Abstract
Background
Bacteria of the genus Xanthomonas cause economically significant diseases in various crops. Their virulence is dependent on the translocation of type III effectors (T3Es) into plant cells by the type III secretion system (T3SS), a process regulated by the master response regulator HrpG. Although HrpG has been studied for over two decades, its regulon across diverse Xanthomonas species, particularly beyond type III secretion, remains understudied.
Results
In this study, we conducted transcriptome sequencing to explore the HrpG regulons of 17 Xanthomonas strains, encompassing six species and nine pathovars, each exhibiting distinct host and tissue specificities. We employed constitutive expression of plasmid-borne hrpG*, which encodes a constitutively active form of HrpG, to induce the regulon. Our findings reveal substantial inter- and intra-specific diversity in the HrpG* regulons across the strains. Besides 21 genes directly involved in the biosynthesis of the T3SS, the core HrpG* regulon is limited to only five additional genes encoding the transcriptional activator HrpX, the two T3E proteins XopR and XopL, a major facility superfamily (MFS) transporter, and the phosphatase PhoC. Interestingly, genes involved in chemotaxis and genes encoding enzymes with carbohydrate-active and proteolytic activities are variably regulated by HrpG*.
Conclusions
The diversity in the HrpG* regulon suggests that HrpG-dependent virulence in Xanthomonas might be achieved through several distinct strain-specific strategies, potentially reflecting adaptation to diverse ecological niches. These findings enhance our understanding of the complex role of HrpG in regulating various virulence and adaptive pathways, extending beyond T3Es and the T3SS.
Similar content being viewed by others
Background
In phytopathogenic Xanthomonas bacteria, HrpG is a conserved response regulator belonging to the OmpR family of two-component regulatory systems [1]. Two-component regulatory systems are one of the major mechanisms used by bacteria to perceive and adapt their physiology to changing environmental conditions [2]. These systems typically rely on a phosphorelay between a sensor kinase and a response regulator, which becomes active upon phosphorylation. In Xanthomonas, HrpG regulates the expression of hrpX, which encodes a transcriptional regulator. HrpX binds to the plant-inducible promoter (PIP) box [3], a DNA sequence present in the cis-elements of various pathogenicity genes, including type III effector proteins (T3Es) and the hrp (hypersensitive response and pathogenicity) genes, which encode the type III secretion system (T3SS) [4, 5]. This system is one of the main virulence factors of Xanthomonas and it enables the direct delivery of bacterial T3Es into the plant cellular environment, where they interfere with plant immunity and alter plant physiology to facilitate disease [6]. Disruption of any key structural component of the T3SS results in a complete loss of pathogenicity for most Xanthomonas species [4].
The Xanthomonas genus comprises 33 species of Gram-negative 𝛾-proteobacteria [7] causing diseases on more than 400 different monocot and dicot plants including economically-important crops [8]. Xanthomonas species are further divided into pathovars that can exhibit distinct host specificities and tissue tropisms, causing a variety of symptoms such as wilting, necrosis or blight [8]. Phylogenetic group-I Xanthomonas include monocot pathogens such as X. translucens pv. translucens (Xtt) [9], causal agent of bacterial leaf streak of barley. On the other hand, phylogenetic group-II Xanthomonas can infect both monocot and dicot plants. For example, X. oryzae pv. oryzae (Xoo) causes bacterial blight on rice, a monocot, while X. phaseoli pv. phaseoli (Xpp) and X. citri pv. fuscans (Xcf) belonging to phylogenetically distinct species are both able to cause common blight of common bean, a dicot. Other economically important Xanthomonas pathovars include X. citri pv. mangiferaeindicae (Xcm), causal agent of mango bacterial black spot, X. citri pv. citri (Xci), responsible for Asiatic citrus canker and X. euvesicatoria pv. alfalfae (Xea), which causes disease on legumes. The genus also comprises X. campestris pv. campestris (Xcc) and X. campestris pv. raphani (Xcr), the causal agents of black rot disease and bacterial spot disease of Brassicaceae respectively. Xcc, Xoo and Xtt are known to infect the plant vasculature through wounds or hydathodes while Xci, Xcm, Xea and Xcr colonize the mesophyll through stomata. Xcf and Xpp colonize their host plant by both ways [10,11,12,13].
Since its discovery, research has shown that HrpG regulates a broad array of genes beyond T3Es and the T3SS. This broader regulatory role was initially identified by comparing the transcriptomes of wild-type versus hrpG mutant strains in hrp-inducing media [14] or wild-type strains versus strains expressing an auto-active gain-of-function form of HrpG, such as HrpGE44K, commonly named HrpG* [15,16,17]. Additional studies have supported these claims, showing HrpG could regulate the expression of genes other than those associated with type III secretion, including genes associated with chemotaxis and motility, and genes encoding extracellular proteases and cell wall degrading enzymes associated with the Xps type II secretion system (T2SS) [18,19,20,21]. However, this part of the HrpG regulon remains understudied and appears to be poorly conserved between Xanthomonas species.
To investigate the conservation of the HrpG regulon across Xanthomonas species, we performed a comprehensive transcriptomic and genomic analysis of 17 Xanthomonas strains transiently expressing the constitutively active hrpG* variant [15]. The HrpG* regulons identified in the different strains varied considerably in their size, ranging from 137 to 2,355 genes. Interestingly, the core HrpG* regulon across these 17 strains comprises only 26 genes, mainly involved in the biogenesis of the T3SS itself. Moreover, it was found that the full extent of genes and processes regulated by HrpG* is remarkably diverse across the 17 strains. These findings suggest that HrpG-dependent pathogenicity in Xanthomonas species can be achieved through diverse strategies.
Results
Transcriptome-based structural annotation of 17 Xanthomonas genomes
17 Xanthomonas strains belonging to nine pathovars were selected to study the diversity and conservation of the HrpG regulon (Fig. 1A). These strains represent pathogens associated with diverse host plants, were sampled over nearly a hundred years in ten countries, and have various lifestyles (Additional file 1: Table S1A; Fig. 1B). Genome sequences of strains XttCFBP2054, XccCN05, XcmLG56−10 and XcmLG81−27 were determined using either short- (Illumina) or long-read (PacBio) sequencing (Additional file 1: Table S1B; S1C). High-quality genome sequences were readily available for the 13 other strains.
Experimentally-based and homogeneous annotation of genomes is vital for cross-species comparative transcriptomic analyses. Such annotations were previously made for Xcc8004 and XcrCFBP5828R, which were based on various transcriptomic datasets and the EuGene-PP pipeline [17, 22]. The same strategy was used to annotate the remaining genomes in combination with different RNA-Seq libraries (Additional file 1: Table S1E), including those from conjugates carrying an empty vector or a vector harboring hrpG*. For Xci and Xcm strains, empty vector conjugates were not generated, and RNA-Seq libraries from wild-type strains were used for annotation instead. Small RNA sequencing was performed for several strains in order to identify small RNAs and determine transcriptional start sites. The newly annotated genomes consist of 4066 (XttCFBP2054) to 5421 (XcfCFBP7767) protein-coding genes (Additional file 1: Table S1C; Data S1 https://doi.org/10.57745/9OTYNJ). The 17 homogeneously annotated genomes were used for all downstream analyses.
