Abstract
Background
The breeder rooster has played a pivotal role in poultry production by providing high-quality semen. Typically, fertility peaks between 30 and 40 weeks of age and then declines rapidly from 45 to 55 weeks of age. Research into improving fertility in aging roosters is essential to extend their productive life. While progress has been made, enhancing fertility in aging roosters remains a significant challenge.
Methods
To identify the genes related to promoting sperm remodeling in aged Houdan roosters, we combined changes in testis and semen quality with transcriptome sequencing (RNA-seq) to analyze the synchrony of semen quality and testis development. In this study, 350-day-old Houdan breeder roosters were selected for RNA-seq analysis in testis tissues from induced molting roosters (D group) and non-induced molting roosters (47DG group). All analyses of differentially expressed genes (DEGs) and functional enrichment were performed. Finally, we selected six DEGs to verify the accuracy of the sequencing by qPCR.
Results
Compared with the 47DG group, sperm motility (P < 0.05), sperm density (P < 0.01), and testis weight (P < 0.05) were significantly increased in roosters in the D group. Further RNA-seq analysis of the testis between the D group and 47DG group identified 61 DEGs, with 21 up-regulated and 40 down-regulated. Functional enrichment analysis showed that the DEGs were primarily enriched in the cytokine-cytokine receptor interaction, Wnt signaling pathway, MAPK signaling pathway, TGF-β signaling pathway, and focal adhesion pathway. The qRT-PCR results showed that the expression trend of these genes was consistent with the sequencing results. WNT5A, FGFR3, AGTR2, TGFβ2, ROMO1, and SLC26A7 may play a role in testis development and spermatogenesis. This study provides fundamental data to enhance the reproductive value of aging roosters.
Similar content being viewed by others
Introduction
The reproductive performance of Breeder roosters plays a crucial role in poultry production. Breeder roosters experience a significant decline in semen quality as they age, putting them at risk of being culled in the subsequent years [1]. The fertility of roosters typically peaks at 12 months and then rapidly declines [2,3,4,5]. This decline was accompanied by a steady decrease in the quantity and quality of sperm per ejaculation [6, 7]. Aging is a highly intricate and irreversible process that takes place in all cells, tissues, and organs of living organisms [8]. It is one of the primary factors leading to the deterioration of various organs, including the reproductive system [9, 10]. Research has shown that even under better breeding conditions, aging still leads to a gradual loss of reproductive function and behavior in roosters [11, 12]. The testes are the primary reproductive organs in roosters responsible for sperm production and testosterone synthesis [13, 14]. There is ample evidence that the reduction in the activity of the gonadal axis associated with declining reproductive behavior is related to the aging phenomenon [15]. During the aging process, the morphological changes that occur in the testes are mainly characterized by a decrease in the volume and number of germ cells, as well as a decrease in semen volume and quality [5]. Roosters experience a decline in reproductive performance as they age, producing fewer viable sperm cells. This leads to a decrease in hatchability and significant economic losses in the poultry industry [13, 16, 17].
Molting is a natural and energy-demanding life history process of birds that helps them adapt to complex environmental changes [18]. Under natural conditions, hens begin to molt gradually after about 12 months of egg production [19]. Natural molting typically lasts for 3–4 months, and the molting process is not synchronized in the flock, resulting in lower egg production rates and inconsistent egg quality. This leads to a decrease in the overall productivity of the flock [20]. On the other hand, forced molting can be completed in about 4 weeks [21]. Forced molting is implemented by systematically restricting feed and implementing management protocols, which disrupt the hen’s natural metabolism and aging of the reproductive system [21]. Subsequently, providing water and feed again promotes cell regeneration and rapid development of reproductive organs, enabling the flock to complete molting within a short period and regain synchronous egg production, resulting in higher economic benefits [20]. As the measures implemented on laying hens are gradually lifted and the hens meet their energy requirements, their neural and endocrine functions are reactivated [22]. The survival potential of laying hens gradually recovers during this process [21]. Not only does the post-molt hen’s productivity significantly exceed that before molting [23], but the bacterial abundance in its feces is also more diverse [24], and its bone strength is stronger [25]. Numerous studies indicate that forced molting can prolong the lifespan of aged laying hens. Currently, forced molting of aging roosters to enhance their reproductive potential and extend their productive lifespan has not been tried.
