Abstract
Background
Body weight and size are important economic traits in chickens. While many growth-related quantitative trait loci (QTLs) and candidate genes have been identified, further research is needed to confirm and characterize these findings. In this study, we investigate genetic and genomic markers associated with chicken body weight and size. This study provides new insights into potential markers for genomic selection and breeding strategies to improve meat production in chickens.
Methods
We performed whole-genome resequencing of and Wenshang Barred (WB) chickens (n = 596) and three additional breeds with varying body sizes (Recessive White (RW), WB, and Luxi Mini (LM) chickens; (n = 50)). We then used selective sweeps of mutations coupled with genome-wide association study (GWAS) to identify genomic markers associated with body weight and size.
Results
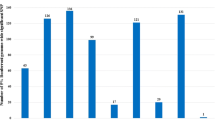
We identified over 9.4 million high-quality single nucleotide polymorphisms (SNPs) among three chicken breeds/lines. Among these breeds, 287 protein-coding genes exhibited positive selection in the RW and WB populations, while 241 protein-coding genes showed positive selection in the LM and WB populations. Genomic heritability estimates were calculated for 26 body weight and size traits, including body weight, chest breadth, chest depth, thoracic horn, body oblique length, keel length, pelvic width, shank length, and shank circumference in the WB breed. The estimates ranged from 0.04 to 0.67. Our analysis also identified a total of 2,522 genome-wide significant SNPs, with 2,474 SNPs clustered around two genomic regions. The first region, located on chromosome 4 (7.41-7.64 Mb), was linked to body weight after ten weeks and body size traits. LCORL, LDB2, and PPARGC1A were identified as candidate genes in this region. The other region, located on chromosome 1 (170.46-171.53 Mb), was associated with body weight from four to eighteen weeks and body size traits. This region contained CAB39L and WDFY2 as candidate genes. Notably, LCORL, LDB2, and PPARGC1A showed highly selective signatures among the three breeds of chicken with varying body sizes.
Conclusion
Overall this study provides a comprehensive map of genomic variants associated with body weight and size in chickens. We propose two genomic regions, one on chromosome 1 and the other on chromosome 4, that could helpful for developing genome selection breeding strategies to enhance meat yield in chickens.
Similar content being viewed by others
Background
Over thousands of years, hundreds of chicken breeds have evolved through natural and artificial selection across different environments [1], leading to significant phenotypic variations in body size, plumage, egg color, and flying ability [2]. Chicken meat has already established itself as one of the most efficient protein sources, accounting for over 30% of global meat products and playing a critical role in food security worldwide [3]. Body weight (BW) is an important economic trait primarily determined by minorgenes that interact with functional genes and serve as molecular markers, which are extensively studied for their association with weight gain.
Genetic analysis and pattern recognition have proven to be useful in identifying the origin of specific breeds or revealing their characteristic traits [4]. A study of commercial broiler populations for selective sweeps revealed numerous loci involved in the selection for muscle mass [5]. Genetic variations present among various chicken breeds have been leveraged to portray specific traits in these breeds [6,7,8,9,10]. As a result, there have been extensive genomic studies on the genetic conditioning of domestic animals such as chickens. Notably, several genes associated with growth and carcass traits in chickens have been identified, including insulin-like growth factors (IGFs) [5, 11] and growth hormone secretagogue receptor (GHSR) [12], lysozyme (LYZ), melanocortin 4 receptor (MC4R), adhesion G protein-coupled receptor G6 (ADGRG6) [13], etc. Furthermore, insulin like growth factor 2 mRNA binding protein 1 (IGF2BP1) has been shown to correlate positively with breast muscle weight and body size in various animals [14,15,16]. A genome-wide association study (GWAS) in F2 progenies of star and silky black-bone chickens also revealed that the LIM domain binding 2 (LDB2) gene was responsible for BW at seven to twelve weeks and weight gain at six to twelve weeks [17]. However, expanding these studies to include more breeds and larger populations is necessary for deeper insights into this research field with current focus on BW and size traits.
The chicken quantitative trait locus (QTL) database (release 45) includes 4,776 QTLs related to growth traits in chicken, such as BW at different ages and average daily gain [18]. However, many of the QTLs, particularly those identified in previous studies, lack precise mapping, leading to broad confidence intervals that encompass several genes. Despite intensive research into the genetics of meat production traits, the knowledge of key genes causing significant phenotypic variations remains limited. This study used a large population of Wenshang Barred (WB) chickens (n = 596) and three other breeds (n = 50) to minimize the risk of biases. It also performed a systematic comparison of the whole genome and a GWAS to identify the genes and genomic regions responsible for BW and size. This study provides new insights into the genetics of chicken selection and promises to facilitate the development of techniques for breeding native chickens.
Materials and methods
Ethics statement
All handling and experimental procedures concerning the chickens used in this study were conducted following ARRIVE guidelines. Ethical approval was granted by Science Research Department of the Shandong Academy of Agricultural Sciences (SAAS) (Jinan, China), with the reference number 2,021,001.
