Abstract
Background
The emergence and spread of multidrug resistant methicillin-resistant Staphylococcus aureus (MDR-MRSA) has serious health consequences in the presence of sub-MIC antibiotics. Therefore, this study was designed to evaluate β-lactamase activity, efflux activity, biofilm formation, and gene expression pattern in Staphylococcus aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 exposed to sublethal concentrations of levofloxacin and oxacillin.
Results
The decreased MICs were observed in S. aureus KACC and S. aureus ATCC when exposed to levofloxacin and oxacillin, while and S. aureus CCARM remained resistance to streptomycin (512 μg/mL) in the presence of levofloxacin and imipenem (>512 μg/mL) in the presence of oxacillin. The considerable increase in extracellular and membrane-bound β-lactamase activities was observed in S. aureus ATCC exposed to oxacillin (>26 μmol/min/mL). The antibiotic susceptibility of all strains exposed to EPIs (CCCP and PAβN) varied depending on the classes of antibiotics. The relative expression levels of adhesion-related genes (clfA, clfB, fnbA, fnnB, and icaD), efflux-related genes (norB, norC, and qacA/B), and enterotoxin gene (sec) were increased more than 5-fold in S. aureus CCARM. The eno and qacA/B genes were highly overexpressed by more than 12- and 9-folds, respectively, in S. aureus CCARM exposed to levofloxacin. The antibiotic susceptibility, lactamase activity, biofilm-forming ability, efflux activity, and gene expression pattern varied with the intrinsic antibiotic resistance of S. aureus KACC, S. aureus ATCC, and S. aureus CCARM exposed to levofloxacin and oxacillin.
Conclusions
This study would provide useful information for better understating of combination therapy related to antibiotic resistance mechanisms and open the door for designing effective antibiotic treatment protocols to prevent excessive use of antibiotics in clinical practice.
Similar content being viewed by others
Background
Over the last several decades, the overuse and misuse of broad-spectrum antibiotics has contributed to the increased emergence of antibiotic resistant pathogens such as methicillin-resistant Staphylococcus aureus (MRSA) [1, 2]. MRSA infections can cause mild to severe diseases, including skin lesion, toxic shock syndrome, endocarditis, osteomyelitis, and meningitis [2, 3]. Both hospital-acquired MRSA and community-acquired MRSA have currently become the leading causes of morbidity and mortality worldwide [3, 4]. Furthermore, MRSA can develop co-resistance to different classes of antibiotics, including fluoroquinolones, aminoglycosides, macrolides, tetracyclines, and β-lactams, known as multidrug resistant (MDR) MRSA [5–7]. The MDR-MRSA can frequently be exposed to subinhibitory concentrations of antibiotics, which leads to gene transfer, biofilm formation, and virulence gene expression [1]. The emergence and spread of MDR-MRSA has serious health consequences in the presence of sub-MIC antibiotics. Therefore, the effective control of MDR-MRSA is a research priority in hospitals and other healthcare facilities.
The different classes of antibiotics are used to improve the treatment of MDR bacterial infections, specifically carbapenem-resistant Enterobacteriaceae (CRE), which is known as combination therapy [8]. The benefits of using the combination therapy include the extension of antibiotic spectrum, synergistic enhancement of antibiotic activity, and decrease in the frequency of antibiotic resistance [8, 9]. Compared to the mono-therapy, the combination therapy can reduce the excessive use of antibiotics. However, controversially, there are also risks associated with the combination therapy. The selection of antibiotic resistance varies depending on the concentrations exposed to antibiotics [10]. Bacteria exposed to sublethal concentrations are likely to have a wide range of mutation variance compared to those exposed to lethal concentrations of antibiotics [1]. Relatively, few studies have investigated the mechanisms of resistance in MRSA under combination therapy. Therefore, in this study, we evaluated the physiological and molecular responses of MRSA to different classes of antibiotics in the presence of oxacillin and levofloxacin as measured by β-lactamase activity, efflux activity, biofilm formation, and gene expression pattern.
Methods
Bacterial strains and culture conditions
Strains of S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 were obtained from American Type Culture Collection (ATCC, Manassas, VA), Korean Agricultural Culture Collection (KACC, Suwon, Korea), and Culture Collection of Antibiotic Resistant Microbes (CCARM, Seoul, Korea), respectively. All strains were cultured in tryptic soy broth (TSB; BD, Becton, Dickinson and Co., Sparks, MD) at 37 °C for 20 h. After cultivation, cultures were centrifuged at 3000 × g for 20 min at 4 °C, washed twice with phosphate-buffered saline (PBS, pH 7.2), and then used for assays.
