Abstract
Background
Two-component systems (TCSs) play a crucial role in the growth of Mycobacterium tuberculosis (M. tuberculosis). However, the precise regulatory mechanism of their contribution remain to be elucidated, and only a limited number of studies have investigated the impact of gene mutations within TCSs on the transmission of M. tuberculosis. Therefore, this study aims to explore the relationship between TCSs gene mutation and the global transmission of M. tuberculosis.
Results
A total of 13531 M.tuberculosis strains were enrolled in the study. Most of the M.tuberculosis strains belonged to lineage4 (n=6497,48.0%), followed by lineage2 (n=5136,38.0%). Our results showed that a total of 36 single nucleotide polymorphisms (SNPs) were positively correlated with clustering of lineage2, such as Rv0758 (phoR, C820G), Rv1747(T1102C), and Rv1057(C1168T). A total of 30 SNPs showed positive correlation with clustering of lineage4, such as phoR(C182A, C1184G, C662T, T758G), Rv3764c (tcrY, G1151T), and Rv1747 C20T. A total of 19 SNPs were positively correlated with cross-country transmission of lineage2, such as phoR A575C, Rv1028c (kdpD, G383T, G1246C), and Rv1057 G817T. A total of 41 SNPs were positively correlated with cross-country transmission of lineage4, such as phoR(T758G, T327G, C284G), kdpD(G1755A, G625C), Rv1057 C980T, and Rv1747 T373G.
Conclusions
Our study identified that SNPs in genes of two-component systems were related to the transmission of M. tuberculosis. This finding adds another layer of complexity to M. tuberculosis virulence and provides insight into future research that will help to elucidate a novel mechanism of M. tuberculosis pathogenicity.
Similar content being viewed by others
Background
Tuberculosis is a serious global health problem caused by Mycobacterium tuberculosis (M. tuberculosis), a pathogen that lives and thrives inside human cells [1]. It is a highly contagious and often fatal disease that affects millions of people worldwide, making it a significant burden on public health systems and societies. However, despite its enormous global burden, the factors that contribute to tuberculosis transmission are still poorly understood. Therefore, developing a better understanding of M. tuberculosis transmission is critical for guiding effective tuberculosis control strategies and reducing the disease’s burden on society.
Bacterial two-component systems (TCSs) are the most important sensing mechanisms that respond to a diverse range of ligands, including ions, gases, and metabolites. In pathogenic bacteria, TCSs play a crucial role in promoting pathogenesis by regulating bacterial gene expression in response to hostile host environments or metabolic stresses [2, 3]. The traditional two-component sensing system comprises a sensor kinase located in the cell membrane, which detects an extracellular ligand and subsequently activates through autophosphorylation on a cytoplasmic histidine residue. The M. tuberculosis H37Rv genome contains 190 transcriptional regulators, including 12 pairs of TCSs and 4 orphan proteins that belong to the two-component system family. These regulators play a role in regulating various aspects of M. tuberculosis, such as virulence, dormancy, persistence, and drug resistance. Some studies have suggested that TCSs may regulate the spread of M. tuberculosis through various pathways [4, 5]. For example, they can influence the growth, metabolism and environmental adaptation of the bacterium by regulating cell wall synthesis and degradation, maintaining intracellular redox balance, and modulating metabolic pathways [6, 7]. However, further research is needed to determine the specific regulatory mechanisms of TCSs in the process of M. tuberculosis transmission.
Whole genome sequencing (WGS) technology has significant implications for the study and treatment of M. tuberculosis [8]. This technique provides comprehensive information on the M. tuberculosis genome, including gene structure, function, regulation, and mutations. Such information can provide critical insights into the biological characteristics of the bacterium, its transmission patterns, drug resistance mechanisms, and new therapeutic targets. Additionally, WGS can help us understand M. tuberculosis evolution by identifying genetic differences and correlations between different strains, studying human-host co-adaptation and coevolution, and discovering new drugs and treatments for tuberculosis [9, 10]. In our research, WGS was used to study the influence of gene mutations in two-component systems on the worldwide transmission of M. tuberculosis. Specifically, the genome cluster was used to represent the transmission of M. tuberculosis.
Results
Characteristics of study samples
A total of 13,531 strains were used in this study including 5136(38.0%) strains belonged to lineage2 and 6497(48.0%) belonged to lineage 4. Lineage 2.2.1 was the dominant sub-lineage, accounting for 41.9%, followed by lineage 4.3 (16.6%), lineage 4.1 (13.9%) and lineage 4.8 (9.4%). The highest clustering rate observed within the lineage was lineage 4 at 0.704, while within sub-lineages, lineage 4.3 exhibited the greatest number of clustered strains. Among the clustered strains, lineage 4 had the most strains of cross-country and cross-regional distribution, while within sub-lineages, lineage 2.2.1 had the highest number of such strains (Table 1).
