Abstract
Understanding the genomic features of livestock is essential for successful breeding programs and conservation. This information is scarce for local goat breeds in Egypt. In the current study, genomic regions with selection signatures were identified as well as runs of homozygosity (ROH), genomic inbreeding coefficients (FROH) and fixation index (FST) were detected in Egyptian Nubian, Damascus, Barki and Boer goat breeds. A total of 46,268 SNP markers and 337 animals were available for the genomic analyses. On average, 145.44, 42.02, 87.90 and 126.95 ROHs were detected per individual in the autosomal genome of the respective breeds. The mean accumulative ROH lengths ranged from 46.5 Mb in Damascus to 360 Mb in Egyptian Nubian. The short ROH segments (< 2 Mb) were most frequent in all breeds, while the longest ROH segments (> 16 Mb) were exclusively found in the Egyptian Nubian. The highest average FROH was observed in Egyptian Nubian (~ 0.12) followed by Boer (~ 0.11), while the lowest FROH was found in Damascus (~ 0.05) and Barki breed (~ 0.03). The estimated mean FST was 0.14 (Egyptian Nubian and Boer), 0.077 (Egyptian Nubian and Barki), 0.075 (Egyptian Nubian and Damascus), 0.071 (Barki and Boer), 0.064 (Damascus and Boer), and 0.015 (Damascus and Barki), for each pair of breeds. Interestingly, multiple SNPs that accounted for high FST values were observed on chromosome 6 in regions harboring ALPK1 and KCNIP4. Genomic regions overlapping both FST and ROH harbor genes related to immunity (IL4R, PHF23, GABARAP, GPS2, and CD68), reproduction (SPATA2L, TNFSF12, TMEM95, and RNF17), embryonic development (TCF25 and SOX15) and adaptation (MC1R, KDR, and KIT), suggesting potential genetic adaptations to local environmental conditions. Our results contribute to the understanding of the genetic architecture of different goat breeds and may provide valuable information for effective preservation and breeding programs of local goat breeds in Egypt.
Similar content being viewed by others

Introduction
Goat (Capra hircus) breeding is of high relevance in several countries because of their high adaptability to diverse environmental conditions compared to other livestock species [1]. Egypt has about 3.4 million live goats [1] that are mainly raised for meat production with minor importance for milk production (except in the coastal regions and oases) [2]. They constitute about 6% of the total red meat production in Egypt, in addition to socioeconomic impacts in rural areas as cash resources when needed [2]. Of all local breeds, Egyptian Nubian (also known as Zaraibi) has the highest potential for prolificacy and milk production [3] and is one of the progenitors of the Anglo-Nubian British breed [4]. Barki is a popular breed in the western desert of Egypt, while the other breeds are mainly present in the Nile Delta [5]. Damascus goat is considered one of the best dual-purpose breeds in the middle east, with high production performance [6] and it was introduced to Egypt more than 50 years ago to improve the productivity of local breeds by crossbreeding [2]. Besides, Boer goat is a transboundary breed that has been introduced to several countries worldwide, including Egypt, to improve the productivity of local breeds. Accordingly, it can by hypothesized that the genomes of Egyptian Nubian, Damascus and Boer goats have evolved for high production and reproductive performance under intensive breeding systems, while Barki has evolved to survive under harsh environmental conditions in desert regions [5].
In Egypt, Barki, Damascus, and Boer goat breeds are reared for meat production, while the Egyptian Nubian is usually reared for milk production [2]. Notably, the Egyptian Nubian has a wide range of coat colors, while Damascus and Barki have reddish brown and black coat colors, respectively. Boer goats are usually found in two colors, white body with reddish brown neck or completely reddish-brown. Pictures illustrating each breed included in the study are presented in Supplementary Figure S1.
Recently, the availability of genomic resources for this species as well as the development of high throughput genotyping tools allowed the exploration of the genomic architecture of local breeds for conservation and breeding purposes [7,8,9]. Concerns about local breeds arise from their relatively small population size and lack of proper breeding plans. Thus, mating of related individuals (i.e. inbreeding) increases, leading to a reduction in fitness due to increased homozygosity, which is referred to as inbreeding depression [7]. Therefore, several genome-wide approaches have been proposed to detect inbreeding in different goat breeds, such as the calculation of the genomic inbreeding coefficient (FROH) [8,9,10].
Domestication and selective breeding are main factors for the high diversity of goat breeds, which left genomic footprints called “selection signatures” [11] with particular phenotypes of various economic importance [12]. Selection signatures can be defined as the reduction, elimination or change of genetic variation in genomic regions that are adjacent to causative variants in response to natural or artificial selection pressures [13]. Such variants usually affect several traits and contribute to the shaping of a breed [14,15,16]. Recently, two approaches have been widely used to detect signatures of selection in several goat breeds: Runs of homozygosity (ROH) and Fixation index (FST) [5, 8,9,10]. Runs of homozygosity are long genomic stretches with a homozygous genotype that arise when two haplotypes share a recent common ancestor [16] and in an individual that has undergone the selection process [17, 18]. Runs of homozygosity provide useful population-level information on inbreeding characteristics and locations of selection signatures [19]. FST is a measurement of the differences in allele frequencies between populations [20]. The selection usually increases the frequency of a particular allele in one breed, thereby increasing the heterozygosity of certain loci in a population leading to higher genetic differentiation between breeds [21]. Notably, FST is more suitable for detecting selective events in the distant past, whereas ROH can detect recent selection signatures [22].
Detection of such important genome features in local breeds is important to understand their adaptation ability and to reduce inbreeding [23]. This has been widely applied using SNP arrays in cattle [18, 21, 22, 24, 25], buffaloes [26, 27], sheep [28] and goats [29, 30]. Detection approaches rely on screening the genomes for regions of homozygosity and estimating differences in allele or haplotype frequency between populations [23, 30]. So far, such information is still limited for local goat breeds in Egypt. Only one study conducted by Kim et al. [5] reported that the FROH and the average lengths of ROH were lower in Barki goats compared with exotic breeds, such as Boer. Moreover, higher proportion of individuals lacking long stretches of ROH was observed in Barki compared with the non-native breeds. Therefore, the objective of this study was to evaluate FROH and to detect signatures of selection through ROH analyses and FST-based comparison of Egyptian Nubian, Damascus, Barki and Boer goat breeds, using SNP arrays. This will allow a better understanding of the genomic landscape and the evolutionary history of these breeds, which may be beneficial for future goat breeding programs.
Materials and methods
Study area and sample collection
All animal procedures included in the current study were approved by the Animal Breeding Ethics Committee at the Desert Research Center (DRC) in Egypt (Project ID: 43,213) with approval reference number AB/NO2020. Additionally, an informed consent was granted for Boer animals from the owners for the inclusion of Boer goats in the study.
Four goat breeds were used in this study: Egyptian Nubian (n = 235), Damascus (n = 95), Barki (n = 28), and Boer (n = 26). The Egyptian Nubian, Damascus, and Barki animals belong to Serw, Maryout, and Siwa Research Farms, respectively, and the Boer animals were obtained from a commercial private farm in the North of Egypt (Fig. 1). Blood samples were collected from the jugular vein of each animal included in the study using vacutainer tubes containing EDTA. Samples were transferred in an icebox to the Molecular Genetics laboratory, Desert Research Center in Cairo for DNA extraction. Genomic DNA was extracted using a QIAamp DNA Blood Mini Kit provided by Qiagen® (Germany) following the manufacturer’s protocol.
Genotyping, quality control and filtering
The quantity and quality of extracted DNA was assessed using the Nanodrop spectrophotometer. High-quality DNA samples (≥ 50 ng/µL) were genotyped at the Research Institute for Farm Animal Biology, Dummerstorf, Germany, using the Illumina®inc. Goat_IGGC_65K_v2 Infinium HD SNP chip (Illumina, San Diego, CA, USA). SNP locations reported in this paper are based on the genome version of Capra hircus available from the National Center for Biotechnology Information (ARS1.2, NCBI). The genotyping BeadChip contained 59,727 SNPs in total, evenly distributed throughout the caprine genome. Genotype calling was performed using GenomeStudio software (Illumina Inc., San Diego, CA, USA) according to the manufacturer’s protocols.