Gene orthology analysis of the 17 Xanthomonas strains used in this study. A: phylogenetic tree of all strains, each represented with a distinct coloured symbol. B: Lifestyle indicates if the pathogen infects primarily leaf mesophyll (light brown), the vascular tissues (dark brown) or both (burgundy). C: Number of predicted protein-coding genes per genome, including those in orthogroups (dark green) and singletons (light green). D: Number of orthogroups per genome. E: Heatmap showing the number of shared orthogroups between strains. Symbols as in panel A. F: Principal component (PC) analysis plot showing the clustering of the different strains based on the presence or absence of orthogroups against the first three PCs. Shape and color of symbols identifies each of the 17 strains from the nine pathovars studied as in panel A
The Xanthomonas core genome is composed of 2,483 orthogroups
To enable cross species comparative transcriptomics, an orthogroup (OG) database was built with the newly annotated genomes using Orthofinder. Collectively, the 17 genomes contained 88,480 genes, of which 81,490 were predicted to be protein-coding. Out of these, 78,225 were assigned to a total of 6,899 orthogroups (Fig. 1C). 2,483 orthogroups were represented by at least one ortholog in each strain, representing the Xanthomonas core genome in this study (Fig. 1D; Data S2 https://doi.org/10.57745/9OTYNJ). A phylogenetic tree including all strains was built using the STAG algorithm and rooted using STRIDE (Fig. 1A). As expected, group-I Xanthomonas strain XttCFBP2054 was located at the root of the tree. To assess the overall similarity in orthogroup content among the strains, a heatmap depicting the number of shared orthogroups and a principal component analysis (PCA) based on a binary presence/absence matrix of all orthogroups in the dataset were used (Fig. 1E, F). The high number of shared orthogroups between related strains in the heatmap, and the clustering of related strains in the PCA is consistent with the genetic relationships of the Xanthomonas strains.
The accessory HrpG* regulons are highly diverse
The HrpG* regulons were identified by sequencing cDNAs of either wild-type strains with or without an empty plasmid or a plasmid carrying hrpG*. The biological reproducibility of each triplicate per genotype was evaluated through MultiQCs on mapping statistics, and through PCA, MA (M = log fold change, A = mean of normalized counts) and volcano plots of the DESeq2 output (Data S3, https://doi.org/10.57745/9OTYNJ). The reproducibility across all samples was consistent except for one Xtt replicate, which was excluded from subsequent differential expression analysis. As expected for a direct HrpG target, expression of the hrpX gene was significantly upregulated in all hrpG* samples (Fig. 2A, B). This demonstrates the presence of biologically active HrpG in all hrpG* samples, thereby validating its use for the analysis of HrpG regulons.
Features of the HrpG* regulons in 17 Xanthomonas strains. A: Phylogenetic tree of all strains as shown in Fig. 1A. B: Log2FC of hrpX in hrpG* strains compared to wild-type. C: Proportion of differentially-expressed predicted protein-coding genes per genome (AdjPval < 0.05). D: Number of DEGs (|Log2FC| > 2). E: Percentage of DEGs (|Log2FC| > 2) in the core genome only considering protein-coding genes. F: Percentage of DEGs (|Log2FC| > 2) in the accessory genome only considering protein-coding genes. G: Principal components analysis plot showing the first three principal components based on the average Log2FC of genes within each orthogroups of the core genome
The number of differentially-expressed genes (DEGs, AdjPval < 0.05) varied across species from 137 to 2,355 genes (Fig. 2C; Additional file 2: Table S2A). Considering a threshold of |Log2FC| > 2, the number of DEGs ranged from 59 to 429 (Fig. 2D). Notably, in all strains but XcfCFBP7767 and XcfCFBP6996R, the proportion of HrpG* regulated protein-coding genes in the core genome was significantly lower than in the accessory genome (Chi-square, BH FDR, AdjPval < 0.05, Fig. 2E, F). Furthermore, the regulation of orthogroups by HrpG* within the core genome was highly variable, as highlighted by a PCA (Fig. 2G). In addition, the strains showed substantial differences in enriched Gene Ontology (GO) terms amongst DEGs considering a threshold of |Log2FC| > 2 (Fig. 3). Notably, only GO terms associated with protein secretion and protein secretion by the type III secretion system were enriched in the regulons of all strains, illustrating that the functions of the accessory HrpG regulon are highly diverse (Fig. 3).
GO term enrichment in the HrpG* regulons in 17 Xanthomonas strains. Only GO terms enriched amongst regulated genes (|Log2FC| > 2) identified in at least three strains are depicted in the table to highlight the main commonalities across the strains (62 GO terms). A total of 197 unique GO terms were found to be enriched in at least one strain as detailed (additional file 2: Table S2B)
The HrpG* core regulon is limited to only 26 orthogroups
To determine the HrpG* core regulon, orthogroups of the core genome of which at least one ortholog was differentially regulated in hrpG* samples across all strains were identified (Table 1). The identified HrpG* core regulon comprises one orthogroup coding for HrpX, 21 orthogroups encoding structural components of the T3SS and two orthogroups coding for the T3Es XopR and XopL. Additionally, the core regulon also includes two orthogroups coding for a putatively Sec/SPI-secreted acid phosphatase of the PAP2 superfamily (annotated as PhoC) and a major facilitator superfamily (MFS) transporter. Here, hrpG upregulation was ignored as sequence reads predominantly originated from plasmid-borne hrpG* transcripts (Data S4 https://doi.org/10.57745/9OTYNJ). Thus, the identified HrpG* core regulon is almost exclusively associated with type III secretion.
There is compelling evidence that group-I Xanthomonas acquired the type III secretion system cluster independently of the group-II Xanthomonas species, potentially resulting in the existence of different HrpG regulons [23]. As the majority of strains used in this study belong to group-II Xanthomonas species, their core regulon was also identified. The identified group-II core regulon includes an additional 12 orthogroups compared to the Xanthomonas core regulon (Table 2). These include orthogroups encoding several known virulence determinants, such as a chorismate mutase and LipA whose function is independent of the T3SS and represent conserved and ancestral virulence mechanisms [24].
Expression of most but not all T3Es encoding genes is controlled by HrpG*
Previous studies have shown that T3Es, both with and without a PIP-box motif in their promoters (TTCGB-N15-TTCGB), can be under positive regulation by HrpG or HrpX (3,14,16,17,21). To investigate the regulation of T3Es by HrpG* in the 17 strains analysed, we used the Effectidor software [25] to identify orthogroups encoding T3Es. A total of 72 orthogroups were predicted to contain orthologs coding for T3Es (Additional file 3: Table S3A). From these, 12 orthogroups corresponding to xopA, hrpW, hpaA and other T3SS associated genes, which are generally not considered T3Es, were excluded (Additional file 3: Table S3C). Additionally, Effectidor identified 15 singletons that are predicted to encode T3Es. Consistent with previous findings, our observations show that the majority of T3E genes are under the control of HrpG* (Additional file 3: Table S3B; Fig. 4). However, the expression of few predicted effectors was not HrpG*-dependent, as observed for xopAW. As for the core T3E gene xopM, its expression was HrpG*-dependent except for both Xcm strains. Interestingly, most orthologs encoding transcription activator-like effectors (TALEs, OG000009) were positively regulated by HrpG*, although Xci TALEs were previously reported as expressed in a HrpG/HrpX-independent manner [14, 21]. We thus conclude that the expression of most, but not all of the predicted T3E genes is under the positive control of HrpG*.
Regulation of orthogroups (OG) encoding putative T3Es in Xanthomonas strains expressing hrpG*. Only significantly DEGs (AdjPval < 0.05) are colored. Missing orthologs are marked in gray. Orthogroup annotation is given on top of the figure, orthogroup number at bottom of the figure. Numbers in blue indicate protein-coding genes which are singletons
Chemotaxis and motility are differentially regulated by HrpG* at both inter- and intra-specific levels
The PCA, based on the average HrpG*-dependent Log2FC of expression of orthogroups within the core genome, indicated that the regulatory network of HrpG* within the core genome differed considerably between strains, even for those of the same pathovar (Fig. 2G). Orthogroups that correlated strongly with the first three principal components were enriched for GO terms associated with the biological process of chemotaxis (Additional file 4). We therefore investigated the regulation of orthogroups comprising genes encoding methyl-accepting chemotaxis proteins (MCPs), Che signalling genes and structural components of the flagellum and the type-IV pilus. Interestingly, the regulation of these orthogroups was HrpG*-dependent in half the strains and both HrpG*-dependent upregulation and downregulation of these processes could be observed, depending on the strain (Fig. 5A). For example, XcmLG56−10 showed strong upregulation of genes in motility- and chemotaxis-related orthogroups while XcmLG81−27 did not. Similarly, XcrCFBP5828R showed strong upregulation of genes belonging to the motility- and chemotaxis-related orthogroups and to a lesser extent in XccCN14 while a strong repression was measured in Xcc8004 and XccCN05. Interestingly, in vitro swimming motilities were reduced in HrpG* mutants of strains Xcc8004, XccCN14 and XccCN05 compared to their wild types (Fig. 5B), while increased for strain XcrCFBP5828R. Those results are essentially consistent with the transcriptomic results except for strain XccCN14 in which expression of chemotaxis-related OGs was essentially unaltered. Altogether, these findings evidence diverse scenarios of HrpG*-dependent regulation of chemotaxis and motility in the Xanthomonas genus.
