rate at the lowest caffeine concentration, they were considered as the potential target strains. Colony characteristics and microscopic structure of strain No. 5 were showed in Additional file 2: Figure S1 and S2, colony characteristics and microscopic structure of strain No. 1 were showed in Additional file 2: Figure S3 and S4. Through molecular identification (Additional file 3: Figure S5), strain No. 5 was identified as Aspergillus sydowii NRRL250 with 99.8% homology, strain No. 1 was identified as Aspergillus niger NCBT110A with 99.8% homology (Fig. 2).
Biodegradation of caffeine by A. sydowii NRRL250 and A. niger NCBT110A
For the biodegradation reaction in a liquid medium, the selected strains (A. sydowii NRRL250 and A. niger NCBT110A) were inoculated into the nutrient medium with the presence of caffeine. During the fermentation, fungal dry mass and caffeine content were determined. In addition, the caffeine degradation rates were calculated by the Eq. (1) to investigate the degrading capability of selected strains. For A. niger NCBT110A, caffeine was no significantly degraded with about 1.2, 3.3, 3.8% of caffeine degraded and theophylline was not detected in the fermentation. Caffeine degradation capability of A. niger NCBT110A was limited in the liquid medium, which showed that A. niger NCBT110A could use caffeine as a potential carbon source when glucose and other nutrients were limited or absent. For A. sydowii NRRL250, caffeine was almost completely degraded at 600 mg/L of caffeine (Table 2). In 15 d, caffeine had been degraded completely. As the degradation product, theophylline was determined by HPLC and increased observably from 40.4 ± 1.0 mg/L on 5 d to 262.6 ± 20.7 mg/L on 15 d. As A. sydowii NRRL250 has ability to degrade caffeine, it will be used to conduct the biodegradation products analysis of caffeine in below.
Biodegradation products analysis of caffeine by A. sydowii NRRL250
A. sydowii NRRL250 was inoculated into a liquid medium with 1200 mg/L of caffeine for analysis of its biodegraded products. And caffeine, theophylline and 3-methylxanthine were determined by HPLC during 3, 6, 9, 12 and 15 d in the fermentation. Caffeine, theophylline and 3-methylxanthine contents as well as caffeine degradation rates were showed in Table 3. Degradation of approximately 7.1, 33.0, 52.8, 68.7 and 90.1% were observed in 3, 6, 9, 12 and 15 d, respectively. Both theophylline and 3-methylxanthine were detected in the fermentation, which showed that theophylline and 3-methylxanthine were the main degradation products from caffeine. Theophylline was first detected on day 3 and it increased obviously with the caffeine degradation, which showed that theophylline was a direct degradation product from caffeine through demethylation. 3-Methylxanthine was not detected on day 3 and first detected on day 6, which indicated that 3-methylxanthine might be a direct degradation product from theophylline instead of caffeine. As the secondary product, 3-methylxanthine content was far below theophylline and only 178.7 ± 10.8 mg/L was produced in 15 d.
Effects of substrate concentration, reaction temperature and pH on the kinetic parameters for caffeine degradation by A. sydowii NRRL250
Microorganism metabolism and degradation capability were influenced by substrate concentration, reaction temperature and pH. In this paper, effects of substrate concentration, reaction temperature and pH on fungal dry mass and kinetic parameters of caffeine degradation by A. sydowii NRRL250 were investigated (Tables 4, 5 and 6, respectively). A. sydowii NRRL250 was inoculated into a liquid medium with increasing caffeine concentrations (600, 1200 and 1800 mg/L, respectively), and the flasks were incubated in an orbital shaker for 15 d (130 rpm, 30 °C). Fungal dry mass and the kinetic parameters, including final caffeine concentration (Ccaffeine,f), final theophylline concentration (Ctheophylline,f), final 3-methylxanthine concentration (C3-methylxanthine,f), the volumetric rate of caffeine degradation (Qcaffeine), the volumetric rate of theophylline production (Qtheophylline), the yield of theophylline (Ytheophylline/caffeine) and caffeine degradation rate (% of caffeine degraded) in different substrate concentrations were showed in Table 4. There was no significant difference in fungal dry mass (p > 0.05). The final concentrations of theophylline and 3-methylaxthine increased significantly (p < 0.05), caffeine degradation rate decreased significantly (p < 0.05) in higher initial caffeine concentrations. Only about 62.9% of caffeine was degraded in 1800 mg/L caffeine concentration. By comparing the results, 1200 mg/L was an appropriate substrate concentration with the high caffeine degradation rate and the high theophylline production.