The whole dataset containing all genotyped individuals was filtered for quality control (QC) with PLINK v1.9 software [31], using the following parameters: (i) genotype call rate < 0.99 for markers and < 90% for individuals; (ii) SNPs located on unknown or the same chromosomal positions and SNPs on sex chromosomes were also excluded from the subsequent analyses. Deviation from Hardy–Weinberg Equilibrium (HWE) was not applied here as a filtration criterion because inbreeding is a possible reason for deviation, which may influence the analysis [32]. Linkage Disequilibrium (LD) pruning was not applied in this study because LD is related to various evolutionary forces (e.g. inbreeding, artificial and natural selection), which are investigated by the ROH analysis [33]. Likewise, the MAF filtering was not implemented in the current study to avoid ignoring large numbers of homozygous regions [34].
Principal component analysis (PCA) was performed using the common filtered SNPs using the ggplot2 package [35] implemented in RStudio software [36].
Runs of homozygosity (ROH) detection
ROH were identified in each goat using the consecutive runs method of the R package detectRUNS [37] with the main following parameters: (1) the minimum number of consecutive SNPs in an ROH was 20; (2) the minimum ROH length was set to 250 kb; (3) the maximum gap between consecutive homozygous SNPs was 1 Mb; (4) the maximum number of opposite genotypes in the run was set to 1; and (5) the maximum number of missing genotypes allowed was 1. The sum of ROH values per goat was calculated and ROH estimates were classified into five categories for each goat: 0–2 Mb, 2–4 Mb, 4–8 Mb, 8–16 Mb and above 16 Mb [33]. For each breed, the numbers of ROH length categories and the proportion of ROH on each autosome were calculated. The consecutive method was preferred over the sliding window approach in order to avoid the detection of artificial ROH shorter than the window described above (20 SNPs) [38]. The total number of ROH, the average number of ROH per individual, the average ROH length, the number of ROH per breed per chromosome, and the number of ROH per length class of length were estimated for each breed in the study.
Estimation of the genomic inbreeding coefficient (FROH)
The genomic inbreeding coefficient (FROH) is the estimation of the fraction of the genome in ROH, where identical-by-descent chromosome copies coalesce in a recent ancestor and ranges from 0 to 1 [39]. FROH was estimated as ROH-based inbreeding for each goat as follows [40]: FROH= LROH/Lauto, where LROH is the total length of ROHs in the genome of each individual and Lauto is the total size of the autosomes of goat genome covered by SNPs, which was 2.46 Gb [41].
Selection signatures
Selection signatures within each breed were investigated based on the estimation of the occurrences of ROH across the genome. The percentage of SNP occurrences (%) was calculated for each breed to identify the genomic regions with a high frequency of ROH (ROH hotspots) and were plotted against chromosomes in Manhattan plots using the qqman package [42] in R. In agreement with Jiang et al. [43], the threshold considered in the current study was 40% of ROH occurrence for each breed, including only the genomic regions that contained a minimum number of 20 consecutive SNPs.
Additionally, the pairwise comparisons between breeds were performed by calculating FST at each SNP. These values were calculated with the Weir and Cockerham FST [44] implemented in PLINK v1.9 [31].
Functional annotation, candidate genes and gene enrichment analysis
For each ROH region above the threshold, functional annotation of genes was obtained from BioMart at the Ensembl Genome Browser (https://www.ensembl.org/biomart/martview) [45]. Gene function and protein domain were identified by the UniProt and OMIA (Online Mendelian Inheritance in Animals) and the GeneCards databases [46]. Likewise, the top 10 SNPs with the highest FST values in all pairwise comparisons were investigated for annotated genes in the goat reference genome (ARS1.2) release 102, within a region spanning ± 0.5 Mb from each SNP [47, 48]. We explored the genes that overlapped with the identified genomic intervals to determine the functional categories that are over-represented and, therefore, are likely under selection in the studied Egypt goat breeds. ShinyGO v. 0.77 [49] software was used to perform the functional enrichment analysis based on gene ontology (GO) [50] and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways [51] against the goat gene set ontologies. The default parameters of the program were applied, and the results were adjusted to the false discovery rate (FDR < 0.05).
Results
Quality control filtering and population structure
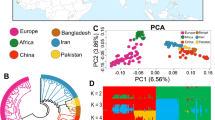
The filtering steps removed 47 animals and 13,459 SNPs, leaving 46,268 variants and 337 animals (210 Egyptian Nubian, 82 Damascus, 21 Barki and 24 Boer). In the PCA, the first and the second principal components (Fig. 2) accounted for 10.44% and 3.54% of the genetic variation across all individuals of the four breeds, respectively. The PCA revealed that the studied breeds were clearly distinguished, with each breed representing a distinct cluster. A few individuals that may share common haplotypes across Damascus and Boer breeds and Damascus and Barki breeds were observed. The Egyptian Nubian individuals were isolated in a separate cluster.
A principal component analysis (PCA) plot representing the genetic landscape of the studied goat population extended across the first and second components (PC1 and PC2) derived from eigenvectors and eigenvalues obtained from eigen decomposition of a genotypic (co)variance matrix between all individuals. The four breeds are presented in different colours, and each point represents one sample
ROH statistics and FROH estimation
The total number of ROH segments detected in Egyptian Nubian, Damascus, Barki and Boer were 30,544, 3446, 1846, and 3047 with an average number of 145.44, 42.02, 87.9, and 126.95, per individual, respectively. Mean ROH number and length in the studied goat population is presented in Table 1. Considering the length category distribution (Fig. 3), the shortest ROH segments (0–2 Mb) were the most common in all studied breeds. The percentage of ROH segments length less than 2 Mb were 71%, 64%, 96% and 63% in Egyptian Nubian, Damascus, Barki and Boer, respectively. The distribution of the 2–4 and 4–8 Mb ROH length classes were similar across the studied breeds. The frequencies of the long ROH segments (8–16 Mb) were found in Barki and Boer, while this class was absent in Damascus breed. The longest ROH segments (> 16 Mb) were only present in the Egyptian Nubian breed (0.01%). The ROH segments were identified in all breeds (Supplementary excel file), with mean accumulative lengths ranging from 46.5 Mb in Damascus to 306.0 Mb in Egyptian Nubian breed (Table 1). The maximum individual length of an ROH segment (16.33 Mb) and the maximum number of SNPs on a segment were found in Egyptian Nubian goats (n = 346 SNPs), while the Damascus breed displayed the lowest maximum values (4.1 Mb and 113 SNPs). Likewise, the mean accumulative ROH lengths were 154.4 and 261.7 in Barki and Boer, respectively. The individual patterns of ROH segment were similar in the Egyptian Nubian breed, while Barki and Boer showed a large individual variation in both ROH number and genomic coverage (Fig. 4).
The average values of FROH were 0.034, 0.057, 0.11 and 0.12 for Barki, Damascus, Boer and Egyptian Nubian breeds, respectively. The highest individual levels of FROH were found in Damascus (FROH = 0.36), Boer (FROH = 0.26), Barki (FROH = 0.18), and Egyptian Nubian breed (FROH = 0.23), while the lowest individual level of FROH were close to 0 and found in Boer goat (FROH = 0.0009). Inbreeding per chromosome estimated from the proportion of the chromosome covered by ROH for the 29 autosomes in the studied goat population is presented in Fig. 5. Across the studied goat population, chromosome 6 showed the highest FROH value (> 0.2) in Egyptian Nubian followed by chromosomes 9 and 26 in Barki (FROH ~ 0.2) and chromosome 25 in Boer (FROH <0.2), while chromosome 18 showed the lowest FROH value (< 0.05) in Damascus breed.