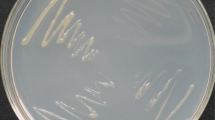
Regulation of chemotaxis and motility in Xanthomonas strains expressing hrpG*. A: Regulation of orthogroups (OG) involved in chemotaxis and motility. Only significantly DEGs (AdjPval < 0.05) are colored. Missing orthologs are marked in gray. Orthogroup annotation is given on top of the figure, orthogroup number at bottom of the figure. Due to the extensive number of orthogroups potentially involved in chemotaxis, only those orthogroups that are regulated by HrpG* in at least two strains are shown, except for the secondary flagellar cluster specific to Xcm strains. Additionally, for both the flagellar and pilus gene clusters, only the regulation of key representative genes that encode various structural components of the flagella and pili are shown. MCPs: methyl-accepting chemotaxis proteins. B: Motility assay showing swimming abilities after 48 h on 0.3% agar plates for four Xanthomonas strains. The plot represents the ratio of swimming halo of HrpG* mutant against wild-type (WT) strain for three biological replicates. Representative pictures are shown
Strain-dependent variability in HrpG*-mediated regulation of candidate T2SS substrates
The HrpG* regulon of different strains exhibited significant enrichment in genes linked to GO terms associated with carbohydrate-active enzymatic activity and proteolytic activity (Fig. 3). Proteins encoded by such genes can be secreted to the extracellular space by the T2SS and play a crucial role in Xanthomonas virulence due to their involvement in the degradation of plant cell wall components, which facilitates nutrient acquisition and the efficient translocation of T3Es [20, 26]. Prediction of type two-dependent secretion across the outer membrane is complex [20, 26]. Yet, T2SS substrate proteins often possess a Sec/SPI signal peptide which mediates the first transport step across the inner membrane. Such signal peptides were predicted in 546 of the 3351 protein-coding genes of the pan HrpG* regulon, representing a significant enrichment for the presence of a signal peptide amongst regulated genes (|Log2FC| > 2, Chi-square, p < 0.0001, Additional file 2: Table S2C). Increase in the expression of the T2SS genes was limited to both Xcm strains and to a lesser extent to XooBAI3, similar to previous reports in Xanthomonas euvesicatoria (Xeu) [20] (Additional file 5: Fig. S1). Yet those observations cannot be generalized to other Xanthomonas species.
Though HrpG is known to regulate the expression of genes encoding proteins with carbohydrate active enzymatic and/or proteolytic activity [16, 18,19,20, 27, 28], such regulation can either be positive or negative without any obvious pattern [29]. We thus examined the regulation of those genes in the transcriptomes of the 17 Xanthomonas strains (Figs. 6 and 7). Once more, various HrpG*-dependent regulatory patterns were observed. For example, orthologs in OG0003431, encoding putative S53 family serine proteases, were generally upregulated except in XooBAI3. Conversely, expression of orthologs in OG0000136, encoding putative S1 family serine proteases, were mainly downregulated, while XooBAI3 strongly upregulated one specific ortholog. Genes encoding proteins with putative pectin-lyase activity were also variably regulated by HrpG*. For example, orthologs in OG0000700, corresponding to genes encoding putative secreted GH28 family pectin lyases, were strongly upregulated in all strains except XooBAI3 and XttCFBP2054. In contrast, orthologs in OG0000100 and OG0000105, corresponding to genes encoding putative secreted PL1 family pectin lyases, showed considerable downregulation, especially in Xanthomonas campestris pathovars, but not in others such as XppCFBP6545R. These results are in line with previous reports, highlighting the differential regulation of genes encoding proteins with specific carbohydrate active enzymatic and/or proteolytic activity by HrpG* across different Xanthomonas strains. Nonetheless, most orthogroups showed consistent HrpG*-dependent regulation across the majority of strains, evidencing the existence of conserved HrpG*-mediated regulatory patterns for genes encoding specific families of carbohydrate active enzymes and/or proteases in the Xanthomonas genus.
Regulation of orthogroups (OG) encoding carbohydrate active enzymes in Xanthomonas strains expressing hrpG*. Only genes with an AdjPval < 0.05 are colored. Absent orthologs are marked in gray. Orthogroup annotations are given on top of the panel according to their CAZy family. Orthogroup numbers are given at the bottom of the panel. Due to the extensive number of orthogroups encoding carbohydrate active enzymes, only orthogroups regulated in at least two strains are shown. The cross, star and triangle symbols indicate the presence of a signal peptide within the protein, as predicted by SignalP6
Regulation of orthogroups (OG) encoding proteins with proteolytic activity in Xanthomonas strains expressing hrpG*. Only genes with an AdjPval < 0.05 are colored. Absent orthologs are marked in gray. Orthogroup annotation is given on top of the figure, orthogroup number is given at the bottom of the figure. Orthogroups are annotated according to their MEROPS identifiers. Due to the extensive number of orthogroups encoding proteins with proteolytic activity, only orthogroups regulated in at least two strains are shown. The cross, star and triangle symbols indicate the presence of a signal peptide within a gene, as predicted by SignalP6
The HrpG* regulon members prepare Xanthomonas cells for the degradation of plant-derived phenolic compounds
The degradation of plant cell walls mediated by T2SS substrates in Xanthomonas species leads to the release of diverse phenolic compounds, including hydroxycinnamic acids, vanillic acid and 4-hydroxybenzoic acid (4-HBA). Interestingly, the HrpG* core regulon comprises an MFS transporter (OG0001168) involved in 4-HBA uptake in Xcc8004 (Table 1) [30, 31]. In addition, the group-II Xanthomonas regulon comprises orthogroups coding different proteins involved in the uptake (VanK/PcaK, OG0000558) and putative degradation (two subunits of a PCA dioxygenase, OG0001327, OG0001328) of phenolic compounds [30, 31] as well as a PcaQ-like transcriptional regulator (OG0001326), which regulates phenolic compound metabolism in other plant-associated bacteria (Table 2) [32, 33]. Therefore, a broader survey of orthogroups relevant for phenolic compound metabolism was conducted (Fig. 8). We observed that expression of numerous genes involved in the import and degradation of such compounds was upregulated, while some genes putatively involved in their efflux were downregulated. Notably, many orthogroups involved in these processes were absent from the XttCFBP2054 genome. Collectively, these results suggest that HrpG* prepares the group-II Xanthomonas metabolism for the import and degradation of plant-derived phenolic compounds.