With the development of sequencing technology, transcriptomes have been shown to have high accuracy in detecting differentially expressed genes (DEGs) related to important economic traits in chickens [21], They have been widely applied in the study of chicken growth and development [26, 27], reproductive traits [27, 28], and disease resistance [29]. Numerous studies have reported that light fasting or intermittent fasting was beneficial to the health of humans and animals, enhancing systemic immune function and slowing down aging [30, 31]. In chickens, study found that molting can stimulate testis growth and development in roosters [1]. However, the underlying molecular mechanism associated with the fasting-induced molting in roosters remains unclear, necessitating further investigation into the genetic networks responsible for this phenotype. Research into fasting-induced molting is gaining momentum. Our objective is to implement fasting-induced molting in Houdan breeder roosters at 350 days of age, aiming to enhance semen quality and, consequently, extend their economic life span. We plan to utilize transcriptome sequencing to identify DEGs subsequent to fasting-induced forced molting, thereby investigate its influence on the reproductive performance of the roosters.
Results and analysis
Effect of induced molting on semen quality and testis development of aged Houdan roosters
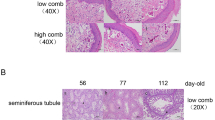
To study the effects of induced molting on semen quality and testicular development in aged roosters, we statistically analyzed the changes in sperm vitality, sperm density, and testicular weight in the D group and the 47DG group of aged Houdan roosters. The results indicated that on the 32nd day of recovery following food restriction-induced molting, sperm density (Fig. 1a), the sperm motility (Fig. 1b), and testis weight (Fig. 1c) of aging Houdan roosters in the D group were all better than 47DG group. The difference in sperm vitality was significant (P < 0.05), and the differences in sperm density and testicular weight were highly significant (P < 0.01). These findings indicate that feed restriction-induced molt can improve testis weight, sperm motility, and sperm density in aging Houdan roosters.
Transcriptome sequencing data analysis
The results of the transcriptome sequencing were shown in Table 1. Q20 was greater than 97%, Q30 was greater than 92%, and the percentage of GC content was above 50%. No separation phenomena were observed, indicating that the quality of the sequencing data was high and met the sequencing requirements, such that the next step could be carried out.
Analysis of differentially expressed genes
The transcriptome sequencing data of the testis tissues of the D group and 47DG group on the 47th day were analyzed using DESeq2 software (Fig. 2). The results showed that compared with the 47DG group, the D group obtained a total of 61 significantly DEGs, including 21 significantly up-regulated genes and 40 significantly down-regulated genes.
Genetic relationship between different group
The principal component analysis (PCA) plot showed that the D group and 47DG group were clearly separated with main principal component (PC) scores as follows: PC1 = 46.33% and PC2 = 21.99% (Fig. 3). Heatmaps based on generated from six samples in the D group and 47DG group. Results showed that were clearly separated (Fig. 4).
GO and KEGG pathway enrichment analysis
GO functional enrichment analysis revealed that DEGs were enriched in three categories: biological process (BP), cellular component (CC), and molecular function (MF). A total of 518 significantly enriched GO terms were identified, including 443 in BP, 24 in CC, and 51 in- MF. As shown in Appendix 1, in the biological process category, DEGs were mainly involved in multicellular organism processes, positive regulation of response to stimulus, MAPK cascade regulation, extrinsic apoptotic signaling pathway, regulation of phosphorus metabolic process, cell adhesion, cell morphogenesis, cell growth, mesenchymal cell differentiation, and kidney system development, with 20, 10, 6, 3, 8, 7, 5, 4, 3, and 3 genes, respectively. In the molecular function category, DEGs were mainly involved in membrane, intrinsic component of membrane, extracellular region, plasma membrane, and vesicle, with 27, 20, 17, 16, and 8 genes, respectively. In the cellular component category, DEGs were mainly involved in signal receptor binding, receptor ligand activity, and growth factor activity, with 9, 5, and 2 genes, respectively.
KEGG pathway enrichment analysis revealed a total of 23 significantly enriched pathways. As shown in Fig. 5, the pathways significantly enriched in DEGs included cytokine-cytokine receptor interaction, neuroactive ligand-receptor interaction, ECM-receptor interaction, TGF-beta signaling pathway, melanogenesis, mTOR signaling pathway, Wnt signaling pathway, focal adhesion, calcium signaling pathway, and MAPK signaling pathway (Fig. 5).