ROH hotspot, functional annotation, candidate genes and gene enrichment analysis
The percentage of SNP occurrences was calculated for each breed to identify the genomic regions with a high frequency of ROH (ROH hotspots), potentially important for selection and/or conservation and plotted against the position of the SNP across autosomes. Manhattan plots of the distribution of ROH in the four goat breeds (Egyptian Nubian, Damascus, Barki and Boer) showed the frequency (%) at which each SNP was observed in ROH across individuals (Fig. 6). Chromosome 18 had the highest peak in Egyptian Nubian, Barki and Boer breeds, but also chromosomes 6 showed high ROH occurrence in Damascus, Egyptian Nubian and Boer goats. Chromosomes 12, 13 and 19 presented high ROH peaks in one or more of the studied breeds. Applying the abovementioned threshold of 40%, 21 ROH hotspots were detected in total across all breeds (Table 2); the highest number of genomic regions identified was found in Egyptian Nubian breed with 11 ROH hotspots, followed by Boer, Barki and Damascus breed with 7, 2 and 1 ROH hotspots, respectively. The longest ROHs hotspots were found in Boer (1.5 Mb), followed by Egyptian Nubian (1.4 and 1.3 Mb), and Barki (1 Mb), while the shortest ROH was found in Damascus (~ 3 Kb). Genomic regions on Chromosome 18 (at 60 and 15 Mb) and Chromosome 6 (at 70 and 71 Mb) were found common in two or more breeds. The number of SNPs within these common regions ranged from 15 (Barki) to 22 (Boer) and from 6 (Damascus) to 37 (Egyptian Nubian) for the corresponding chromosomes, respectively.
The gene enrichment analysis identified 39 significantly enriched (FDR < 0.05) biological pathways using a list of the identified candidate genes in the current study (Supplementary excel file). The identified candidate genes were significantly enriched in biological pathways related to immunity and regulation of the immune response (Fig. 7); for instance, the positive regulation of type 2 immune response (FDR = 0.046) and pigmentation (FDR = 0.045).
Genetic differentiation between breeds
The highest average FST between pairs of the breeds was 0.14 for Egyptian Nubian and Boer followed by 0.077 for Egyptian Nubian and Barki, 0.075 for Nubian and Damascus, 0.071 for Barki and Boer, 0.064 for Damascus and Boer, and the lowest was 0.015 for Damascus and Barki (Table 3). The SNP-specific FST values were further plotted against the autosomes of goat (Supplementary Figure S2). Analysis of breed specific differentiation between each pair of goat breeds included in the study resulted in several putative regions of selection and are presented in the Supplementary excel file. The top 10 SNPs that accounted for highest FST values between breeds in each of the pairwise comparisons are presented in Supplementary Tables S1, S2, S3, S4, S5 and S6. The SNP that accounted for the highest genetic differentiation (FST = 0.96) value was observed on chromosome 6 at 13.9 Mb (Nubian vs. Boer) while the SNP that accounted for the lowest genetic differentiation (FST = 0.35) was observed on chromosome 6 at 14.1 Mb (Damascus vs. Barki). Multiple overlapping SNPs residing in multiple ROH hotspots and FST genomic windows on two autosomes (chromosomes 6 and 18) was detected in the studied goat breeds.
Discussion
Considering the native habitat of Barki breed in the Egyptian desert, the breed was expected to cluster independently from the other breeds that predominate in the Nile Delta. Nonetheless, they have partially clustered together because of breeders’ efforts to improve growth and milk performance of Barki by crossing with Damascus [6]. The PCA results showed that Damascus and Boer goats are genetically connected, indicating gene flows between these breeds, in contrast to the Egyptian Nubian that was genetically distinct and has a separate genetic structure due to the application of specific breeding programs [52]. Boer goats were shown to be quite scattered in PCA plot despite implementing some sort of breeding programs. We speculate that this may be because those samples were taken from private farms, in which breeders apply uncontrolled crossing with Damascus, which has quite similar color and body characteristics as Boer. However, PCA clearly classified the four breeds based on SNPs information into distinct clusters. In agreement, Barki and Boer goats were differentiated in the PCA plot from the non-native breeds in independent clusters [5].
The average estimate of inbreeding measured by homozygosity (FROH) showed higher values (0.12 and 0.11) for the breeds under intensive selection of important traits (Egyptian Nubian and Boer, respectively), compared to low values (0.05 and 0.03) for breeds that are not managed under specific breeding programs (Damascus and Barki, respectively) [53]. This shows that maintaining the local breeds with low population size is possible through adopting a breeding scheme that aim at less intensive selection for production traits [54], allowing a higher diversity in their genomes. The highest individual FROH estimate in the current study (FROH = 0.36) was higher than that reported in local Russian goat (FROH = 0.27). Likewise, the average FROH in the current study (FROH = 0.03 to 0.12) were higher than those reported in local Russian goats (FROH = 0.033 to 0.077) [55]. The average FROH in Nubian and Boer are lower than that estimated in Chinese goats (FROH = 0.13) [32] and consistent with those estimated at the worldwide scale in the global (FROH = 0.12) goat breeds [30]. Likewise, the average FROH in Barki (0.03) and Boer (0.11) in the current study were higher than those reported previously [5] for the same breeds (0.03 vs. 0.02 and 0.11 vs. 0.09), respectively. This may indicate that Damascus and Barki goats have higher genetic variability than Egyptian Nubian, Boer and the global goat breeds and therefore, have a greater chance to be conserved as a genetic resource [32].
The length of ROH regions is an important aspect of ROH as the longer ROHs refer to more recent inbreeding or artificial selection [7, 8], while shorter ROHs indicate ancient inbreeding [56]. The longest ROH category (> 16 Mb) was only present in Nubian goat, which indicate recent inbreeding and possible higher incidence of inbreeding depression in the future [56]. On the contrary, Damascus breed had the shortest mean length of ROH in their genomes. Interestingly, the shortest ROH segments were most common Boer goats in agreement with other studies of goats from Uganda [8], Russia [55], China [32] and Italy [57]. Furthermore, Kim et al. [5] reported larger ROHs length in Boer compared with Barki goats. There, the high frequency of shorter ROH segments in the breed genome was attributed to the presence of ancestral family relatedness [55].
The total number of ROH estimated in this study, which ranged from 30,544 (Egyptian Nubian) to 1846 (Barki) was lower than those detected in GX (n = 44,422) and GF (n = 16,598) Chinese goat breeds [32], while it was comparable to those identified in the Italian goat breeds (n = 28,383) [57]. Likewise, the average ROH number ranged from 145.44 (Nubian) to 42.02 (Damascus) and was lower than those detected in the corresponding Chinese breeds (n = 277.60 and 207.50), respectively [32]. In contrary, this number was higher than those reported in Italian goat breeds (n = 3.02–38.83) [58]. The analysis of patterns of homozygosity provides insight into demographic history [55]. The average accumulative ROH length estimated here in Egyptian Nubian (306 Mb) and Boer (261.7 Mb) were higher than those estimated in Turkish breeds (210.64 Mb), Central Asian breeds (260.64 Mb) [32], and Russian local breeds (79.42–183.94 Mb) [55].
The average ROH number estimated in the current study in Damascus (42.02 ROH) was lower than those in Turkish breeds (60 ROH) and Central Asian breeds (90 ROH) [30] [32], but higher than those detected in Russian local breeds (18–41 ROH) [55]. However, our study reported higher ROH numbers in Egyptian Nubian (145.44 ROH) and Boer (126.95 ROH) than those reported in the previous studies [59]. Moreover, Kim et al. [5] reported slightly shorter mean ROH length in Barki (1.7 vs. 1.48 Mb) and longer in Boer goats (2.06 vs. 10.61 Mb).
