Abstract
Backgroud
It is important to expound the opposite clinical outcomes between children and adulthood for eradicate malaria. There remains unknown about the correlation between adaptive immune response and age-related in malaria.
Methods
4 and 8-week-old mice were used to mimic children and adulthood, respectively. Parasitemia and the survival rate were monitored. The proportion and function of Th1 and Th2 cells were detected by FACS. The levels of IFN-γ, IL-4, total IgG, IgG1, IgG2a and Plasmodium yoelii MSP-1-specific IgG were measured by ELISA.
Results
The adult group showed greater resistance to P. yoelii 17XL infection, with lower parasitemia. Compared with 4-week-old mice, the percentage of CD4+T-bet+IFN-γ+ Th1 cells as well as IFN-γ production were significantly increased on day 5 p.i. in the 8-week-old mice after P. yoelii 17XNL infection. The percentage of CD4+GATA3+IL-4+ Th2 cells and CD4+CXCR5+ Tfh cells, and IL-4 production in the 8-week-old mice significantly increased on day 5 and day 10 after P. yoelii 17XNL infection. Notably, the levels of total IgG, IgG1, IgG2a and P. yoelii MSP-1-specific IgG were also significantly increased in the 8-week-old mice. PD-1, a marker of exhaustion, was up-regulated on CD4+ or activated CD4+ T cells in the 8-week-old mice as compared to the 4-week-old group.
Conclusions
Thus, we consider that enhanced cellular and humoral adaptive immunity might contribute to rapid clearance of malaria among adults, likely in a PD-1-dependent manner due to induction of CD4+ T cells exhaustion in P. yoelii 17XNL infected 8-week-old mice.
Similar content being viewed by others
Background
Malaria is a serious infectious disease, which cause of mortality and morbidity in tropical countries [1]. According to WHO report, malaria transmission was found in 91 countries,with Africa experiencing disproportionately high malaria cases (90% of the total) and accounting for 91% of total malaria deaths worldwide [2]. Notably, children under the age of 5 years are particularly vulnerable to plasmodium infection. More than two-thirds of malaria deaths (70%) occur in this age group [3]. Of the five Plasmodium species that infect humans, Plasmodium falciparum and Plasmodium vivax are the most common, and P. falciparum is the most virulent and responsible for the majority of deaths [2, 3]. In addition, the multiplicity of infection (MOI) varies depending on the overall prevalence of infection in the population, and the age of the individual [4, 5]. The young children are highly susceptible to clinical illness and high parasitemia, whereas the adults are highly resistant [4], resulting in a major difference in the spectrum of disease manifestations between children and adults [6]. Therefore, understanding the immunological mechanisms involved in susceptibility to different virulent Plasmodium species during infection in children or adulthood could contribute to the development of an immunologically based control strategy to prevent or treat this devastating disease.
Upon infection, anti-parasite immunity plays a pivotal role in removing the parasite from the blood. Firstly innate immunity is activated via complement system, innate lymphoid cells and dendritic cells (DCs), act to limit the acute phase of parasitemia, but are insufficient to clear the infection [7,8,9]. When DCs present the processed antigen, adaptive immunity is activated. Direct cell cytotoxicity, cytokine secretion as well as anti-malarial antibody work together for effective parasite clearance [10,11,12,13]. Childhoods and young children are more susceptible to malaria infection than adults worldwide [4]. Age-related changes in immune systems increased prevalence of asthma, nasal polyps and lung injury [14, 15]. However, whether differences in cellular and humoral immunity lead to this age-related infection profile remains unknown. Therefore, we used different virulent Plasmodium (lethal P.y17XL and non-lethal P.y17XNL) strains to infect4-week-old and 8-week-old BALB/c mice to mimic infancy and adulthood, respectively, in order to characterize the relationship between immune cell responses and age-related malaria infection among different age groups, and understand the mechanism of malaria immunity. We propose that the dynamics of MOI can be explained by a model of increasing acquired immunity to blood-stage infection with age.
Results
Comparison of different species of Plasmodium infection course in 4-week-old and 8-week-old BALB/c mice
To investigate the relationship between age-related host immunity against malaria infection, we used BALB/c mice of different age groups to mimic infancy and adulthood, and monitored parasitemia and the survival rate at different time points after lethal P.y17XL and non-lethal P.y17XNL infections. Within 20 days after P.y17XNL infection, 96% of the 8-week-old mice successfully survived whereas only 78% of the 4-week-old mice survived (Fig. 1a). In accordance with the survival rate, the parasitemia peaked at 12% in the 4-week-old mice on day 11 p.i. while it was only 7% in the 8-week-old group, although the onset of parasitemia was similar in both groups on day 3 p.i. (Fig. 1b). Similarly, parasitemia peaked at 80% in the 4-week-old mice on day 8 p.i., and all mice died; however, in the 8-week-old group, parasitemia peaked at 75% on day 8 p.i., subsequently declined, and all mice died on day 11 p.i. (Fig. 1d). Therefore, children were more susceptible to parasite infection, whereas the adult group seemed to be relatively resistant.