Differential expression of orthogroups (OG) encoding proteins putatively involved in plant phenolic compounds metabolism. Only genes with an AdjPval < 0.05 are colored. Absent orthologs are marked in gray. Orthogroup annotation is given on top of the panel, orthogroup number is given at the bottom of the figure. Pca: protocatechuate. Hp: hypothetical protein
HrpG* induces the expression of genes involved in cytochrome C maturation in most Xanthomonas strains
GO terms associated with “iron binding”, “heme transport” and “siderophore uptake” were significantly enriched in the HrpG* regulons of different strains (Fig. 3) and are commonly associated with iron homeostasis. Orthologs of TonB-dependent receptors involved in iron uptake in Xcc [34] were differentially regulated in some strains expressing HrpG* (Additional file 5: Fig. S2) but did not explain the observed enrichment of those three GO terms in all strains. Upon further investigation we found that the enrichment in these GO terms originated mainly from orthogroups comprising genes putatively involved in cytochrome C maturation (Ccm genes). Cytochrome C maturation complexes can have multiple roles in bacteria, including respiration [35], resistance to antimicrobial phenazines [36], or virulence as shown for Xcc [37]. Interestingly, the identified regulated Ccm genes were located in an evolutionary-conserved gene cluster composed of a hypothetical protein, the sigma-factor RpoE4, another hypothetical protein with a zinc-finger domain and a S8A protease with a predicted Sec/SPI secretion signal (Fig. 9). Most genes in this cluster were regulated positively by HrpG* except in XppCFBP6546R and XttCFBP2054. These results indicate that HrpG* regulates cytochrome C maturation in most Xanthomonas strains.
Discussion
This comparative transcriptomics study has provided a genus-wide overview of the evolutionarily-conserved processes regulated by HrpG*, as well as a glimpse into the intra- and inter-specific diversity of the regulon (Fig. 10). The regulons of the strains investigated here encompass hundreds, if not thousands of genes, including a variety of known virulence and adaptive pathways with notable enrichment for putative T2SS substrates. However, these regulons differ considerably, both within and between species. This variation is illustrated by a small HrpG* core regulon (26 orthogroups for the Xanthomonas genus and 38 for group-II Xanthomonas) and by the fact that only two gene ontology terms (GO0009306: protein secretion, and GO0030254: protein secretion by type III secretion system), were significantly enriched among the regulated genes across all strains. Additionally, our analysis revealed that the proportion of regulated protein-coding genes in the accessory genomes of most strains was significantly higher than that in the core genome. Collectively, these findings illustrate that the HrpG regulon from one Xanthomonas strain cannot be inferred from another related Xanthomonas strain. The diversity of the HrpG regulon could be the result of long-term evolution. Indeed, HrpG and HrpX were acquired prior to the divergence of group I and II Xanthomonas and the acquisition of the T3SS [38] suggestive of an ancestral role of the HrpG regulon for other biological functions. How the T3SS became part of the core regulon of HrpG remains to be elucidated.
Schematic representation of the key processes identified to be regulated by HrpG* in this study. Red symbols annotated in bold indicate processes that are part of the Xanthomonas core regulon. Other red symbols indicate terms part of the group-II Xanthomonas core regulon. Symbols in purple highlight other regulated orthogroups which have been discussed. Dashed lines indicate hypothesized interactions, whereas continuous lines indicate interactions which are thought to be direct. T3SS: type III secretion system; T3Es: type III effectors; T2SS: Xps type II secretion system; MCPs: methyl-accepting chemotaxis proteins
The acid phosphatase PhoC belongs to the Xanthomonas HrpG* core regulon
The acid phosphatase PhoC (OG0000410) from the PAP2 superfamily belongs to the HrpG* core regulon and exhibits high conservation across Xanthomonas species. Of the 657 publicly available Xanthomonas genomes that contain a hrp T3SS gene cluster (Data S6, https://doi.org/10.57745/9OTYNJ), 650 harbour a phoC ortholog. Among these, 606 orthologs have a predicted Sec/SPI secretion signal whilst all others have a Sec/SPII secretion signal, indicating a strong conservation of Sec-dependent transport to the periplasm (Additional file 6: Table S5A). The ortholog of phoC in Xeu has been shown to be upregulated during the interaction with tomato although knockout mutants were not affected in virulence [39]. Additionally, the Xcc phoC ortholog was not important for fitness inside cauliflower hydathodes nor for Xcc virulence on cabbage [40, 41]. Despite the strong conservation of this orthogroup, its molecular or enzymatic functions remain elusive. In contrast, the functions of the T3Es XopL and XopR, both member of the HrpG* core regulon, have extensively been studied [42]. The xopL orthologs encode atypical E3 ubiquitin ligases which can contribute to Xanthomonas virulence in several distinct mechanistical ways, depending on the species [43,44,45,46,47]. This, together with significant interspecific variation in amino acid sequence, in planta subcellular localizations and host specific cell death-inducing capability indicate that this ancestral effector has undergone significant diversification [47]. In Xoo, XopR is an effector which localizes to the plasma membrane, where it associates with various receptor-like cytoplasmic kinases [48,49,50]. In both Xoo and Xanthomonas axonopodis pv. manihotis XopR is thought to contribute to virulence by interfering with PTI [48, 51].
The group-II Xanthomonas HrpG* core regulon mediates ancestral T3SS-independent virulence mechanisms
The regulation of 12 orthogroups was identified to be HrpG*-dependent specifically in the group-II Xanthomonas species tested in this study. Among them, orthogroups encoding VirK, a chorismate mutase, and the LipA/LesK lipase have been previously identified by comparative genomic analyses to be potential conserved virulence determinants in Xanthomonas species [24]. Orthologs of virK are conserved across various lineages of plant-associated bacteria and are present in the genomes of all 657 Xanthomonas strains with a T3SS (Data S6, https://doi.org/10.57745/9OTYNJ). While the function of VirK has not yet been experimentally addressed, in silico analyses suggest that it interacts with structural components of both the T3SS and the Xps T2SS, and it is hypothesized to be secreted by the T2SS [24]. Orthologs of the chorismate mutase are also found in all genomes of Xanthomonas species with a T3SS, showing high levels of amino acid sequence similarity, including a Sec/SPI/SPII signal secretion signal (Additional file 6: Table S5B; Data S6, https://doi.org/10.57745/9OTYNJ). Although its molecular functions remain undefined, it contributes to Xoo virulence, possibly by interfering with salicylate-dependent immunity [24, 52]. LipA/LesK orthologs are conserved with a Sec/SPI secretion signal in nearly all Xanthomonas species with a T3SS but absent from non-pathogenic Xanthomonas (Additional file 6: Table S5C; Data S6, https://doi.org/10.57745/9OTYNJ) [24]. Interestingly, while an ortholog is present in XttCFBP2054 genome, it lacks a PIP-box promoter motif, likely explaining the HrpG*-independent expression of LipA/LesK in this Group-I Xanthomonas. Xeu, Xci and Xoo LipA/LesK contribute to virulence on tomato [53], citrus [24] and rice [54], respectively. Importantly, the ortholog of LipA/LesK is also a virulence mechanism for Xylella fastidiosa on grapevine [55] suggestive of an ancestral virulence function in the genera Xanthomonas, Xylella and Burkholderia [24] before its co-optation in the HrpG regulon of Group-II Xanthomonas.
The group-II Xanthomonas HrpG* regulon relies on a transcriptional regulatory cascade
In addition to HrpX, the group-II core regulon comprises three additional transcriptional regulators, namely a MarR transcription factor, HpaR and a PcaQ-like transcriptional activator. The Xci MarR ortholog is essential for pathogenicity on Rangpur lime [56] although its gene targets are unknown. The transcriptional regulator HpaR is essential for Xcc virulence and thought to regulate the expression of various virulence mechanisms including extracellular proteases secreted by the Xps T2SS [18]. As for the PcaQ-like transcriptional activator, it is hypothesized to be the main regulator of genes involved in protocatechuate degradation pathway, which is known to be important for Xcc virulence [30, 31]. Interestingly, in XttCFBP2054, the PcaQ-like transcriptional activator is absent and the expression of the MarR transcription factor and hpaR is HrpG*-independent. This could explain the great divergence observed between the regulons in XttCFBP2054 and those in group-II Xanthomonas. These results thus suggest that a complex cascade defines the full extent of HrpG* regulons in group-II Xanthomonas, which could be refined by determining the transcriptomes of the corresponding single and multiple mutants in a hrpG* mutant background. In the absence of such experimental evidence, it is difficult to determine which the HrpG regulon members are directly placed under the control of HrpG or under the control of other regulators such as HrpX.