Birds and sample collection
A total of 596 WB chickens obtained from Jinqiu Agriculture and Animal Husbandry Co., Ltd. (Wenshang, Shandong, China) were used in this study. The chickens were raised in accordance with the breeding and management protocols for WB chickens. The experimental chickens remained in cages throughout the entire process, including the brooding stage from 0 to 7 weeks of age, the growing stage from 8 to 16 weeks of age, and were then transferred to the laying house at approximately 15 weeks of age. The chickens were housed individually in cages and had unrestricted access to food and water, along with regular immunization. Chickens were kept under natural light during the growing stage, followed by 16 h:8 h light:dark cycle after growing stage. The chicken coop maintained temperature control through the use of a fan humidification curtain, and feeding and manure cleaning processes were mechanized. The BW and body size were measured at 0–18 weeks. The measurement methods for some traits were as follows: Body oblique length (BOL): the distance between the shoulder joints and the sciatic tuberosity was measured along the animal’s body surface with a leather ruler. Chest breadth (CB): the distance between the two shoulder joints measured on the body surface with a caliper. Chest depth (CD): the distance from the first thoracic vertebra to the anterior edge of the keel was measured with a caliper on the body surface. Thoracic horn (TH): the angle of the thorax on both sides was measured with a thoracic corrector at the anterior edge of the keel. Keel length (KL): the distance from the anterior end of the keel eminence to the end of the keel was measured on the body surface with a caliper. Shank length (SL): the straight line distance from the upper tibial joint to the third and fourth toes measured with a caliper. Shank circumference (SC): circumference of the middle of the tibia. Pelvic width (PW): distance between the two sciatic tuberosities measured with calipers. All the phenotypic data were distributed within the range of the mean ± 3 standard deviations and passed quality control for subsequent GWAS analysis.
Genetic materials, DNA extraction and sequencing
We collected blood samples from the wing vein of 596 chickens (supplementary Table S1) and extracted genomic DNA using the phenol-chloroform method. The DNA quality was assessed by agarose gel electrophoresis, and paired-end (2 × 150 bp) DNA libraries were constructed for each sample. The DNBSEQ sequencing platform (BGI Genomics, Shenzhen, China) was used to obtain sequence data for all libraries. Notably, the sequencing data of three chicken breeds (n = 50), including those from our previous research on local chicken breeds (Recessive White (RW), WB, and Luxi Mini (LM) chickens) were also used in this study. RW broilers are classified as a specialized line for meat production, known for their large body size and well-developed pectoral muscles. WB chickens, on the other hand, are versatile and utilized for both meat and egg production, characterized by medium-sized bodies. The LM chicken is a small ornamental breed that originates from China, known for its compact size. At 5 months of age, adult hens of this breed typically weigh around 0.86 kg, while adult cocks weigh approximately 1.2 kg. The accession number for these data is CRA006685 in the GSA database [19]. This study analyzed a total of 646 chickens from three breeds.
Variant calling, quality control
Sequencing raw data was filtered with SOAPnuke (v1.5.6) [20] by removing reads containing sequencing adapter; removing low-quality data with read quality value < 20; remove reads whose unknown base (N base) ratio > 10%, and remove the reads with low quality accounting for more than 50%. The Burrows–Wheeler aligner (BWA) software [21] was used to align clean data to the chicken reference genome (http://ftp.ensembl.org/pub/release-106/fasta/gallus_gallus/), and the Samtools software [22] was used to sort the aligned sequences according to the coordinates on the genome. The Qualimap 2 tool [23] was used to obtain summary statistics to assess the effectiveness of read mapping and alignment quality, and Samtools software was used to filter out the reads with quality values less than 30. Single nucleotide polymorphisms (SNPs) were called using the GATK HaplotypeCaller v3.3 [24] with the SNP filtering conditions based on the following: Quality by Depth (QD) < 2.0, Fisher Strand (FS) > 60.0, root mean square of Mapping Quality (MQ) < 40.0, MQRankSum < -12.5, HaplotypeScore > 13.0, and ReadPosRankSum < -8.0. The SNPs obtained by preliminary filtration were selected for subsequent analysis according to the following quality control standards: SNPs with minor allele frequency (MAF) > 0.05 and missing rate < 0.1. A total of 9,406,362 biallelic SNPs were retained for subsequent analysis.
Population genetics analysis
Principal component analysis (PCA) was performed using the software PLINK v1.9. The population structure of different admixture proportions was evaluated using the program ADMIXTURE v1.3. Three solutions (2 < k < 4) were selected for genetic clustering, and the software FigTree v1.4.0 (tree.bio.ed.ac.uk/software/figtree/) was used to visualize the phylogenetic trees.
Analysis of nucleotide diversity, linkage disequilibrium (LD) decay
To further evaluate the genetic characteristics among different species, we determined the genetic diversity by measuring the fixation-index (Fst) using VCFtools v0.1.13 [25]. The linkage disequilibrium (LD) decay level was calculated and plotted using the PopLDdecay software [26], with a maximum distance of 500 kb.
Detection of selective sweeps
We detected candidate divergent regions (CDRs) by searching the genome for regions with high Fst (top 1%) values. First, we calculated the Fst value along the autosomes in sliding 40-kb windows with 10-kb steps using VCFtools software and in-house scripts, by comparing values among WB, RW and LM chickens. We restricted our CDR descriptions to the top 1% most significant windows in Fst values, as these windows represented the extreme ends of the distributions.