Parasitemia (a, c) and survival rate (b, d) of Py17.XNL or Py17.XL infection in 4-week-old and 8-week-old BALB/c mice. Parasitemia was calculated by counting the number of parasite-infected erythrocytes per 1000 erythrocytes. Mortality was checked daily and Mantel-Cox test analyzed the difference of survival rate (Fig. A χ2 = 3.580, p = 0.059, by Mantel-Cox test, Fig. C χ2 = 1.483, p = 0.22, by Mantel-Cox test). The data are representative of two separate experiments
Comparison of Th1 immune response in different species of Plasmodium-infected 4-week-old and 8-week-old BALB/c mice
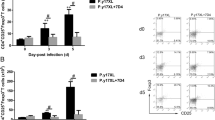
Next, the relationship between Th1 cell responses and age during the early stage of plamodium infection was determined. The percentage of CD4+ T-bet+ IFN-γ + Th1 cells was determined by flow cytometry, and the level of IFN-γ in splenocytes was measured by ELISA. Compared with 4-week-old mice, the frequency and absolute number of Th1 cells were significantly increased in P.y17XNL-infected 8-week-old mice on day 5 p.i. (Fig. 2a-c) (p < 0.05). The level of IFN-γ in P.y17XNL-infected 8-week-old mice on day 5 p.i. had the same trend as Th1 cells (Fig. 2g). Interestingly, in P.y17XL-infected 8-week-old mice, the frequency and absolute number of Th1 cells peaked on day 3 p.i. (Fig. 2d-f) (p < 0.05), then subsequently decreased, but remained higher than normal control on day 5 p.i. (p < 0.05). Notably, the level of IFN-γ in lethal P.y17XL-infected mice was significantly increased on day 5 p.i. (p < 0.05), but there was no obvious difference between the 4-week-old mice and 8-week-old mice (Fig. 2h). This data suggested that enhanced Th1 cell responses might be associated with age-related non-lethal P.y17XNL infection and resistance during the early stage of lethal P.y17XL infection.
Flow cytometric and ELISA analysis demonstrated Th1 immune response in different species of Plasmodium-infected 4-week-old and 8-week-old BALB/c mice. Two-dimensional contour map (upper-left panel) (a), column diagram: upper-left panel, the proportion of Th1 cells in CD4+ T cells (B) and absolute cell number (c) of CD4+T-bet+IFN-γ+ Th1 cells in 4-week-old and 8-week-old BALB/c mice after Py17XL infection are displayed; Representative dot plots (lower-left panel) (d), column diagram:lower-left panel, the proportion of Th1 cells in CD4+ T cells (e) and absolute cell number (f) of CD4+T-bet+IFN-γ+ Th1 cells in 4-week-old and 8-week-old BALB/c mice after Py17XNL infection are displayed. Level of IFN-γ (g, h) in spleen cell supernatants in Py17XNL/Py17XL-infected BALB/c mice were measured. Results are presented as arithmetic mean of 9 mice each group ± SE. Single asterisk (*) and double asterisks (**) indicate p < 0.05 and p < 0.01, respectively, as compared with control mice. A single pound sign (#) and double pound sign (##) indicate p < 0.05 and p < 0.01, respectively, as compared with 8-week-old mice. Normal control group: 0 day group
Comparison of Th2 immune response in different species of Plasmodium-infected 4-week-old and 8-week-old BALB/c mice
To assess the characteristics of Th2 cell responses and their relationship with age during the late stage of plasmodium infection, we evaluated the percentage and absolute number of CD4+GATA3+IL-4+ Th2 cells and interleukin-4 (IL-4) production. The proportion and absolute number of Th2 cells were elevated in both groups on day 5 p.i. and day 10 p.i. after P.y17XNL infection as compared to normal control (Fig. 3a-c) (p < 0.05). Following, we found that there was obvious difference in the percentage and absolute number of Th2 cells on day 5 and 10 p.i. between the 4-week-old and 8 week-old BALB/c mice (Fig. 3b) (p < 0.05). Consistently, the level of IL-4 production in P.y17XNL-infected 8-week-old mice was significantly increased as compared to the 4-week-old mice on day 5 p.i. and day 10 p.i. (Fig. 3d) (p < 0.05). In addition, we detected the percentage and absolute number of CD4+CXCR5+ Tfh cells, recognized as specificized providers of cognate B cell help. The percentage and absolute number of CD4+CXCR5+ Tfh cells peaked on day 5 p.i., and then decreased to normal level on day 10 p.i. in the 4-week-old mice. However, in the 8-week-old mice, the percentage and absolute number of CD4+CXCR5+ Tfh cells were significantly increased on day 10 p.i. as compared to the 4-week-old mice (Fig. 3e, f, 3G) (p < 0.05). As expected, the percentage and absolute number of Th2 cells, and CD4+CXCR5+ Tfh cells, and the level of IL-4 were significantly elevated in P.y17XNL-infected 8-week-old group during the late stage of malaria infection. These results indicated that an enhanced Th2 immunity during non-lethal P.y17XNL infection might contribute to rapid clearance of Plasmodium in adults.