The studied breeds are dual-purpose breeds for dairy and meat production, so it was expected that the identified differentiated regions across breeds may contain more genes associated with such traits. However, most of the identified genes were related to immune response, adaptation and reproduction, which may imply that these animals tended to be selected for their higher adaptation performance as they live in extremely hard environmental condition characterized by shortage of feed supply and water resources, especially in desert [5, 8].
Within the identified top ROH hotspots, five genes were detected (CHR18, 15.73 Mb): SPG7 (SPG7 Matrix AAA Peptidase Subunit, Paraplegin) and CPNE7 (Copine 7) genes are playing a role in diverse cellular processes including membrane trafficking and intracellular motility [60, 61]; the SPATA2L (Spermatogenesis Associated 2 Like) gene is involved in sperm formation [62]; TCF25 is a member of the basic helix-loop-helix (bHLH) family of transcription factors that are important in embryonic development and cell death control [63]; and the MC1R (Melanocortin1 Receptor) gene is known to be involved in pigmentation and hair color [64].
Another important ROH hotspot was detected on CHR19 at 26.67 Mb, which harbored candidate genes involved in (1) immunity: PHF23 (PHD Finger Protein 23), GABARAP (GABA Type A Receptor-Associated Protein), CD68 (CD68 Molecule) and GPS2 (G Protein Pathway Suppressor2) [65,66,67,68], (2) sperm formation and reproduction: TMEM95 (Transmembrane Protein 95), SPEM1 (Spermatid Maturation 1) [69, 70], and (3) embryonic development, cell growth, morphogenesis, tissue repair and muscle growth: FGF11 (Fibroblast Growth Factor 11) and SOX15 (SRY-Box Transcription Factor 15) genes [71, 72].
The detection of genetic markers that influence reproduction traits (e.g., litter size) in goats has taken a lot of interest. Two candidate gene were detected on CHR6 at 70.74 and 71.14 Mb: (1) the KIT (KIT Proto-Oncogene, Receptor Tyrosine Kinase) gene, which plays an essential role in the regulation of cell survival and proliferation, hematopoiesis, stem cell maintenance, gametogenesis, mast cell development, migration and function, melanogenesis [73], and a possible pleotropic role in reproductive performance in livestock. The KIT and its ligand (i.e., KITLG), are two well-known pigmentation genes in livestock [52, 74], and selection signals have been detected in many different goat [75] and sheep populations [76]. Interestingly, the same gene was identified to differentiate between the Egyptian Nubian and Boer (FST = 0.89), which may explain their difference in the dominant hair colors. In addition to (2) the KDR (Kinase Insert Domain Receptor) gene is important in survival, migration and morphogenesis [77]. The KDR gene identified here in Egyptian Nubian and Damascus breeds was previously reported in wild and domestic sheep [28] and was associated with proliferation, survival, migration, and tubular morphogenesis in mammals.
Importantly, the same candidate genes and genomic regions on CHR6 (70.74 Mb, KIT) and CHR18 (15.73 Mb, SPG) were recently identified by whole genome sequencing as top ROH hotspots in Jianchang Black goats [52]. Furthermore, the RNF17 (Ring Finger Protein 17) gene that encodes a testis-specific protein containing a RING finger domain and may be involved in spermiogenesis [78] was detected on CHR12 (50.04 Mb).
Two candidate genes that are playing a role in immune regulation were identified on CHR13 (41.73 Mb): CST3 (Cystatin C) and CST7 (Cystatin F) [79, 80]. Additionally, two candidate genes were identified on CHR6 (13.49 Mb): ALPK1 (Alpha Kinase 1) and TIFA (TRAF Interacting Protein with Forkhead Associated Domain), which are involved in the innate immune response, adaptive immunity and DNA damage response [81]. The 44.42 Mb on CHR6 harbors the DHX15 (DEAH-Box Helicase 15) and PI4K2B (Phosphatidylinositol 4-Kinase Type 2 Beta) genes, which play a key role in antiviral innate immunity [82] and early T cell activation [83], respectively.
A considerable overlap of selection signatures was observed between results of ROH and FST approaches implemented in the current study. The common variants from both approaches were observed on CHR6 (at 13.49, 34.13, and 70.74 Mb) and CHR18 (at 15.73 and 60.12 Mb). The region on CHR6 at 13.49 Mb (FST = 0.74) harbored two immunity-relate genes: TIFA (TRAF Interacting Protein with Forkhead Associated Domain) and ALPK1 (Alpha Kinase 1). The region on CHR6 at 44.42 Mb (FST = 0.69) harbored two potential genes, PI4K2B (Phosphatidylinositol 4-Kinase Type 2 Beta) and CCK1 (cholecystokinin1 receptor) that are involved in the regulation of vesicular trafficking [84] and food intake [85], respectively. The same region was reported by Kim et al. [5] in multiple goat breeds including Barki and Boer.
The FST value estimated in the pairwise comparison of Egyptian Nubian vs. Barki (FST = 0.077) was lower than reported by Agha et al. [86] using microsatellite markers (FST = 0.17). The high overlap observed between FST and ROH results suggests that the selection on genes related to immunity and adaptation is not breed-specific [8]. Multiple overlapping SNPs residing in multiple ROH hotspots and FST genomic windows on two autosomes (CHR6 and 18) between the studied goat populations were detected. Interestingly, one ROH hotspot on CHR12 (at 50.04 Mb) was common in the global goat breeds [30], which harbored genes related to vision (GJA3) and hearing (GJB2 and GJB6) and are essential for adaptive evolution in goats [32].
The pairwise differentiation identified genetic variants harboring candidate genes that may control the breed-specific traits. For instance, three potential candidate genes appeared to differentiate between Damascus and Boer located at 31.98 Mb on CHR29 (FST = 0.68): (1)ETS1 (ETS Proto-Oncogene1), which is involved in stem cell development and death and the differentiation, survival and proliferation of lymphoid cells [87], (2)IGSF9B (Immunoglobulin Superfamily Member 9B), which is involved in immune response [88], and (3)SPATA19 (Spermatogenesis Associated 19), which plays an important role in sperm motility and correct sperm midpiece assembly [89].
The region at 36.49 Mb on CHR6 harbored three candidate genes to differentiate between Egyptian Nubian and Barki (FST = 0.68) and Nubian and Boer (FST = 0.95): (1)HERC5 (HECT And RLD Domain Containing E3 Ubiquitin Protein Ligase 5), which is important in the antiviral immune response [90], (2)PPM1K (Protein Phosphatase, Mg2+/Mn2 + Dependent 1 K), which is essential for cellular survival and development [91], and (3)SPP1 (Secreted Phosphoprotein 1), which acts as a cytokine involved in enhancing production of interferon-gamma and interleukin-12 and reducing production of interleukin-10 [92].
Results of GO and KEGG for the list of candidate genes identified in the current study support the assumption that pathways associated with adaptation mechanisms to harsh environmental conditions, such as regulation of immune response may be under positive selection in Egyptian goat breeds. These findings agree with previous reports in Egyptian [5] and Ugandan [8] goat breeds and indicates that adaptation to local environmental conditions requires a complex network of multiple genes [93], which is an important feature of indigenous breeds to increase their adaptation capacity.
Summarizing, a detailed picture of the genetic structure and the ROH landscape of Egyptian Nubian, Barki, Damascus, and Boer goat breeds was given using SNP markers. The investigated animals came from different breeding systems and geographical backgrounds, in which different selection processes are applied. Additionally, the ROH hotspots revealed that many candidate genes are putatively under selection for adaptation, immunity, growth and reproduction in the investigated breeds, which is compatible with our previous rationale hypothesis.
