Abstract
Background
The genetics of male fertility is complex and not fully understood. Male subfertility can adversely affect the economics of livestock production. For example, inadvertently mating bulls with poor fertility can result in reduced annual liveweight production and suboptimal husbandry management. Fertility traits, such as scrotal circumference and semen quality are commonly used to select bulls before mating and can be targeted in genomic studies. In this study, we conducted genome-wide association analyses using sequence-level data targeting seven bull production and fertility traits measured in a multi-breed population of 6,422 tropically adapted bulls. The beef bull production and fertility traits included body weight (Weight), body condition score (CS), scrotal circumference (SC), sheath score (Sheath), percentage of normal spermatozoa (PNS), percentage of spermatozoa with mid-piece abnormalities (MP) and percentage of spermatozoa with proximal droplets (PD).
Results
After quality control, 13,398,171 polymorphisms were tested for their associations with each trait in a mixed-model approach, fitting a multi-breed genomic relationship matrix. A Bonferroni genome-wide significance threshold of 5 × 10− 8 was imposed. This effort led to identifying genetic variants and candidate genes underpinning bull fertility and production traits. Genetic variants in Bos taurus autosome (BTA) 5 were associated with SC, Sheath, PNS, PD and MP. Whereas chromosome X was significant for SC, PNS, and PD. The traits we studied are highly polygenic and had significant results across the genome (BTA 1, 2, 4, 6, 7, 8, 11, 12, 14, 16, 18, 19, 23, 28, and 29). We also highlighted potential high-impact variants and candidate genes associated with Scrotal Circumference (SC) and Sheath Score (Sheath), which warrants further investigation in future studies.
Conclusion
The work presented here is a step closer to identifying molecular mechanisms that underpin bull fertility and production. Our work also emphasises the importance of including the X chromosome in genomic analyses. Future research aims to investigate potential causative variants and genes in downstream analyses.
Similar content being viewed by others
Background
Northern Australia represents a critical region for the Australian beef breeding industry, and bull fertility is an important contributor to profitability [1,2,3]. However, bull fertility has yet to benefit from the advancements in genomics and selective breeding, which has further contributed to improving female fertility [4]. The Bull Breeding Soundness Evaluation (BBSE) provides a comprehensive assessment of male fertility-related traits linked to the number of calves a sire produces in the subsequent mating season [5, 6]. The BBSE traits which consist of assessment of body conformation, testicular development and sperm motility and morphology assessment, are heritable and can be used for selection and genetic improvement programs [7]. Previous genome-wide association studies (GWAS) have identified candidate genes for Scrotal Circumference (SC) and semen traits which are recorded in BBSE [8,9,10,11]. Identifying these critical genomic regions expands the current understanding of the underlying genetics of bull fertility and can also be used to inform genomic predictions and improve their accuracy [12].
Previous work used medium or high-density SNP arrays, such as the Illumina 50 K panel or the BovineHD chip. Thus, genetic variants associated with GWAS are usually not causal mutations but are single nucleotide polymorphisms (SNP) in Linkage Disequilibrium (LD) to causal variants [13]. With advancements in genome sequencing and imputation methodologies, lower-density panels can be accurately imputed to sequence level [14, 15]. This allows the genome to be viewed in finer detail, which increases our chances of detecting a causal variant. This study aimed to conduct GWAS on seven BBSE traits to identify genetic variants and candidate genes underpinning bull fertility. The variants identified in this analysis could be incorporated into genomic predictions to improve the rate of genetic improvement in bull fertility and production traits.