Relationship between TCSs gene mutation and lineage transmission
We studied the relationship of gene mutation in the two-component system and lineage transmission. The random forest and gradient boosting decision tree models of lineage 1 were successfully established. For further details see Additional file 2: Table S9 and Additional file 1: Fig. S6. Subsequently, a generalized linear mixed model was established to analyze 60 variables that represented the intersection of random forest and gradient boosting decision tree (Additional file 2: Tables S4 and S5). A total of 31 SNPs showed a positive correlation with clustering of lineage1(P < 0.05), including 15 synonymous SNPs and 16 nonsynonymous SNPs, such as Rv3764c (tcrY, T1354C, OR,1.975; 95%CI,1.456–2.680), Rv1747(C980T, OR, 2.344; 95%CI,1.723–3.19), Rv1057(C177T, OR,1.539, 95%CI, 1.24–1.91), and Rv3245c (mtrB, C831T, G300T) (Additional file 2: Table S24). The results showed that 31 SNPs increased the risk of lineage1 transmission. For lineage2, the random forest and gradient boosting decision tree models were successfully established (Table 2; Fig. 1). Subsequently, a generalized linear mixed model was established to examine 60 variables that contributed to the gradient boosting decision tree and random forest models (Additional file 2: Tables S4 and S5). A total of 36 SNPs showed a positive correlation with clustering of lineage2(P < 0.05), including 12 synonymous SNPs and 24 nonsynonymous SNPs, such as Rv0758 (phoR, C820G), Rv1747 T1102C, Rv1057 C1168T, Rv3764c (tcrY, C284A), Rv0982(mprB, G910A), Rv2247(accD6, G567A, T600C), Rv1027c (KdpE, G178A, C626A), Rv3245c (mtrB, A971G, G1110A), and Rv3765c (tcrX, G293C) (Additional file 2: Table S25). The results showed that 36 SNPs increased the risk of lineage2 transmission. For lineage3, the random forest and gradient boosting decision tree models of lineage 3 were successfully established (Additional file 2: Table S10 and Additional file 1: Fig. S7). Subsequently, a generalized linear mixed model was established to analyze 60 variables that represented the common features from both gradient boosting decision tree and random forest models (Additional file 2: Tables S4 and S5). A total of 29 SNPs showed a positive correlation with clustering of lineage3(P < 0.05), including 11 synonymous SNPs and 18 nonsynonymous SNPs, such as Rv0758 (phoR, G448T, G694T), Rv3764c (tcrY, C278T), Rv1747(G2188A, C460T), Rv3765c (tcrX, G415A), Rv1057 G186A, Rv0982(mprB, G1477A), Rv1032c (trcS, T946C), Rv1027c (KdpE, C45T), and Rv3245c (mtrB, C24T) (Additional file 2: Table S23). The results showed that 29 SNPs increased the risk of transmission of lineage3. For lineage4, the random forest and gradient boosting decision tree models of lineage 4 were successfully established (Additional file 2: Table S11 and Additional file 1: Fig. S8). Subsequently, a generalized linear mixed model was established to analyze a total of 60 variables that represented the intersection of random forest and gradient boosting decision tree (Additional file 2: Tables S4 and S5). A total of 30 SNPs showed a positive correlation with clustering of lineage4(P < 0.05), including 15 synonymous SNPs and 15 nonsynonymous SNPs, such as Rv0758(phoR, C182A, C1184G, C662T, T758G), Rv3764c (tcrY, G1151T), Rv1747 C20T, Rv3765c (tcrX, C45G), Rv1057 C585A, Rv1032c (trcS, G977T), Rv2247(accD6, G957A), and Rv3245c (mtrB, T33C) (Additional file 2: Table S7). The results showed that 30 SNPs increased the risk of transmission of lineage4.
Relationship between TCSs gene mutation and sub-lineage transmission
The random forest and gradient boosting decision tree models of lineage 2.2.1, lineage 2.2.2, lineage 4.1, lineage 4.2, lineage 4.4 and lineage 4.8 were successfully established (Additional file 2: Tables S4, S5, S12–S18) (Additional file 1: Figs. S9–S15). The results of the generalized linear mixed model showed that 30 SNPs were positively correlated with clustering of lineage2.2.1(P < 0.05), including 10 synonymous SNPs and 20 nonsynonymous SNPs, such as Rv2027c (dosT, T874C), Rv1028c (kdpD, G2453A), Rv1057 C29G, Rv0982 (mprB, G1477A), Rv1032c (trcS, T946C), Rv1027c (KdpE, G178A, C626A), Rv3245c (mtrB, A971G), and Rv2247(accD6, G567A) (Additional file 2: Table S28). The results showed that 30 SNPs increased the risk of transmission of lineage2.2.1. For lineage2.2.2, a total of 16 SNPs showed a positive correlation with clustering (P < 0.05), including 8 synonymous SNPs and 8 nonsynonymous SNPs, such as Rv2027c (dosT, C215T), Rv1028c (kdpD, G2085), Rv0982 (mprB, G910A), and Rv3245c (mtrB, T33C) (Table 3). The results showed that 16 SNPs increased the risk of transmission of lineage2.2.2.