In order to compare the kinetic parameters in different reaction temperatures, A. sydowii NRRL250 was inoculated into an identical liquid medium with the initial caffeine concentration of 1200 mg/L, and the flasks were incubated in an orbital shaker with different reaction temperatures (25, 30 and 35 °C, respectively) for 15 d. The fungal dry mass and the kinetic parameters were showed in Table 5. In the temperature range between 25 and 30 °C, there were no significant differences in fungal dry mass, the final caffeine concentration, the volumetric rate of caffeine degradation, and caffeine degradation rate (p > 0.05). In 35 °C, there was no significant decline (p > 0.05) in fungal mass. And the final caffeine concentration, the volumetric rate of caffeine degradation and caffeine degradation rate declined significantly (p < 0.05). However, the theophylline concentration, the volumetric rate of theophylline production and the yield of theophylline increased significantly (p < 0.05) in 35 °C. The optimal temperature for caffeine degradation was 30 °C. And higher temperature promotes theophylline production.
In order to compare the kinetic parameters in different pH, phosphate buffer was used to adjust the pH of liquid medium. The fungal mass and the kinetic parameters were showed in Table 6. pH had remarkable effects on fugal dry mass and the kinetic parameters. In pH of 5, the growth and caffeine catabolism of A. sydowii NRRL250 were restricted. Through comparisons, pH of 6 was the optimum pH for caffeine degradation with the highest fugal dry mass, caffeine degradation rate and theophylline production.
Applications of A. sydowii NRRL250 and A. niger NCBT110A in SMF of tea infusion
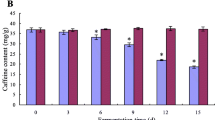
Due to the caffeine degradation characteristic, A. sydowii NRRL250 was suitable to produce decaffeinated and high-theophylline tea through an inoculated fermentation. In this paper, A. sydowii NRRL250 was inoculated into the sterile tea infusion for SMF, caffeine and theophylline contents were determined by HPLC during 0, 3, 6, 9, 12 and 15 d. The final fungal dry mass was also determined. In addition, the SMF inoculated by A. niger NCBT110A, natural treatment and sterile treatment (control) were carried out to explore the influence of microorganism on caffeine. Changes of caffeine and theophylline contents were showed in Fig. 3. Fungal dry mass and the kinetic parameters of caffeine degradation were showed in Table 7.
There were no significant changes of caffeine and theophylline contents in sterile treatment (control) (p > 0.05). During SMF by A. niger NCBT110A, caffeine increased significantly (p < 0.05), which showed that the caffeine degradation capability of A. niger NCBT110A was limited in the presence of carbohydrate and other nutriment. During SMF by A. sydowii NRRL250, most of caffeine (985.1 mg/L) was degraded (Fig. 3a, Table 7). As a main degradation product, theophylline increased sharply from 24.7 mg/L to 501.2 mg/L in the SMF by A. sydowii NRRL250 (Fig. 3b, Table 7). Because the existence of A. sydowii NRRL250, in natural treatment, caffeine decreased significantly with about 256.3 mg/L of caffeine degraded and theophylline increased significantly with 74.7 mg/L in 15 d (p < 0.05) (Fig. 3). Therefore, A. sydowii NRRL250 was appropriate for the production of decaffeinated tea or high-theophylline tea through an inoculated fermentation.
Discussion
Although several effective strains were selected from the soil of tea and coffee gardens to degrade caffeine [26,27,28], the functional strain selected from tea was not reported. In this paper, 11 fungi were isolated from PETSSF, and 7 strains could survive in a solid medium with caffeine alone. But only 2 strains had a high growth rate at the lowest caffeine concentration, which suggested that those 2 strains used caffeine as a carbon source directly or indirectly. The two superior strains were identified as A. niger NCBT110A and A. sydowii NRRL250 by molecular identification method.
The substrate tests in the liquid medium with caffeine found that the caffeine degradation capability of A. niger NCBT110A was limited in the presence of glucose and other nutrients. A. niger NCBT110A could use caffeine as a potential carbon source when the absence of glucose. A. sydowii NRRL250 could degrade caffeine completely in a liquid medium with 600 mg/L of caffeine. Therefore, A. sydowii NRRL250 was a potentially effective strain to degrade caffeine.