Estimation of genetic parameters
SNP-based heritability (h2 SNP) was calculated using the GCTA v1.93.2 beta software [27] based on the genetic relationship matrix (GRM) between pairs of individuals [28]. The restricted maximum likelihood (REML) method was used for genetic parameter estimation. The genetic-statistical model was defined as follows:
where \( {Y}_{i}\) is a vector of clutch traits; \( {X}_{i}\) and \( {Z}_{i}\) are incidence matrices for \( {b}_{i}\) and \( {u}_{i}, \)respectively; bi is a vector of fixed effect; \( {u}_{i}\) is a vector of polygenic effects with a variance-covariance structure of \( u{\sim} N\left(0,G{\sigma }_{u}^{2}\right);\) G is the GRM between individuals; \( {\sigma }_{u}^{2} \)is the polygenic variance; \( {e}_{i}\) is a vector of random residual effects with \( {e}_{i}{\sim}N(0,I{\sigma }_{e}^{2})\); I is an identity matrix of dimension n × n (with a sample size n= 596).
Genome-wide association study for body weight and body size in Wenshang Barred chicken
WB chickens were selected based on meat production traits over multiple generations, and SNP information and phenotypic records were comprehensively collected. To investigate the genetic basis of BW and body size, association analysis of BW, chest breadth (CB), chest depth (CD), thoracic horn (TH), body oblique length (BOL), keel length (KL), pelvic width (PW), shank length (SL) and shank circumference (SC) was performed using the linear mixed model in the Genome-wide Efficient Mixed Model Association (GEMMA) software (v0.98.4) based on chickens genotyped by whole-genome sequencing. After quality control (-- mind 0.1, --maf 0.05) using the PLINK v1.9 software, a total of 9,406,362 SNPs were retained, and GWAS was performed as follows:
where y denotes the vector of phenotypic values; W represents the vector of covariates, including a column of 1 s; α is the vector of the corresponding coefficients including the intercept; x represents the vector of marker genotypes; β denotes the effect size of the marker; u represents the vector of random polygenic effects; e is the vector of errors. The Wald test was used as a criterion to select SNPs associated with metabolizable efficiency traits. Similarly, the whole-genome and suggestive significance thresholds were corrected by the Bonferroni test (0.05/9,406,362 and 0.01/9,406,362, respectively). Additionally, Manhattan and quantile-quantile (Q-Q) plots were visualized using the CMplot package in the R environment. The LD blocks of target regions were performed using the Haploview v4.2 software.
Statistical analysis
Statistical analyses were performed using SPSS 25.0 software (IBM Corporation, Armonk, NY, USA) or R environment.
Results
Whole-genome sequencing and variation
Following standardized procedures for library construction and whole-genome sequencing using the BGISEQ platform, we obtained 4.15 Tb of raw data (supplementary Table S1) for 596 individuals, with a mean coverage of 7.03X (supplementary Table S1). After performing quality control, the total reads per individual were 51,113,663, with a mean mapping ratio of 99.73%. These data satisfied the requirements for subsequent analyses.
Phylogenetic and demographic analyses
We conducted a comprehensive analysis of the genetic relationships among three different chicken breeds with varied body sizes. First, we used QC SNPs and performed PCA on the three breeds, which revealed a significant genetic difference among RW, WB, and LM chickens (Fig. 1a). Second, we used pairwise genetic distances to construct a neighbor-joining tree (Fig. 1b). Third, genetic coancestry analysis was performed by assuming different number of ancestral populations (K = 2–4, Fig. 1c) to classify the chickens into groups. It was found that the LD decay distance was different among the three breeds (Fig. 1d). As expected, the fast-growing RW, WB, and LM chickens were genetically distant from each other.
Population genetic diversity and demographic history inferences (a) PCA plot with three chicken breeds (b) Neighbor-joining tree constructed by genetic distance among three chicken breeds (c) Population structure analysis of three body size of chickens, where the number of ancestral clusters were set from K = 2–4. (d) LD decay in three body size of chickens. (e) Detection of selective sweep windows in purebred chickens. The red dash line indicates the top 1% threshold of Fst values. (f) Putative selected windows and genes on the chromosome 27:6.05–6.09 Mb region. RW: recessive white chickens; WB: Wenshang Barred chickens; LM: Luxi mini chickens
Genomic signatures in purebred WB chickens
We performed the Fst test based on allele frequency differentiation with a 40 kb window size and a step size of 10 kb to identify the genomic loci that underwent selective sweeps among the three chicken breeds (Fig. 1e). By overlapping the results of the Fst analysis, we identified 287 protein-coding genes in the RW and WB populations and 241 protein-coding genes in the LM and WB populations (Fig. 1e, supplementary Table S2–3). Notably, we detected a lead signal on chromosome 5 in the RW breed, which annotated the INS and IGF2 genes that play key roles in the skeletal muscle development process [29]. We also examined the IGF2BP1 gene and found significant differences in the Fst values among the RW, WB, and LM chickens (Fig. 1f). Subsequently, we identified the list of genes harboring the top selective sweep windows (Table S4). For instance, the known growth factors HBEGF, VEGFA, FGF23, and FGF6 play a crucial role in body development. Additionally, TBX20 acts as a transcriptional activator and repressor required for cardiac development and is responsible for maintaining functional and structural phenotypes in adult heart. TOLLIP is a Toll-interacting protein and an essential component of the signaling pathway of IL1B and Toll-like receptors. Also, TBX5 is involved in heart development and limb pattern formation. In addition, we also conducted KEGG pathway and GO term enrichment analyses on the gene sets from the 453 selective sweep genes in all chickens (Table S5 and S6, Fig. S1). Based on the phenotype or physiological process, the GO terms were classified into several clusters, including autophagy (e.g., GO:0006995, GO:0006914 and GO:0016236), energy metabolism (e.g., GO:0009060, GO:0022900, GO:0006091, GO:0007005, GO:0055114), and growth (e.g., GO:0071363, GO:0007169, GO:0007167).