For lineage4.1, a total of 22 SNPs showed a positive correlation with clustering (P < 0.05), including 12 synonymous SNPs and 10 nonsynonymous SNPs, such as Rv1028c (kdpD, G943A, G2136A), Rv2027c (dosT, G1256A), Rv1032c (trcS, G857A), and Rv1747 C20T (Additional file 2: Table S29). Our results showed that these 22 SNPs increased the risk of transmission of lineage4.1. For lineage4.2, the result of the generalized linear mixed model showed that 7 SNPs were positively correlated with clustering (P < 0.05), including 2 synonymous SNPs and 5 nonsynonymous SNPs, such as Rv0758 (phoR, C182A), Rv0930(pstA1, G895T, C913T), Rv3245c (mtrB, C1113A), and Rv2247(accD6, G957A, T600C) (Additional file 2: Table S30). The results showed that 7 SNPs increased the risk of transmission of lineage4.2. For lineage4.4, the result of the generalized linear mixed model showed that 18 SNPs were positively correlated with clustering (P < 0.05), including 9 synonymous SNPs and 9 nonsynonymous SNPs, such as Rv0982(mprB, G901A, G230C), Rv1028c (kdpD, C1102T), Rv0758 (phoR, C662T, T758G, A341C), Rv0982 (mprB, G901A. G230C), and Rv3245c (mtrB, C1083T), (Additional file 2: Table S31). The results showed that 18 SNPs increased the risk of transmission of lineage4.4. For lineage4.8, the result of the generalized linear mixed model showed that 15 SNPs were positively correlated with clustering (P < 0.05), including 5 synonymous SNPs and 10 nonsynonymous SNPs, such as Rv1028c (kdpD, C643T), Rv3764c (tcrY, G1151T), Rv1032c (trcS, C1375G), Rv0758(phoR, T148G), and Rv2247(accD6, T600C), (Additional file 2: Table S32). The results showed that 15 SNPs increased the risk of transmission of lineage4.8.
Relationship between TCSs gene mutation and cluster size
For analyzing the relationship of gene mutation in the two-component system and cluster size, the random forest and gradient boosting decision tree models for lineage2 and lineage4 were successfully established.
The random forest and gradient boosting decision tree models of lineage 2 and lineage4 were successfully established. (Additional file 2: Tables S6, S19, and S20). The results of the generalized linear mixed model indicated that 25 SNPs were positively correlated with cluster size of lineage2(P < 0.05), including 14 synonymous SNPs and 11 nonsynonymous SNPs, such as Rv0758(phoR, C820G), Rv1747 C696A, Rv1028c (kdpD, G383T), Rv1057 C653T, Rv1032c (trcS, C1202T), Rv2247(accD6, G567A), Rv3245c (mtrB, A1660G), Rv0982(mprB, G910A, C780T), and Rv1027c (KdpE, C626A) (Additional file 2: Table S33). The results showed that 25 SNPs increased the risk of small clusters, medium clusters, and large clusters of lineage2. The results of the generalized linear mixed model indicated that a total of 30 significant SNPs were positively correlated with cluster size of lineage4(P < 0.05), including 13 synonymous SNPs and 17 nonsynonymous SNPs, such as Rv0758(phoR, C182A, C1184G, C662T), Rv1028c(kdpD, C2320T, A1982T, G943A, A214G), Rv3132c(devS,C552G), Rv0982(mprB, G901A), Rv3764c(tcrY, G1151T), Rv1747 C20T, Rv0982(mprB, G910A, C1191G), Rv1032c (trcS, G977T, C1445T), Rv2247(accD6, G957A, T600C), and Rv3245c (mtrB, A778G) (Additional file 2: Table S34). The results showed that 30 SNPs increased the risk of small clusters, medium clusters, and large clusters of lineage4.