Materials and methods
Animals and phenotypes
A total of 6,422 animals of six breeds with BBSE measurements of seven phenotypes were used in this study. These animals are from two research populations and four stud herds from the industry. The two-research populations consisted of animal data obtained from the Cooperative Research Centre for Beef Genetic Technologies (Beef CRC) project [16], which included 1,051 Brahman (BRH) and 1,819 Tropical Composite bulls (TRC). Animal data for these four stud herds were contributed by four properties in Queensland, which included 1,288 Santa Gertrudis (SGT), 760 Droughtmasters (DMT), 844 Ultra blacks (UBK), and 660 Belmont Tropical Composite (BTC) [17]. The seven BBSE phenotypes used in this study included four physical measures on the animal and three semen measurements. These measurements were conducted according to the standards prescribed by Australian Cattle Veterinarians [5], which have been covered extensively in the literature [16]. Details on the seven phenotypes can be found in Table 1. Summary statistics and heritabilities of each trait are shown in Table 2. The breed-wise summary statistics for each trait are available in Additional file 6.
Phenotypic measures for all six populations were collected from 2003 to 2020. Each bull was assessed once, and the year of measurement was recorded as the fixed effect - year of birth. The individuals involved in the assessment and collection of phenotypes are different for the two research populations and the four stud herds. Phenotypes for the two research populations were collected and assessed by two experienced veterinarians who worked together throughout the collection period. In the four stud herds, an experienced animal scientist and veterinarian conducted the examinations. For semen morphology traits, semen samples from the two research populations were analysed in the same laboratory. Whereas, sperm samples from the four stud herds were assessed in different accredited labs selected by the producers. The age of the bulls when the phenotypes were collected differed across the six populations. In the Beef CRC, when SC was collected, BRH and TRC bulls were around 360 days old. Sheath score and sperm morphology assessments were conducted at around 700 days old. In the four stud herds, all phenotypes for SGT and DMT bulls were obtained at around 600 days, whereas UBK and BTC bulls had their phenotypes measured around 440 days old and 390 days old, respectively. Phenotypes were pre-adjusted using a generalised linear model analysis (PROC GLM) for their fixed effects (year of birth, breed, property) and covariates (age at measurement, PC1 and PC2) using SAS ® software 9.4 (SAS Inst. Inc.). A subset of this population was previously used in a multi-breed analysis [18].
Genotypes, quality control and genomic relationship matrices
SNP genotype imputation up to whole-genome sequence level was conducted in two rounds. In the first round, the reference population was established using Beef CRC and industry cattle. This reference population consisted of 2,452 animals made of BTC, BRH, DMT, SGT, UBK, Angus, Bonsmara, Boran, Composite, and Tuli breeds that were genotyped with the bovine high-density chip (~ 700 K) and phased using Eagle 2 (v2.4.1) which formed the imputation targets for the next step [19]. In the target population (n = 6422), genotyping was first done using a variety of commercial 50 K SNP chips (Bovine SNP50 v1 or v2 or Neogen Tropical Chip v1 and v2). The genotypes from these animals were also phased with Eagle 2 (v2.4.1) [19], and formed the imputation targets for the next step. Imputation of targets from low to high density using the phased reference was conducted using Minimac3 for the autosomes and Minimac4 for the X chromosome [14]. For imputation to sequence level, genotypes from 668 animals in the 1000 bull genome project run 7 [20] were filtered to keep only bi-allelic markers and minor alleles with at least four copies. The sequence-level reference panel consisted of 668 animals made of BRH, DMT, SGT, Afrikander, Angus, Angus Red, Beefmaster, Boran, Brangus, Charolais, Gir, Hereford, Limousin, Murray Grey, Nelore, Senepol, Shaiwal, Shorthorn and Tuli breeds. The data generated in the first round was then used to impute genotypes to sequence level (~ 25 million) in the second round using the same procedure done in the first round. SNPs with an imputation R2 > 0.8, a call rate > 0.9 and a minor allele frequency > 0.01 were kept for further analysis, leaving 13,398,171 SNPs, including 92,134 SNPs mapped onto the X chromosome after quality control for all 6422 animals. The Genomic Relationship Matrices (GRM) were constructed in the software GCTA [21] using a high-density panel, one GRM for the autosomes plus the Pseudo – Autosomal Region of chromosome X (657,563 SNPs for autosomes and 22,775 SNPs for X), and a second GRM using the remaining SNP from the X chromosome. Heritability estimates for each trait were obtained using the restricted maximum likelihood (REML) analysis in GCTA [21].