Single antibiotic susceptibility assay
The susceptibility of S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 to each antibiotic listed in Additional file 1: Table S1 was evaluated according to the Clinical Laboratory Standards Institute (CLSI) procedure with minor modification [11]. All antibiotic stock solutions were prepared by dissolving in distilled water (ampicillin, cefoxitin, ceftazidime, ceftriaxone, gentamicin, meropenem, oxacillin, streptomycin, and vancomycin), ethaol (chloramphenicol and tetracycline), glacial acetic acid (ciprofloxacin, levofloxacin, and norfloxacin), dimethyl sulfoxide (DMSO; imipenem) to obtain a final concentration of 10.24 mg/mL. Each stock solution (100 μL) was serially (1:2) diluted from 512 μg/mL with TSB in 96-well microtiter plates (BD Falcon, San Jose, CA). All strains were inoculated at a level of 106 CFU/mL and incubated at 37 °C for 18 h. Minimum inhibitory concentration (MIC) was determined at the lowest concentration of each antibiotic at which there is no visible growth. MIC breakpoints were used to define susceptible (S), intermediate (I), and resistant (R) strains [12, 13].
Combination antibiotic sensitivity test
The susceptibility of S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 to each antibiotic was also evaluated in the presence of oxacillin or levofloxacin. All strains (105 CFU/mL each) were inoculated in 96-well microtiter plates containing serial (1:2) antibiotic dilutions and basal antibiotic (oxacillin or levofloxacin; 1/2 MIC). MICs were determined as above mentioned.
β-lactamase activity assay
The ability of β-lactamase to hydrolyze nitrocefin was evaluated by using a spectrophotometric assay with minor modifications [14]. S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 cells exposed to 1/2 MIC of oxacillin or levofloxacin at 37 °C for 20 h were centrifuged at 3000 × g for 20 min at 4 °C. The cells suspended in PBS and cell-free supernatants were mixed with 20 μL of 1.5 mM nitrocefin and incubated at 37 °C for 30 min. The absorbance was measured every 5 min at 515 nm [15].
Ethidium Bromide (EtBr) cartwheel method
The cultured strains were centrifuged and then rinsed with PBS. The harvested cells were suspended in PBS with and without EPIs (CCCP, 0.5 μg/mL; PAβN, 8 μg/mL) [16, 17]. TSA plates containing EtBr (1 μg/mL) were prepared under darkness and divided into 9 sectors with cartwheel pattern. The prepared cells were swabbed on EtBr-agar plates and then incubated at 37 °C for 16 h. After incubation, the swabbed EtBr-agar plates were observed under UV illumination (Gel-doc XR System; Bio-Rad, Hertfordshire, UK).
Competitive efflux pump inhibition assay
The efflux pump activity of S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 was evaluated in the absence and presence of efflux pump inhibitors (EPIs), carbonyl cyanide-m-chlorophenyl hydrazone (CCCP) and phenylalanine-arginine-β-naphthylamide (PAβN). The changes in antibiotic susceptibility of S. aureus ATCC 15564, S. aureus KACC 10778, and S. aureus CCARM 3080 exposed to EPIs were determined as above mentioned.
Biofilm-forming ability assay
The biofilm formation potential by S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 was evaluated in the absence and presence of oxacillin or levofloxacin, which was based on the ability of strains to attach on 12-well polystyrene microtiter plate surface. All strains (106 CFU/mL each) were inoculated in TSB containing 1/2 MIC of oxacillin or levofloxacin and incubated at 37 °C for 24 h. After cultivation, each well was gently washed with PBS to remove loosely adhered cells. The adhered cells were harvested by using a cell scraper (Thermo Scientific Nunc, Rochester, NY). The collected cells were dispersed in PBS (1 mL) and then serially diluted (1:10) with PBS. The proper dilutions were plated on trypticase soy agar (TSA) using an Autoplate Spiral Plating System (Spiral Biotech Inc., Norwood, MA, USA). The plates were incubated at 37 °C for 24–48 h to enumerate adhered cells using a QCount Colony Counter (Spiral Biotech Inc.).