Flow cytometric and ELISA analysis demonstrated Th2 immune response in different species of Plasmodium-infected 4-week-old and 8-week-old BALB/c mice. Representative dot plots (upper-left panel) (a), column diagram:upper-left panel, the proportion of Th2 cells in CD4+ T cells (b) and absolute cell number (c) of CD4+GATA3+IL-4+ Th2 cells in 4-week-old and 8-week-old BALB/c mice after P.y17XNL infection are displayed. Level of IL-4 (d) in spleen cell supernatants in P.y17XNL-infected BALB/c mice were measured. Representative dot plots (upper-left panel) (e), The proportion (f) and absolute cell number (g) of CD4+CXCR5+Tfh cells were measured by flow cytometry in 4-week-old and 8-week-old BALB/c mice after Py17XNL infection. Results are presented as arithmetic mean of 9 mice each group ± SE. Single asterisk (*) and double asterisks (**) indicate p < 0.05 and p < 0.01, respectively, as compared with control mice. A single pound sign (#) and double pound sign (##) indicate p < 0.05 and p < 0.01, respectively, as compared with 8-week-old mice. Normal control group: 0 day group
PD-1 signal regulated immune response in different species of Plasmodium-infected 4-week-old and 8-week-old BALB/c mice
PD-1 signaling plays an essential role in regulating immune cell exhaustion. To explore whether PD-1 signaling mediated effector T cell exhaustion and facilitated persistent infection in infancy or adulthood, we detected the expression of PD-1 on CD4+ or activated CD4+ T cells after lethal P.y17XL and non-lethal P.y17XNL infection by flow cytometry. The expression of PD-1 on CD4+ or activated CD4+ T cells was significantly increased on day 5 and day 10 p.i. after lethal P.y17XL and non-lethal P.y17XNL infections. Compared with 8-week-old mice, the expression of PD-1 on CD4+ or activated CD4+ T cells after non-lethal P.y17XNL infection on day 10 p.i. was significantly raised in the 4-week-old mice (Fig. 4a-c) (p < 0.05). Interestingly, in lethal P.y17XL infection, the expression of PD-1 on CD4+ or activated CD4+ T cells was higher in the 8-week-old mice than in the 4-week-old mice on day 10 p.i. (Fig. 4d-f).
Flow cytometric analysis demonstrated PD-1 signaling promoted immune response in different species of Plasmodium-infected 4-week-old and 8-week-old BALB/c mice. Representative dot plots (upper-left panel) (a) and column diagram:upper-left panel, the proportion of CD4+PD-1+ cells in splenocytes (b) and absolute cell number (c) of CD4+PD-1+ cells in 4-week-old and 8-week-old BALB/c mice after P.y17XNL infection are displayed. Representative dot plots (lower-left panel) (d) and column diagram:lower-left panel, the proportion of CD4+PD-1+ cells in splenocytes (e) and absolute cell number) (f) of CD4+PD-1+ cells in 4-week-old and 8-week-old BALB/c mice after P.y17XL infection are displayed. Results are presented as arithmetic mean of 9 mice each group ± SE. Single asterisk (*) and double asterisks (**) indicate p < 0.05 and p < 0.01, respectively, as compared with control mice. A single pound sign (#) and double pound sign (##) indicate p < 0.05 and p < 0.01, respectively, as compared with 8-week-old mice. Normal control group: 0 day group
The levels of total and P.y MSP-1-specific antibody in P.y17XNL-infected 4-week-old and 8-week-old BALB/c mice
Protection from clinical malaria has been reported to be associated with both the breadth and magnitude of the antibody responses to merozoite antigens [16]. ELISA of B cell-related total IgG, IgG1 and IgG2a also showed a significant difference in antibody production in adult mice as compared to children mice on day 10 p.i. (Fig. 5a, b, c). Interestingly, compared with 4-week-old mice, P.y MSP-1-specific IgG antibody production was increased in the 8-week-old mice during malaria infection (Fig. 5d) (p < 0.05).
ELISA analysis demonstrated the levels of total and P.y MSP-1-specific antibodies in spleen supernatants of P.y17XNL-infected 4-week-old and 8-week-old BALB/c mice. IgG (a), IgG1 (b), IgG2a (c) and P.y MSP-1-specific IgG (d) were measured in supernatants of P.y17XNL-infected 4-week-old and 8-week-old BALB/c mice by ELISA. Results are presented as arithmetic mean of 9 mice each group ± SE. Single asterisk (*) and double asterisks (**) indicate p < 0.05 and p < 0.01, respectively, as compared with control mice. A single pound sign (#) and double pound sign (##) indicate p < 0.05 and p < 0.01, respectively, as compared with 8-week-old mice. Normal control group: 0 day group
Discussion
Malaria infection is known to be age-related, with children being more susceptible than adults [17,18,19,20]. This study aimed to investigate whether the susceptibility to malaria infection in children and adulthood is associated with cellular and humoral immune responses, using a mouse model of lethal P.y17XL and non-lethal P.y17XNL infections in different age groups. Children mice were found to be more susceptible to P.y17XNL infection, with higher parasitemia at various time points. The adult group was more resistant to P.y17XL infection with lower parasitemia during the early stage of malaria infection. Importantly, enhanced cellular and humoral immunity, especially MSP-1 specific antibody, might contribute to rapid clearance of malaria in the adult group.
Malaria infections have various clinical symptoms, including febrile, anemia, acidosis and end-organ failure. To be mentioned, the difference of clinical phenotypic correlated with parasite proliferation rates, which can be controled by erythrocyte and hemoglobin polymorphisms [21]. In addition, disease profile can be determined by the strain and host [22]. In this study, 4-week-old and 8-week-old mice were used to mimic infancy and adulthood, respectively. We successfully established the age-related malaria infection mouse model to study the age-related anti-malaria immunity. Compared with 8-week-old group, the survival rate and parasitemia at different time points indicated that the 4-week-old group was same to both lethal and non-lethal parasite infections. After non-lethal P.y17XNL infection, parasitemia was significantly higher in the 4-week-old mice than the 8-week-old mice during the acute and chronic stages of infection. After lethal P.y17XL infection, a significant difference in parasitemia was observed in the early stage of infection. In accordance with the parasitemia, enhanced Th1 immune responses were only observed in the early stage in adult mice after lethal P.y17XL infection and enhanced adaptive immune responses (Th1 and Th2) were detected in adult mice during non-lethal P.y17XNL infection. These data suggested that the difference in response to non-lethal and lethal Plasmodium infections was associated with the pattern of immune cell responses in the host. Thus, clinical phenotypes of malaria infections can be determined by age and immune states from host.