Genome-wide association analysis and quantitative trait loci analysis
The first two principal components calculated PLINK 1.9 [22], and the GRMs, were used to account for the underlying genetic structure of the multi-breed population under study. As bias could be introduced when a tested SNP is also included in the GRM that is fitted in the model [23], the Leave One Chromosome Out (LOCO) approach to GWAS was also implemented by building a different GRM when testing each chromosome, leaving out any SNPs that are on the tested chromosome [24]. The MLM method implemented in GCTA is as follows:
Where y represents the phenotype in question, a represents the mean, b represents the additive genetic effect of the tested SNP, x represents the SNP genotype indicator variable which is coded as 0, 1 and 2, g – represents the joined effect of all variants, excluding any variants on which the chromosome of the tested SNP is located, and e is the residual variance. A genome-wide significance threshold of 5 × 10− 8 was used, which is a conservative Bonferroni correction. After the first round of GWAS was completed, the most significant SNPs in each chromosome were refitted as a discrete covariate in the second round of GWAS for each trait in GCTA [21]. This was done to determine if the most significant SNP in each chromosome could account for the entire peak for that chromosome. GWAS Manhattan plots were created in R [25] using the Scattermore package [26]. Using bedtools [27], SNPs within 50 Kbp that met the significance threshold (5 × 10− 8) were merged into a Quantitative Trait Loci (QTL). Using GALLO [28], genes found in each region were reported using gene annotation data of the Bos taurus ARS UCD 1.2 genome assembly obtained from Ensembl version 105 [29]. The find_genes_qtls_around_markers function was used to identify the genes located in each region. The following parameters were used: the method was set to gene, marker was set to haplotype, and the interval was set to 0.
Similarly, the same regions were used to identify any previously reported QTL in the Animal QTL database (https://www.animalgenome.org/cgi-bin/QTLdb/BT/index) [30] that overlapped with regions reported in this study. Ensembl Variant effect prediction (VEP) was conducted on all significant SNPs to ascertain the impact of each variant [31]. Pairwise LD was calculated between the high impact variants and the top variant for their respective QTL using PLINK 1.9 [22]. We considered variants that meet the R2 threshold of 0.4 to be in LD. Finally, the percentage of genetic variance explained by each SNP was calculated using a formula made available in a previous report [32]:
Where pi and qi are the SNP’s allele frequencies \(\widehat{{a}_{i}^{2}}\) is the estimated additive effect of the trait studied, and \({\sigma }_{g}^{2}\) is the estimated genetic variance.
Results and discussion
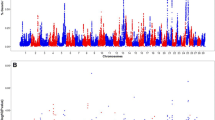
In this study, we conducted GWAS using sequence-level genotypes and targeted seven bull fertility and production traits measured in a multi-breed population of 6,422 bulls. This section discusses important regions and candidate genes identified through GWAS and QTL analysis. A summary of GWAS results for the most significant genomic region discovered for each trait is provided in Table 3. A complete table of GWAS summary statistics for all tested SNP and each trait is available in Additional file 1. Additional file 2 contains SNP that were significant for at least one trait. Manhattan plots for GWAS in SC and Sheath are shown in Figs. 1 and 2. The remaining Manhattan plots can be found in Additional file 3. A vast number of previously published QTL were identified for some traits. As such, we have summarised these results in Figs. 3 and 4. The sperm morphology traits (PNS, PD, and MP) did not have normally distributed residuals. This is not ideal for GWAS, but it is expected as sperm morphological abnormalities affect only some bulls. The majority of breeding bulls present a high percentage of normal sperm.