In the perspective of physiology of tea tree (C. sinesis (L.) O. Kuntze), caffeine is synthesized in the root. Theobromine (3, 7-dimethyxanthine) is a direct precursor of caffeine anabolism and a major rate-limiting step in caffeine synthesis [29]. Theophylline (1, 3-dimethyxanthine) and 3-methylxanthine are the main degradation products in caffeine catabolism [30]. In addition, theophylline is a rate-limiting step of caffeine catabolism in the physiology of tea tree (C. sinesis (L.) O. Kuntze) and coffee tree (Coffea arabica L.). And demethylase is an important enzyme which catalyzes the reaction from caffeine to theophylline. In microbial secondary metabolites, the degradation products and degradation pathways of caffeine were not completely clear. In the substrate tests with caffeine of A. sydowii NRRL250, theophylline and 3-methlxanthine were detected. And theophylline and 3-methlxanthine increased significantly (p < 0.05) with the degradation of caffeine. Caffeine catabolism in secondary metabolites of A. sydowii NRRL250 was similar to the metabolites in the physiology of tea tree (C. sinesis (L.) O. Kuntze), theophylline and 3-methlxanthine were the main degradation products from caffeine by demethylation.
In this study, the optimum substrate concentration, reaction temperature and pH of A. sydowii NRRL250 were investigated. The optimum conditions of caffeine degradation were 1) substrate concentration of 1200 mg/L, 2) reaction temperature at 30 °C, and 3) pH of 6. The optimum conditions provided the relevant information for the application of A. sydowii NRRL250 in caffeine degradation.
In previous researches, A. sydowii is an important industrial and medical microorganism, which could produce monosaccharide and indole alkaloids [31,32,33]. In addition, A. sydowii could be used in biodegradation of methyl parathion [25]. Due to the caffeine degradation characteristic, A. sydowii NRRL250 would be applied in the production of decaffeinated tea or high-theophylline tea. In SMF, 985.1 mg/L of caffeine was degraded, and 501.2 mg/L of theophylline was produced in 15 d. Further research could be conducted in related to the caffeine degradation pathway and productive technology of decaffeinated tea by A. sydowii NRRL250.
Conclusions
The purpose of this research was to screen and identify the strains which able to degrade caffeine during the PET fermentation process. The results of the research show that strain Aspergillus sydowii NRRL250 and strain A. niger NCBT110A could use caffeine as a potential carbon source when glucose and other nutrients were limited or absent. A. sydowii NRRL250 was an effective strain to degrade caffeine, which could be applied in the production of decaffeinated or high-theophylline tea. In addition, theophylline and 3-methlxanthine were the main degradation products from caffeine in secondary metabolites of A. sydowii NRRL250.
Abbreviations
- CFU:
-
Colony forming units
- DNA:
-
Deoxyribonucleic acid
- HPLC:
-
High performance liquid chromatography
- ITS:
-
Internal transcribed spacer
- MEGA:
-
Molecular evolutionary genetics analysis
- NCBI:
-
National Center for Biotechnology Information
- PCR:
-
Polymerase chain reaction
- PDA:
-
Potato dextrose agar
- PET:
-
Pu-erh tea
- PETSSF:
-
Pu-erh tea solid-state fermentation
- RNA:
-
Ribonucleic acid
- rRNA:
-
Ribosomal ribonucleic acid
- SMF:
-
Submerged fermentation
- SSF:
-
Solid-state fermentation
References
Wei K, Wang LY, Zhou J, He W, Zeng JM, Jiang YW, Cheng H. Comparison of catechins and purine alkaloids in albino and normal green tea cultivars (Camellia sinensis L.) by HPLC. Food Chem. 2012;130:720–4.
Wang LY, Wei K, Jiang YW, Cheng H, Zhou J, He W, Zhang CC. Seasonal climate effects on flavanols and purine alkaloids of tea (Camellia sinensis L.). Eur Food Res Technol. 2011;233:1049–55.
Zhu YC, Luo YH, Wang PP, Zhao MY, Li L, Hu XS, Chen F. Simultaneous determination of free amino acids in pu-erh tea and their changes during fermentation. Food Chem. 2016;194:643–9.
Sari F, Velioglu YS. Changes in theanine and caffeine contents of black tea with different rolling methods and processing stages. Eur Food Res Technol. 2013;237:229–36.