HrpG* regulates motility and chemotaxis in unpredictable ways
Motility and chemotaxis are known virulence determinants in Xanthomonas, primarily associated with the initial stages of infection [57,58,59,60]. As HrpG expression gradually increases during infection [22, 61], it seems likely that HrpG could mediate the suppression of motility and chemotaxis, thereby potentially limiting PTI induced by flagellar components [29]. So far, reports in Xci have indeed shown that HrpG negatively regulates motility and chemotaxis, likely in a HrpX-independent manner [14]. However, we report the variable regulation of these processes. For instance, HrpG*-dependent downregulation was observed in XooBAI3, while no regulation was observed in XcmLG81−27 and, remarkably, upregulation was measured in XcmLG56−10. These findings are intriguing, especially considering the crucial role of motility and chemotaxis in the virulence of Xanthomonas species, and suggest the presence of complex inter- and intra-specific virulence mechanisms that require further investigation.
Conclusion
In conclusion, we demonstrate that Xanthomonas species not only differ by their gene contents (Fig. 1), but also by their gene expression profiles, which are diverse even at the intra-pathovar scale. These observations are consistent with a general evolutionary context where virulence factors important in some hosts are often directly or indirectly recognised as immune elicitors in other hosts and therefore undergo diversifying selection. The observed differential expression within the HrpG regulon may limit recognition of some of these genes while diversifying the strains encountered by plants. Such adaptative transcriptional regulation would have the advantage of being transient, plastic and environment-dependent thus facilitating the emergence of novel virulence properties.
Materials and methods
Bacterial strains, plasmids and growth conditions
Bacterial strains and plasmids used in this study are listed (Additional file 1: Table S1). Xanthomonas strains were grown at 28 °C in MOKA medium [34]. Escherichia coli cells were grown on LB medium at 37 °C. For solid media, agar was added at a final concentration of 1.5% (w/v). Antibiotics were used when appropriate at the following concentrations: 50 µg/mL kanamycin, 50 µg/mL rifampicin, 40 µg/mL spectinomycin.
Genome sequencing and assembly
Genomic DNA was extracted from bacterial cells grown overnight in MOKA-rich medium using the Wizard genomic DNA purification kit (Promega) or as described [62] for short- or long-read sequencing, respectively. Shotgun sequencing of genomic DNA was performed either on HiSeq2000 Illumina platform [63] or PacBio [64]. Genome assembly was performed as described for short read assemblies [63]. FLYE Assembler (version 2.9) was used for long-read assemblies [65]. The GenBank accession numbers for the genomes generated in this study are given in the additional file 1.
Cloning of pBBR-hrpG* plasmid
The hrpG* (E44K) coding sequences were amplified from Xcc strain 8004 hrpG* [66] and Xanthomonas vesicatoria pv. euvesicatoria strain 85 − 10 hrpG [1] and cloned as described [17] into pBBR1-MCS-2 [67] under the control of the constitutive lac promoter (Additional file 1: Table S1D). Plasmids were introduced into E. coli by electroporation. The plasmids were introduced into the different Xanthomonas strains by triparental mating using pRK2073 as helper plasmid [68, 69] (Additional file 1: Table S1D).
RNA extraction, rRNA and tRNA depletion and cDNA pyro-sequencing
RNAs were extracted from Xanthomonas strains grown at exponential phase (OD600 nm between 0.5 and 0.7) in MOKA rich medium. For each strain, RNA was extracted from at least three independent replicates of both the wild-type or empty vector conjugates and the HrpG* conjugates as described [22]. Specific Xanthomonas probes were employed to deplete rRNA and tRNA molecules [17] after which RNAs were fractionated into short (< 200 nt) and long RNAs using Zymo Research RNA Clean & Concentrator TM-5 columns (Proteigene). Strand-specific RNA sequencing was performed on an Ilumina HiSeq2000 platform, as described [22]. A detailed overview of all generated and used RNA-Seq libraries and their NCBI Sequence Read Archive (SRA) accessions is provided in additional file 1 (Additional file 1: Table S1E).
Structural annotation of Xanthomonas genomes
For each strain, the different RNA-Seq libraries available were merged in order to experimentally support the structural annotation of the genomes using Eugene-PP [70]. The used RNA-Seq libraries are detailed in additional file 1. The annotations used and generated in this study are provided in Data S1 (https://doi.org/10.57745/9OTYNJ).
Differential expression analyses of the RNA-Seq results
RNA-Seq reads were pseudo mapped onto the reference genomes using Salmon (version 1.4.0) with standard parameters [71]. An overview of which RNA-Seq datasets were used is given in additional file 1 (Additional file 1: Table S1E). Overall mapping quality was assessed using multiQC [72]. DEGs were identified using DESeq2 with Salmon’s count tables using standard parameters [73]. To assess sample reproducibility, PCA and MA plots were generated using DESeq2’s plotPCA and plotMA functions. Volcano plots for each strain were made using EnhancedVolcano [74]. The PCA on the expression of all orthogroups in the core genome across all strains was based on a matrix with a single average Log2FC value for all genes within an orthogroup. Genes with non-significant Log2FC were assigned a Log2FC of 0. Session information, appropriate raw data and relevant R scripts to reproduce the results are available in a code repository (Data S5, https://doi.org/10.57745/9OTYNJ).
Identification of the core HrpG* regulon
An orthogroup database constructed using Orthofinder (version 2.4.0) with standard parameters [75] was used to compare the regulation of genes by HrpG* across different strains. The species trees in Fig. 1 were made using Orthofinder’s STAG algorithm [76] and rooted using STRIDE [77]. Orthogroups, for which across all strains, at least one ortholog was differentially regulated in the wild-type or empty vector strains compared to the hrpG* strains (AdjPval < 0.05) were considered orthogroups part of the core regulon. To investigate the regulation of the endogenous hrpG by HrpG*, we investigated whether reads mapping to hrpG originated from the endogenous hrpG gene or the exogenous hrpG* gene by mapping RNA-Seq reads to both endogenous and HrpG* sequences. Reads that did not map to these two sequences or which mapped multiple times were then removed. The coverage at the polymorphic site of hrpG/hrpG* for the remaining reads was then assessed. A Chi-square test was used to test for equal proportions of HrpG*-dependent regulation of predicted protein-coding genes within the core and accessory genomes. Multiple testing correction was performed using the Benjamini–Hochberg procedure [78].
Conservation of the core HrpG regulon across all publicly available genomes
To investigate the conservation of orthogroups identified in this study across plant pathogenic Xanthomonas strains with a T3SS, an additional orthogroup database was built using publicly available genomes of Xanthomonas species with a T3SS (Data S6, https://doi.org/10.57745/9OTYNJ). This database was built on available genomes having less than 206 contigs and a Busco score of over 94.9% (657 genomes in total representing 25 Xanthomonas species and at least 67 different pathovars, Data S6). The conservation of relevant orthogroups identified in this study was studied for each corresponding orthogroup in the orthology analysis constructed with the 657 genomes.
Gene ontology and signal peptide prediction
Enrichment of specific GO terms in various gene lists was investigated using the TOPGO R package [79]. Signal peptides were predicted by signalP6.0 [80]. A Chi-square test was used to test for an enrichment of genes with Sec/SPI amongst regulated genes. When appropriate, promoter sequences were scanned for PIP-box motifs using the TTCGB-N15-TTCGB consensus [3] allowing for one mismatch.
Regulation of type three effector gene expression
T3Es were predicted using Effectidor [25] using the advanced mode, which makes use of the genomes GFF3 files to more accurately predict T3Es. Each orthogroup of which at least one ortholog was annotated as a true T3E by Effectidor was considered a true T3E orthogroup. True T3E orthogroups were further manually annotated and curated by integrating BLASTP, paperblast [81] and the EUROXANTH database [42]. Because genes encoding TALEs are often poorly assembled due to their repetitive sequences [82], only TALE orthologs for which the complete N-terminal domain upstream of the repeat region was present were considered.