The Manhattan plot in A shows associations for SC in GWAS LOCO, whereas the Manhattan plot in B shows associations for SC after fitting the most significant SNP in each chromosome as a fixed effect. The inverse log p – values for each SNP are plotted along the y-axis for each chromosome on the x-axis. The dotted line represents the genome-wide significance threshold of 5 × 10− 8
The Manhattan plot in A shows associations for Sheath in GWAS LOCO, whereas the Manhattan plot in B shows associations for Sheath after fitting the most significant SNP in each chromosome as fixed effects. The inverse log p – values for each SNP are plotted along the y-axis for each chromosome on the x-axis. The dotted line represents the genome-wide significance threshold of 5 × 10− 8
Heritability estimates of individual traits
Heritability estimates across traits range from low (0.07, CS) to high (0.59, Sheath) (Table 2). The estimates we report for our traits were similar to those published in tropical beef cattle populations [8, 33, 34]. Our estimates for SC were similar to those measured in TRC bulls at 12 months (0.46) and slightly higher than those measured at 24 months (0.44) [34].
Single trait associations
The number of associated SNP varied enormously, depending on the target trait (Table 3). A total of 30 significant SNPs were detected for CS, while more than 135 thousand SNP were significant for Sheath. The strongest SNP association for Weight was located at 23.3 Mb of BTA 14 (p = 2.27 × 10− 10). This result is somewhat expected because previous GWAS in datasets containing Bos Taurus and Bos Indicus breeds have been reporting a QTL in BTA 14 for stature and weight traits [35,36,37].
The strongest SNP association for CS (p = 1.79 × 10 − 9) was located at 6.7 Mb of BTA 23. This is a new discovery as there are no CS QTL in BTA 23 currently recorded in the cattle QTL database (https://www.animalgenome.org/cgi-bin/QTLdb). The strongest SNP association for SC (p = 1.15 x − 79) was located at 79 Mb on the X chromosome. This is not the first time we detect SNP associations on X for SC, and so this result confirms previous GWAS carried out with smaller datasets [8, 9, 11]. The strongest association (p = 1.98 × 10− 288) for Sheath was located at 47.8 Mb of BTA 5. This finding is consistent with previous GWAS work in sheath score that used a subset of the data included in this study [32]. The subset of data contained only BRH and TRC bulls, which differs from our multi-breed analyses [32]. In short, the larger dataset is expanding on the initial findings and in subsequent sections of this discussion, we detail the QTL, genes, and variants uncovered with sequence-level data.
Similarly, the current dataset enhanced our ability to detect associations for the three semen traits: PNS, PD and MP. The strongest SNP association (p = 3.35 × 10 − 14) for PNS was located at 46.4 Mb at BTA 5. Previously, we had only identified SNPs on X for PNS [8, 9, 11]. A recent study on American cattle did identify SNP associations in chromosome 5 for PNS, corroborating our new finding in tropical breeds [38]. The strongest SNP association (p = 1.98 × 10 − 13) for PD was located at 46 Mb of BTA 5. A total of 173 significant SNP associations were detected for MP. The strongest SNP association (p = 2.77 × 10 − 10) was located at 6.2 Mb of the X chromosome. This multibreed dataset confirmed that chromosome X harbors SNP associations for semen traits as expected [8, 9, 11]. It also allowed the discovery of significant SNP on BTA 5, pointing to new candidate genes (described below).
The most significant SNP for a trait may not account for all the variation at a particular locus, and multiple causal variants may exist at a given locus [39]. As such, we verified the most significant SNPs for each chromosome in each trait by refitting these SNPs back into the mixed model. In general, the most significant SNP in each chromosome accounted for the entire variation in that locus for most traits, as seen in Figs. 1 and 2. However, in Sheath, the most significant SNP did not account for all the variation in BTA 5 (Fig. 2). Perhaps more than one causal SNP exists in that BTA 5 region and this is important because it overlaps with significant QTL discovered for SC and semen traits. The SNP associations across traits found in BTA 5 are discussed in more detail below (see Table 4).