GO and KEGG pathway enrichment analysis. (a) Top 20 GO Term enrichment of DEGs, the bubble size represents the number of DEGs, and the bubble color represents the Q-value. (b) Top 20 KEGG pathway enrichment of DEGs, the bubble size represents the number of DEGs, and the bubble color represents the Q-value
Verification of RNA-seq results using qPCR
We performed qRT-PCR analysis the expression of 6 DEGs (BPIFCB, SLC2A12, ROMO1, WNT5A, SLC26A7, TGFβ2) (Fig. 6). Compared with 47DG group, the data from transcriptome sequencing showed that the 6 genes were significantly change in D group. The differential fold change was converted using log2(fold change), Our results showed that the expression profiles of the candidate unigenes revealed by the qRT-PCR data were consistent with those derived from transcriptome sequencing (Fig. 6), which indicated that the RNA-Seq analysis was reliable and provided a valuable gene sequence for biological analysis.
Discussion
There is evidence to suggest a correlation between testis weight and semen quality capacity [1, 6]. Meanwhile, the testis is an important parameter for evaluating male reproductive capacity [32]. From our results, it can be perceived that the testis weight and semen quality of D Group roosters have been improved, which is consistent with previous research results [1]. In this study, 61 DEGs were identified by transcriptome sequencing of testis tissues from 350-day-old Houdan roosters, which underwent induced molting and recovery compared to those that did not undergo induced molting and recovery. GO functional and KEGG pathway enrichment analysis showed that these DEGs played important roles in the testis development and sperm generation process of roosters after induced molting. The DEGs were enriched in pathways related to cytokine-cytokine receptor interactions, neuroactive ligand-receptor interactions, ECM-receptor interactions, TGF-β signaling pathway, melanogenesis, mTOR signaling pathway, Wnt signaling pathway, focal adhesion, calcium signaling pathway, and MAPK signaling pathway. The DEGs detected in chicken testis tissue samples were mainly significantly enriched in steroid biosynthesis, lipid metabolism and glycerol lipid metabolism [33]. Li et al. found that cytokines and testosterone synergistically regulated the primary spermatocytes in sperm formation, sperm development, and the blood-testis barrier [34, 35]. Xiao et al. found that intercellular adhesion molecules had an antagonistic effect on the tight junction barrier of supporting cells, participated in the reconstruction of the supporting cell-blood-testis barrier, and regulated sperm adhesion in the epithelial cycle [36]. It was discovered by researchers Zhu et al. that the sperm motility and reproductive capacity of roosters were enhanced after induced molting. Through transcriptome sequencing analysis of rooster testicles, it was found that the regulation of cellular cytoskeleton is closely associated with spermatogenesis [1]. Cytokines play an important role in establishing and maintaining the immune characteristics of the testis [37, 38]. Testis cells produce various anti-inflammatory cytokines, which are important for the development of the testis and sperm generation.
The MAPK signaling pathway is one of the classical and important pathways in the eukaryotic signal transduction network. It is a key signaling pathway involved in cell proliferation, differentiation, apoptosis, and stress responses under normal and pathological conditions [39]. In this study, FGFR3 and TGFβ2 DEGs were significantly enriched in the MAPK signaling pathway in testis tissues. Several other studies [40,41,42] have shown that this pathway is involved in cell proliferation, differentiation, and apoptosis, making it an important signaling pathway. In mammals, MAPK can be translated as “mitogen-activated protein kinase” Regulating cell proliferation, differentiation, movement, and survival is one of the important pathways affecting testis development and spermatogenesis [43]. This suggests that the MAPK signaling pathway plays a critical role in regulating rooster testis development, spermatogenesis, and sperm quality. David M Ornitz et al. found that FGFR3 is a signal protein secreted by the FGF family, which mediates the intracellular signaling pathway with MAPK through the interaction between FGF ligands and their signal receptors [44]. FGF family members play a role in early embryonic development and organ development, and secreted FGF factors regulate basic cellular processes, including positive and negative regulation of proliferation, survival, migration, differentiation, and metabolism [44, 45]. FGFs also play an important role in adult tissues, often mediating metabolic functions, tissue repair, and regeneration by reactivating developmental signaling pathways [44, 46]. Elia Escasany et al. found that the transforming growth factor β (TGFβ) family of cytokines is a secreted multifunctional protein that is involved in cell proliferation and differentiation, wound healing, and fibrosis [46]. Study by Yang F et al. support the promoting role of TGF-β in neovascularization, which may be direct or indirect [47]. The Wnt signaling pathway plays an important role in development, regeneration, tumor development, and tissue repair [48]. The Wnt signaling pathway is usually divided into the canonical Wnt signaling pathway and the non-canonical Wnt signaling pathway, and Wnt ligands activate these two pathways by binding to different receptors on the cell surface [49]. The signals are transmitted to the cell nucleus through the Wnt and Ca2+ signaling pathways, activating downstream signaling molecules and regulating cell growth, morphology changes, and other processes [50]. WNT5A was typically involved in activating the non-canonical pathway and may inhibit the canonical pathway, thus participating in male germ cell development as a representative ligand involved in regulating male germ cell differentiation, and it was also a necessary ligand for the proliferation of testis cells [51]. In mammals, anti-Müllerian hormone (AMH) was produced by Sertoli cells that differentiate from the testis and by granulosa cells after birth [52]. Although AMH in birds is not identical to that in mammals [53, 54], it plays an important role in chicken gonadal development and maintaining testis morphology. This may also play an important role in the process of testis redevelopment and sperm production in roosters after fasting-induced molting in this study, but its specific functional role needs to be further explored.