Relationship between TCSs gene mutation and cross-country transmission
Random forest and gradient boosting decision tree models were successfully implemented to analyze the cross-country transmission of M. tuberculosis via gene mutations in TCSs, specifically focusing on lineage2 and lineage4. (Additional file 2: Tables S7, S2, and S22; Additional file 1: Figs. S16 and S17)
The results of the generalized linear mixed model showed that a total of 19 SNPs were positively correlated with cross-country transmission of lineage2(P < 0.05), including 6 synonymous SNPs and 13 nonsynonymous SNPs, such as Rv0758(phoR, A575C), Rv1028c (kdpD, G383T, G1246C), Rv1057 G817T, Rv0982(mprB, G910A), Rv1747 T373G. Rv0982(mprB, G910A, C1317G), Rv1027c (KdpE, G178A) (Additional file 2: Table S35). The results showed that 19 SNPs increased the risk of cross-country transmission of lineage2. A total of 41 SNPs were positively correlated with cross-country transmission of lineage4 (P < 0.05), including 20 synonymous SNPs and 21 nonsynonymous SNPs, such as Rv0758(phoR, T758G, T327G, C284G), Rv1028c(kdpD, G1755A, G625C), Rv1057 (C980T, Rv1747 T373G), Rv3764c T736C, Rv0982(mprB, G1323A), Rv2247(accD6, G181A, C700T, G36A), Rv1027c (KdpE, G381A), Rv1032c (trcS, T188G, G977T, G571A),and Rv3245c(mtrB, T354C, G1011A) (Additional file 2: Table S36). The results showed that 41 SNPs increased the risk of cross-country transmission of lineage4.
Relationship between TCSs gene mutation and cross-regional transmission
The random forest and gradient boosting decision tree models were successfully established for analyzing the cross-regional transmission of M. tuberculosis through gene mutations in TCSs within lineage 2 and lineage 4. (Table 4, Additional file 2: Tables S8 and S23) (Additional file 1: Figs. S18 and S19).
The results of the generalized linear mixed model showed that a total of 22 SNPs were positively correlated with cross-regional transmission of lineage2(P < 0.05), including 9 synonymous SNPs and 13 nonsynonymous SNPs, such as Rv0758(PhoR, C385A), Rv1057(G817T, A1136G), Rv2027c (dosT, C1343T), Rv1028c (kdpD, G383T), Rv1747(T373G). Rv0982(mprB, G910A, C1317G), Rv1027c (KdpE, G178A), and Rv1032c (trcS, A886G, G748A, G561A) (Additional file 2: Table S37). The results showed that 22 SNPs increased the risk of cross-regional transmission of lineage2. A total of 34 SNPs were positively correlated with cross-regional transmission of lineage4(P < 0.05), including 13 synonymous SNPs and 21 nonsynonymous SNPs, such as Rv0758(phoR, T758G, C805T, C294A, C1184G), Rv1057 C980T, Rv1028c (kdpD, G1755A, G1266C, G625C), Rv1747 C2112T, Rv2247(accD6, G36A), Rv1027c (KdpE, G381A), Rv1032c (trcS, T188G, G977T, G571A), and Rv3245c (mtrB, G390A), (Additional file 2: Table S38). The results showed that 34 SNPs increased the risk of cross-regional transmission of lineage4.
The above findings revealed that synonymous SNPs and nonsynonymous SNPs in PhoR, mprB and Rv1747 were significantly related to the transmission of various lineages of M.tuberculosis, including cross-country and cross-regional transmission. In addition, missense mutations in KdpD and trcS, as well as synonymous mutations in Rv3245c (mtrB), Rv2247 (accD6) and Rv1027c (KdpE), were also significantly related to the transmission of various lineages of M.tuberculosis, including cross-country and cross-regional transmission (Fig. 2). These mutations increased the risk of transmission of M.tuberculosis.
Discussion
The transmission factors of pathogenic bacteria have always been elusive, and the transmission factors of M. tuberculosis were also very complicated. Until now, there has been no research on whether the gene mutation in TCSs is related to the transmission of M. tuberculosis. Our research showed that there were SNPs in the genes of the two-component system, which increased the risk of the transmission of M. tuberculosis. With the continuous discovery of new two-component systems, these findings showed that the gene mutation of TCSs has universal and extensive significance for the transmission of M. tuberculosis.
Our study revealed that SNPs in PhoR increased the risk of transmission of M. tuberculosis, including C820G, G448T, G694T, C182A, C1184G, C662T, T758G, C820G, C182A, C1184G, and C662T. PhoP, part of the two-component system PhoR-PhoP, is the response regulator protein that activates or represses the genes of the regulon [11]. The PhoR gene encodes a kinase and is considered one of the main signaling pathways involved in regulating phosphate metabolism in M. tuberculosis. This gene senses changes in extracellular phosphate concentration, activating the PhoP-PhoR two-component system to regulate the adaptive response of M. tuberculosis [12, 13]. The increased risk of M. tuberculosis transmission associated with multiple SNPs in PhoR can be attributed to their impact on gene expression, thereby influencing crucial cellular processes including metabolism, virulence, and adaptation in M. tuberculosis [14]. Furthermore, several studies have indicated that mutations occurring at specific sites in the PhoR gene can affect various biological aspects of M. tuberculosis, such as growth, metabolism, and drug resistance, thus indirectly influencing the pathogen’s survival rate and infectivity within the host [15, 16].