Similar to other infectious diseases, accumulating evidences have indicated that CD4+ T cells are essential to control malaria infection [23,24,25,26]. Numerous studies have highlighted the role of Th1/Th2 cells or related signaling mechanisms in controlling malaria infection [27,28,29,30,31]. In this study, enhanced Th1 and Th2 responses were displayed in 8-week-old mice after malaria infection. Significantly higher percentage of Th1/Th2 cells and level of IFN-γ/IL-4 were observed in the 8-week-old mice as compared to the 4-week-old mice. In vitro studies also showed an enhanced Th1 cell response, which indicated an important role of Th1/Th2 cell-mediated age-related anti-malarial response. However, many studies suggested a shift from Th2 to Th1 cell responses with age. Li et al. found that IFN-γ level increased with age but not Th-related transcription factors, while IL-4 expression in plasma and CD4+ splenocytes declined with age [32]. A shift from Th2 towards Th1 immune responses was also observed in children with tertian or tropical malaria infection [33]. These studies partly supported our conclusion that enhanced Th1 cells might contribute to malaria clearance during the early stage of plasmodium infection. However, we observed enhanced Th2 cells during the late stage/chronic stage of malaria infection. Further studies are needed to investigate if any shift exists during the early stage of malaria infection. In addition, follicular T helper (Tfh) cells are essential for Plasmodium infection clearance by activating germinal center B cell responses [34,35,36,37]. Relative research found that the preferential localization of Tfh cells in the germinal center (GC) suggests a unique, intimate relationship between the Tfh cell and the B cell. Cytokines and cell-surface receptors provided by Tfh cells, as a kind of auxillary signal incompletely to keep GC B cells alive and proliferation via CD40L, IL-21 and IL-4 help [38, 39]. In this study, the percentage and absolute number of CD4+CXCR5+ Tfh cells peaked on day 5 p.i., and then decreased to normal level on day 10 p.i. in the 4-week-old mice. However, in the 8-week-old mice, the percentage and absolute number of CD4+CXCR5+ Tfh cells were significantly increased on day 10 p.i. as compared to the 4-week-old mice (Fig. 3e, f). Relative studies found that the addition of Tfh cells induces GC collapse, result for damage of B cells. Thus we speculated that GC perhaps has collapsed in young mice during the early stage of plasmodium infection, because Tfh cells increased rapidly. In addition, the GC is the primary site of B cell affinity maturation [39]. These studies supported our findings that the impaired function of antibody-secreting B cells and Tfh cells in childhood and children may account for their susceptibility to malaria infection.
We also observed a dampening of PD-1 signaling on activated CD4+ T cells after non-lethal P.y17XNL infection but not lethal P.y17XL infection in the 8-week-old mice. PD-1 co-inhibitory signaling was reported to regulate helper T cell differentiation and anti-Plasmodium humoral immunity [39], and PD-1 deficiency could enhance humoral immunity during malaria infection [40]. PD-1 was also a marker of T-cell exhaustion [41]. Several studies have also proven that chronic malaria infection drives T cell exhaustion through PD-1 signaling [42, 43]. Therefore, we speculated that during non-lethal infection, humoral immunity plays an essential role in the late stage of malaria clearance, perhaps correlated with enhanced PD-1 signaling on activated CD4+ T cells, which may help to drive CD4+ effector T cell exhaustion and promote persistent infection in children. Therefore, differences in PD-1 signaling could be observed in different age groups after non-lethal but not lethal malaria infection.
Several studies have confirmed that immune effector mechanisms are required to eliminate malarial parasites, and B cells secrete specific antibodies supported by Th2 cells, which can effectively remove the parasites to prevent the recidivation and recrudescence [44, 45]. Similarly, infusion of malaria hyperimmune serum resulted in rapid clearance of parasitized erythrocytes [45]. Merozoites proliferation from RBCs can be prevented by Anti-Plasmodium antibodies, depended on blocking cytoadherence to endotheliar capillary of iRBCs and promoting phagocytosis by mononuclear cells [46,47,48]. However, researchers found that levels of antimalarial antibodies continue to increase significantly resulting from chronic exposure to infection [49], perhaps correlated with impaired establishment of B cell memory [50]. Thus in young children,we can found the short-lived antibody responses [51,52,53,54]. In this study, we detected the levels of B cell-related total IgG, IgG1 and IgG2a in P.y17XNL-infected BALB/c mice. The results showed a difference in antibody production between adult and children mice, and the levels of total antibody might contribute to rapid clearance of malarial parasites in the adult group during the chronic stage of non-lethal P.y17XNL infection. Moreover, IgG1 and IgG3 antibodies against merozoite surface proteins (MSPs) are thought to be instrumental in protection, which is considered as a major vaccine candidate [55]. Therefore, we detected the levels of P.y MSP-1 specific antibody. Consistently, the dynamics of P.y MSP-1 specific antibody was the same as total antibody. These data implied that an enhanced antibody response during chronic stage of non-lethal P.y17XNL infection might contribute to rapid clearance of malaria in the adult group.