Descriptive statistics of traits
We calculated the descriptive statistics for the traits related to BW and body size (Table 1). The coefficients of variation of these traits in the population ranged from 4.62 to 12.94%. The SNP-based heritability estimates for the BW traits (0.47 - 0.67) and shank traits (0.33 - 0.59) were high, but they were relatively low (0.04 - 0.05) for TH traits, breast muscle traits, and body size traits.
GWAS and fine-mapping for body weight and body size traits
This study mainly focused on analyzing BW and body size traits. The Manhattan plots and significant SNPs are shown in Figs. 2, 3, 4 and 5, and the corresponding Tables 2, 3, 4 and 5. The Q-Q plots are shown in Fig. S3, S3, S4, and S4, and Table S7 shows the significant SNPs for all phenotypes. The additive effects of lead SNPs estimated by GEMMA are shown in Table S8.
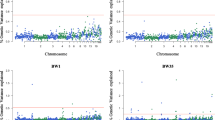
We identified two significant regions, one on chromosome 1 (170.4-171.5 Mb) and the other on chromosome 4 (74.1–76.4 Mb), for the ten BW traits. The chromosome 1 region was found to be correlated with BW during the entire growth stage. In particular, the largest region associated with BW at 16 weeks contained 218 significant SNPs, implicating genes such as RB1, RCBTB2, CAB39L, SETDB2, PHF11, ARL11, KPNA3, SPRYD7, RNASEH2B, and WDFY2. In contrast, the chromosome 4 region was associated with BW traits after 10 weeks of age. The most significant interval was correlated with BW at 16 weeks of age, comprising 1,076 significant SNPs, involving genes such as PPARGC1A, KCNIP4, SLIT2, LCORL, LDB2, and LAP3.
We also identified breast muscle size traits on chromosome 4 (74.4–76.2 Mb). Specifically, 167 significant SNPs were associated with the CB trait, and 343 significant SNPs were correlated with the CD trait, involving the following genes: SLIT2, KCNIP4, LAP3, LCORL, and LDB2.
Manhattan plots of GWAS for breast muscle size traits in WB chicken. Each dot represents a SNP in the dataset. The horizontal red and blue lines indicate the thresholds for genome-wide significance (P value = 1.07e-09) and suggestive significance (P value = 5.32e-09), respectively. CB: chest breadth; CD: chest depth; TH: thoracic horn
For body size traits, 99 significant SNPs found to be associated with the 18-week BOL trait were located on the SLIT2 and LCORL genes. The 167, 19, and 175 significant SNPs located on chromosomes 1, 2 and 4, respectively, were associated with the 18-week KL trait, and involved the genes ITM2B, CAB39L, SETDB2, PHF11, ARL11, KPNA3, SPRYD7, YES1, COLEC12, SLIT2, LCORL, and LDB2. A total of 24 significant SNPS located in the 75.07 -76.24 Mb region of chromosome 4 were correlated with PW traits, and the annotated genes included SLIT2, LCORL and LDB2.
Manhattan plots of GWAS for body size traits in WB chicken. Each dot represents a SNP in the dataset. The horizontal red and blue lines indicate the thresholds for genome-wide significance (P value = 1.07e-09) and suggestive significance (P value = 5.32e-09), respectively. BOL: body oblique length; KL: keel length; PW: pelvic width
For the SL traits, 1,505 significant SNPs were located on chromosomes 1, 4 and 27. The 21 significant SNPs were clustered within a 48.25-kb region (chromosome 1:170.56–171.04 Mb), 1,458 SNPs were clustered within a 2.31-Mb region (chromosome 4:74.1–76.1 Mb) and 26 SNPs were clustered within a 191-kb region (chromosome 27:5.95–6.14 Mb). The genes PHF11, SPRYD7, PPARGC1A, SLIT2, KCNIP4, LCORL, LAP3, QDPR, LDB2, IGF2BP1, and GIP were annotated in the above significant regions. For the SC traits at 8 weeks and 18 weeks, 780 and 1,931 significant SNPs, respectively, were located in the 73.87-76.45 Mb region of chromosome 4 with a total of 2.57 Mb, involving the genes PPARGC1A, KCNIP4, SLIT2, LCORL, LAP3, LDB2, and TAPT1.
Manhattan plots of GWAS for SL and SC traits in WB chicken. Each dot represents a SNP in the dataset. The horizontal red and blue lines indicate the thresholds for genome-wide significance (P value = 1.07e-09) and suggestive significance (P value = 5.32e-09), respectively. SL: shank length; SC: shank circumference
The LCORL, LDB2 and PPARGC1A gene is a potential causal gene for body weight and body size
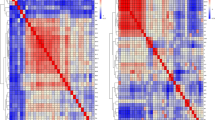
The LCORL, LDB2, and PPARGC1A genes were significantly correlated with 13, 12, and 8 traits, respectively, based on the results of the above analysis. In this section, we focus on these three genes and analyze their polymorphism in the three chicken breeds (RW, WB, and LM) with varied body sizes. The Fst analysis results indicated that the significant SNPs present on these genes showed apparent differences among breeds with pronounced differences in BW and body size (Fig. 6a, b and c). Furthermore, we observed that the related SNPs showed a strong linkage in the WB chicken breed (Fig. 6d, e and f). These findings suggest the possibility that the genome regions of these three genes might have undergone natural selection during the development of different chicken breeds.