Chen YS, Liu BL, Chang YN. Bioactivities and sensory evaluation of pu-erh teas made from three tea leaves in an improved pile fermentation process. J Biosci Bioeng. 2010;109(6):557–63.
Oh HW, Kim BC, Lee KH, Kim DY, Park DS, Park HM, Bae KS. Paenibacillus camelliae sp. nov., isolated from fermented leaves of Camellia sinensis. J Microbiol. 2008;46:530–4.
Su XQ, Zhang GJ, Ma Y, Chen M, Chen SH, Duan SH, Wan JQ, Hashimoto F, Lv HP, Li JH, Lin Z, Zhao M. Isolation, identification, and biotransformation of teadenol a from solid sate fermentation of pu-erh tea and in vitro antioxidant activity. Appl Sci. 2016;6:161–73.
Zhang W, Yang RJ, Fang WJ, Yan L, Lu J, Sheng J, Lv J. Characterization of thermophilic fungal community associated with pile fermentation of Pu-erh tea. Int J Food Microbiol. 2016;227:29–33.
Lyu CY, Chen CY, Ge F, Liu DQ, Zhao SL, Chen D. A preliminary metagenomic study of puer tea during pile fermentation. J Sci Food Agr. 2013;93:3165–74.
Zhao M, Xiao W, Ma Y, Sun TT, Yuan WX, Na T, Zhang DL, Wang YX, Li YL, Zhou HJ, Cui XD. Structure and dynamics of the bacterial communities in fermentation of the traditional Chinese post-fermented pu-erh tea revealed by 16S RNA gene clone library. World J Microb Biot. 2013;29:1877–84.
Zhao ZJ, Tong HR, Zhou L, Wang EX, Liu QJ. Fungal colonization of pu-erh tea in Yunnan. J Food Safety. 2010;30:769–84.
Abe M, Takaoka N, Idemoto Y, Takagi C, Imai T, Nakasaki K. Characteristic fungi observed in the fermentation process for Puer tea. Int J Food Microbiol. 2008;124:199–203.
Zhao M, Zhang DL, Su XQ, Duan SM, Wan JQ, Yuan WX, Liu BY, Ma Y, Pan YH. An integrated metagenomics/metaproteomics investigation of the microbial communities and enzymes in solid-state fermentation of pu-erh tea. Sci Rep. 2015;5:10117. http://www.nature.com/articles/srep10117.
Qin JH, Li N, Tu PF, Ma ZZ, Zhang L. Change in tea polyphenol and purine alkaloid composition during solid-state fungal fermentation of post-fermented tea. J Agr Food Chem. 2012;60:1213–7.
Haas D, Pfeifer B, Reiterich C, Partenheimer R, Reck B, Buzina W. Identification and quantification of fungi and mycotoxins from Pu-erh tea. Int J Food Microbiol. 2013;166:316–22.
Wang WN, Zhang L, Wang S, Shi SP, Jiang Y, Li N, Tu PF. 8-CN-ethyl-2-pyrrolidinone substituted flavan-3-ols as the marker compounds of Chinese dark teas formed in the post-fermentation process provide significant antioxidative activity. Food Chem. 2014;152:539–45.
Zhang L, Li N, Ma ZZ, Tu PF. Comparison of the chemical constituents of aged pu-erh tea, ripened pu-erh tea, and other teas using HPLC-DAD-ESI-MSn. J Agr Food Chem. 2011;59:8754–60.
Lv HP, Zhang YJ, Lin Z, Liang YR. Processing and chemical constituents of Pu-erh tea: a review. Food Res Int. 2013;53:608–18.
Wang D, Xiao R, Hu XT, Xu KL, Hou Y, Zhong Y, Meng J, Fan BL, Liu LG. Comparative safety evaluation of Chinese Pu-erh green tea extract and Pu-erh black tea extract in Wistar rats. J Agr Food Chem. 2010;58:1350–8.
Wang D, Xu KL, Zhang Y, Luo X, Xiao R, Hou Y, Bao W, Yang W, Yan H, Yao P, Liu LG. Acute and subchronic oral toxicities of Pu-erh black tea extract in Sprague-Dawley rats. J Ethnopharmacol. 2011;134:156–64.
Wang XG, Wan XC, Hu SX, Pan CY. Study on the increase mechanism of the caffeine content during the fermentation of tea with microorganisms. Food Chem. 2008;107:1086–91.