Conclusion
Taken together, the findings of this study revealed that in non-lethal P.y17XNL infection, higher burden of parasitemia in children mice were associated with weakened Th1 cellular immune responses, down-regulated humoral immunity with decreased percentage and number of Th2 and Tfh cells as well as lower level of antibody secretion and enhanced PD-1 signaling on activated CD4+ T cells. Higher resistance to lethal P.y17XL infection in the early stage in adult mice was associated with enhanced Th1 cellular immune responses and weakened PD-1 signaling on activated CD4+ T cells. These results provide a new insight on immune responses in malaria infection.
However, we have to consider a question: the expression of PD-1 on activated CD4+ T cells induced depletion of immune cells. On the one hand, depletion of immune cells induced down-regulation of anti-malaria immune response; on the other hand, exhaust of immune cells reduces the immunopathological injury in malaria-infected hosts. It is very difficult to regulate the dynamic balance between them. So there are many questions to deal with about using PD-1 antagonists to treat malaria infections.
Methods
Mice, parasite and experimental infection
The 4-week-old (90 mice) and 8-week-old (90 mice) female BALB/c mice were purchased from Beijing Animal Institute. P.y17XL and P.y17XNL strains were provided by Dr. Motomi Torii (Department of Molecular Parasitology, Ehime University Graduate School of Medicine, Ehime, Japan). Infections were initiated by intraperitoneal (i.p.) injection of 1 × 106 P.y 17XL or 1 × 106 P.y 17XNL parasitized erythrocytes in BALB/c mice. All animal procedures were conducted in compliance with the Regulations for the Administration of Affairs Concerning Experimental Animals (1988.11.1), and humanely treated. The experimental mice were matched for age and sex. Parasitemia was examined by light microscopy of Giemsa-stained, tail blood smears. Mortality was monitored daily. All experiments were performed in compliance with local animal ethics committee requirements. The animals were not submitted to euthanasia during the process of plasmodium infection. Other mice were submitted to euthanasia during detecting the relative index in indicated time points, the way to do it is posterior cervical dislocation after eyeball blood extraction.
Spleen cell culture
Spleen cell culture was prepared as previously described [56]. Briefly, we aseptically removed spleen from each mouse, and then passed through a sterile fine-wire mesh with 10 ml of RPMI1640 including 5% heat-inactivated fetal calf serum (FCS) (Hyclone Laboratories, Inc.), 25 mM Hepes (Life Technologies), 0.12% gentamicin (Schering, Montreal, Quebec, Canada) and 2 mM glutamine (Life Technologies). Cell suspensions were centrifuged at 350×g for 10 min at room temperature (RT). Using cold 0.17 M NH4Cl to lysed Erythrocytes. Following the cells were washed twice with fresh medium, and then viability of the spleen cells was confirmed by trypan blue exclusion, and was always > 90%. Spleen cells were adjusted to a final concentration of 107cells/ml in RPMI1640 supplemented with 10% heat-inactivated FCS. Aliquots (500 μl/well) of the cell suspension were incubated in 24-well flat-bottom culture plates (FALCON) in triplicate for 48 h at 37 °C in a humidified 5% CO2 incubator. Then, the plates were centrifuged at 350×g for 10 min at RT, supernatants were collected and stored at − 80 °C until they were assayed for the levels of IFN-g, IL-4, IgG, IgG1, IgG2a and P. y MSP-1-specific IgG.
Cytokine analysis
Commercial enzyme-linked immunosorbent assay (ELISA) kit smeasured levels of IFN-γ and IL-4 according to the manufacturer’s protocols (R&D Systems, Minneapolis, MN). Using a microplate reader read the OD values at 450 nm. The concentrations of cytokines in samples were calculated against the standard curve generated using recombinant IFN-g and IL-4, respectively.
Multiplex assay for antibody determination
Levels of total serum IgG, IgG1, IgG2a and P.y MSP-1-specific IgG were measured by ELISA as previously described with some modifications [57]. Briefly, Maxisorp flat-bottomed, 96-well microplates were coated overnight at 4 °C with 50 μg of P.y MSP-1 antigens in a carbonate-bicarbonate buffer (pH 9.6). The plates were washed with PBS-Tween (PBS-T) and blocked with 0.05% bovine serum albumin (BSA)-PBS-T. Next, 100 μl of plasma dilutions in 0.05% BSA-PBS-T (1:50 for P.y MSP-1 IgG) were added in duplicate and incubated at RT for 2 h. After washing with PBS-T, the plates were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (Sigma, USA) at a dilution of 1:5000. The OD values were read in a microplate reader at 490 nm.
Cell surface/intracytoplasmicstaining and flow cytometry
To assess the function of CD4+ T cells, we detected Tfh (CD4+CXCR5+cells), CD4+PD-1+cells and CD4+CD62−PD-1+cells, spleen cells from BALB/c mice infected with P.y17XL/P.y17XNL at different time points were double-stained with FITC-conjugated anti-CD4 (clone GK1.5, BD), BV421-conjugated anti-PD-1 (clone J43, BD), PE-conjugated anti-CXCR-5 (clone 2G8, BD) and APC-conjugated anti-CD62L (MEL-14, BD), followed by two washes, staining and analysis by flow cytometry.