Association results of the candidate region on chromosome 4 for BW and body size traits. (a, b, c) Putative selected SNPs in the LCORL, LDB2 and PPARGC1A genes. RW: recessive white chickens; WB: Wenshang Barred chickens; LM: Luxi mini chickens. (d, e, and f) Linkage disequilibrium (LD) analysis of the overlap significant SNPs on the LCORL, LDB2 and PPARGC1A genes
Discussion
The domestic chicken is an ideal model to investigate the genetics of phenotypic evolution [30]. Evolving poultry genetics and breeding have led to a diverse range of phenotypes and demographic history in local breeds [31, 32]. Domestication has also limited phenotypic differences among local breeds by selecting for genetic variants that favor traits leading to improved production [33]. Among these traits, animal body size plays a critical role in the profitability of poultry meat. Therefore, optimizing this trait has been an important goal during domestication [33, 34]. Selection for particular traits is the decisive factor behind the substantial rise in productivity, accounting for more than 90% of the improvement [35]. We conducted a comprehensive genetic diversity study and selective sweep analysis in fast-growing (RW chickens), local chicken (WB chicken), and mini chicken (LM chicken). The selective sweep analysis identified 455 protein-coding genes that underwent positive selection and played a critical role in domestication and breeding processes for phenotypes associated with muscle growth, reproduction, brain development, growth factors, and disease resistance. The significant GO enrichment terms were related to the insulin and GnRH signaling pathways in chickens.
Chicken breeds exhibit great variation in size in response to natural and/or artificial selection [36]. Understanding the genetic mechanisms underlying this variability in chicken body size is still inadequate. The chicken body size primarily reflects the growth of muscles and bones [37, 38], making growth a crucial selection criterion in chicken breeding. Genetically, chicken body size is a complex trait influenced by several genes on autosomal and sex chromosomes. Hundreds of QTLs have been mapped on autosomes for body size-related traits, such as SL, KL, and BW [37, 39,40,41,42,43,44].
A genome-wide association study was conducted to analyze the body size of Asian pheasants and Asian bantams. The study found a region on chromosome 4 (GGA4:17.3–21.3 Mb) that contained a total of 60 genes. Two notable genes in this region are myotubularin 1 (MTM1) and secreted frizzled-related protein 2 (SFRP2), both of which are potential candidate genes associated with body size traits [44]. The previous GWAS study included 541 chickens from 23 regional breeds in Italy, with each breed consisting of 20 to 24 chickens. Significant SNPs were found in the genome-wide association study, specifically associated with dwarfism in the dwarf breeds. These breeds shared a candidate genomic region on chromosome 1, where significant SNPs were found within the LEMD3 and HMGA2 genes [45]. This study examined the genome-wide association of 10 BW traits and 16 body size traits. We confirmed a total of 2,522 genome-wide significant SNPs, most of which were present on chromosomes 1 and 4. The 72 \( {\sim}\) 76 Mb region of chromosome 4 contained genes, such as PPARGC1A, KCNIP4, SLIT2, LCORL, LDB2, and LAP3. The GWAS of F2 progenies showed that the LDB2 gene was associated with BW at 7 \( {\sim}\) 12 weeks and average daily gain at 6 \( {\sim}\) 12 weeks [17]. A previous GWAS study was conducted using the chicken 60 K SNP panel on 1,328 Korean native chickens to analyze body weight (BW) traits.The results identified twelve single nucleotide polymorphisms (SNPs) associated with BW at the suggestive significance level.These SNPs were found near or within 11 candidate genes, specifically WDR37, KCNIP4, SLIT2, PPARGC1A, MYOCD, and ADGRA3 [46]. Some of the genes overlapped with the results of this study. The NCAPG-LCORL locus is widely believed to impact human height in human studies. In the GWAS of cattle and horses and the whole-genome selective sweep analysis of pigs and dogs, the NCAPG-LCORL locus was found to be significantly associated with body length and BW [47]. Furthermore, PPARGC1A has been shown to facilitate mitochondrial biogenesis and modulate skeletal muscle metabolism by mediating the flux of glycolysis and the tricarboxylic acid (TCA) cycle, which drives the transformation of fast-twitch myofibers to slow-twitch myofibers, thus increasing chicken skeletal muscle mass [48]. In another region, the CAB39L and WDFY2 genes were identified as candidates on chromosome 1 (170.5-171.5 Mb) and associated with BW from 4 to 18 weeks of age and body size traits. The CAB39L gene can be considered a novel candidate gene for chicken growth and development [49]. The WDFY2 may be a candidate susceptibility gene located downstream of TP63 in the network of limb development [50]. Other genes, such as IGF2BP1 and GIP, were found to be associated with the SL trait. The GIP gene encodes an incretin hormone that induces insulin secretion [51] and mediates appetite and energy intake [52].
In domestic animals, loci with a significant positive effect on favorable traits tend to undergo strong selection and fixation. In this study, we investigated the genomic variations of the LCORL, LDB2, and PPARGC1A genes in the chromosome 4:72 \( {\sim}\) 76 Mb region by performing selective sweep analysis. The results indicated that these genes not only were associated with BW and body size traits, as revealed by the GWAS, but also were strongly selected and fixed among breeds with differences in BW and size. Thus, it is likely that the chromosome 4:72 \( {\sim}\) 76 Mb region harbors loci that significantly impact BW and body size.