Quantitative PCR assay
The RNA extraction was carried out using RNeasy Protect Bacteria Mini kit (Qiagen, Hilden, Germany). Briefly, S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 cells (0.5 mL each) exposed to 1/2 MIC of oxacillin or levofloxacin at 37 °C for 20 h were mixed with 1 ml of RNAprotect Bacteria Reagent. The mixtures were centrifuged at 5000 × g for 10 min, and the collected cells were lysed with a buffer containing lysozyme. The lysates were mixed with ethanol to extract RNA using an RNeasy mini column. In order to synthesize cDNA, the RNA extracts were rinsed with a Wipe buffer to remove the genomic DNA and then mixed a master mixture of reverse transcriptase, RT buffer, and RT primer mix and then incubated at 42 °C for 15 min followed by 95 °C for 3 min. The PCR mixture (20 μl) containing 10 μl of 2× QuantiTect SYBR Green PCR Master, 2 μl of each primer, and 2 μl of cDNA, and 4 μl of RNase-free water was denatured at 95 °C for 30 s, followed by 45 cycles of 95 °C for 5 s, 55 °C for 20 s, and 72 °C for 15 s using an iCycler iQ™ system (Bio-Rad Laboratories, Hemel Hempstead, UK). The custom-synthesized oligonucleotides using IDT (Integrated DNA Technologies Inc., Coralville, IA, USA) as primers of S. aureus are listed in Additional file 2: Table S2. The relative gene expression levels were estimated using the comparative method [18]. The CT values of target genes in S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 cells exposed to 1/2 MIC of oxacillin or levofloxacin were compared to the CT values obtained from the control cells, respectively. The reference gene (16S ribosomal RNA) was used for normalization of target gene expression.
Statistical analysis
Data were analyzed by the Statistical Analysis System (SAS) software. All analyses were carried out in duplicate for three replicates. The general linear model (GLM) and Fisher’s least significant difference (LSD) procedures were used to determine significant mean differences at p < 0.05.
Results
Antibiotic susceptibility of S. aureus
The MICs of selected antibiotic against S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 were determined in absence and presence of antibiotics (levofloxacin and oxacillin) (Table 1). S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 were classified on the basis of MIC breakpoints as antibiotic-sensitive, intermediate, and antibiotic-resistant strains, respectively. S. aureus KACC 10778 and S. aureus ATCC 15564 were relatively sensitive to most antibiotics when compared to S. aureus CCARM 3080 which was resistant to all antibiotics with the exception of chloroamphenicol and vancomycin. The MIC values of most antibiotics against all strains tested were decreased in the presence of levofloxacin and oxacillin. However, no changes were observed in susceptibilities of S. aureus KACC 10778 to chloramphenicol when exposed to levofloxacin, gentamicin exposed to oxacillin, tetracycline exposed to levofloxacin and oxacillin and S. aureus ATCC 15564 to ceftazidime exposed to levofloxacin and tetracycline exposed to levofloxacin and oxacillin. The reduced susceptibility of S. aureus CCARM 3080 to streptomycin was observed in the presence of levofloxacin.
Lactamase activity
The extracellular and membrane-bound β-lactamase activities were measured in S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 exposed to levofloxacin and oxacillin. No significant change in β-lactamase activities was observed in S. aureus KACC 10778 and S. aureus CCARM 3080 exposed to levofloxacin. The highest extracellular and membrane-bound β-lactamase activities were observed in S. aureus ATCC 15564 exposed to oxacillin, increased to 33 and 26 μmol/min/mL, respectively (Fig. 1).
Hydrolyzing activity of extracellular β-lactamase a and membrane-bound β-lactamase b produced by Staphylococcus aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 exposed to a half MIC of oxacillin or levofloxacin. Means with different letters (a–c) on the bars are significantly different at p < 0.05
Efflux activity
The efflux activity of S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 was evaluated on TSA agar plates containing EtBr (Fig. 2). The level of fluorescence intensity was increased in S. aureus ATCC 15564 exposed to CCCP and PAβN compared to the control. The highest efflux activity was observed in S. aureus CCARM 3080 regardless of the presence of efflux pump inhibitors. The role of efflux pumps in the antibiotic resistance was evaluated in S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 exposed to efflux pump inhibitors, CCCP and PAβN (Fig. 3). The antibiotic susceptibility patterns of all strains exposed to efflux pump inhibitors varied in the types of antibiotics. The antibiotic activity of imipenem against S. aureus KACC 10778 was increased in the presence of efflux pump inhibitors, whereas the resistance of S. aureus KACC 10778 to streptomycin and tetracycline was increased in the presence of efflux pump inhibitors. The sensitivity of S. aureus ATCC 15564 to ampicillin, ciprofloxacin, and imipenem was increased in the presence of CCCP and PAβN. The sensitivity of S. aureus CCARM 3080 to imipenem, oxacillin, and streptomycin was increased in the presence of CCCP and PAβN.