To assess dynamics of Th1(CD4+T-bet+IFN-γ+) cells and Th2 (CD4+GATA3+IL-4+) cells, spleen cells from BALB/c mice infected with P.y17XL/P.y17XNL at different time points were triple-stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4 (clone GK1.5), PE-conjugated anti-T-bet (clone eBio4B10, eBioscience), APC-conjugated anti-IFN-γ (XMG1.2, BD) for Th1 cells, and FITC-conjugated anti-CD4 (clone GK1.5), PE-conjugated anti-GATA-3 (clone L50–823, BD), APC-conjugated anti-IL-4 (clone 11B11, BD) for Th2 cells. After stimulation for 2 h with PMA and ionomycin at 37 °C, Golgi Stop (BD Bioscience) was added to each reaction (1:500, vol/vol). After co-culture for 4 h at 37 °C, the cells were washed with 3% FCS and then resuspended in 100 μl of 3% FCS. FITC-anti-CD4, PE-anti-T-bet and PE-anti-GATA3 were added for surface staining. Then, the cells were fixed and permeabilized, and intracytoplasmic staining was performed using allophycocyanin (APC)-anti-IFN-γ. We used the isotype control antibodies as follows: Table 1. All antibodies were purchased from BD Pharmingen.
Statistical analysis
All analyses were performed using GraphPad Prism version 6.0 (GraphPad Software, La Jolla, CA). Data are presented as mean ± standard error of the mean (SEM). Survival analysis was performed using the Kaplan-Meier log-rank test. Statistical significance of differences between the two groups was assessed by unpaired Student’s t-tests. P-values were calibrated using Bonferroni correction, and were considered statistically significant if they were less than 0.05.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- P.y17XL :
-
Plasmodium yoelii 17XL
- P.y17XNL :
-
Plasmodium yoelii 17XNL
- DCs:
-
Dendritic cells
- IL-4:
-
Interleukin-4
- Tfh:
-
Follicular T helper cells
- RBCs:
-
Red blood cells
- iRBCs:
-
infected RBCs
- MSPs:
-
Merozoite surface proteins
- i.p.:
-
intraperitoneal
- FCS:
-
Fetal calf serum
References
Beri D, Balan B, Tatu U. Commit, hide and escape: the story of Plasmodium gametocytes. Parasitolog. 2018;16:1–11.
WHO World Malaria Report. 2017. https://www.worldaware.com/article/blog/global-malaria-report-2017.
Miller LH, Baruch DI, Marsh K, Rooth I, Färnert A, Davenport MP. The pathogenic basis of malaria. Nature. 2002;415:673–9.
Pinkevych M, Petravic J, Bereczky S, Ingegerd R, Anna F, Miles PD. Understanding the relationship between Plasmodium falciparum growth rate and multiplicity of infection. J Infect Dis. 2015;211(7):1121–7.
Soulama I, Nébié I, Ouédraogo A, Gansane A, Diarra A, Tiono AB, Bougouma EC, Konaté AT, Kabré GB, Taylor WR, Sirima SB. Plasmodium falciparum genotypes diversity in symptomatic malaria of children living in an urban and a rural setting in Burkina Faso. Malar J. 2009;8:135.
Wassmer SC, Taylor TE, Rathod PK, Mishra SK, Mohanty S, Arevalo-Herrera M, Duraisingh MT, Smith JD. Investigating the pathogenesis of severe malaria: a multidisciplinary and cross-geographical approach. Am J Trop Med Hyg. 2015;93(3 Suppl):42–56.
Boyle MJ, Reiling L, Feng G, Langer C, Osier FH, Aspeling-Jones H, Cheng YS, Stubbs J, Tetteh KK, Conway DJ, McCarthy JS, Muller I, Marsh K, Anders RF, Beeson JG. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity. 2015;42:580–90.
Mauduit M, See P, Peng K, Rénia L, Ginhoux F. Dendritic cells and the malaria pre-erythrocytic stage. Immunol Res. 2012;53:115–1126.
Palomo J, Quesniaux V, Togbe D, Reverchon F, Ryffel B. Unravelling the Roles of Innate Lymphoid Cells in Cerebral Malaria Pathogenesis. Parasite Immunol. 2018;40(2) https://doi.org/10.1111/pim.12502.
Berg A, Otterdal K, Patel S, Reverchon F, Ryffel B. Complement activation correlates with disease severity and contributes to cytokine responses in Plasmodium falciparum malaria. J Infect Dis. 2015;212:1835–40.
Chen L, He Z, Qin L, Li Q, Shi X, Zhao S, Chen L, Zhong N, Chen X. Antitumor effect of malaria parasite infection in a murine Lewis lung cancer model through induction of innate and adaptive immunity. PLoS One. 2011;6:e24407.
Claser C, Chang ZW, Russell B, Rénia L. Adaptive immunity is essential in preventing recrudescence of Plasmodium yoelii malaria parasites after artesunate treatment. Cell Microbiol. 2017;19(11) https://doi.org/10.1111/cim.12763.
Mandala WL, Msefula CL, Gondwe EN, Drayson MT, Molyneux ME, MacLennan CA. Cytokine Profiles in Malawian Children Presenting with Uncomplicated Malaria, Severe Malarial Anemia, and Cerebral Malaria. Clin Vaccine Immunol. 2017;24(4):e00533–16.
Cho SH, Kim DW, Lee SH, Kolliputi N, Hong SJ, Suh L, Norton J, Hulse KE, Seshadri S, Conley DB, Kern RC, Tan BK, Peters A, Grammer LC, Schleimer RP. Age-related increased prevalence of asthma and nasal polyps in chronic rhinosinusitis and its association with altered IL-6 trans-signaling. Am J Respir Cell Mol Biol. 2015;53:601–6.
Linge HM, Lee JY, Ochani K, Koga K, Kohn N, Ojamaa K, Powell SR, Miller EJ. Age influences inflammatory responses, hemodynamics, and cardiac proteasome activation during acute lung injury. Exp Lung Res. 2015;41:216–27.