Fibroblast growth factor receptor 3 (FGFR3), belonging to the fibroblast growth factor family, can specifically bind to the fibroblast growth factor receptor complex on the membrane of kidney and kidney tubular epithelial cells and regulate the reabsorption of calcium and phosphorus by activating the MAPK signaling pathway [36]. Ortiz-García CI et al. found that ROS produced by members of the NOX family play an important role in physiological processes such as sperm capacitation and acrosome reaction, and the ROS produced by NADPH oxidase (NOX) are associated with the activation of calcium-dependent proteinases and calmodulin-dependent proteinases [55]. Liu et al. found that ROS produced by NADPH oxidase can regulate the TGF-β signaling pathway through pathways including the MAPK pathway and the GTP-binding protein pathway [56]. Jankowska, A found that calcium-binding protein B (S100B) is present in most spermatogonia and sperm cells, and the Ca2+-regulated membrane-bound guanylate cyclase transduction mechanism plays a role in the biochemical action, molecular mechanism, and functional characteristics of spermatogenesis in the testis, mainly through S100B expression, transducing Ca2+-driven membrane-bound guanylate cyclase expression in germ cells [57]. This may be related to the significant improvement in sperm motility observed in this study. In summary, DEGs such as WNT5A, FGFR3, AMH, TGFβ2, S100B and NOX4 may play important roles in the process of testis redevelopment in aging laying hens after fasting-induced molting.
Conclusion
In this study, we performed induced molting experiment was conducted on 350-day-old Houdan roosters. Compared with the control group, the experimental group showed a significant increase in sperm motility, sperm density, and testis weight. Transcriptome sequencing analysis identified 61 DEGs, functional annotation revealed that genes such as WNT5A, FGFR3, AMH, TGFβ2, S100B and NOX4 play important roles in testis development and spermatogenesis in elderly Houdan roosters after induced molting. This study provides basic data for further enhancing the breeding value of aging roosters and testis development after induced molting.
Materials and methods
Ethics statement
All the animal experiments were approved by the Animal Care Committee of the College of Animal Science and Technology, Henan Agricultural University, and were performed following the protocol approved by the Institutional Animal Care and Use Committee (IACUC) of China. All efforts were made to minimize animal suffering.
Experimental animals and sample collection
In this experiment, seventy elderly aged Houdan roosters (fifty weeks of age) from the germplasm resources field of Henan Agricultural University were selected as experimental animals. They were divided into two groups: Group D and Group 47DG, with 35 roosters in each group. Group 47DG had free access to feed and water, while Group D underwent induced molting (Table 2: Feed Composition and Nutrient Content). The 47DG group was fed normally while the D group underwent induced molting (Table 2: Feed Coposition and Nutrient Content). The experiment lasted 47 days. The induction process included a 15-day fasting phase followed by a 32-day recovery phase. During the fasting phase, the roosters were deprived of food for 15 days, and water was withheld for 3 days (the first three days of the fasting period), with exposure to 8 h of light each day. During the recovery phase, the roosters began to gradually resume feeding starting from the 16th day of the experiment (30 g per day on days 16 and 17, 60 g per day on days 18 and 19, 90 g per day on days 20 and 21, and 120 g per day on days 22 and 23, after which they had free access to food and water), with an additional 0.5 h of light exposure added each day. On the 47th day of the experiment, three roosters from each group were randomly selected and euthanized by venous bloodletting after a 12-hour fast. Testicular tissues were promptly collected after slaughter, placed in RNA-free freezing tubes, and stored in liquid nitrogen. The testis transcriptome sequencing was outsourced to Nanjing Parsecno Gene Technology Co., Ltd.
Semen quality determination
The collected semen was diluted 500-fold with a 0.9% sodium chloride solution. A 10 µL mixed solution was taken, observed, and counted under a 40X microscope (Motic China) using a blood cell counting plate. Sperm counts were taken from the four corners and the central square of the counting chamber. (Sperm pressed against the boundary of the square were counted as left, not right, and up, not down.) The number of forward-moving sperm was counted to calculate the sperm density and sperm motility. All counting work was performed by the same person. Sperm density was calculated as the total number of sperm/80 × 500 × 400 × 10,000, and sperm motility (%) was calculated as the number of sperm with linear movement/total number of sperm × 100.