Observations of the same individual at multiple time points are called longitudinal traits and provide a better representation of growth and production in farm animals than single data records [53,54,55,56,57]. The BW of chickens at different weeks of age is a classic example of a longitudinal trait. In this study, we performed GWAS independently for each time point to identify the genetic basis of BW. However, a more effective strategy would be to fit the growth curve and use the fitted parameters to conduct the association analysis. This approach would better reflect the growth trajectory and provide a novel insight into the genetic underpinnings of BW in chickens.
Conclusion
In conclusion, our GWAS identified 2,522 SNPs with genome-wide significance, the majority of which are being reported for the first time. Several SNP effects overlapped with previously reported QTL regions, supporting the validation of QTL effects. Using a combination of GWAS and FST-based approaches, we identified three genes (LCORL, LDB2, and PPARGC1A) in the Chinese WB chicken associated with BW and body size traits. Our study provides important insights into the evolution and genetic basis of Chinese local chickens, which may be beneficial for both domestic and international chicken breeders. This study may also contribute to the development of genome-scale selective breeding strategies aimed at increasing chicken meat yield.
Data availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive in National Genomics Data Center, China National Center for Bioinformation / Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA011183) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa.
Abbreviations
- BOL:
-
body oblique length
- BW:
-
body weight
- CB:
-
chest breadth
- CD:
-
chest depth
- CDRs:
-
candidate divergent regions
- Fst:
-
fixation-index
- GRM:
-
genetic relationship matrix
- GWAS:
-
genome-wide association study
- KL:
-
keel length
- LD:
-
linkage disequilibrium
- LM:
-
luxi Mini chickens
- MAF:
-
minor allele frequency
- PCA:
-
principal component analysis
- PW:
-
pelvic width
- QTLs:
-
quantitative trait loci
- REML:
-
restricted maximum likelihood
- RW:
-
recessive White chickens
- SC:
-
shank circumference
- SL:
-
shank length
- SNPs:
-
single nucleotide polymorphisms
- TH:
-
thoracic horn
- WB:
-
wenshang Barred chickens
References
Athrey G. Chap. 18 - Poultry genetics and breeding. In: Animal Agriculture Edited by Bazer FW, Lamb GC, Wu G: Academic Press; 2020: 317–330.
West B, Zhou B-X. Did chickens go North? New evidence for domestication. J Archaeol Sci. 1988;15(5):515–33.
Tan X, Liu R, Zhao D, He Z, Li W, Zheng M, Li Q, Wang Q, Liu D, Feng F et al. Large-scale genomic and transcriptomic analyses elucidate the genetic basis of high meat yield in chickens. J Adv Res 2023.
Jeong H, Kim K, Caetano-Anollés K, Kim H, Kim BK, Yi JK, Ha JJ, Cho S, Oh DY. Whole genome sequencing of Gyeongbuk Araucana, a newly developed blue-egg laying chicken breed, reveals its origin and genetic characteristics. Sci Rep. 2016;6:26484.
Rubin CJ, Zody MC, Eriksson J, Meadows JR, Sherwood E, Webster MT, Jiang L, Ingman M, Sharpe T, Ka S, et al. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature. 2010;464(7288):587–91.
Sun Y, Liu R, Zhao G, Zheng M, Sun Y, Yu X, Li P, Wen J. Genome-wide linkage analysis and association study identifies loci for polydactyly in chickens. G3 (Bethesda). 2014;4(6):1167–72.
Sun Y, Zhao G, Liu R, Zheng M, Hu Y, Wu D, Zhang L, Li P, Wen J. The identification of 14 new genes for meat quality traits in chicken using a genome-wide association study. BMC Genomics. 2013;14:458.
Chang CS, Chen CF, Berthouly-Salazar C, Chazara O, Lee YP, Chang CM, Chang KH, Bed’Hom B, Tixier-Boichard M. A global analysis of molecular markers and phenotypic traits in local chicken breeds in Taiwan. Anim Genet. 2012;43(2):172–82.
Dorshorst B, Okimoto R, Ashwell C. Genomic regions associated with dermal hyperpigmentation, polydactyly and other morphological traits in the silkie chicken. J Hered. 2010;101(3):339–50.
Freese NH, Lam BA, Staton M, Scott A, Chapman SC. A novel gain-of-function mutation of the proneural IRX1 and IRX2 genes disrupts axis elongation in the Araucana rumpless chicken. PLoS ONE. 2014;9(11):e112364.
Wang MS, Otecko NO, Wang S, Wu DD, Yang MM, Xu YL, Murphy RW, Peng MS, Zhang YP. An evolutionary genomic perspective on the breeding of dwarf chickens. Mol Biol Evol. 2017;34(12):3081–8.
Fang M, Nie Q, Luo C, Zhang D, Zhang X. Associations of GHSR gene polymorphisms with chicken growth and carcass traits. Mol Biol Rep. 2010;37(1):423–8.
Yang X, Sun J, Zhao G, Li W, Tan X, Zheng M, Feng F, Liu D, Wen J, Liu R. Identification of major loci and candidate genes for meat production-related traits in broilers. Front Genet. 2021;12:645107.