Biofilm-forming ability
The biofilm-forming ability of S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 was evaluated in the presence of levofloxacin and oxacillin (Fig. 4). Compared to the control, the number of biofilm-forming cells of S. aureus KACC 10778 was reduced by approximately 2 log CFU/mL in the presence of oxacillin, whereas those of S. aureus ATCC 15564 and S. aureus CCARM 3080 were reduced by 0.5-1 log CFU/mL in the presence of levofloxacin and oxacillin.
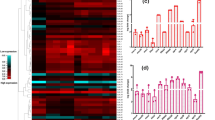
Differential gene expression
The relative expression of adhesion-related genes (clfA, clfB, eno, fib, fnbA, fnnB, and icaD), efflux-related genes (mdeA, norB, norC, and qacA/B), and enterotoxin gene (sec) were observed in S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 grown in the absence and presence of levofloxacin and oxacillin (Fig. 5). The relative expression levels of most selected genes were increased more than 5-fold in antibiotic-resistant S. aureus CCARM 3080 (Fig. 5a). The clfB, fnbB, norB, and qacA/B genes were overexpressed in S. aureus KACC 10778 grown in the presence of oxacillin (>3-fold), whereas the relative expression levels of eno, icaA, and icaD were decreased more than 5-fold in both levofloxacin and oxacillin (Fig. 5b). Most of genes in S. aureus ATCC 15564 were slightly overexpressed in the presence of levofloxacin and oxacillin (Fig. 5c). As shown in Fig. 5d, the eno and qacA/B genes were overexpressed by more than 12- and 9-fold, respectively, in S. aureus CCARM 3080 grown in the presence of levofloxacin. The norB was slightly overexpressed in S. aureus CCARM 3080 grown in the presence of levofloxacin and oxacillin.
Discussion
This study describes the antibiotic susceptibility and gene expression dynamics of S. aureus with different antibiotic resistance profiles when exposed to sub-MICs of levofloxacin and oxacillin. As antibiotic-resistant pathogens are frequently exposed to sublethal concentrations of antibiotic prescribed in hospitals, this study sheds light on the understanding of antibiotic resistance mechanisms and the effectiveness of combination therapy. This study investigated the relationship between phenotypic and genotypic properties of S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 exposed to a half MIC of levofloxacin or oxacillin.
The susceptibilities of S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 to most antibiotics were increased in the presence of levofloxacin and oxacillin, whereas no difference in the susceptibilities of S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 to ceftazidime, chloramphenicol, gentamicin, and vancomycin were observed in the presence of levofloxacin and oxacillin (Table 1). S. aureus CCARM 3080 showed the decreased susceptibility to streptomycin. This is in good agreement with a previous report that MRSA exhibited the enhanced resistance to other classes of antibiotics, leading to multidrug resistance [19]. Compared to S. aureus KACC 10778 and S. aureus ATCC 15564, the decreased oxacillin susceptibility was observed in S. aureus CCARM 3080 (MIC > 512 μg/mL), which may be attributed to the activation of penicillin-binding protein (PBP2a) encoded by mecA, but not due to the activation of β-lactamase [20, 21]. MRSA can acquire additional resistance to ciprofloxacin, causing a frequent failure in antibiotic treatment [6]. The successive antibiotic treatment may be not effective against bacterial infections because of the increase in bacterial adaptation to initial antibiotic exposure. Thus, the combination therapy is commonly used to broaden antibiotic spectrum and achieve synergistic effect in life-threatening infections [8, 9, 22]. As shown in Table 1, S. aureus CCARM 3080 exposed to levofloxacin was more susceptible to most classes of antibiotics than that exposed to oxacillin with the exception of streptomycin and vancomycin. Interestingly, the susceptibility of S. aureus CCARM 3080 to streptomycin, however, was decreased in the presence of levofloxacin. The combination therapy can lead to cross-resistance to different classes of antibiotics. The antibiotic susceptibility of S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 exposed to levofloxacin and oxacillin depends on the additional resistance mechanisms. Therefore, the systematic investigation is needed to understand the mechanisms underlying cross-resistance in combination therapy.