The construction of cDNA library and sequencing
Total RNA was extracted from three left testis tissues of the experimental and control groups using Trizol reagent (Invitrogen Life Technologies, Shanghai, China). Afterward, the concentration, quality, and integrity were determined using a NanoDrop spectrophotometer (Thermo Fisher Scientific). After testing the samples, mRNA was purified using magnetic beads and oligo(dT) to bind the polyA tail of mRNA. Subsequently, the mRNA was fragmented using a fragmentation buffer. Single-stranded cDNA was generated using random hexamer primers from the mRNA template, and double-stranded cDNA was prepared using dNTPs and DNA polymerase I. The purified double-stranded cDNA underwent end repair, A-tailing, and adapter ligation, followed by size selection using AMPure XP beads. Subsequently, the final cDNA library was prepared by PCR amplification of the fragments. The sequencing library was then sequenced on the NovaSeq 6000 platform (Illumina, San Diego, CA) by Shanghai Personal Biotechnology Co. Ltd.
Transcriptome sequencing data analysis and quality control
After sequencing, image files were obtained and converted using the sequencing platform’s built-in software to generate the original data in FASTQ format (raw data). Sequencing data contained a number of connectors and low-quality reads. Therefore, we utilized Cutadapt (v1.15) software to filter the sequencing data and obtain high-quality sequences (Clean Data) for subsequent analysis. All subsequent analyses were based on the clean data for high-quality analysis.
Differentially expressed gene screening and functional enrichment
We used HTSeq (v0.9.1) statistics to compare the read count values for each gene as the baseline gene expression. The gene expression was normalized using FPKM (Fragments Per Kilobase per Million fragments). The differential expression of genes was analyzed using DESeq (v1.30.0) with the following criteria: a fold change in expression |log2FoldChange| > 1 and a significant P-value < 0.05. GO and KEGG pathway enrichment analyses were conducted using the topGO R package and the Orthology-Based Annotation System (KOBAS) software, respectively.
Real-time PCR validation
To verify the accuracy of the transcriptome data, six DEGs screened during the experiment were randomly selected. The expression of testis differentially expressed genes was detected using qPCR with GAPDH used as the housekeeping gene. The primers for the target genes and internal reference gene (Table 3) were synthesized by Shangya Biotechnology Co., Ltd.The RT-qPCR protocol was as follows: one microgram of RNA was reverse transcribed to cDNA, and qPCR was performed in a 10 µL volume containing 1.0 µL of cDNA, 5.0 µL of SYBR Premix Ex Taq II (TaKaRa, Dalian, China), 0.5 µL of each primer (10 µmol/L), and 3 µL of RNase-free water. A LightCycler96 real-time PCR system (Roche Applied Science, Indianapolis, IN) was utilized. The amplification conditions were as follows: 94 °C for 5 min; 35 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s; followed by a final 10-min extension at 72 °C. The relative expression of genes was calculated using the 2−ΔΔCT method [58].
Statistical analysis
Data were analyzed using one-way analysis of variance followed by Duncan’s multiple range test in SPSS version 23.0. P-values less than 0.05 were considered statistically significant. The data were presented as the average value ± standard error. GraphPad Prism version 8.0 was used for plotting.
Data availability
No datasets were generated or analysed during the current study.
References
Zhu T, Liang W, He Y, Zhang B, Liu C, Wang D, Deng L, Li D, Li W, Yan F, et al. Transcriptomic analysis of mechanism underlying the effect of induced molting on semen quality and reproductive performance in aged Houdan roosters. Poult Sci. 2023;102(10):102935.
Avital-Cohen N, Heiblum R, Argov-Argaman N, Rosenstrauch A, Chaiseha Y, Mobarkey N, Rozenboim I. Age-related changes in gonadal and serotonergic axes of broiler breeder roosters. Domest Anim Endocrinol. 2013;44(3):145–50.
Oliveira LBT, Butolo JEG, Butolo EAF, Reis RS, Travençolo BAN, Beletti ME. L-arginine supplementation minimizes aging-induced changes in the sperm chromatin of roosters. Poult Sci. 2023;102(8):102805.
Johnston SD, López-Fernández C, Pappin E, Hampe A, Doneley R, Lierz M, Gosálvez J. Assessment of avian sperm DNA fragmentation using the sperm chromatin dispersion assay. Reprod Fertil Dev. 2020;32(10):948–52.