Conclusion
Multiple signatures of selection were detected in four goat breeds in Egypt using runs of homozygosity (ROH) and fixation index (FST) approaches. The identified chromosomal regions harbor candidate genes that encode proteins involved in the innate and adaptive immunity, vesicular trafficking, feed intake, sperm motility, cell development and embryonic growth and development, and adaptation. This implies that the genomes of Egyptian local goat breeds are shaped by adaptation to hard arid environmental conditions. Our results revealed recent genomic inbreeding in the local Egyptian Nubian goat, probably due to closed breeding in small populations, with possible inbreeding depression in the future. To protect this precious goat germplasm resource in Egypt, more scientific conservation strategies and efficient management systems should be established. Our findings provided a better understanding of the genomic landscape and evolutionary history of local Egyptian goat breeds and will benefit future breeding programs in local goat.
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the OSF repository using the following link: https://osf.io/7q6j8/ and can be downloaded upon request from the corresponding author (ahmedsallam2@gmail.com).
References
FAOSTAT. FAOSTAT. Food and Agriculture Organization of the United Nations. 2019. https://www.fao.org/faostat/en/#data/QCL.
Galal S, Abdel-Rasoul F, Shaat I, Anous M. On-station characterization of small ruminant breeds in Egypt. Characterization of Small Ruminant Breeds in West Asia and North Africa. Luis Inigez, editor. Aleppo; 2005. https://scholar.google.com/citations?view_op=view_citation&hl=en&user=FMLU8PMAAAAJ&citation_for_view=FMLU8PMAAAAJ:2osOgNQ5qMEC.
Alsheikh SM. Influence of age and live body weight on daily milk yield of Zaraibi and Shami goats in Sinai, Egypt. Annals of Agricultural Sciences. 2013;58(1):1–3. https://doi.org/10.1016/j.aoas.2013.01.001.
Latif MGA, Abdelsalam MM, Abd El-Aziz NM. Meat production characteristics of egyptian Baladi and Angora goats. Meat Sci. 1987;20(3):211–6. https://doi.org/10.1016/0309-1740(87)90012-X.
Kim E-S, Elbeltagy AR, Aboul-Naga AM, Rischkowsky B, Sayre B, Mwacharo JM, et al. Multiple genomic signatures of selection in goats and sheep indigenous to a hot arid environment. Heredity (Edinb). 2016;116:255–64.
Nowier AM, Darwish HR, Ramadan SI, Othman OE. Polymorphism of lactoferrin gene in egyptian goats and its association with milk composition traits in Zaraibi breed. Trop Anim Health Prod. 2020;52(3):1065–71. https://doi.org/10.1007/s11250-019-02099-3.
Hedrick PW, Garcia-Dorado A. Understanding Inbreeding Depression, purging, and genetic rescue. Trends Ecol Evol. 2016;31(12):940–52. https://doi.org/10.1016/j.tree.2016.09.005.
Onzima RB, Upadhyay MR, Doekes HP, Brito LF, Bosse M, Kanis E, Onzima RB, Upadhyay MR, Doekes HP, Brito LF, Bosse M, Kanis E, Groenen MAM, Crooijmans RPMA. Genome-wide characterization of selection signatures and runs of homozygosity in Ugandan Goat Breeds. Front Genet. 2018;9:9. https://doi.org/10.3389/fgene.2018.00318.
Rodríguez-Ramilo ST, Elsen JM, Legarra A. Inbreeding and effective population size in french dairy sheep: comparison between genomic and pedigree estimates. J Dairy Sci. 2019;102(5):4227–37. https://doi.org/10.3168/jds.2018-15405.
Cortellari M, Negro A, Bionda A, Grande S, Cesarani A, Carta A, et al. Using pedigree and genomic data toward Better Management of Inbreeding in italian dairy Sheep and Goat Breeds. Animals. 2022;12:2828.
Randhawa IAS, Khatkar MS, Thomson PC, Raadsma HW. A meta-assembly of selection signatures in cattle. PLoS One. 2016;11:e0153013-. https://doi.org/10.1371/journal.pone.0153013.
Almeida OAC, Moreira GCM, Rezende FM, Boschiero C, de Oliveira Peixoto J, Ibelli AMG, Almeida OAC, Moreira GCM, Rezende FM, Boschiero C, de Oliveira Peixoto J, Ibelli AMG, Ledur MC, de Novais FJ, Coutinho LL. Identification of selection signatures involved in performance traits in a paternal broiler line. BMC Genomics. 2019;20(1):449. https://doi.org/10.1186/s12864-019-5811-1.
Sabeti PC, Schaffner SF, Fry B, Lohmueller J, Varilly P, Shamovsky O, et al. Positive natural selection in the human lineage. Science. 1979;2006(312):1614–20.
Qanbari S, Simianer H. Mapping signatures of positive selection in the genome of livestock. Livest Sci. 2014;166:133–43.
Saravanan K, Panigrahi M, Kumar H, Bhushan B, Dutt T, Mishra BP. Selection signatures in livestock genome: a review of concepts, approaches and applications. Livest Sci. 2020;241: 104257.
Ferenčaković M, Sölkner J, Curik I. Estimating autozygosity from high-throughput information: effects of SNP density and genotyping errors. Genet Selection Evol. 2013;45: 42.
Xie R, Liu S, Deng T, Wang L, et al. Genome-wide scan for runs of homozygosity identifies candidate genes in three Pig Breeds. Animals. 2019;9:518.
Forutan M, Ansari Mahyari S, Baes C, Melzer N, Schenkel FS, Sargolzaei M. Inbreeding and runs of homozygosity before and after genomic selection in North American Holstein cattle. BMC Genomics. 2018;19(1):98. https://doi.org/10.1186/s12864-018-4453-z.
Colpitts J, McLoughlin PD, Poissant J. Runs of homozygosity in Sable Island feral horses reveal the genomic consequences of inbreeding and divergence from domestic breeds. BMC Genomics. 2022;23:501. https://doi.org/10.1186/s12864-022-08729-9.
Wright S. The genetical structure of populations. Ann Eugen. 1949;15:323–54.
Makina SO, Muchadeyi FC, van Marle-Köster E, Taylor JF, Makgahlela ML, Maiwashe A. Genome-wide scan for selection signatures in six cattle breeds in South Africa. Genet Selection Evol. 2015;47:92.
Maiorano AM, Lourenco DL, Tsuruta S, Ospina AMT, Stafuzza NB, Masuda Y, Maiorano AM, Lourenco DL, Tsuruta S, Ospina AMT, Stafuzza NB, Masuda Y, Filho AEV, Cyrillo JNDSG, Curi RA, Silva JAIIdV. Assessing genetic architecture and signatures of selection of dual purpose Gir cattle populations using genomic information. PLoS ONE. 2018;13(8): e0200694. https://doi.org/10.1371/journal.pone.0200694.
Eydivandi S, Roudbar MA, Karimi MO, Sahana G. Genomic scans for selective sweeps through haplotype homozygosity and allelic fixation in 14 indigenous sheep breeds from Middle East and South Asia. Sci Rep. 2021;11:2834. https://doi.org/10.1038/s41598-021-82625-2.
Zhao F, McParland S, Kearney F, Du L, Berry DP. Detection of selection signatures in dairy and beef cattle using high-density genomic information. Genet Selection Evol. 2015;47:49.
Freitas PHF, Wang Y, Yan P, Oliveira HR, Schenkel FS, Zhang Y, Freitas PHF, Wang Y, Yan P, Oliveira HR, Schenkel FS, Zhang Yi, Xu Q, Brito LF. Genetic diversity and signatures of selection for thermal stress in cattle and other two bos species adapted to divergent climatic conditions. Front Genet. 2021;12:12. https://doi.org/10.3389/fgene.2021.604823.
Deng TX, Ma XY, Lu XR, Duan AQ, Shokrollahi B, Shang JH. Signatures of selection reveal candidate genes involved in production traits in chinese crossbred buffaloes. J Dairy Sci. 2022;105(2):1327–37. https://doi.org/10.3168/jds.2021-21102.
Mokhber M, Moradi-Shahrbabak M, Sadeghi M, Moradi-Shahrbabak H, Stella A, Nicolzzi E, et al. A genome-wide scan for signatures of selection in Azeri and Khuzestani buffalo breeds. BMC Genomics. 2018;19:449. https://doi.org/10.1186/s12864-018-4759-x.