QTL analysis
The GWAS literature includes accounts of false positives: QTL or SNP associations that are seen once and not validated (winners curse) [40]. To mitigate this issue, we focused on reporting QTL regions that overlap with known QTL from previous work. We used the QTL database [30] to identify consensus between our current analyses and published work. The number of significant QTL identified per trait and the total sum of these QTL can be found in Table 4.
A total of 1,120 previously reported QTL overlapped with the regions identified in this study for weight. While most of the identified QTL were associated with weight-related traits, some of these regions were also important female traits such as milk fat yield, health-related traits, and reproductive traits.
A total of 20,095 previously reported QTL overlapped with the significant regions reported for SC. Most QTL were associated with SC reported in Canchim bulls [41], and the age of puberty was reported in our previous study with TC bulls [11]. This result is not surprising as a bull is considered to have reached puberty after achieving a SC of 26 cm [42].
In Sheath, 2,671 previously reported QTL overlapped with the significant regions in this study. Most QTL were associated to female traits such as milk protein percentage or milk yield. However, QTL were also associated to male traits such as inhibin hormone levels and SC. Inhibin hormone levels are considered an early indicator of sexual development, and genes such as INHBE and INHBC are located in BTA 5 [11, 43]. Of note, the GWAS on blood hormone levels of Inhibin used 50 K genotype data for Brahman and TRC cohorts that were included in the current larger dataset [11].
Previously reported QTL for PNS mirrored the result for SC (Figs. 3 and 4). This is not surprising given that both BTA 5 and chromosome X were associated with both traits, and a positive genetic correlation has been reported in a previous study between the two traits [16]. For PD, previously reported QTL were associated with reproduction traits such as inhibin level and SC, whereas most QTL were associated to meat and carcass and female traits for MP. Recent studies in dairy populations reported a QTL in BTA 6 associated with sperm abnormality traits in Brown Swiss bulls [44, 45]. Studies in Holstein bulls identified regions in BTA 1, 2, 4, 6, 7, 8, 16, 23 and 26 associated with progressive and total motility [46]. However, none of these regions overlapped with the QTL reported in our studied population. The dissimilarities in QTL reported could be due to genetic differences between beef and dairy cattle at a genome-wide level [47].
Significant QTL mapping to the X chromosome for SC, PNS and sperm abnormalities highlights its importance in male fertility and spermatogenesis. The X chromosome is a candidate region for species divergence genes which are highly expressed in the testis of mice and humans [48]. Sexual antagonism and sex-chromosome meiotic drive have been suggested as a possible reason for the large number of genes associated with spermatogenesis found in the X chromosome [8].
Overlapping regions across traits
Due to the vast number of genes detected for some traits, we have included the list of genes in each associated region for each trait in Additional file 4. To facilitate further use of our findings, we have included a list of all genes across associated regions in Additional file 5. A list of genes across associated regions that map at least four traits is shown in Table 5.
Across traits, we observed that BTA 5 is an important region for male fertility in bulls. Regions in BTA 5 that have overlapping results point to SNP and genes associated with five out of the seven studied traits: SC, Sheath, PNS, PD and MP (Table 5). 16 candidate genes were identified within these significant regions as associated with at least four traits. Next, we reviewed the literature to discuss how the known function of these genes could be related to SC, Sheath, or sperm morphology traits.
Three candidate genes (DYRK2, CAND1, and GRIP1) listed in Table 5 have known biological roles linking them with spermatogenesis. Spermatogenesis is likely to underpin most bull fertility traits, so these genes warrant further discussion. The DYRK family of kinases displayed high expression in the testis and was suggested to play a role in the later stages of spermatogenesis [49]. The CAND1 protein is highly expressed in the brain and testis in humans and has been reported to be highly expressed in spermatozoa of fertile men [29, 50]. In mice, GRIP1 is necessary for the adhesion of Sertoli cells to germ cells and plays an important role in efficient spermatogenesis [51]. Mice without GRIP1 appeared to suffer from impaired fertility due to abnormalities in the testis [51]. However, little is known about the role of GRIP1 in bull fertility, although its gene and protein expression in different stages of the oestrous cycle have been covered previously [52]. Perhaps these genes are similarly involved with spermatogenesis in bulls. However, further research is required to ascertain their effects on bovine spermatogenesis and testicular function.