Nemati Z, Dehgani P, Besharati M, Amirdahri S. Dietary carob fruit (Ceratonia siliqua L.) supplementation improves spermatogenesis, semen quality and embryonic death via antioxidant effect in aging broiler breeder roosters. Anim Reprod Sci. 2022;239:106967.
Sarabia Fragoso J, Pizarro Díaz M, Abad Moreno JC, Casanovas Infesta P, Rodriguez-Bertos A, Barger K. Relationships between fertility and some parameters in male broiler breeders (body and testicular weight, histology and immunohistochemistry of testes, spermatogenesis and hormonal levels). Reprod Domest Anim = Zuchthygiene. 2013;48(2):345–52.
Ottinger MA, Abdelnabi M, Li Q, Chen K, Thompson N, Harada N, Viglietti-Panzica C, Panzica GC. The Japanese quail: a model for studying reproductive aging of hypothalamic systems. Exp Gerontol. 2004;39(11–12):1679–93.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243–78.
Ye N, Lv Z, Huang Z, Cheng Y, Wei Q, Shi F. Dietary folic acid supplementation improves semen quality and spermatogenesis through altering autophagy and histone methylation in the testis of aged broiler breeder roosters. Theriogenology. 2022;181:8–15.
Lagares MA, Ecco R, Martins N, Lara L, Rocha J, Vilela D, Barbosa VM, Mantovani PF, Braga J, Preis IS, et al. Detecting reproductive system abnormalities of broiler breeder roosters at different ages. Reprod Domest Anim = Zuchthygiene. 2017;52(1):67–75.
Adeldust H, Farzinpour A, Farshad A, Rostamzadeh J, López Béjar M. Effect of orally administrated letrozole on reproduction performance and gene expression of FOXJ1, LPR2 and PVRL3 in reproductive tract in aged roosters. Theriogenology. 2021;161:131–9.
Sabzian-Melei R, Zare-Shahneh A, Zhandi M, Yousefi AR, Rafieian-Naeini HR. Effects of dietary supplementation of different sources and levels of selenium on the semen quality and reproductive performance in aged broiler breeder roosters. Poult Sci. 2022;101(10):101908.
Rosenstrauch A, Degen AA, Friedländer M. Spermatozoa retention by sertoli cells during the decline in fertility in aging roosters. Biol Reprod. 1994;50(1):129–36.
Wilson FD, Johnson DI, Magee DL, Hoerr FJ. Testicular histomorphometrics including sertoli cell quantitation for evaluating hatchability and fertility issues in commercial breeder-broiler roosters. Poult Sci. 2018;97(5):1738–47.
Gao S, Zhao BX, Long C, Heng N, Guo Y, Sheng XH, Wang XG, Xing K, Xiao LF, Ni HM et al. Natural Astaxanthin Improves Testosterone Synthesis and Sperm Mitochondrial Function in Aging Roosters. Antioxidants (Basel, Switzerland) 2022, 11(9).
Adeldust H, Farzinpour A, Farshad A, Rostamzadeh J, Lopez-Bejar M. Increased sperm cell production in ageing roosters by an oral treatment with an aromatase inhibitor and a natural herbal extract designed for improving fertility. Reprod Domest Anim = Zuchthygiene. 2017;52(Suppl 4):58–60.
Weil S, Rozenboim I, Degen AA, Dawson A, Friedländer M, Rosenstrauch A. Fertility decline in aging roosters is related to increased testicular and plasma levels of estradiol. Gen Comp Endocrinol. 1999;115(1):23–8.
Kiat Y, Vortman Y, Sapir N. Feather moult and bird appearance are correlated with global warming over the last 200 years. Nat Commun. 2019;10(1):2540.
Berry WD. The physiology of induced molting. Poult Sci. 2003;82(6):971–80.
Mishra R, Mishra B, Kim YS, Jha R. Practices and issues of moulting programs for laying hens: a review. Br Poult Sci. 2022;63(5):720–9.
Zhang T, Chen Y, Wen J, Jia Y, Wang L, Lv X, Yang W, Qu C, Li H, Wang H et al. Transcriptomic analysis of laying hens revealed the role of aging-related genes during forced molting. Genes 2021, 12(11).
Laporta L, Micera E, Surdo NC, Moramarco AM, Di Modugno G, Zarrilli A. A functional study on L-type calcium channels in granulosa cells of small follicles in laying and forced molt hens. Anim Reprod Sci. 2011;126(3–4):265–70.