Dash SS, Gummadi SN. Biodegradation of caffeine by Pseudomonas sp. NCIM 5235. Res J Microbiol. 2006;1:115–23.
Wang QP, Gong JS, Chisti Y, Sirisansaneeyakul S. Fungal isolates from a pu-erh type tea fermentation and their ability to convert tea polyphenols to theabrownins. J Food Sci. 2015;80:M809–17.
Tan HP, Xu WP, Zhao AP, Zhou LL, Liu MD, Tan FY, Zou Y, Wang YJ. Determination of catechins and purine alkaloids in tea by high performance liquid chromatography. Anal Lett. 2012;45:2530–7.
Alvarenga N, Birolli WG, Seleghim Mirna HR, Porto A. Biodegradation of methyl parathion by whole cells of marine-derived fungi Aspgillus sydowii and Penicillium decaturense. Chemosphere. 2014;117:47–52.
Algharrawi Khalid HR, Summers Ryan M, Sridhar G, Mani S. Direct conversion of theophylline to 3-methylxanthine by metabolically engineered E. coli. Microb Cell Factories. 2015;14:203–15.
Sánchez GG, Roussos S, Augur C. Effect of caffeine concentration on biomass production, caffeine degradation, and morphology of Aspergillus tamari. Folia Microbiol. 2013;58:195–200.
Gokulakrishnan S, Chandraraj K, Gummadi Sathyanarayana N. A preliminary study of caffeine degradation by Pseudomonas sp. GSC1182. Int J Food Microbiol. 2007;113:346–50.
Mohanpuria P, Kumar V, Ahuja PS, Yadav SK. Producing low-caffeine tea through post-transcriptional silencing of caffeine synthase mRNA. Plant Mol Biol. 2011;75:523–34.
Xia ZZ, Ni YN, Kokot S. Simultaneous determination of caffeine, theophylline and theobromine in food samples by a kinetic spectrophotometric method. Food Chem. 2014;141:4087–93.
Matkar K, Chapla D, Divecha J, Nighojkar A, Madamwar D. Production of cellulase by a newly isolated strain of Aspergillus sydowii and its optimization under submerged fermentation. Int Biodeter Biodeg. 2013;78:24–33.
He F, Sun YL, Liu KS, Zhang XY, Qian PY, Wang YF, Qi SH. Indole alkaloids from marine-derived fungus Aspergillus sydowii SCSIO00305. J Antibiot. 2012;65:109–11.
Song XQ, Zhang X, Han QJ, Li XB, Li G, Li RJ, Jiao Y. Xanthone derivatives from Aspergillus sydowii, an endophytic fungus from the liverwort Scapania ciliate S. Lac and their immunosuppressive activities. Phytochem Lett. 2013;6:318–21.
Sirisansaneeyakul S, Wannawilai S, Chisti Y. Repeated fed-batch production of xylitol by Candida magnoliae TISTR 5663. J Chem Technol Biot. 2013;88:1121–9.
Acknowledgements
We thank Kunming Dapu Tea CO., LTD, and Yunnan Institute of Microbiology for their assistance in sample collection and microorganism identification.
Funding
This work was supported by Modern Agricultural Industry Technology System of China (CARS-23) and the national natural science found of China (C161104). The funding bodies had no role in the design of the study, in data collection, analysis or interpretation, or in writing the manuscript.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Contributions
BXZ, CQM, XT: participated in research design; BXZ, CQM: participated in the writing of the paper; CQM, HZW: participated in the performance of the research; CQM, BXZ: participated in data analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Heating method effects on microbial count and main chemical components of tea infusion. Note: All date are presented as mean ± SD, A-Bp < 0.05 in the same column, ND: not detectable, TPs is the abbreviation of tea polyphenols. (DOCX 15 kb)
Additional file 2:
Figure S1. Colony characteristics of strain No. 5 on culture medium. Figure S2. Conidia structure of strain No.5 under optical microscope. Figure S3. Colony characteristics of strain No.1 on culture medium. Figure S4. Conidia structure of strain No.1 under optical microscope. (DOCX 3679 kb)
Additional file 3:
Figure S5. ITS sequences data of the target strains. (DOCX 16 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhou, B., Ma, C., Wang, H. et al. Biodegradation of caffeine by whole cells of tea-derived fungi Aspergillus sydowii, Aspergillus niger and optimization for caffeine degradation. BMC Microbiol 18, 53 (2018). https://doi.org/10.1186/s12866-018-1194-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-018-1194-8