The Rv1027c-Rv1028c genes in M. tuberculosis are predicted to encode the kdpDE two-component system, which exhibits a high degree of conservation among bacterial species [17]. This system has been extensively studied and found to regulate virulence and stress resistance in various human pathogens. Our results reveal that multiple SNPs in kdpD increase the risk of transmission of M. tuberculosis. These SNPs may change the function of kdpD protein by affecting the domain of kdpD, and trigger the expression regulation of kdpD. This regulation of gene expression further promotes the adaptive response of kdpDE system [18]. Additionally, research has demonstrated the essential role of kdpD in the pathogen’s survival within the host, and a mutant strain with kdpDE exhibited a hyper-virulent phenotype in SCID mice [19, 20]. The expression of the trcR-trcS two-component system is induced upon the adaptation of the organism to the intracellular milieu and potentially during extracellular replication of M. tuberculosis within the liquefaction cavity after rupture of the wall granuloma [21]. Our results revealed that multiple SNPs in trcS increased the risk of transmission of M. tuberculosis. The trcS gene in M. tuberculosis serves multiple functions, acting as a regulatory gene encoding a sensor kinase protein involved in the two-component signal transduction system. This system enables the bacterium to detect and respond to environmental changes. Moreover, studies have demonstrated that deletion of the trcS gene in this bacterium reduces its survival rate in mouse lungs and increases host clearance [22, 23]. Furthermore, mutations in the trcS gene may be associated with drug resistance in M. tuberculosis by regulating intracellular metabolic and virulence processes.
Moreover, our research also elucidates the association between SNPs in other two-component system genes and the dissemination of M.tuberculosis. These genetic mutations have the potential to alter diverse physiological functions of the bacterium that are intricately linked to its transmission. Mutations of these gene may change various physiological functions of the bacterium related to their transmission. It is worth noting that although we have confirmed the impact of these SNPs on the transmission of M. tuberculosis, further research is still needed to determine how these mutations affect the function of the TCSs and the mechanism through which they influence the transmission of M. tuberculosis. Additionally, it should be noted that factors influencing the transmission of M. tuberculosis are highly complex, involving not only genetic mutations but also various aspects such as the environment, host immune system, and genotype. Therefore, in formulating prevention and treatment strategies, it is necessary to comprehensively consider all possible factors, objectively evaluate their contributions to disease transmission, and thus more effectively control and prevent the occurrence and transmission of tuberculosis.
Conclusion
The two-component system is a widely distributed signal transduction system in bacteria that regulates a variety of biological processes, including metabolism, virulence, pathogenicity, and adaptation. The SNPs in TCSs gene increase the risk of transmission of M.tuberculosis, which reflects the important role of TCSs in the life activities of M.tuberculosis. Therefore, in-depth research on the function and regulatory mechanism of these genes can help us better understand the molecular biology characteristics of M. tuberculosis, providing new ideas and methods for the prevention and control of tuberculosis. In summary, this study provides new clues for us to understand the transmission mechanism of M. tuberculosis and also serves as a reference for related research. In the future, we will further deepen research in this area to provide more effective means for controlling tuberculosis.
Method
Sample Collection
A total of 1550 M. tuberculosis culture-positive cases were collected from two medical institutions from 2011 to 2018 in China: Shandong Public Health Clinical Research Center (SPHCC) and Weifang Respiratory Clinical Hospital (WRCH). The study did not include M. tuberculosis culture-positive cases who had previously undergone evaluation and were subsequently being treated.
DNA extraction and sequencing
Genomic DNA was extracted from 1447 strains using Cetyltrimethylammonium Bromide (CTAB) and underwent quality control (QC). In total, 103 strains of M.tuberculosis were excluded because of improper handling during the DNA extraction and poor quality of extracted DNA. The genomes were sequenced using the Illumina HiSeq 4000 system, and the resulting sequence data were deposited in the National Center for Biotechnology Information (NCBI) under BioProject PRJNA1002108. In addition, this study included 13,267 strains of M. tuberculosis from 52 countries and 18 regions around the world [24,25,26,27,28,29,30,31,32]. We utilized BWA-MEM (version 0.7.17-r1188) to accurately map the reference genome of the standard isolate M. tuberculosis H37Rv. Our analysis only included samples exhibiting a coverage rate of 98% or higher and a minimum depth of at least 20% [33]. Finally, a total of 13,531 genomes were analyzed, please refer to Additional file 2: Tables S1-S2 for the specific sample numbers.
Single nucleotide polymorphism (SNP) analysis
After variant calling using Samclip (version 0.4.0) and SAMtools (version 1.15), we applied further filtering to the resulting variants via Free Bayes (version 1.3.2) and Bcftools (version 1.15.1). We excluded Single Nucleotide Polymorphisms (SNPs) located within repeat regions, including polymorphic GC-rich sequences (PE/PPE genes) and direct repeat SNPs, as well as repeat bases identified through the use of Tandem Repeat Finder (version 4.09) and RepeatMask (version 4.1.2-P1) [34, 35]. Finally, SNP annotation was conducted via SnpEff v 4.1 l, with the resulting output obtained utilizing the Python programming language [36].