Huo S, Li Y, Guo Y, Zhang S, Li P, Gao P. Improving effects of Epimedium flavonoids on the selected reproductive features in layer hens after forced molting. Poult Sci. 2020;99(5):2757–65.
Han GP, Lee KC, Kang HK, Oh HN, Sul WJ, Kil DY. Analysis of excreta bacterial community after forced molting in aged laying hens. Asian-Australasian J Anim Sci. 2019;32(11):1715–24.
Onbaşılar EE, Kahraman M, Güngör ÖF, Kocakaya A, Karakan T, Pirpanahi M, Doğan B, Metin D, Akan M, Şehu A, et al. Effects of cage type on performance, welfare, and microbiological properties of laying hens during the molting period and the second production cycle. Trop Anim Health Prod. 2020;52(6):3713–24.
Meyer MM, Bobeck EA. Dietary inositol-stabilized arginine silicate numerically reduced woody breast severity in male Ross 708 broilers without altering growth. Poult Sci. 2023;102(5):102589.
Sun X, Wang Y, Wang C, Wang Y, Ren Z, Yang X, Yang X, Liu Y. Genome analysis reveals hepatic transcriptional reprogramming changes mediated by enhancers during chick embryonic development. Poult Sci. 2023;102(4):102516.
Surugihalli C, Farley LS, Beckford RC, Kamkrathok B, Liu HC, Muralidaran V, Patel K, Porter TE, Sunny NE. Remodeling of Hepatocyte Mitochondrial Metabolism and De Novo Lipogenesis during the embryonic-to-neonatal transition in chickens. Front Physiol. 2022;13:870451.
Guo LX, Nie FR, Huang AQ, Wang RN, Li MY, Deng HY, Zhou YZ, Zhou XM, Huang YK, Zhou J, et al. Transcriptomic analysis of chicken immune response to infection of different doses of Newcastle disease vaccine. Gene. 2021;766:145077.
Han K, Singh K, Rodman MJ, Hassanzadeh S, Wu K, Nguyen A, Huffstutler RD, Seifuddin F, Dagur PK, Saxena A, et al. Fasting-induced FOXO4 blunts human CD4(+) T helper cell responsiveness. Nat Metabolism. 2021;3(3):318–26.
Ezzati A, Rosenkranz SK, Horne BD. Importance of intermittent fasting regimens and selection of adequate therapy on inflammation and oxidative stress in SARS-CoV-2 infection. Nutrients 2022, 14(20).
Amann RP. Lessons for the poultry industry gleaned from experiences with other commodity species. Poult Sci. 1999;78(3):419–27.
Elokil AA, Abouzaid M, Magdy M, Xiao T, Liu H, Xu R, Li S. Testicular transcriptome analysis under the dietary inclusion of l-carnitine reveals potential key genes associated with oxidative defense and the semen quality factor in aging roosters. Domest Anim Endocrinol. 2021;74:106573.
Li MW, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring events during spermatogenesis in the testis: an emerging concept of regulation. Cytokine Growth Factor Rev. 2009;20(4):329–38.
Wen Q, Tang EI, Xiao X, Gao Y, Chu DS, Mruk DD, Silvestrini B, Cheng CY. Transport of germ cells across the seminiferous epithelium during spermatogenesis-the involvement of both actin- and microtubule-based cytoskeletons. Tissue Barriers. 2016;4(4):e1265042.
Xiao X, Mruk DD, Cheng CY. Intercellular adhesion molecules (ICAMs) and spermatogenesis. Hum Reprod Update. 2013;19(2):167–86.
Mullaney BP, Skinner MK. Transforming growth factor-beta (beta 1, beta 2, and beta 3) gene expression and action during pubertal development of the seminiferous tubule: potential role at the onset of spermatogenesis. Mol Endocrinol (Baltimore Md). 1993;7(1):67–76.
Hazra R, Corcoran L, Robson M, McTavish KJ, Upton D, Handelsman DJ, Allan CM. Temporal role of sertoli cell androgen receptor expression in spermatogenic development. Mol Endocrinol (Baltimore Md). 2013;27(1):12–24.
Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biology Reviews: MMBR. 2011;75(1):50–83.
Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92(2):689–737.
Luo D, He Z, Yu C, Guan Q. Role of p38 MAPK Signalling in Testis Development and Male Fertility. Oxidative medicine and cellular longevity 2022, 2022:6891897.
Luo D, Qi X, Xu X, Yang L, Yu C, Guan Q. Involvement of p38 MAPK in Leydig cell aging and age-related decline in testosterone. Front Endocrinol. 2023;14:1088249.