The remaining candidate genes from Table 5, do not have a known function that directly links them to spermatogenesis. However, they are ubiquitously expressed in reproductive tissues. The CPNE (copines) gene group of membrane-bound proteins have multiple functions in membrane transport, signal transduction and cancer [53]. CPNE8 is a gene expressed ubiquitously in the prostate, testis, heart, and brain tissues [53, 54]. It was previously suggested that CPNE8 might be an important gene for prostate regulation and development [54]. The PTPRR gene may have a tumour-suppressive function in prostate cancer, and prostate cancer samples often contain lower levels of PTPRR compared to regular tissue samples [55, 56]. In addition, the PTPRB gene was expressed in porcine and equine spermatozoa and found mainly in the plasma membrane of sperm heads, acrosome, and tail [57]. The expression of PTPRB mainly in the tail of spermatozoa, suggests its involvement in sperm motility regulation [57]. Previous literature has highlighted the different functions of tyrosine phosphorylation in spermatozoa, which are crucial for successful fertilisation [58,59,60]. The expression of the BEST3 gene in the form of bestrophin 3 is ubiquitous in human muscle but found in low levels in the bone marrow, testis and retina [61]. At the same time, BEST3 plays a role in regulating cell proliferation and apoptosis, both of which are important features in mammalian spermatogenesis [62,63,64,65]. Most of these genes appear to be involved in cancer literature, which is consistent with reproductive physiology that often involves cell proliferation [66].
Notably, three genes within BTA 5 regions (Table 5) play an important role in tropical adaptation, which is expected in this cattle population. Between 47.3 Mb and 47.9 Mb is a common region in BTA 5 that contains several genes, including HELB, which is suggested to influence tropical cattle adaptation, which helps cattle cope with harsh temperatures and high intensity of ultraviolet light [67]. IRAK3 is suggested to be involved in intramuscular fat disposition and systemic inflammation regulation, HMGA2 regulates body size. The region containing HMGA2 has been previously associated with navel length in Nellore cattle and has also been reported to regulate body size [68, 69]. A copy number variant (CNV) in the HMGA2 gene has been proposed to be a functional variant associated with naval length [68]. This CNV is within a detected QTL and may play a role in sheath score, SC, PNS, and PD in the studied population. We conducted a preliminary analysis to observe the same region (5:47,840,005-47846215, reference genome ARS UCD 1.2) and explored whether this CNV segregates in our population. Using 138 whole genome sequenced cattle, that were part of the reference panel for the SNP imputation, we observed in 79 of them an increased coverage depth which likely indicates the presence of a CNV. Future studies are required to confirm whether this region of increased coverage depth is due to a CNV segregating in our population and whether this CNV is the same as previously described. Additional efforts should be made to impute this CNV for the entire multibreed population and verify it’s contribution to these traits. Regions in BTA5 have been consistently reported in previous studies. BTA 5 is evidently harbouring important regions for fertility traits and production traits. Dissecting the genes and mutations implicated in fertility as opposed to heat tolerance or growth could further inform selective breeding.
Variant effect prediction (VEP): candidate genes
For the most significant variant in each trait (Table 3), VEP did not reveal any variants that will have a moderate or high functional impact on a protein. Instead, most variants were labelled as modifiers which either have effects that are difficult to predict or have little evidence of protein impact.