Regulation of genes relevant for motility, carbohydrate active enzymes and proteases
Orthogroups involved in motility were identified using the Eugene-PP genome annotations and manually curated. Orthogroups encoding proteins with carbohydrate active enzyme activity were predicted using dbCAN3 [83] and further curated and annotated using the Carbohydrate-Active enZYmes (CAZY) Database [84]. Genes encoding enzymes with protease activity were identified using the Eugene-PP genome annotations and manually curated and annotated using the MEROPS database [85].
Visualization of regulation against phylogeny
Several figures were generated in order to explore a potential relationship between strain phylogeny and the regulation of specific orthogroups under HrpG*. The HrpG*-dependent Log2FC of expression all genes within an orthogroup were plotted against the phylogenetic tree as made by Orthofinder. The Log2FC of genes for which either Log2FC or AdjPval were assigned NA values by DESeq were set to 0. Figures were built using GGtree with equal branch length for the species tree [86].
Motility assays
Swimming motility was assessed as described [22]. In short, 2 µl of bacterial suspensions (5.108 cfu/mL) were spotted on 0.3% agar plates and incubated 48 h at 28 °C. Swimming motility was measured as the diameter of the white halo minus the colony diameter.
Data availability
All genomes sequenced and assembled in this study have been made publicly accessible. For a comprehensive list of their DOIs, please see Additional File 1. The transcriptome sequencing reads are available in the NCBI Sequence Read Archive (SRA), with the specific SRA accession numbers provided in Additional File 1. Supplementary data files can be found on recherche.data.gouv.fr under the DOI: https://doi.org/10.57745/9OTYNJ. This includes generated genome annotation for the 17 strains as Data S1, and comparative statistics and orthogroup analyses for these genomes, performed using OrthoFinder, as Data S2. R scripts utilized for analyzing the data, are stored in a code repository in Data S5. Comparative statistics and orthogroup information for 675 publicly available Xanthomonas genomes which include a type T3SS are detailed in Data S6, along with their respective genome accession numbers.
References
Wengelnik K, Van Den Ackerveken G, Bonas U. HrpG, a key hrp regulatory protein of Xanthomonas campestris Pv. Vesicatoria is homologous to two-component response regulators. Mol Plant Microbe Interact. 1996;9(8):704–12.
Stock AM, Robinson VL, Goudreau PN. Two-Component Signal Transduction. Annu Rev Biochem. 2000;69(1):183–215.
Koebnik R, Krüger A, Thieme F, Urban A, Bonas U. Specific binding of the Xanthomonas campestris Pv. Vesicatoria AraC-type transcriptional activator HrpX to plant-inducible promoter boxes. J Bacteriol. 2006;188(21):7652–60.
Büttner D, Bonas U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol Rev. 2010;34(2):107–33.
Tampakaki AP, Skandalis N, Gazi AD, Bastaki MN, Sarris PF, Charova SN, et al. Playing the harp: evolution of our understanding of hrp/hrc genes. Annu Rev Phytopathol. 2010;48(347–70):347–70.
White FF, Potnis N, Jones JB, Koebnik R. The type III effectors of Xanthomonas. Mol Plant Pathol. 2009;10(6):749–66.
Parte AC, Carbasse JS, Meier-Kolthoff JP, Reimer LC, Göker M. List of prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. Int J Syst Evol Microbiol. 2020;70:5607–12.
Ryan RP, Vorhölter FJ, Potnis N, Jones JB, Van Sluys MA, Bogdanove AJ, et al. Pathogenomics of Xanthomonas: understanding bacterium–plant interactions. Nat Rev Microbiol. 2011;9(5):344–55.
Young JM, Park DC, Shearman HM, Fargier E. A multilocus sequence analysis of the genus Xanthomonas. Syst Appl Microbiol. 2008;31(5):366–77.
Zaumeyer WJ, Thomas HR, Zaumeyer WJ, Thomas HR. A Monographic Study of Bean Diseases and Methods for Their Control. 1957 [cited 2024 Feb 22];(169625). https://ageconsearch.umn.edu/record/169625
Jacques MA, Josi K, Darrasse A, Samson R. Xanthomonas axonopodis Pv. Phaseoli var. Fuscans is aggregated in stable Biofilm Population sizes in the phyllosphere of field-grown beans. Appl Environ Microbiol. 2005;71(4):2008–15.
De O, Carvalho A, Da Cunha M, Rodrigues R, Sudré CP, Santos IS, Fernandes KVS, et al. Ultrastructural changes during early infection of Vigna unguiculata and Phaseolus vulgaris leaves by Xanthomonas axonopodis Pv. Phaseoli and an unexpected association between chloroplast and mitochondrion. Acta Physiol Plant. 2011;33(5):2025–33.
Almeida IMGD, Rodrigues LMR, Beriam LOS. Xanthomonas fuscans subsp. fuscans causing wilt symptoms in bean plants (Phaseolus vulgaris) in Brazil. Arq Inst Biol [Internet]. 2015 Apr 7 [cited 2024 Feb 22];82(0). http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1808-16572015000100300&lng=en&tlng=en
Guo Y, Figueiredo F, Jones J, Wang N. HrpG and HrpX play global roles in coordinating different virulence traits of Xanthomonas axonopodis Pv. Citri. Mol Plant Microbe Interact. 2011;24(6):649–61.
Wengelnik K, Rossier O, Bonas U. Mutations in the regulatory gene hrpG of Xanthomonas campestris pv. vesicatoria result in constitutive expression of all hrp genes. J Bacteriol. 1999;181(21):6828–31.
Noel L, Thieme F, Nennstiel D, Bonas U. cDNA-AFLP analysis unravels a genome-wide hrpg-regulon in the plant pathogen Xanthomonas campestris Pv. Vesicatoria. Mol Microbiol. 2001;41(6):1271–81.
Roux B, Bolot S, Guy E, Denancé N, Lautier M, Jardinaud MF, et al. Genomics and transcriptomics of Xanthomonas campestris species challenge the concept of core type III effectome. BMC Genomics. 2015;16:975.
Wei K, Tang DJ, He YQ, Feng JX, Jiang BL, Lu GT, et al. hpaR, a putative marR Family Transcriptional Regulator, is positively controlled by HrpG and HrpX and involved in the Pathogenesis, hypersensitive response, and extracellular protease production of Xanthomonas campestris Pathovar Campestris. J Bacteriol. 2007;189(5):2055–62.
Wang L, Rong W, He C. Two Xanthomonas Extracellular polygalacturonases, PghAxc and PghBxc, are regulated by type III secretion regulators HrpX and HrpG and are required for virulence. MPMI. 2008;21(5):555–63.
Szczesny R, Jordan M, Schramm C, Schulz S, Cogez V, Bonas U, et al. Functional characterization of the xcs and xps type II secretion systems from the plant pathogenic bacterium Xanthomonas campestris Pv Vesicatoria. New Phytol. 2010;187(4):983–1002.
Kogenaru S, Yan Q, Guo Y, Wang N. RNA-seq and microarray complement each other in transcriptome profiling. BMC Genomics. 2012;13(1):629.
Luneau CA, Roux B, Carrère S, Jardinaud MF, Gaillac A, et al. Xanthomonas transcriptome inside cauliflower hydathodes reveals bacterial virulence strategies and physiological adaptations at early infection stages. Mol Plant Pathol. 2022;23(2):159–74.
Merda D, Briand M, Bosis E, Rousseau C, Portier P, Barret M, et al. Ancestral acquisitions, gene flow and multiple evolutionary trajectories of the type three secretion system and effectors in Xanthomonas plant pathogens. Mol Ecol. 2017;26(21):5939–52.
Assis RDAB, Polloni LC, Patané JSL, Thakur S, Felestrino ÉB, Diaz-Caballero J, et al. Identification and analysis of seven effector protein families with different adaptive and evolutionary histories in plant-associated members of the Xanthomonadaceae. Sci Rep. 2017;7(1):16133.