Osier FH, Fegan G, Polley SD, Murungi L, Verra F, Tetteh KK, Lowe B, Mwangi T, Bull PC, Thomas AW, Cavanagh DR, McBride JS, Lanar DE, Mackinnon MJ, Conway DJ, Marsh K. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun. 2008;76:2240–8.
Boutlis CS, Weinberg JB, Baker J, Bockarie MJ, Mgone CS, Cheng Q, Anstey NM. Nitric oxide production and nitric oxide synthase activity in malaria-exposed Papua new Guinean children and adults show longitudinal stability and no association with parasitemia. Infect Immun. 2004;72(12):6932–8.
Cox MJ, Kum DE, Tavul L, Narara A, Raiko A, Baisor M, Alpers MP, Medley GF, Day KP. Dynamics of malaria parasitaemia associated with febrile illness in children from a rural area of Madang, Papua New Guinea. Trans R Soc Trop Med Hyg. 1994;88:191–7.
Rogier C, Commenges D, Trape JF. Evidence for an age-dependent pyrogenic threshold of Plasmodium falciparum parasitemia in highly endemic populations. Am J Trop Med Hyg. 1996;54:613–9.
Smith T, Genton B, Baea K, Taime J, Narara A, Al-Yaman F, Beck HP, Hii J, Alpers M. Relationships between Plasmodium falciparum infection and morbidity in a highly endemic area. Parasitology. 1994;109:539–49.
Weatherall DJ, Miller LH, Baruch DI, Marsh K, Doumbo OK, Casals-Pascual C, Roberts DJ. Malaria and the red cell. Hematology (Am Soc Hematol Educ Program). 2002;1:35–57.
Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58:283–92.
Couper KN, Phillips RS, Brombacher F, Alexander J. Parasite-specific IgM plays a significant role in the protective immune response to asexual erythrocytic stage Plasmodium chabaudi ASinfection. Parasite Immunol. 2005;27:171–80.
Seixas E, Ostler D. Plasmodium chabaudi chabaudi (AS): differential cellular responses to infection in resistant and susceptible mice. Exp Parasitol. 2005;110:394–405.
Taylor-Robinson AW, Phillips RS. B cells are required for the switch from Th1 to Th2 regulated immune responses to Plasmodium chabaudi infection. Infect Immun. 1994;62:490–8.
Yazdani SS, Mukherjee P, Chauhan VS, Chitnis CE. Immune responses to asexual blood-stages of malaria parasites. Curr Mol Med. 2006;6:187–203.
Fauconnier M, Palomo J, Bourigault ML, Meme S, Szeremeta F, Beloeil JC, Danneels A, Charron S, Rihet P, Ryffel B, Quesniaux VF. IL-12R beta 2 is essential for the development of experimental cerebral malaria. J Immunol. 2012;188:1905–14.
Haque A, Best SE, Montes de Oca M, James KR, Ammerdorffer A, Edwards CL, de Labastida RF, Amante FH, Bunn PT, Sheel M, Sebina I, Koyama M, Varelias A, Hertzog PJ, Kalinke U, Gun SY, Rénia L, Ruedl C, MacDonald KP, Hill GR, Engwerda CR. Type I IFN signaling in CD8- DCs impairs Th1-dependent malaria immunity. J Clin Invest. 2014;124:2483–96.
Maneekan P, Leaungwutiwong P, Misse D, Luplertlop N. T helper (Th) 1 and Th2 cytokine expression profile in dengue and malaria infection using magnetic bead-based bio-plex assay. Southeast Asian J Trop Med Public health. 2013;44:31–6.
Perez-Mazliah D, Langhorne J. CD4 T-cell subsets in malaria: TH1/TH2 revisited. Front Immunol. 2014;5:671.
Tatfeng YM, Agbonlahor DE, Amegor OF. Measurement of Th1, Th2 cytokines and white cell count in childhood haemoglobinopathies with uncomplicated malaria infection. Hematology. 2012;17:47–50.
Xia Y, Yang J, Wang G, Li C, Li Q. Age-related changes in DNA methylation associated with shifting Th1/Th2 balance. Inflammation. 2016;39:1892–903.
Khodzhaeva NM. Age-related cytokine regulation in children with malaria. Med Parazitol. 2011;2:25–8.
Figueiredo MM, Costa PAC, Diniz SQ, Henriques PM, Kano FS, Tada MS, Pereira DB, Soares IS, Martins-Filho OA, Jankovic D, Gazzinelli RT, Antonelli LRDV. T follicular helper cells regulate the activation of B lymphocytes and antibody production during Plasmodium vivax infection. PLoS Pathog. 2017;13:e1006484.
Hansen DS, Obeng-Adjei N, Ly A, Ioannidis LJ, Crompton PD. Emerging concepts in T follicular helper cell responses to malaria. Int J Parasitol. 2017;47:105–10.
Perez-Mazliah D, Nguyen MP, Hosking C, McLaughlin S, Lewis MD, Tumwine I, Levy P, Langhorne J. Follicular Helper T Cells are essential for the elimination of Plasmodium infection. EBioMedicine. 2017;24:216–30.
Salles EM, Menezes MN, Siqueira R, Borges da Silva H, Amaral EP, Castillo-Méndez SI, Cunha I, Cassado ADS, Vieira FS, Olivieri DN, Tadokoro CE, Alvarez JM, Coutinho-Silva R, D’Iimpério-Lima MR. P2X7 receptor drives Th1 cell differentiation and controls the follicular helper T cell population to protect against Plasmodium chabaudi malaria. PLoS Pathog. 2017;13:e1006595.
Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621–63.
Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41(4):529–42.
Obeng-Adjei N, Portugal S, Tran TM, Yazew TB, Skinner J, Li S, Jain A, Felgner PL, Doumbo OK, Kayentao K, Ongoiba A, Traore B, Crompton PD. Circulating Th1-cell-type Tfh cells that exhibit impaired B cell help are preferentially activated during acute malaria in children. Cell Rep. 2015;13:425–39.
Zander RA, Obeng-Adjei N, Guthmiller JJ, Kulu DI, Li J, Ongoiba A, Traore B, Crompton PD, Butler NS. PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity. Cell Host Microbe. 2015;17(5):628–41.
Liu T, Lu X, Zhao C, Zhao T, Xu W. PD-1 deficiency enhances humoral immunity of malaria infection treatment vaccine. Infect Immun. 2015;83:2011–7.
Liu J, Zhang S, Hu Y, Yang Z, Li J, Liu X, Deng L, Wang Y, Zhang X, Jiang T, Lu X. Targeting PD-1 and Tim-3 pathways to reverse CD8 T-cell exhaustion and enhance ex vivo T-cell responses to autologous dendritic/tumor vaccines. J Immunother. 2016;39:171–80.
Horne-Debets JM, Faleiro R, Karunarathne DS, Liu XQ, Lineburg KE, Poh CM, Grotenbreg GM, Hill GR, MacDonald KP, Good MF, Renia L, Ahmed R, Sharpe AH, Wykes MN. PD-1 dependent exhaustion of CD8+ T cells drives chronic malaria. Cell Rep. 2013;5:1204–13.
Wykes MN, Horne-Debets JM, Leow CY, Karunarathne DS. Malaria drives T cells to exhaustion. Front Microbiol. 2014;5:249.
Maestre A, Carmona-Fonseca J. Immune responses during gestational malaria: a review of the current knowledge and future trend of research. J Infect Dev Countries. 2014;8(4):391–402.
White NJ. Malaria parasite clearance. Malar J. 2017;16(1):88.
Beeson JG, Osier FH, Engwerda CR. Recent insights into humoral and cellular immune responses against malaria. Trends Parasitol. 2008;24:578–84.
Taylor-Robinson AW. Regulation of immunity to Plasmodium: implications from mouse models for blood stage malaria vaccine design. Exp Parasitol. 2010;126:406–14.
Wipasa J, Elliott S, Xu H, Good MF. Immunity to asexual blood stage malaria and vaccine approaches. Immunol Cell Biol. 2002;80:401–14.
Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–32.
Illingworth J, Butler NS, Roetynck S, Mwacharo J, Pierce SK, Bejon P, Crompton PD, Marsh K, Ndungu FM. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol. 2013;190:1038–47.
Cavanagh DR, Elhassan IM, Roper C, Robinson VJ, Giha H, Holder AA, Hviid L, Theander TG, Arnot DE, McBride JS. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J Immunol. 1998;161:347–59.
Dorfman JR, Bejon P, Ndungu FM, Langhorne J, Kortok MM, Lowe BS, Mwangi TW, Williams TN, Marsh K. B cell memory to 3 Plasmodium falciparum blood-stage antigens in a malaria-endemic area. J Infect Dis. 2005;191:1623–30.
Akpogheneta OJ, Duah NO, Tetteh KK, Dunyo S, Lanar DE, Pinder M, Conway DJ. Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect Immun. 2008;76:1748–55.
Ma SH, Zheng L, Liu YJ, Guo SY, Feng H, Chen G, Li DM, Wang JC, Cao YM. Plasmodium yoelii: influence of antimalarial treatment on acquisition of immunity in BALB/c and DBA/2 mice. Exp Parasitol. 2007;116(3):266–72.
Mehrizi AA, Asgharpour S, Salmanian AH, Djadid ND, Zakeri S. IgG subclass antibodies to three variants of Plasmodium falciparum merozoite surface protein-1 (PfMSP-1(19)) in an area with unstable malaria transmission in Iran. Acta Trop. 2011;119:84–90.
Acknowledgements
We thank Dr. Motomi Torii (Ehime University Graduate School of Medicine, Ehime, Japan) for his guidance in this research and providing malaria parasite strains of P.y17XL and P.y17XNL.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81101278; 81429004); Outstanding youth program of Taizhou university (Z2020080); and doctor launching fund project of Liaoning province (20180540019). The funders had no role in the design of the study or in the collection analysis or interpretation of the data.
Author information
Authors and Affiliations
Contributions
YM.C authored the manuscript. G. C and W. P designed the experiments. QB. W, YT.D F. L, XD. S and S. X performed all experiments. YT. D provided critical support for all data analysis. G. C critically reviewed the manuscript. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The 4-week-old and 8-week-old female BALB/c mice were used for all experiments and protocols complied with the China medical University Animal Ethics Committee requirements. This study does not involve the use of human data or tissue. All authors have read and approved the manuscript.
Consent for publication
Not applicable.
Competing interests
No potential conflict of interest was reported by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Qb., Du, Yt., Liu, F. et al. Adaptive immune responses mediated age-related Plasmodium yoelii 17XL and 17XNL infections in 4 and 8-week-old BALB/c mice. BMC Immunol 22, 6 (2021). https://doi.org/10.1186/s12865-020-00391-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12865-020-00391-8