The extracellular and membrane-bound β-lactamase activities were influenced by the intrinsic antibiotic resistance of S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 when exposed to levofloxacin and oxacillin. The production of β-lactamase was considerably increased in S. aureus ATCC 15564 when exposed to oxacillin, suggesting that the stabilities of β-lactam antibiotics were enhanced against staphylococcal β-lactamases (Fig. 1). Methicillin, oxacillin, cephalothin are less susceptible to hydrolysis by staphylococcal β-lactamases [23]. The resistance of S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 to β-lactam antibiotics may be associated with low-affinity PBPs and membrane permeability [21, 23, 24]. On the other hand, the results imply that the inappropriate selection of antibiotics for combination therapy can lead to the induction of β-lactamases.
S. aureus KACC 10778 exhibited low efflux activity in the absence and presence of inhibitors (CCCP and PAβN), whereas the efflux activity of S. aureus ATCC 15564 was effectively inhibited by CCCP and PAβN (Fig. 2). The highest efflux activity was observed in S. aureus CCARM 3080, which was not even reduced by efflux pump inhibitors. This suggests that there exist the inhibitor-insensitive efflux pump systems in S. aureus CCARM 3080, resulting in multidrug resistance. The enhanced efflux activity is a main cause of multidrug resistance in S. aureus CCARM 3080 [17].
S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 were exposed to efflux pump inhibitors to characterize the substrate specificity of multidrug efflux pumps (Fig. 3). The MICs of imipenem against S. aureus KACC 10778, ampicillin, ciprofloxacin, and imipenem against S. aureus ATCC 15564, and imipenem, oxacillin, and streptomycin against S. aureus CCARM 3080 were considerably decreased in the presence of efflux pump inhibitors (CCCP and PAβN), suggesting the antibiotic resistance is associated with the proton motive force and substrate competition-dependent efflux systems [25]. The plasma membrane is depolarized in the presence of CCCP, which collapses proton electrochemical gradient [25]. PAβN, a substrate of efflux pumps, acts as an inhibitor competing with antibiotics [14]. The antibiotics inducing PAβN-susceptible efflux can act as potential competitors for multidrug efflux pump systems. The multidrug resistance in bacteria is directly related to the activity of efflux pumps [26, 27]. The reduced susceptibility to the different classes of antibiotics in resistant S. aureus CCARM 3080 was due to the interacting resistance mechanisms. As shown in Fig. 3, the MICs of ceftazidime to all strains tests were increased in the presence of PAβN, which corresponds to Table 1 and Fig. 3. The efflux pumps are stimulated by β-lactams. The efflux-mediated antibiotic resistance mechanism can affect the β-lactam uptake, resulting in the increase in β-lactam susceptibility [24]. In contrast, no changes in antibiotic susceptibility between absence and presence of efflux pump inhibitors were observed in S. aureus KACC 10778 (ciprofloxacin, levofloxacin, meropenem, and oxacillin), S. aureus ATCC 15564 (levofloxacin and meropenem), and S. aureus CCARM 3080 (ciprofloxacin and levofloxacin). The observations imply that there are various types of efflux pump systems in S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 [28]. Unlike PAβN, CCCP can decrease the accumulation of antibiotics, resulting in the elevated MICs in S. aureus KACC 10778 (ampicillin and streptomycin), S. aureus ATCC 15564 (ampicillin and oxacillin), and S. aureus CCARM 3080 (ampicillin, imipenem, meropenem, and oxacillin) [28].
The reduction in the number of biofilm cells was more noticeable in S. aureus KACC 10778 exposed to oxacillin than the multiple antibiotic-resistant S. aureus ATCC 15564 and S. aureus CCARM 3080 (Fig. 4). The antibiotic-resistant strains are more likely to obtain cross-protection against various stresses such as acid, heat, and antibiotics [29]. The exposure to certain antibiotics can positively associated with the formation of bacterial biofilms [30]. The degree of biofilm formation depends on the type of antibiotics, including biofilm-inducing (ampicillin, vancomycin, and ceftizoxime) and biofilm-noninducing antibiotics (gentamycin) [30]. The enhanced resistance of biofilms to environmental stresses and antibiotics play an important role in the pathogenesis of bacterial infections [31]. Therefore, the combination therapy needs to take into account the risk factors for multiple antibiotic-resistant bacterial infections.