Phylogenetic analysis
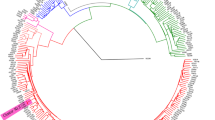
The strains were classified into different lineages according to Coll et al. [37](Additional file 2: Tables S1-S2). Construction of the maximum likelihood phylogenetic tree was conducted through the IQ-TREE software package (version 1.6.12), utilizing the JC nucleotide substitution model and gamma model of rate heterogeneity, with 100 bootstrap replicates included [38]. Mycobacterium canettii CIPT140010059 was deemed to be an outlier. The resultant phylogenetic tree was visualized through the utilization of iTOL (https://itol.embl.de/) (Fig. 3, Additional file 1: Figs. S1–S7).
Propagation analysis
Cluster analysis was utilized to investigate the influence of two-component system gene mutations on the transmission of M. tuberculosis [39]. Based on a previous study [40], we applied clustering to define transmission clusters and used a threshold of less than 25 SNPs. In addition, we chose the threshold of 25 SNPs because our isolates were spread in terms of location and time (1991–2019) and because we were probably missing several intermediary isolates (and cases) in our collection. (Additional file 2: Tables S1-S2). Additionally, according to the classification of transmission clusters by scholars, we also divided transmission clusters into large, medium, or small (large, over 75th percentile; medium, between 25th and 75th percentile; and small, under 25th percentile) [14]. To enhance understanding of the global distribution patterns and conduct an extensive analysis of the transmission dynamics of M.tuberculosis strains, we classified them into cross-country and within-country clusters. Furthermore, we categorized the M. tuberculosis strains into cross-regional and within-regional clusters based on geographic location utilizing the United Nations standard regions (UN M.49).
Acquisition of two-component system genes
A total of 45 two-component system genes were obtained according to NCBI and literature search [2, 7, 41]. Python was utilized to detect mutations in genes associated with TCSs (Additional file 2: Table S3).
Modeling and statistical analysis
Prediction models including gradient boosting decision tree and random forest were established by machine learning using the Scikit-learn Python package. We randomly divided all samples into training and test sets at a ratio of 7:3. Each of the models was evaluated with the metrics of Kappa, sensitivity, specificity, accuracy, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NLR) and area under curve (AUC) [42]. After the model was fitted, we evaluated the importance of the input variables on the model. To enhance the precision of predicting risk factors, we utilized the score to assess the influence of each input feature of the models, and take the intersection of both conditions and obtain the top-performing accessions as the important features [43, 44]. Subsequently, we established the generalized linear mixed model by using the statsmodels.api Python package to further analyze the important features and obtain the final influencing factors. All statistical analyses were performed using SPSS 26.0. All statistical tests were two-tailed, and P values less than 0.05 were considered statistically significant.
Data Availability
The whole genome sequences have been submitted to the NCBI under the accession number PRJNA1002108.
Change history
08 January 2024
A Correction to this paper has been published: https://doi.org/10.1186/s12864-023-09914-0
Abbreviations
- M. tuberculosis :
-
Mycobacterium tuberculosis
- TCSs:
-
Two-component systems
- WGS:
-
Whole genome sequencing
- SPHCC:
-
Shandong Public Health Clinical Research Center
- WRCH:
-
Weifang Respiratory Clinical Hospital
- CTAB:
-
Cetyltrimethylammonium Bromide
- QC:
-
Quality control
- SNP:
-
Single nucleotide polymorphism
- SNPs:
-
Single nucleotide polymorphisms
- NCBI:
-
National Center for Biotechnology Information
- PPV:
-
Positive Predictive Value
- NPV:
-
Negative Predictive Value
- PLR:
-
Positive Likelihood Ratio
- NLR:
-
Negative Likelihood Ratio
- AUC:
-
Area Under Curve
References
World Health Organization. Global Tuberculosis report 2022. Geneva: World Health Organization; 2022.
Parish T. Two-Component Regulatory Systems of Mycobacteria. Microbiol Spectr. 2014;2(1):MGM2-0010-2013.
Zhou PF, Long QX, Zhou YX, Wang HH, Xie J. Mycobacterium tuberculosis two-Component systems and implications in novel vaccines and Drugs. Crit Rev Eukar Gene Expr. 2012;22:37–52.
Kusebauch U, Ortega C, Ollodart A, Rogers RS, Sherman DR, Moritz RL, et al. Mycobacterium tuberculosis supports protein tyrosine phosphorylation. Proc Natl Acad Sci U S A. 2014;111:9265–70.
Buglino JA, Sankhe GD, Lazar N, Bean JM, Glickman MS. Integrated sensing of host stresses by inhibition of a cytoplasmic two-component system controls M. Tuberculosis acute lung Infection. eLife. 2021;10:e65351.