Wagner N, Avram O, Gold-Binshtok D, Zerah B, Teper D, Pupko T. Effectidor: an automated machine-learning-based web server for the prediction of type-III secretion system effectors. Bioinformatics. 2022;38(8):2341–3.
Alvarez-Martinez CE, Sgro GG, Araujo GG, Paiva MRN, Matsuyama BY, Guzzo CR, et al. Secrete or perish: the role of secretion systems in Xanthomonas biology. Comput Struct Biotechnol J. 2021;19:279–302.
Yamazaki A, Hirata H, Tsuyumu S. HrpG regulates type II secretory proteins in Xanthomonas axonopodis Pv. Citri. J Gen Plant Pathol. 2008;74(2):138–50.
Furutani A, Takaoka M, Sanada H, Noguchi Y, Oku T, Tsuno K, et al. Identification of novel type III secretion effectors in Xanthomonas oryzae Pv. Oryzae. Mol Plant Microbe Interact. 2009;22(1):96–106.
Teper D, Pandey SS, Wang N. The HrpG/HrpX regulon of Xanthomonads—An insight to the complexity of regulation of virulence traits in Phytopathogenic Bacteria. Microorganisms. 2021;9(1):187.
Wang JY, Zhou L, Chen B, Sun S, Zhang W, Li M, et al. A functional 4-hydroxybenzoate degradation pathway in the phytopathogen Xanthomonas campestris is required for full pathogenicity. Sci Rep. 2015;5(1):18456.
Chen B, Li R, Zhou L, Qiu J, Song K, Tang J, et al. The phytopathogen Xanthomonas campestris utilizes the divergently transcribed pobA / pobR locus for 4-hydroxybenzoic acid recognition and degradation to promote virulence. Mol Microbiol. 2020;114(5):870–86.
Parke D. Characterization of PcaQ, a LysR-type transcriptional activator required for catabolism of phenolic compounds, from Agrobacterium tumefaciens. J Bacteriol. 1996;178(1):266–72.
MacLean AM, Haerty W, Golding GB, Finan TM. The LysR-type PcaQ protein regulates expression of a protocatechuate-inducible ABC-type transport system in Sinorhizobium meliloti. Microbiology. 2011;157(9):2522–33.
Blanvillain S, Meyer D, Boulanger A, Lautier M, Guynet C, Denancé N et al. R Redfield editor 2007 Plant Carbohydrate scavenging through TonB-Dependent receptors: A Feature Shared by Phytopathogenic and aquatic Bacteria. PLoS ONE 2 2 e224.
Hederstedt L. Diversity of Cytochrome C Oxidase Assembly Proteins in Bacteria. Microorganisms. 2022;10(5):926.
Wu J, Pan X, Xu S, Duan Y, Luo J, Zhou Z, et al. The critical role of cytochrome c maturation (CCM) system in the tolerance of Xanthomonas campestris Pv. Campestris to phenazines. Pestic Biochem Physiol. 2019;156:63–71.
Chen L, Wang M, Huang L, Zhang Z, Liu F, Lu G. XC_0531 encodes a c-type cytochrome biogenesis protein and is required for pathogenesis in Xanthomonas campestris Pv. Campestris. BMC Microbiol. 2017;17(1):142.
Pena MM, Bhandari R, Bowers RM, Weis K, Newberry E, Wagner N et al. Genetic and Functional Diversity Help Explain Pathogenic, Weakly Pathogenic, and Commensal Lifestyles in the Genus Xanthomonas. Ma LJ, editor. Genome Biology and Evolution. 2024;16(4):evae074.
Tamir-Ariel D, Navon N, Burdman S. Identification of genes in Xanthomonas campestris Pv. Vesicatoria Induced during its Interaction with Tomato. J Bacteriol. 2007;189(17):6359–71.
Qian W, Jia Y, Ren SX, He YQ, Feng JX, Lu LF et al. Comparative and functional genomic analyses of the pathogenicity of phytopathogen Xanthomonas campestris pv. campestris. Genome Res. 2005;15(6):757–67.
Luneau BM, Quiroz Monnens T, Carrère S, Bouchez O, Jardinaud MF, et al. Genome-wide identification of fitness determinants in the Xanthomonas campestris bacterial pathogen during early stages of plant infection. New Phytol. 2022;236(1):235–48.
Costa J, Pothier JF, Bosis E, Boch J, Kölliker R, Koebnik R. A community-curated DokuWiki Resource on Diagnostics, Diversity, pathogenicity, and Genetic Control of Xanthomonads. MPMI. 2024;37(3):347–53.
Jiang W, Jiang BL, Xu RQ, Huang JD, Wei HY, Jiang GF, et al. Identification of six type III effector genes with the pip box in Xanthomonas campestris Pv. Campestris and five of them contribute individually to full pathogenicity. Mol Plant Microbe Interact. 2009;22(11):1401–11.
Popov G, Fraiture M, Brunner F, Sessa G. Multiple Xanthomonas euvesicatoria type III effectors inhibit flg22-Triggered immunity. MPMI. 2016;29(8):651–60.
Soni M, Mondal KK. Xanthomonas axonopodis Pv. Punicae uses XopL effector to suppress pomegranate immunity: XopL effector of Xap suppresses pomegranate immunity. J Integr Plant Biol. 2018;60(4):341–57.
Leong JX, Raffeiner M, Spinti D, Langin G, Franz-Wachtel M, Guzman AR, et al. A bacterial effector counteracts host autophagy by promoting degradation of an autophagy component. EMBO J. 2022;41(13):e110352.
Ortmann S, Marx J, Lampe C, Handrick V, Ehnert TM, Zinecker S et al. A conserved microtubule-binding region in Xanthomonas XopL is indispensable for induced plant cell death reactions. Mackey D, editor. PLoS Pathog. 2023;19(8):e1011263.
Akimoto-Tomiyama C, Furutani A, Tsuge S, Washington EJ, Nishizawa Y, Minami E, et al. XopR, a type III Effector secreted by Xanthomonas oryzae Pv. Oryzae, suppresses Microbe-Associated Molecular Pattern-Triggered immunity in Arabidopsis thaliana. MPMI. 2012;25(4):505–14.
Wang S, Sun J, Fan F, Tan Z, Zou Y, Lu D. A Xanthomonas oryzae pv. Oryzae effector, XopR, associates with receptor-like cytoplasmic kinases and suppresses PAMP-triggered stomatal closure. Sci China Life Sci. 2016;59(9):897–905.
Verma G, Mondal KK, Kulshreshtha A, Sharma M. XopR T3SS-effector of Xanthomonas oryzae Pv. Oryzae suppresses cell death-mediated plant defense response during bacterial blight development in rice. 3 Biotech. 2019;9(7):272.
Medina CA, Reyes PA, Trujillo CA, Gonzalez JL, Bejarano DA, Montenegro NA, et al. The role of type III effectors from Xanthomonas axonopodis Pv. Manihotis in virulence and suppression of plant immunity: role of type III effectors from Xam. Mol Plant Pathol. 2018;19(3):593–606.
Degrassi G, Devescovi G, Bigirimana J, Venturi V. Xanthomonas oryzae Pv. Oryzae XKK.12 contains an AroQ γ Chorismate Mutase that is involved in Rice Virulence. Phytopathology®. 2010;100(3):262–70.
Tamir-Ariel D, Rosenberg T, Navon N, Burdman S. A secreted lipolytic enzyme from Xanthomonas campestris Pv. Vesicatoria is expressed in planta and contributes to its virulence: Xanthomonas campestris Pv. Vesicatoria LipA. Mol Plant Pathol. 2012;13(6):556–67.
Jha G, Rajeshwari R, Sonti RV. Functional interplay between two Xanthomonas oryzae pv. Oryzae Secretion systems in modulating virulence on Rice. MPMI. 2007;20(1):31–40.