Most of the overexpressed adhesion-, efflux pump-, and enterotoxin genes were observed in S. aureus CCARM 3080 compared to S. aureus KACC 10778 (Fig. 5a). The increased expression levels of adhesion-related genes in S. aureus ATCC 15564 and S. aureus CCARM were directly related to the enhanced biofilm-forming ability [32]. The genes encoding clumping factor, laminin-, and fibronectin-binding proteins were overexpressed in S. aureus ATCC 15564 and S. aureus CCARM when exposed to levofloxacin and oxacillin, suggesting sublethal concentrations of antibiotics can improve the surface adhesion properties of bacteria [1]. The increased resistance of S. aureus CCARM 3080 to multiple antibiotics may be mediated by the overexpression of mdeA, norB, norC, and qacA/B genes [33–36]. The expression level of sec gene was increased more than 20-fold in S. aureus CCARM 3080, suggesting that staphylococcal enterotoxin can be a major cause of staphylococcal infections [37]. Most genes were overexpressed in resistance strains (S. aureus ATCC 15564 and S. aureus CCARM) when exposed to levofloxacin and oxacillin (Fig. 5c-d). The multidrug resistance of S. aureus ATCC 15564 and S. aureus CCARM was attributed to the overexpression of efflux pump-related genes (mdeA, norB, norC, and qacA/B) [17]. However, the different expression levels of genes encoding efflux pump system in S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 were observed between exposures to levofloxacin and oxacillin. The expression of efflux pump-related genes is induced depending on the exposure to different classes of antibiotics as substrates. Ciprofloxacin is a common substrate of efflux pump systems (NorA, NorB, and NorC) and cationic lipophilic drugs are preferential substrates of QacA/B, a major facilitator superfamily (MFS) [38, 39]. MRSA carries qacA and qacB in higher rate than MSSA [36]. Kanamycin, linezolid, and lincomycin are the substrates of LmrS multidrug efflux pump, which is similar to EmrB of Escherichia coli and FarB of Neisseria gonorrhoeae [7]. The major substrate of MdeA multidrug efflux pump is norfloxacin [39]. Tetracycline, fluoroquinolone, and macrolides are the substrates of TetA(K), SdrM, and Mdf(A) efflux pump systems, respectively [39, 40]. The overexpression of the efflux pump systems contributes to the enhanced resistance to their substrates. Bacteria exposed to sublethal concentration can become resistant to multiple antibiotics in a high frequency, whereas lethal concentrations can induce single mutation [1]. Therefore, a proper combination therapy needs to be designed on the basis of antibiotic resistance mechanisms and profiles of target pathogens, which can increase the susceptibility to combination therapy and prevent the spread of newly acquired antibiotic resistance.
Conclusions
This study highlights the varied and interactive characteristics of antibiotic resistance in S. aureus KACC 10778, S. aureus ATCC 15564, and S. aureus CCARM 3080 exposed to sub-MICs of levofloxacin and oxacillin. The phenotypic and genotypic properties of S. aureus with different antibiotic resistance profiles varied in terms of lactamase activity, efflux activity, biofilm-forming ability, and gene expression pattern. The antibiotic-resistant S. aureus can acquire cross resistance between different classes of antibiotics when exposed to sublethal concentration, ascribed to differential activity of β-lactamase and efflux pump systems. The results obtained in this study indicate that monitoring antibiotic susceptibility patterns is essential to effectively treat antibiotic-resistant bacterial infections in case of continuous antibiotic exposure, and appropriate combination therapy is required in treating pathogens with different levels of antibiotic resistance. In addition, the characteristic changes in phenotypic and genotypic expression can be used to accurately detect antibiotic-resistant bacteria, which plays an important role in designing antibiotic treatment plan and developing new diagnostic technique for antibiotic resistance. Further systematic studies taking into account molecular approaches are needed to demonstrate the multiple antibiotic resistance mechanisms of pathogens exposed to various antibiotics through combination therapy.
Abbreviations
CCCP, carbonyl cyanide-m-chlorophenyl hydrazine; CFU, colony forming unit; CRE, carbapenem-resistant Enterobacteriaceae; EPI, efflux pump inhibitor; MDR, multidrug resistance; MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; PAβN, phenylalanine-arginine-β-naphthylamide
References
Andersson DI, Hughes D. Microbiological effects of sublethal levels of antibiotics. Nat Rev Micro. 2014;12(7):465–78.