Supply P, Magdalena J, Himpens S, Locht C. Identification of novel intergenic repetitive units in a mycobacterial two-component system operon. Mol Microbiol. 1997;26:991–1003.
Bretl DJ, Demetriadou C, Zahrt TC. Adaptation to Environmental Stimuli within the host: Two-Component Signal Transduction Systems of Mycobacterium tuberculosis. Microbiol Mol Biol Rev. 2011;75:566–82.
Takiff HE, Feo O. Clinical value of whole-genome sequencing of Mycobacterium tuberculosis. Lancet Infect Dis. 2015;15:1077–90.
Walker TM, Kohl TA, Omar SV, Hedge J, Del Ojo Elias C, Bradley P, et al. Whole-genome sequencing for prediction of Mycobacterium tuberculosis drug susceptibility and resistance: a retrospective cohort study. Lancet Infect Dis. 2015;15:1193–202.
Köser CU, Bryant JM, Becq J, Török ME, Ellington MJ, Marti-Renom MA, et al. Whole-genome sequencing for rapid susceptibility testing of M. Tuberculosis. N Engl J Med. 2013;369:290–2.
Waturuocha UW, Krishna MS, Malhotra V, Dixit NM, Saini DK. A low-prevalence single-nucleotide polymorphism in the Sensor kinase PhoR in Mycobacterium tuberculosis suppresses its autophosphatase activity and reduces pathogenic fitness: implications in Evolutionary Selection. Front Microbiol. 2021;12:724482.
Mendes MV, Tunca S, Antón N, Recio E, Sola-Landa A, Aparicio JF, et al. The two-component phor-phop system of Streptomyces natalensis: inactivation or deletion of phoP reduces the negative phosphate regulation of pimaricin biosynthesis. Metab Eng. 2007;9:217–27.
De Maio F, Berisio R, Manganelli R, Delogu G. PE_PGRS proteins of Mycobacterium tuberculosis: a specialized molecular task force at the forefront of host-pathogen interaction. Virulence. 2020;11:898–915.
Chiner-Oms Á, Sánchez-Busó L, Corander J, Gagneux S, Harris SR, Young D et al. Genomic determinants of speciation and spread of the Mycobacterium tuberculosis complex. Sci Adv. 2019.
Vashist A, Malhotra V, Sharma G, Tyagi JS, Clark-Curtiss JE. Interplay of PhoP and DevR response regulators defines expression of the dormancy regulon in virulent Mycobacterium tuberculosis. J Biol Chem. 2018;293:16413–25.
Ryndak M, Wang S, Smith I. PhoP, a key player in Mycobacterium tuberculosis virulence. Trends Microbiol. 2008;16:528–34.
Agrawal R, Saini DK. Rv1027c-Rv1028c encode functional KdpDE two–component system in Mycobacterium tuberculosis. Biochem Biophys Res Commun. 2014;446:1172–8.
Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84.
Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during Infection. Proc Natl Acad Sci U S A. 2003;100:12989–94.
Parish T, Smith DA, Kendall S, Casali N, Bancroft GJ, Stoker NG. Deletion of two-component regulatory systems increases the virulence of Mycobacterium tuberculosis. Infect Immun. 2003;71:1134–40.
Haydel SE, Benjamin WH, Dunlap NE, Clark-Curtiss JE. Expression, autoregulation, and DNA binding properties of the Mycobacterium tuberculosis TrcR response regulator. J Bacteriol. 2002;184:2192–203.
Wernisch L, Kendall SL, Soneji S, Wietzorrek A, Parish T, Hinds J, et al. Analysis of whole-genome microarray replicates using mixed models. Bioinformatics. 2003;19:53–61.
Haydel SE, Dunlap NE, Benjamin WH. In vitro evidence of two-component system phosphorylation between the Mycobacterium tuberculosis TrcR/TrcS proteins. Microb Pathog. 1999;26:195–206.
Chen X, He G, Wang S, Lin S, Chen J, Zhang W. Evaluation of whole-genome sequence method to Diagnose Resistance of 13 anti-tuberculosis Drugs and characterize resistance genes in clinical Multi-drug Resistance Mycobacterium tuberculosis isolates from China. Front Microbiol. 2019;10:1741.
Yang C, Luo T, Shen X, Wu J, Gan M, Xu P, et al. Transmission of multidrug-resistant Mycobacterium tuberculosis in Shanghai, China: a retrospective observational study using whole-genome sequencing and epidemiological investigation. Lancet Infect Dis. 2017;17:275–84.
Koster KJ, Largen A, Foster JT, Drees KP, Qian L, Desmond E, et al. Genomic sequencing is required for identification of Tuberculosis transmission in Hawaii. BMC Infect Dis. 2018;18:608.
Hicks ND, Yang J, Zhang X, Zhao B, Grad YH, Liu L, et al. Clinically prevalent mutations in Mycobacterium tuberculosis alter propionate metabolism and mediate multidrug tolerance. Nat Microbiol. 2018;3:1032–42.