Li X, Yang J, Shen M, Xie X-L, Liu G-J, Xu Y-X, Li X, Yang Ji, Shen M, Xie X-L, Liu G-J, Xu Y-X, Lv F-H, Yang H, Yang Y-L, Liu C-B, Zhou P, Wan P-C, Zhang Y-S, Gao L, Yang J-Q, Pi W-H, Ren Y-L, Shen Z-Q, Wang F, Deng J, Xu S-S, Salehian-Dehkordi H, Hehua E, Esmailizadeh A, Dehghani-Qanatqestani M, Štěpánek O, Weimann C, Erhardt G, Amane A, Mwacharo JM, Han J-L, Hanotte O, Lenstra JA, Kantanen J, Coltman DW, Kijas JW, Bruford MW, Periasamy K, Wang X-H, Li M-H. Whole-genome resequencing of wild and domestic sheep identifies genes associated with morphological and agronomic traits. Nat Commun. 2020;11(1):2815. https://doi.org/10.1038/s41467-020-16485-1.
Henkel J, Saif R, Jagannathan V, Schmocker C, Zeindler F, Bangerter E, et al. Selection signatures in goats reveal copy number variants underlying breed-defining coat color phenotypes. PLoS Genet. 2019;15:e1008536-. https://doi.org/10.1371/journal.pgen.1008536.
Bertolini F, Cardoso TF, Marras G, Nicolazzi EL, Rothschild MF, Amills M, et al. Genome-wide patterns of homozygosity provide clues about the population history and adaptation of goats. Genetics Selection Evolution. 2018;50:59. https://doi.org/10.1186/s12711-018-0424-8.
Chang CC, Chow CC, Tellier LCAM, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:s13742-015-0047–8. https://doi.org/10.1186/s13742-015-0047-8.
Li G, Tang J, Huang J, Jiang Y, Fan Y, Wang X et al. Genome-Wide Estimates of Runs of Homozygosity, Heterozygosity, and Genetic Load in Two Chinese Indigenous Goat Breeds. Front Genet [Internet]. 2022;13. Available from: https://www.frontiersin.org/articles/https://doi.org/10.3389/fgene.2022.774196.
Dixit SP, Singh S, Ganguly I, Bhatia AK, Sharma A, Kumar NA, Dixit SP, Singh S, Ganguly I, Bhatia AK, Sharma A, Kumar NA, Dang AK, Jayakumar S. Genome-wide runs of homozygosity revealed selection signatures in Bos indicus. Front Genet. 2020;11:11. https://doi.org/10.3389/fgene.2020.00092.
Schiavo G, Bovo S, Bertolini F, Tinarelli S, Dall’Olio S, Nanni Costa L, Schiavo G, Bovo S, Bertolini F, Tinarelli S, Dall’Olio S, Nanni Costa L, Gallo M, Fontanesi L. Comparative evaluation of genomic inbreeding parameters in seven commercial and autochthonous pig breeds. Animal. 2020;14(5):910–20. https://doi.org/10.1017/S175173111900332X.
Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag; 2016.
RStudio Team. RStudio: Integrated Development for R. RStudio, PBC. URL http://www.rstudio.com/. Boston, MA.; 2020.
Biscarini F, Cozzi P, Gaspa G, Marras G, detectRUNS. Detect runs of homozygosity and runs of heterozygosity in diploid genomes. 2018. https://www.semanticscholar.org/paper/detectRUNS%3A-Detect-runs-of-homozygosity-and-runs-of-Biscarini-Cozzi/ccc3091c370ae99f7c1b56aa5521b80cd47a4258.
Fabbri MC, Dadousis C, Tiezzi F, Maltecca C, Lozada-Soto E, Biffani S, et al. Genetic diversity and population history of eight italian beef cattle breeds using measures of autozygosity. PLoS ONE. 2021;16:e0248087.
Kardos M, Nietlisbach P, Hedrick PW. How should we compare different genomic estimates of the strength of inbreeding depression? Proc Natl Acad Sci U S A. 2018;115(11):E2492–3.
McQuillan R, Leutenegger A-L, Abdel-Rahman R, Franklin CS, Pericic M, Barac-Lauc L, McQuillan R, Leutenegger A-L, Abdel-Rahman R, Franklin CS, Pericic M, Barac-Lauc L, Smolej-Narancic N, Janicijevic B, Polasek O, Tenesa A, MacLeod AK, Farrington SM, Rudan P, Hayward C, Vitart V, Rudan I, Wild SH, Dunlop MG, Wright AF, Campbell H, Wilson JF. Runs of homozygosity in european populations. Am J Hum Genet. 2008;83(3):359–72. https://doi.org/10.1016/j.ajhg.2008.08.007.
Cardoso TF, Amills M, Bertolini F, Rothschild M, Marras G, Boink G, et al. Patterns of homozygosity in insular and continental goat breeds. Genet Selection Evol. 2018;50:56.
Turner D. qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. J Open Source Softw. 2018;3: 731.
Jiang Y, Li X, Liu J, Zhang W, Zhou M, Wang J, Jiang Y, Li X, Liu J, Zhang W, Zhou M, Wang J, Liu L, Su S, Zhao F, Chen H, Wang C. Genome-wide detection of genetic structure and runs of homozygosity analysis in Anhui indigenous and western commercial pig breeds using PorcineSNP80k data. BMC Genomics. 2022;23(1):373. https://doi.org/10.1186/s12864-022-08583-9.
Weir BS, Cockerham CC. Estimating F-Statistics for the analysis of Population structure. Evolution (N Y). 1984;38:1358.
Kinsella RJ, Kähäri A, Haider S, Zamora J, Proctor G, Spudich G, et al. Ensembl BioMarts: a hub for data retrieval across taxonomic space. Database. 2011;2011:1–9.
Safran M, Rosen N, Twik M, BarShir R, Stein TI, Dahary D et al. The GeneCards Suite. In: Abugessaisa I, Kasukawa T, editors. Practical Guide to Life Science Databases. Singapore: Springer Nature Singapore; 2021. p. 27–56. https://doi.org/10.1007/978-981-16-5812-9_2.
Bickhart DM, Rosen BD, Koren S, Sayre BL, Hastie AR, Chan S, Bickhart DM, Rosen BD, Koren S, Sayre BL, Hastie AR, Chan S, Lee J, Lam ET, Liachko I, Sullivan ST, Burton JN, Huson HJ, Nystrom JC, Kelley CM, Hutchison JL, Zhou Y, Sun J, Crisà A, Ponce de León FA, Schwartz JC, Hammond JA, Waldbieser GC, Schroeder SG, Liu GE, Dunham MJ, Shendure J, Sonstegard TS, Phillippy AM, Van Tassell CP, Smith TPL. Single-molecule sequencing and chromatin conformation capture enable de novo reference assembly of the domestic goat genome. Nat Genet. 2017;49(4):643–50. https://doi.org/10.1038/ng.3802.
Han H, Yang M-M, Dan J, Chao S-Y, Zhang X-J, Wei Q et al. Selection signatures in goats reveal a novel deletion mutant underlying cashmere yield and diameter. Gigascience. 2022;1:1–14.
Ge SX, Jung D, Yao R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics. 2020;36(8):2628–9. https://doi.org/10.1093/bioinformatics/btz931.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25(1):25–9. https://doi.org/10.1038/75556.
Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51:D587-592.
Sun X, Guo J, Li L, Zhong T, Wang L, Zhan S, Sun X, Guo J, Li Li, Zhong T, Wang L, Zhan S, Lu J, Wang D, Dai D, Liu GE, Zhang H. Genetic diversity and selection signatures in Jianchang Black Goats revealed by whole-genome sequencing data. Animals. 2022;12(18): 2365. https://doi.org/10.3390/ani12182365.