Anerillas C, Altés G, Gorospe M. MAPKs in the early steps of senescence implemEMTation. Front cell Dev Biology. 2023;11:1083401.
Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdisciplinary Reviews Dev Biology. 2015;4(3):215–66.
Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol. 2006;291(2):325–39.
Escasany E, Lanzón B, García-Carrasco A, Izquierdo-Lahuerta A, Torres L, Corrales P, Rodríguez Rodríguez AE, Luis-Lima S et al. Martínez Álvarez C, Javier Ruperez F : Transforming growth factor β3 deficiency promotes defective lipid metabolism and fibrosis in murine kidney. Disease models & mechanisms 2021, 14(9).
Yang F, Sun Y, Bai Y, Li S, Huang L, Li X. Asthma promotes Choroidal Neovascularization via the transforming growth factor Beta1/Smad Signalling Pathway in a mouse model. Ophthalmic Res. 2022;65(1):14–29.
Chawengsaksophak K, Svingen T, Ng ET, Epp T, Spiller CM, Clark C, Cooper H, Koopman P. Loss of Wnt5a disrupts primordial germ cell migration and male sexual development in mice. Biol Reprod. 2012;86(1):1–12.
Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24(22):2517–30.
Kessenbrock K, Smith P, Steenbeek SC, Pervolarakis N, Kumar R, Minami Y, Goga A, Hinck L, Werb Z. Diverse regulation of mammary epithelial growth and branching morphogenesis through noncanonical wnt signaling. Proc Natl Acad Sci USA. 2017;114(12):3121–6.
He N, Wang Y, Zhang C, Wang M, Wang Y, Zuo Q, Zhang Y, Li B. Wnt signaling pathway regulates differentiation of chicken embryonic stem cells into spermatogonial stem cells via Wnt5a. J Cell Biochem. 2018;119(2):1689–701.
Oreal E, Pieau C, Mattei MG, Josso N, Picard JY, Carré-Eusèbe D, Magre S. Early expression of AMH in chicken embryonic gonads precedes testicular SOX9 expression. Dev Dynamics: Official Publication Am Association Anatomists. 1998;212(4):522–32.
Estermann MA, Major AT, Smith CA. Genetic regulation of Avian Testis Development. Genes 2021, 12(9).
Hirst CE, Major AT, Smith CA. Sex determination and gonadal sex differentiation in the chicken model. Int J Dev Biol. 2018;62(1–2–3):153–66.
Ortiz-García CI, Salgado-Lucio ML, Roa-Espitia AL, Muñoz-Sánchez AA, Cordero-Martínez J, Hernández-González EO. Calpain regulates reactive Oxygen species production during Capacitation through the activation of NOX2 and NOX4. Int J Mol Sci 2023, 24(4).
Liu RM, Desai LP. Reciprocal regulation of TGF-β and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol. 2015;6:565–77.
Jankowska A, Burczynska B, Duda T, Warchol JB, Sharma RK. Calcium-modulated rod outer segment membrane guanylate cyclase type 1 transduction machinery in the testes. J Androl. 2007;28(1):50–8.
Xu L, Sun X, Wan X, Li K, Jian F, Li W, Jiang R, Han R, Li H, Kang X, et al. Dietary supplementation with Clostridium butyricum improves growth performance of broilers by regulating intestinal microbiota and mucosal epithelial cells. Anim Nutr (Zhongguo Xu Mu Shou Yi Xue Hui). 2021;7(4):1105–14.
Acknowledgements
Not applicable.
Funding
Financial support of this study was provided by the Key Research Project of the Shennong Laboratory (SN01-2022-05), the Scientific Studio of Zhongyuan Scholars (234400510023).
Author information
Authors and Affiliations
Contributions
Wenjie Liang and Yuehua He conceived the project and designed the experiments. Tingqi Zhu, Binbin Zhang, Shuangxing Liu, Haishan Guo and Pingquan Liu performed the animal experiments and sample collection. Huayuan Liu, Donghua Li, Xiangtao Kang provided valuable suggestion and comments to improve the manuscript with contributions from all other authors. Wenting Li and Guirong Sun discussed the results. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal experiments were performed according to the Regulations for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, 2004). The protocols and guidelines were approved by the Institutional Animal Care and Use Committee of Henan Agricultural University, China. All sections of this study adhere to the ARRIVE Guidelines for reporting animal research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
There are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liang, W., He, Y., Zhu, T. et al. Dietary restriction promote sperm remodeling in aged roosters based on transcriptome analysis. BMC Genomics 25, 680 (2024). https://doi.org/10.1186/s12864-024-10544-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10544-3