DeLeo FR, Chambers HF. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clin Invest. 2009;119(9):2464–74.
Decousser J-W, Desroches M, Bourgeois-Nicolaos N, Potier J, Jehl F, Lina G, et al. Susceptibility trends including emergence of linezolid resistance among coagulase-negative staphylococci and meticillin-resistant Staphylococcus aureus from invasive infections. Int J Antimicrob Agent. 2015;46(6):622–30.
Tesařová M, Horká M, Moravcová D, Svojanovská L, Mlynarikova K, Růžička F. SDS-PAGE and gel IEF: Tool for differentiation of methicillin-resistant and methicillin-sensitive strains of Staphylococcus aureus. Curr Microbiol. 2015;72(3):315–20.
Munier A-L, de Lastours V, Barbier F, Chau F, Fantin B, Ruimy R. Comparative dynamics of the emergence of fluoroquinolone resistance in staphylococci from the nasal microbiota of patients treated with fluoroquinolones according to their environment. Int J Antimicrob Agent. 2015;46(6):653–9.
Weber SG, Gold HS, Hooper DC, Karchmer AW, Carmeli Y. Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg Infect Dis. 2003;9(11):1415–22.
Floyd JL, Smith KP, Kumar SH, Floyd JT, Varela MF. LmrS is a multidrug efflux pump of the major facilitator superfamily from Staphylococcus aureus. Antimicrob Agent Chemother. 2010;54(12):5406–12.
Ahmed A, Azim A, Gurjar M, Baronia AK. Current concepts in combination antibiotic therapy for critically ill patients. Ind J Crit Care Med. 2014;18(5):310–4.
Vestergaard M, Paulander W, Marvig RL, Clasen J, Jochumsen N, Molin S, et al. Antibiotic combination therapy can select for broad-spectrum multidrug resistance in Pseudomonas aeruginosa. Int J Antimicrob Agent. 2016;47(1):48–55.
Sandegren L. Selection of antibiotic resistance at very low antibiotic concentrations. Upsala J Med Sci. 2014;119(2):103–7.
CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, Approved standard M07-A8. 2009.
Hamilton-Miller JMT, Shah S. Activity of glycylcyclines CL 329998 and CL 331002 against minocycline-resistant and other strains of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1996;37(6):1171–5.
CLSI. Performance standards for antimicrobial susceptibility testing; Twenty-fourth informational supplement. Fifteenth informational supplement M100-S24 Clinical and Laboratory Standards Institute, Wayne, PA, USA. 2014.
Matsumoto Y, Hayama K, Sakakihara S, Nishino K, Noji H, Iino R, et al. Evaluation of multidrug efflux pump inhibitors by a new method using microfluidic channels. PLoS ONE. 2011;6(4):e18547.
Sharma S, Ramnani P, Virdi JS. Detection and assay of β-lactamases in clinical and non-clinical strains of Yersinia enterocolitica biovar 1A. J Antimicrob Chemother. 2004;54(2):401–5.
Costa SS, Falcão C, Viveiros M, Machado D, Martins M, Melo-Cristino J, et al. Exploring the contribution of efflux on the resistance to fluoroquinolones in clinical isolates of Staphylococcus aureus. BMC Microbiol. 2011;11(1):1–12.
Martins M, Viveiros M, Couto I, Costa SS, Pacheco T, Fanning S, et al. Identification of efflux pump-mediated multidrug-resistant bacteria by the ethidium bromide-agar cartwheel method. In Vivo. 2011;25(2):171–8.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT Method. Method. 2001;25(4):402–8.
Shahkarami F, Rashki A, Rashki GZ. Microbial susceptibility and plasmid profiles of methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus. Jundishapur. J Microbiol. 2014;7(7):e16984.
Jung J-S, Shin W-S, Kim S-K, Park Y-S. Different responses of MSSA and MRSA to oxacillin of their respective MICs. J Bacteriol Virol. 2009;39:287–94.
Stapleton PD, Taylor PW. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci Prog. 2002;85(Pt 1):57–72.
Rybak MJ, McGrath BJ. Combination antimicrobial therapy for bacterial infections. Drugs. 2012;52(3):390–405.
Hartman BJ, Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984;158(2):513–6.
Pages J-M, Lavigne J-P, Leflon-Guibout V, Marcon E, Bert F, Noussair L, et al. Efflux pump, the masked side of ß-lactam resistance in Klebsiella pneumoniae clinical isolates. PLoS ONE. 2009;4(3):e4817.