Neumann GB, Korkuć P, Arends D, Wolf MJ, May K, König S, Neumann GB, Korkuć P, Arends D, Wolf MJ, May K, König S, Brockmann GA. Genomic diversity and relationship analyses of endangered german black Pied cattle (DSN) to 68 other taurine breeds based on whole-genome sequencing. Front Genet. 2023;13:13. https://doi.org/10.3389/fgene.2022.993959.
Gutiérrez-Reinoso MA, Aponte PM, García-Herreros M. A review of inbreeding depression in dairy cattle: current status, emerging control strategies, and future prospects. J Dairy Res. 2022;89(1):3–12. https://doi.org/10.1017/S0022029922000188.
Deniskova TE, Av D, Selionova MI, Reyer H, Sölkner J, Fornara MS, et al. SNP-Based genotyping provides insight into the west asian origin of russian local goats. Front Genet. 2021;12:708740.
Makanjuola BO, Maltecca C, Miglior F, Schenkel FS, Baes CF. Effect of recent and ancient inbreeding on production and fertility traits in canadian holsteins. BMC Genomics. 2020;21(1):605. https://doi.org/10.1186/s12864-020-07031-w.
Cortellari M, Bionda A, Negro A, Frattini S, Mastrangelo S, Somenzi E, et al. Runs of homozygosity in the italian goat breeds: impact of management practices in low-input systems. Genet Selection Evol. 2021;53:92.
Mastrangelo S, Di Gerlando R, Sardina MT, Sutera AM, Moscarelli A, Tolone M, Mastrangelo S, Di Gerlando R, Sardina MT, Sutera AM, Moscarelli A, Tolone M, Cortellari M, Marletta D, Crepaldi P, Portolano B. Genome-wide patterns of homozygosity reveal the Conservation Status in five italian Goat populations. Animals. 2021;11(6): 1510. https://doi.org/10.3390/ani11061510.
Brito LF, Kijas JW, Ventura RV, Sargolzaei M, Porto-Neto LR, Cánovas A, Brito LF, Kijas JW, Ventura RV, Sargolzaei M, Porto-Neto LR, Cánovas A, Feng Z, Jafarikia M, Schenkel FS. Genetic diversity and signatures of selection in various goat breeds revealed by genome-wide SNP markers. BMC Genomics. 2017;18(1):229. https://doi.org/10.1186/s12864-017-3610-0.
Giudice T, Lombardi F, Santorelli FM, Kawarai T, Orlacchio A. Hereditary spastic paraplegia: Clinical-genetic characteristics and evolving molecular mechanisms. Exp Neurol. 2014;261:518–39.
Tang H, Pang P, Qin Z, Zhao Z, Wu Q, Song S, Tang H, Pang P, Qin Z, Zhao Z, Wu Q, Song S, Li F. The CPNE Family and their role in cancers. Front Genet. 2021;12:12. https://doi.org/10.3389/fgene.2021.689097.
Zhao J, Zhao J, Xu G, Wang Z, Gao J, Cui S, Zhao J, Zhao J, Xu G, Wang Z, Gao J, Cui S, Liu J. Deletion of Spata2 by CRISPR/Cas9n causes increased inhibin alpha expression and attenuated fertility in male mice†. Biol Reprod. 2017;97(3):497–513. https://doi.org/10.1093/biolre/iox093.
Olsson M, Durbeej M, Ekblom P, Hjalt T. Nulp1, a novel basic helix-loop-helix protein expressed broadly during early embryonic organogenesis and prominently in developing dorsal root ganglia. Cell Tissue Res. 2002;308(3):361–70. https://doi.org/10.1007/s00441-002-0544-9.
Wolf Horrell EM, Boulanger MC, D’Orazio JA. Melanocortin 1 receptor: structure, function, and Regulation. Front Genet. 2016;7:7. https://doi.org/10.3389/fgene.2016.00095.
Chan S, Smith E, Gao Y, Kwan J, Blum BC, Tilston-Lunel AM, et al. Loss of G-Protein pathway suppressor 2 promotes Tumor Growth through activation of AKT Signaling. Front Cell. Dev Biol. 2021;8:608044.
Zhang J, Li S, Liu F, Yang K. Role of CD68 in tumor immunity and prognosis prediction in pan-cancer. Sci Rep. 2022;12(1):7844. https://doi.org/10.1038/s41598-022-11503-2.
Lee JH, Rho SB, Chun T. GABAA receptor-associated protein (GABARAP) induces apoptosis by interacting with DEAD (asp-Glu-Ala-Asp/His) box polypeptide 47 (DDX 47). Biotechnol Lett. 2005;27(9):623–8. https://doi.org/10.1007/s10529-005-3628-2.
Wang Z, Hu J, Li G, Qu L, He Q, Lou Y, Wang Z, Hu J, Li Ge, Qu L, He Q, Lou Y, Song Q, Ma D, Chen Y. PHF23 (plant homeodomain finger protein 23) negatively regulates cell autophagy by promoting ubiquitination and degradation of E3 ligase LRSAM1. Autophagy. 2014;10(12):2158–70. https://doi.org/10.4161/auto.36439.
Lamas-Toranzo I, Hamze JG, Bianchi E, Fernández-Fuertes B, Pérez-Cerezales S, Laguna-Barraza R, Lamas-Toranzo I, Hamze JG, Bianchi E, Fernández-Fuertes B, Pérez-Cerezales S, Laguna-Barraza R, Fernández-González R, Lonergan P, Gutiérrez-Adán A, Wright GJ, Jiménez-Movilla M, Bermejo-Álvarez P. TMEM95 is a sperm membrane protein essential for mammalian fertilization. Elife. 2020;9:9. https://doi.org/10.7554/eLife.53913.
Zheng H, Stratton CJ, Morozumi K, Jin J, Yanagimachi R, Yan W. Lack of Spem1 causes aberrant cytoplasm removal, sperm deformation, and male infertility. Proc Natl Acad Sci U S A. 2007;104:6852–7.
Yun Y-R, Won JE, Jeon E, Lee S, Kang W, Jo H, Yun Y-R, Won JE, Jeon E, Lee S, Kang W, Jo H, Jang J-H, Shin US, Kim H-W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J Tissue Eng. 2010;1(1): 218142. https://doi.org/10.4061/2010/218142.
Sarkar A, Hochedlinger K. The Sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12(1):15–30. https://doi.org/10.1016/j.stem.2012.12.007.
Lennartsson J, Jelacic T, Linnekin D, Shivakrupa R. Normal and oncogenic forms of the receptor tyrosine kinase kit. Stem Cells. 2005;23(1):16–43. https://doi.org/10.1634/stemcells.2004-0117.
Fontanesi L, D’Alessandro E, Scotti E, Liotta L, Crovetti A, Chiofalo V, et al. Genetic heterogeneity and selection signature at the KIT gene in pigs showing different coat colours and patterns. Anim Genet. 2010;41:478–92.
Guo J, Zhong J, Li L, Zhong T, Wang L, Song T, et al. Comparative genome analyses reveal the unique genetic composition and selection signals underlying the phenotypic characteristics of three chinese domestic goat breeds. Genet Selection Evol. 2019;51:70.
Kijas JW, Lenstra JA, Hayes B, Boitard S, Porto Neto LR, San Cristobal M, et al. Genome-wide analysis of the World’s Sheep Breeds reveals high levels of historic mixture and strong recent selection. PLoS Biol. 2012;10: e1001258.
Qian Y, Ding L, Cao L, Yu Z, Shao X, Wang L, Qian Y, Ding Li, Cao L, Yu Z, Shao X, Wang L, Zhang M, Wang Q, Che X, Jiang Na, Yan H, Fang W, Jin Y, Huang J, Gu A, Ni Z. Gene polymorphisms of VEGF and KDR are associated with initial fast peritoneal solute transfer rate in peritoneal dialysis. BMC Nephrol. 2022;23(1):365. https://doi.org/10.1186/s12882-022-02975-5.