Wang K, Hu H, Tian Y, Li J, Scheben A, Zhang C, Li Y, Wu J, Yang L, Fan X, et al. The Chicken Pan-genome reveals Gene Content Variation and a promoter region deletion in IGF2BP1 affecting body size. Mol Biol Evol. 2021;38(11):5066–81.
Zhou Z, Li M, Cheng H, Fan W, Yuan Z, Gao Q, Xu Y, Guo Z, Zhang Y, Hu J, et al. An intercross population study reveals genes associated with body size and plumage color in ducks. Nat Commun. 2018;9(1):2648.
Sutter NB, Bustamante CD, Chase K, Gray MM, Zhao K, Zhu L, Padhukasahasram B, Karlins E, Davis S, Jones PG, et al. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007;316(5821):112–5.
Gu X, Feng C, Ma L, Song C, Wang Y, Da Y, Li H, Chen K, Ye S, Ge C, et al. Genome-wide association study of body weight in chicken F2 resource population. PLoS ONE. 2011;6(7):e21872.
Hu ZL, Park CA, Reecy JM. Building a livestock genetic and genomic information knowledgebase through integrative developments of animal QTLdb and CorrDB. Nucleic Acids Res. 2019;47(D1):D701–10.
Wang J, Lei Q-x, Cao D-g, Zhou Y, Han H-x, Liu W, Li D-p, Li F-w, Liu J. Whole genome SNPs among 8 chicken breeds enable identification of genetic signatures that underlie breed features. J Integr Agric 2022.
Chen Y, Chen Y, Shi C, Huang Z, Zhang Y, Li S, Li Y, Ye J, Yu C, Li Z, et al. SOAPnuke: a MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience. 2018;7(1):1–6.
Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26(5):589–95.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–9.
Okonechnikov K, Conesa A, García-Alcalde F. Qualimap 2: advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics. 2016;32(2):292–4.
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, et al. The genome analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303.
Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, et al. The variant call format and VCFtools. Bioinformatics. 2011;27(15):2156–8.
Zhang C, Dong SS, Xu JY, He WM, Yang TL. PopLDdecay: a fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics. 2019;35(10):1786–8.
Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82.
Li Y, Liu X, Bai X, Wang Y, Leng L, Zhang H, Li Y, Cao Z, Luan P, Xiao F, et al. Genetic parameters estimation and genome-wide association studies for internal organ traits in an F(2) chicken population. J Anim Breed Genet. 2022;139(4):434–46.
Liu L, Wang S, Tian W, Xu C, Wei C, Cui K, Jiang L, Wang D. Effect of Zbed6 single-allele knockout on the growth and development of skeletal muscle in mice. Biology (Basel) 2023, 12(2).
Wang MS, Thakur M, Peng MS, Jiang Y, Frantz LAF, Li M, Zhang JJ, Wang S, Peters J, Otecko NO, et al. 863 genomes reveal the origin and domestication of chicken. Cell Res. 2020;30(8):693–701.
Andersson L. Genetic dissection of phenotypic diversity in farm animals. Nat Rev Genet. 2001;2(2):130–8.
Bruford MW, Bradley DG, Luikart G. DNA markers reveal the complexity of livestock domestication. Nat Rev Genet. 2003;4(11):900–10.
Andersson L, Georges M. Domestic-animal genomics: deciphering the genetics of complex traits. Nat Rev Genet. 2004;5(3):202–12.
Mignon-Grasteau S, Boissy A, Bouix J, Faure J-M, Fisher AD, Hinch GN, Jensen P, Le Neindre P, Mormède P, Prunet P, et al. Genetics of adaptation and domestication in livestock. Livest Prod Sci. 2005;93(1):3–14.
Muir WM, Wong GK, Zhang Y, Wang J, Groenen MA, Crooijmans RP, Megens HJ, Zhang H, Okimoto R, Vereijken A, et al. Genome-wide assessment of worldwide chicken SNP genetic diversity indicates significant absence of rare alleles in commercial breeds. Proc Natl Acad Sci U S A. 2008;105(45):17312–7.
Boegheim IJM, Leegwater PAJ, van Lith HA, Back W. Current insights into the molecular genetic basis of dwarfism in livestock. Vet J. 2017;224:64–75.
Gao Y, Feng CG, Song C, Du ZQ, Deng XM, Li N, Hu XX. Mapping quantitative trait loci affecting chicken body size traits via genome scanning. Anim Genet. 2011;42(6):670–4.
Geng AL, Zhang Y, Zhang J, Zeng LC, Chang C, Wang HH, Yan ZX, Chu Q, Liu HG. Effects of light regime on the hatching performance, body development and serum biochemical indexes in Beijing You Chicken. Poult Sci. 2021;100(8):101270.
Hu ZL, Park CA, Wu XL, Reecy JM. Animal QTLdb: an improved database tool for livestock animal QTL/association data dissemination in the post-genome era. Nucleic Acids Res. 2013;41(Database issue):D871–879.
Lyu S, Arends D, Nassar MK, Brockmann GA. Fine mapping of a distal chromosome 4 QTL affecting growth and muscle mass in a chicken advanced intercross line. Anim Genet. 2017;48(3):295–302.
Johnsson M, Henriksen R, Höglund A, Fogelholm J, Jensen P, Wright D. Genetical genomics of growth in a chicken model. BMC Genomics. 2018;19(1):72.
Mebratie W, Reyer H, Wimmers K, Bovenhuis H, Jensen J. Genome wide association study of body weight and feed efficiency traits in a commercial broiler chicken population, a re-visitation. Sci Rep. 2019;9(1):922.