Nascimento R, Gouran H, Chakraborty S, Gillespie HW, Almeida-Souza HO, Tu A, et al. The type II secreted Lipase/Esterase LesA is a key virulence factor required for Xylella fastidiosa Pathogenesis in grapevines. Sci Rep. 2016;6(1):18598.
Laia ML, Moreira LM, Dezajacomo J, Brigati JB, Ferreira CB, Ferro MI, et al. New genes of Xanthomonas citri subsp. citri involved in pathogenesis and adaptation revealed by a transposon-based mutant library. BMC Microbiol. 2009;9(1):12.
Das A, Rangaraj N, Sonti RV. Multiple adhesin-like functions of Xanthomonas oryzae Pv. Oryzae are involved in promoting leaf attachment, entry, and virulence on rice. Mol Plant Microbe Interact. 2009;22(1):73–85.
Pradhan BB, Ranjan M, Chatterjee S. XadM, a novel adhesin of Xanthomonas oryzae Pv. Oryzae, exhibits similarity to Rhs family proteins and is required for optimum attachment, biofilm formation, and virulence. Mol Plant Microbe Interact. 2012;25(9):1157–70.
Kumar Verma R, Samal B, Chatterjee S. Xanthomonas oryzae pv. oryzae chemotaxis components and chemoreceptor Mcp2 are involved in the sensing of constituents of xylem sap and contribute to the regulation of virulence-associated functions and entry into rice. Molecular Plant Pathology. 2018;19(11):2397–415.
Li RF, Ren PD, Liu QQ, Yao JL, Wu L, Zhu GN, et al. McvR, a single domain response regulator regulates motility and virulence in the plant pathogen Xanthomonas campestris. Mol Plant Pathol. 2022;23(5):649–63.
Zhang Y, Callaway EM, Jones JB, Wilson M. Visualisation of hrp gene expression in Xanthomonas euvesicatoria in the tomato phyllosphere. Eur J Plant Pathol. 2009;124(3):379–90.
Mayjonade B, Gouzy J, Donnadieu C, Pouilly N, Marande W, Callot C, et al. Extraction of high-molecular-weight genomic DNA for long-read sequencing of single molecules. Biotechniques. 2016;61(4):203–5.
Bellenot C, Carrère S, Gris C, Noël LD, Arlat M. Genome Sequences of 17 Strains from Eight Races of Xanthomonas campestris pv. campestris. Baltrus DA, editor. Microbiol Resour Announc. 2022;11(7):e00279-22.
Denancé N, Szurek B, Doyle EL, Lauber E, Fontaine-Bodin L, Carrère S, et al. Two ancestral genes shaped the Xanthomonas campestris TAL effector gene repertoire. New Phytol. 2018;219(1):391–407.
Kolmogorov M, Yuan J, Lin Y, Pevzner PA. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 2019;37(5):540–6.
Guy E, Genissel A, Hajri A, Chabannes M, David P, Carrere S et al. Natural Genetic Variation of Xanthomonas campestris pv. campestris Pathogenicity on Arabidopsis Revealed by Association and Reverse Genetics. Guttman D, Ausubel FM, editors. mBio. 2013;4(3):e00538-12.
Kovach ME, Elzer PH, Steven Hill D, Robertson GT, Farris MA, Roop RM, et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166(1):175–6.
Figurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76(4):1648–52.
Ditta G, Stanfield S, Corbin D, Helinski DR. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980;77(12):7347–51.
Sallet E, Roux B, Sauviac L, Jardinaud MF, Carrere S, Faraut T, et al. Next-generation annotation of prokaryotic genomes with EuGene-P: application to Sinorhizobium meliloti 2011. DNA Res. 2013;20(4):339–54.
Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14(4):417–9.
Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32(19):3047–8.
Love MI, Huber W, Anders S. Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
Blighe K. EnhancedVolcano [Internet]. 10.18129/B9.bioc.EnhancedVolcano: Bioconductor; 2018 [cited 2024 Feb 23]. https://bioconductor.org/packages/EnhancedVolcano
Emms DM, Kelly S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 2019;20(1):238.
Emms DM, Kelly S. STAG: Species Tree Inference from All Genes [Internet]. Evolutionary Biology; 2018 Feb [cited 2024 Feb 26]. http://biorxiv.org/lookup/doi/10.1101/267914
Emms DM, Kelly S. STRIDE: Species Tree Root inference from gene duplication events. Mol Biol Evol. 2017;34(12):3267–78.
Benjamini Y, Hochberg Y. Controlling the false Discovery rate: a practical and powerful Approach to multiple testing. J Roy Stat Soc: Ser B (Methodol). 1995;57(1):289–300.
Alexa A, Rahnenfuhrer J. topGO: enrichment analysis for gene ontology. Bioinformatics. 2010;22(13):1600–7.
Teufel F, Almagro Armenteros JJ, Johansen AR, Gíslason MH, Pihl SI, Tsirigos KD, et al. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat Biotechnol. 2022;40(7):1023–5.
Price MN, Arkin AP, PaperBLAST. Text Mining Papers for Information about Homologs. Langille MGI, editor. mSystems. 2017;2(4):e00039-17.
Erkes A, Grove RP, Žarković M, Krautwurst S, Koebnik R, Morgan RD, et al. Assembling highly repetitive Xanthomonas TALomes using Oxford Nanopore sequencing. BMC Genomics. 2023;24(1):151.
Zheng J, Ge Q, Yan Y, Zhang X, Huang L, Yin Y. dbCAN3: automated carbohydrate-active enzyme and substrate annotation. Nucleic Acids Res. 2023;51(W1):W115–21.
Drula E, Garron ML, Dogan S, Lombard V, Henrissat B, Terrapon N. The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res. 2022;50(D1):D571–7.
Rawlings ND, Barrett AJ, Thomas PD, Huang X, Bateman A, Finn RD. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018;46(D1):D624–32.
Yu G. Using ggtree to visualize data on Tree-Like structures. Curr Protocols Bioinf. 2020;69(1):e96.
Acknowledgements
We wish to thank Claudine Zischek for the construction of Xcc strains, Aude Cerutti for helpful advice on ribodepletion, Caroline Bellenot for sharing curated Xanthomonas genomics resources and Naama Waagner for running Effectidor analyses.
Funding
This work was supported by grants from the “Agence Nationale de la Recherche” projects XANTHOMIX (ANR-2010-GENM-013-02 to BR, SC, EC, SC, EL, AD, MA, BS, OP, MAJ, LG, RK and LDN) and XBOX (ANR-19-CE20-JCJC-0014-01 to TQM, LDN and AB). All co-authors are members of the French Network on Xanthomonads (FNX) supported by the INRAE Plant Health division. This study is set within the framework of the “Laboratoires d’Excellences” (LABEX) TULIP (ANR-10-LABX-41) and of the “Ecole Universitaire de Recherche” (EUR) TULIP-GS (ANR-18-EURE-0019).
Author information
Authors and Affiliations
Contributions
The initial XANTHOMIX proposal was written under coordination of MA, OP, MAJ, LDN and RK. BR, SCU, BS, EC, AD, LDN, EL and LG were involved in the sequencing of four Xanthomonas genomes, the construction of the different Xanthomonas conjugates and the preparation and sequencing of cDNAs. SCA handled genome annotations, transcriptomic pseudomapping, and Orthofinder analyses. MFJ performed the initial transcriptomic analyses. TQM performed quality controls on the raw data, conducted the in-depth comparative transcriptomic analyses, prepared all the figures and drafted the manuscript. AB and LDN supervised the analyses, interpreted of the data and wrote the manuscript. All authors read, commented and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Monnens, T.Q., Roux, B., Cunnac, S. et al. Comparative transcriptomics reveals a highly polymorphic Xanthomonas HrpG virulence regulon. BMC Genomics 25, 777 (2024). https://doi.org/10.1186/s12864-024-10684-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10684-6