Pan J, Goodheart M, Chuma S, Nakatsuji N, Page DC, Wang PJ. RNF17, a component of the mammalian germ cell nuage, is essential for spermiogenesis. Development. 2005;132(18):4029–39. https://doi.org/10.1242/dev.02003.
Sawyer AJ, Garand M, Chaussabel D, Feng CG. Transcriptomic profiling identifies neutrophil-specific upregulation of Cystatin F as a marker of Acute inflammation in humans. Front Immunol. 2021;12:12. https://doi.org/10.3389/fimmu.2021.634119.
Zi M, Xu Y. Involvement of cystatin C in immunity and apoptosis. Immunol Lett. 2018;196:80–90. https://doi.org/10.1016/j.imlet.2018.01.006.
Zimmermann S, Pfannkuch L, Al-Zeer MA, Bartfeld S, Koch M, Liu J, Zimmermann S, Pfannkuch L, Al-Zeer MA, Bartfeld S, Koch M, Liu J, Rechner C, Soerensen M, Sokolova O, Zamyatina A, Kosma P, Mäurer AP, Glowinski F, Pleissner K-P, Schmid M, Brinkmann V, Karlas A, Naumann M, Rother M, Machuy N, Meyer TF. ALPK1- and TIFA-Dependent Innate Immune Response triggered by the Helicobacter pylori type IV Secretion System. Cell Rep. 2017;20(10):2384–95. https://doi.org/10.1016/j.celrep.2017.08.039.
Pattabhi S, Knoll ML, Gale M, Loo Y-M. DHX15 is a coreceptor for RLR Signaling that promotes Antiviral Defense against RNA virus infection. J Interferon Cytokine Res. 2019;39(6):331–46. https://doi.org/10.1089/jir.2018.0163.
Griffioen M, van der Meijden ED, Slager EH, Honders MW, Rutten CE, van Luxemburg-Heijs SAP, et al. Identification of phosphatidylinositol 4-kinase type II β as HLA class II-restricted target in graft versus leukemia reactivity. Proc Natl Acad Sci U S A. 2008;105:3837–42.
Alli-Balogun GO, Gewinner CA, Jacobs R, Kriston-Vizi J, Waugh MG, Minogue S. Phosphatidylinositol 4-kinase IIβ negatively regulates invadopodia formation and suppresses an invasive cellular phenotype. Mol Biol Cell. 2016;27(25):4033–42. https://doi.org/10.1091/mbc.E16-08-0564.
Sayegh AI. The role of cholecystokinin receptors in the short-term control of Food Intake. 2013;114:277–316. https://doi.org/10.1016/B978-0-12-386933-3.00008-X.
Agha SH, Pilla F, Galal S, Shaat I, D’Andrea M, Reale S, Agha SH, Pilla F, Galal S, Shaat I, D’Andrea M, Reale S, Abdelsalam AZA, Li MH. Genetic diversity in egyptian and italian goat breeds measured with microsatellite polymorphism. J Anim Breed Genet. 2008;125(3):194–200. https://doi.org/10.1111/j.1439-0388.2008.00730.x.
Hsu T, Trojanowska M, Watson DK. Ets proteins in biological control and cancer. J Cell Biochem. 2004;91(5):896–903. https://doi.org/10.1002/jcb.20012.
Dermody TS, Kirchner E, Guglielmi KM, Stehle T. Immunoglobulin superfamily virus receptors and the evolution of adaptive immunity. PLoS Pathog. 2009;5(11): e1000481. https://doi.org/10.1371/journal.ppat.1000481.
Mi Y, Shi Z, Li J. Spata19 is critical for sperm mitochondrial function and male fertility. Mol Reprod Dev. 2015;82:907–13.
Mathieu NA, Paparisto E, Barr SD, Spratt DE. HERC5 and the ISGylation Pathway: critical modulators of the antiviral Immune response. Viruses. 2021;13(6): 1102. https://doi.org/10.3390/v13061102.
Neumann J, Boknik P, Kirchhefer U, Gergs U. The role of PP5 and PP2C in cardiac health and disease. Cell Signal. 2021;85: 110035. https://doi.org/10.1016/j.cellsig.2021.110035.
Iyer SS, Cheng G. Role of Interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32(1):23–63. https://doi.org/10.1615/CritRevImmunol.v32.i1.30.
Waineina RW, Okeno TO, Ilatsia ED, Ngeno K. Selection signature analyses revealed genes Associated with Adaptation, Production, and Reproduction in selected Goat Breeds in Kenya. Front Genet. 2022;13:13. https://doi.org/10.3389/fgene.2022.858923.
Acknowledgements
The authors acknowledge Mr. Alaa Ghobashy from Albohayrah governorate for providing the majority of Boer samples.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was supported by grants from the Science, Technology & Innovation Funding Authority (STDF), the Egyptian Ministry for Scientific Research, within the activities of the ongoing research project with reference number “43213”.
Author information
Authors and Affiliations
Contributions
A.M.S. analyzed the data, interpreted the results and wrote the manuscript. C.U.B. revised the manuscript and contributed data analysis. F.B. revised the manuscript. H.R. involved in genotyping, data analysis and revised the manuscript. K.W. has critically edited and revised the manuscript, A.A. has critically edited and revised the manuscript and A.E.R contributed to collecting the data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal procedures included in the current study were approved by the Animal Breeding Ethics Committee at the Desert Research Center (DRC) in Egypt (Project ID: 43213) with approval reference number AB/NO2020. All methods were performed in accordance with the relevant guidelines and regulations, and in compliance with the ARRIVE guidelines. In addition, the project does not include euthanasia of the experimental animals. The sample size was decided based on the availability of similar animals with similar physical and physiological status. Additionally, an informed consent was granted for Boer animals from the owners for the inclusion of Boer goats in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Pictures of the investigated goats in the current study. Figure S2. Manhattan plots for SNP-specific pairwise fixation index (FST). Genetic differentiation between individuals of four goat breeds in Egypt(Nubian, Damascus, Barki and Boer). FST estimates are represented on the x-axis and genomic positions on chromosomes on y-axis. Each dot represents a SNP.
Additional file 3: Table S1.
The top 10 single nucleotide polymorphisms (SNP) and annotated genes differentiating between Egyptian Nubian and Damascus goat breeds based on FST estimates.1Chromosome, 2Location in base pairs, 3Minor allele frequency, 4Based on the Ensembl database. Table S2. The top 10 single nucleotide polymorphisms (SNP) and annotated genes differentiating between Egyptian Nubian and Boer goat breeds based on FST estimates.1Chromosome,2Location in base pairs, 3Minor allele frequency, 4Based on the Ensembl database.Table S3. The top 10 single nucleotide polymorphisms (SNP) and annotated genes differentiating between Egyptian Nubian and Barki goat breeds based on FST estimates.1Chromosome, 2Location in base pairs, 3Minor allele frequency, 4Based on the Ensembl database. Table S4. The top 10 single nucleotide polymorphisms (SNP) and annotated genes differentiating between Damascus and Boer goat breeds based on FST estimates.1Chromosome, 2Location in base pairs, 3Minor allele frequency, 4Based on the Ensembl database. Table S5. The top 10 single nucleotide polymorphisms (SNP) and annotated genes differentiating between Damascus and Barki goat breeds based on FST estimates.1Chromosome,2Location in base pairs, 3Minor allele frequency, 4Based on the Ensembl database.Table S6. The top 10 single nucleotide polymorphisms (SNP) and annotated genes differentiating between Barki and Boer goat breeds based on FST estimates. 1Chromosome,2Location in base pairs, 3Minor allele frequency, 4Based on the Ensembl database.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sallam, A.M., Reyer, H., Wimmers, K. et al. Genome-wide landscape of runs of homozygosity and differentiation across Egyptian goat breeds. BMC Genomics 24, 573 (2023). https://doi.org/10.1186/s12864-023-09679-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09679-6