Liu Q, Ma A, Wei L, Pang Y, Wu B, Luo T, et al. China’s Tuberculosis epidemic stems from historical expansion of four strains of Mycobacterium tuberculosis. Nat Ecol Evol. 2018;2:1982–92.
Huang H, Ding N, Yang T, Li C, Jia X, Wang G, et al. Cross-sectional whole-genome sequencing and epidemiological study of Multidrug-resistant Mycobacterium tuberculosis in China. Clin Infect Dis. 2019;69:405–13.
Luo T, Comas I, Luo D, Lu B, Wu J, Wei L, et al. Southern East Asian origin and coexpansion of Mycobacterium tuberculosis Beijing family with Han Chinese. Proc Natl Acad Sci USA. 2015;112:8136–41.
Jiang Q, Liu Q, Ji L, Li J, Zeng Y, Meng L, et al. Citywide transmission of Multidrug-resistant Tuberculosis under China’s Rapid Urbanization: a Retrospective Population-based genomic spatial epidemiological study. Clin Infect Dis. 2020;71:142–51.
Coll F, Phelan J, Hill-Cawthorne GA, Nair MB, Mallard K, Ali S, et al. Genome-wide analysis of multi- and extensively drug-resistant Mycobacterium tuberculosis. Nat Genet. 2018;50:307–16.
Jung Y, Han D, BWA-MEME. BWA-MEM emulated with a machine learning approach. Bioinformatics. 2022;:btac137.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–9.
Liu F, Zhang Y, Zhang L, Li Z, Fang Q, Gao R, et al. Systematic comparative analysis of single-nucleotide variant detection methods from single-cell RNA sequencing data. Genome Biol. 2019;20:242.
Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin). 2012;6:80–92.
Coll F, McNerney R, Guerra-Assunção JA, Glynn JR, Perdigão J, Viveiros M, et al. A robust SNP barcode for typing Mycobacterium tuberculosis complex strains. Nat Commun. 2014;5:4812.
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–74.
Seto J, Wada T, Suzuki Y, Ikeda T, Mizuta K, Yamamoto T, et al. Mycobacterium tuberculosis Transmission among Elderly persons, Yamagata Prefecture, Japan, 2009–2015. Emerg Infect Dis. 2017;23:448–55.
Walker TM, Ip CL, Harrell RH, Evans JT, Kapatai G, Dedicoat MJ, et al. Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: a retrospective observational study. Lancet Infect Dis. 2013;13:137–46.
Li X, Lv X, Lin Y, Zhen J, Ruan C, Duan W, et al. Role of two-component regulatory systems in intracellular survival of Mycobacterium tuberculosis. J Cell Biochem. 2019;120:12197–207.
Luo Y, Xue Y, Song H, Tang G, Liu W, Bai H, et al. Machine learning based on routine laboratory indicators promoting the discrimination between active Tuberculosis and latent Tuberculosis Infection. J Infect. 2022;84:648–57.
Bi X, Xu Q, Luo X, Sun Q, Wang Z. Weighted Random Support Vector machine clusters analysis of resting-state fMRI in mild cognitive impairment. Front Psychiatry. 2018;9:340.
Agarwal G, Saade S, Shahid M, Tester M, Sun Y. Quantile function modeling with application to salinity tolerance analysis of plant data. BMC Plant Biol. 2019;19:526.
Acknowledgements
We thank Shandong Provincial Hospital, Shandong Provincial Chest Hospital, 13 municipal-level and 21 county-level local health departments for drug susceptibility data, demographic, and clinical data.
Funding
This work was supported by the Department of Science & Technology of Shandong Province (CN) (No.2007GG30002033; No.2017GSF218052) and the Jinan Science and Technology Bureau (CN) (No.201704100). The funding body/bodies did not provide any assistance in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
HCL, YL, and YML participated in the study design. YL, HCL, YML, XLK, NNT, and YFL performed data collection and statistical analyses. YL, TTW, YYL, and YWH helped draft the manuscript. YWH, QLH, and YYL overviewed and supervised the project. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study complies with the Declaration of Helsinki, and was approved by the Ethics Committee of Shandong Provincial Hospital, affiliated with Shandong University (SPH), the Ethics Weifang Respiratory Clinical Hospital (WRCH) and the Ethics Committee of Shandong Provincial Chest Hospital (SPCH), which waived informed patient consent because all patient records and information were anonymized and deidentified before the analysis.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: there was an error in affiliation 1 and the Background section of the Abstract had to be revised.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Y., Kong, X., Li, Y. et al. Association between two-component systems gene mutation and Mycobacterium tuberculosis transmission revealed by whole genome sequencing. BMC Genomics 24, 718 (2023). https://doi.org/10.1186/s12864-023-09788-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09788-2