Pagès J-M, Masi M, Barbe J. Inhibitors of efflux pumps in Gram-negative bacteria. Trend Mol Med. 2005;11(8):382–9.
Yasufuku T, Shigemura K, Shirakawa T, Matsumoto M, Nakano Y, Tanaka K, et al. Correlation of overexpression of efflux pump genes with antibiotic resistance in Escherichia coli strains clinically isolated from urinary tract infection patients. J Clin Microbiol. 2011;49(1):189–94.
Costa SS, Viveiros M, Amaral L, Couto I. Multidrug efflux pumps in Staphylococcus aureus: an update. Open Microbiol J. 2013;7:59–71.
Kehrenberg C, de Jong A, Friederichs S, Cloeckaert A, Schwarz S. Molecular mechanisms of decreased susceptibility to fluoroquinolones in avian Salmonella serovars and their mutants selected during the determination of mutant prevention concentrations. J Antimicrob Chemother. 2007;59(5):886–92.
Langsrud S, Sundheim G, Holck AL. Cross-resistance to antibiotics of Escherichia coli adapted to benzalkonium chloride or exposed to stress-inducers. J Appl Microbiol. 2004;96(1):201–8.
Kafil HS, Mobarez AM, Moghadam MF, Zs H, Yousefi M. Gentamicin induces efaA expression and biofilm formation in Enterococcus faecalis. Microb Pathog. 2016;92:30–5.
Fey PD, Olson ME. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol. 2010;5(6):917–33.
Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agent. 2010;35(4):322–32.
Ding Y, Onodera Y, Lee JC, Hooper DC. NorB, an efflux pump in Staphylococcus aureus strain MW2, contributes to bacterial fitness in abscesses. J Bacteriol. 2008;190(21):7123–9.
He X, Ahn J. Differential gene expression in planktonic and biofilm cells of multiple antibiotic-resistant Salmonella Typhimurium and Staphylococcus aureus. FEMS Microbiol Lett. 2011;325(2):180–8.
Truong-Bolduc QC, Strahilevitz J, Hooper DC. NorC, a new efflux pump regulated by MgrA of Staphylococcus aureus. Antimicrob Agent Chemother. 2006;50(3):1104–7.
Ho C-M, Li C-Y, Ho M-W, Lin C-Y, Liu S-H, Lu J-J. High rate of qacA- and qacB-positive methicillin-resistant Staphylococcus aureus isolates from chlorhexidine-impregnated catheter-related bloodstream infections. Antimicrob Agent Chemother. 2012;56(11):5693–7.
Derzelle S, Dilasser F, Duquenne M, Deperrois V. Differential temporal expression of the staphylococcal enterotoxins genes during cell growth. Food Microbiol. 2009;26(8):896–904.
Schumacher MA, Miller MC, Grkovic S, Brown MH, Skurray RA, Brennan RG. Structural mechanisms of QacR induction and multidrug recognition. Science. 2001;294(5549):2158–63.
Andersen JL, He G-X, Kakarla P, Kc R, Kumar S, Lakra WS, et al. Multidrug efflux pumps from Enterobacteriaceae, Vibrio cholerae and Staphylococcus aureus bacterial food pathogens. Int J Environ Res Pub Health. 2015;12(2):1487–547.
Levy SB. Active efflux mechanisms for antimicrobial resistance. Antimicrob Agent Chemother. 1992;36(4):695–703.
Acknowledgements
Not applicable.
Funding
This work supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant number : HI15C-1798-010015).
Availability of data and materials
The data supporting the conclusions are included within the manuscript and also provided in additional files.
Authors’ contributions
AJ conducted all experiments and also contributed to the writing and preparation of the manuscript. JA contributed to the experimental design, data interpretation, and manuscript writing. Both authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Table S1.
Description of antibiotics used in this study. (DOCX 13 kb)
Additional file 2: Table S2.
Primer sequences used in qPCR analysis for Staphylococcus aureus. (DOCX 13 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jo, A., Ahn, J. Phenotypic and genotypic characterisation of multiple antibiotic-resistant Staphylococcus aureus exposed to subinhibitory levels of oxacillin and levofloxacin. BMC Microbiol 16, 170 (2016). https://doi.org/10.1186/s12866-016-0791-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-016-0791-7