Dadousis C, Somavilla A, Ilska JJ, Johnsson M, Batista L, Mellanby RJ, Headon D, Gottardo P, Whalen A, Wilson D, et al. A genome-wide association analysis for body weight at 35 days measured on 137,343 broiler chickens. Genet Sel Evol. 2021;53(1):70.
Lyu S, Arends D, Nassar MK, Weigend A, Weigend S, Wang E, Brockmann GA. High-density genotyping reveals candidate genomic regions for chicken body size in breeds of Asian origin. Poult Sci. 2023;102(1):102303.
Perini F, Cendron F, Wu Z, Sevane N, Li Z, Huang C, Smith J, Lasagna E, Cassandro M, Penasa M. Genomics of Dwarfism in Italian Local Chicken breeds. Genes (Basel) 2023, 14(3).
Cha J, Choo H, Srikanth K, Lee SH, Son JW, Park MR, Kim N, Jang GW, Park JE. Genome-Wide Association Study Identifies 12 Loci Associated with Body Weight at Age 8 weeks in Korean native chickens. Genes (Basel) 2021, 12(8).
Takasuga A. PLAG1 and NCAPG-LCORL in livestock. Anim Sci J. 2016;87(2):159–67.
Ma M, Cai B, Kong S, Zhou Z, Zhang J, Zhang X, Nie Q. PPARGC1A is a moderator of skeletal muscle development regulated by miR-193b-3p. Int J Mol Sci 2022, 23(17).
Wang S, Wang Y, Li Y, Xiao F, Guo H, Gao H, Wang N, Zhang H, Li H. Genome-Wide Association Study and Selective Sweep Analysis Reveal the Genetic Architecture of body weights in a chicken F(2) Resource Population. Front Vet Sci. 2022;9:875454.
Monti P, Ciribilli Y, Foggetti G, Menichini P, Bisio A, Cappato S, Inga A, Divizia MT, Lerone M, Bocciardi R et al. P63 modulates the expression of the WDFY2 gene which is implicated in cancer regulation and limb development. Biosci Rep 2019, 39(12).
Thorens B. Glucagon-like peptide-1 and control of insulin secretion. Diabete Metab. 1995;21(5):311–8.
Lavin JH, Wittert GA, Andrews J, Yeap B, Wishart JM, Morris HA, Morley JE, Horowitz M, Read NW. Interaction of insulin, glucagon-like peptide 1, gastric inhibitory polypeptide, and appetite in response to intraduodenal carbohydrate. Am J Clin Nutr. 1998;68(3):591–8.
Duan X, An B, Du L, Chang T, Liang M, Yang BG, Xu L, Zhang L, Li J. E G: Genome-Wide Association Analysis of Growth Curve Parameters in Chinese Simmental Beef Cattle. Anim (Basel) 2021, 11(1).
Zhang Y, Song Y, Gao J, Zhang H, Yang N, Yang R. Hierarchical mixed-model expedites genome-wide longitudinal association analysis. Brief Bioinform 2021, 22(5).
Ning C, Wang D, Zhou L, Wei J, Liu Y, Kang H, Zhang S, Zhou X, Xu S, Liu JF. Efficient multivariate analysis algorithms for longitudinal genome-wide association studies. Bioinformatics. 2019;35(23):4879–85.
Ning C, Wang D, Zheng X, Zhang Q, Zhang S, Mrode R, Liu JF. Eigen decomposition expedites longitudinal genome-wide association studies for milk production traits in Chinese holstein. Genet Sel Evol. 2018;50(1):12.
Ning C, Kang H, Zhou L, Wang D, Wang H, Wang A, Fu J, Zhang S, Liu J. Performance gains in Genome-Wide Association Studies for Longitudinal Traits via modeling time-varied effects. Sci Rep. 2017;7(1):590.
Acknowledgements
The authors would like to thank Dingli Cao at the Poultry Institute, Shandong Academy of Agricultural Sciences for animal care and sample collection.
Funding
This research was supported by the Agricultural Stock Breeding Project of Shandong Province (2022LZGC013, 2020LZGC013,2019LZGC019), China Agriculture Research System of MOF and MARA (CARS-41, CARS-40), Agricultural Scientific and Technological Innovation Project of Shandong Academy of Agricultural Sciences (CXGC2022C04, CXGC2022D04, CXGC2022E11, CXGC2023F11), Shandong Provincial Natural Science Foundation (ZR2020MC169), The Key Research Program of Shandong province (2021CXGC0010805).
Author information
Authors and Affiliations
Contributions
JW, YZ and DC conceived and designed the study. JW performed statistical analyses and wrote the paper. QL, YZ, HH, and WL participated in data analyses. JW, ZL, JL, FL, HH, SZ, QC and DL participated in the design of the study and contributed to acquisition of data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments were carried out in accordance with the relevant guidelines and adhering to the ARRIVE guidelines (https://arriveguidelines.org/) for reporting animal experiments. The work was approved by the animal experiments performed in this study were approved by the Science Research Department of the Shandong Academy of Agricultural Sciences (SAAS) (Ji’nan, China). (Approval number: 2021001).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Liu, J., Lei, Q. et al. Elucidation of the genetic determination of body weight and size in Chinese local chicken breeds by large-scale genomic analyses. BMC Genomics 25, 296 (2024). https://doi.org/10.1186/s12864-024-10185-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10185-6