When VEP was expanded to include SNPs within candidate regions listed in Table 4, similar results were observed with significant variants categorised as modifiers (Fig. 5). This is logical as most traits examined in this study are complex. As such, the effects of these variants segregating in various loci across the genome have little effect on the protein or phenotype [70]. However, we observed one variant of high functional impact located in IRAK3, which results in a premature stop codon (Table 6). While this SNP may not have an equivalent quantitative effect on the trait compared to the peak SNP, it could still have a high functional impact which should be considered. As mentioned previously, IRAK3 plays a role in immune suppression. A rodent study reported a negative relationship between IRAK3 and TNF-α expression and suggested that IRAK3 is associated with immune suppression during cases of sepsis [71]. IRAK3 may also be a factor produced by Sertoli cells that causes inflammatory effector T-cells to develop regulatory functions which reduce the number of available T-cells [72]. A recent review highlighted that Sertoli cells aid in creating and maintaining an environment that shields germ cells from autoimmune destruction [73]. This is due to the presentation of antigens on the surface of end-stage germ cells, which are detected as foreign, and can lead to autoimmune destruction resulting in suboptimal fertility or sterility [73, 74]. Perhaps, a variant of high impact on IRAK3 may affect the protein’s ability to regulate autoimmune destruction efficiently, leading to decreased fertility. However, further downstream work is required to verify this speculation.
Variants prioritized with the variant effect predictor
We identified 17 high-impact variants, predicted with VEP, as shown in Table 6. High-impact variants are predicted to have a disruptive effect on a protein, which may have a potential downstream impact on the associated phenotypes [31]. Pairwise LD calculation between high-impact variants and the top variants for their respective QTL are available in Additional file 6 (Tables S7 to S10). Among these high-impact variants, 15 variants were in BTA 5, and the remaining two were found in BTA 2 and the X chromosome. All variants were associated with either SC, Sheath, PNS or PD. Seven high-impact variants were in LD with the top variants for their respective QTL with an R2 ranging from 0.41 to 0.97. The high-impact variant rs479267746 lies within the coding region of a gene (IRAK3) which has been previously associated with fertility. The expression of IRAK3 by Sertoli cells, which play an important role in spermatogenesis, has been discussed in detail in the previous section. The high-impact variant rs439285466 lies within the protein-coding region of a gene called RLIM. Although RLIM has not been associated to bull fertility or bull production traits, it has been previously associated with the regulation of cell proliferation which is fundamental process for spermatogenesis [75]. Considering the LD with top QTL variants for SC and other bull traits, together with the VEP results and the known function of IRAK3 and RLIM, we would prioritize the 2 high-impact variants in these genes for future work. These variants should be further tested for their impact on bull fertility.
The remaining 10 high-impact variants, while not in LD (R2 < 0.4) with the top variants of the corresponding QTL, were significantly associated with either SC or Sheath themselves. Some of the high impact variants identified in this study, lie within known genes (NUDT4, SMUG1, KRT77, BIN2, ARHGAP9, and CFAP54) previously not connected with bull traits or male fertility [75,76,77,78,79,80,81,82]. We proposed these 17 variants be further investigated in subsequent analysis to ascertain variant effects in other populations.
Conclusion
This study highlights the importance of BTA5 for bull fertility and production traits and demonstrates the need to include the X chromosome in genomic analyses. We also highlighted candidate genes of relevance across several traits, which should be further investigated in ascertaining gene effects on spermatogenesis and fertility. Finally, we identified several high-impact variants for SC and Sheath, which required further validation in future work.
Data Availability
The raw data on which the conclusions of the paper rely are available from the CSIRO https://www.csiro.au/) under a Data Use Agreement. Summary statistics for every tested SNP are available as supplementary files (Additional files 1 to 5). The datasets can be accessed in ScienceDB using the following link: https://www.scidb.cn/s/7Jbi2e. Addtionally, the unique links for each additional file has been listed in the supporting information section below. Original phenotype data can be obtained from respective producers under circumstances where a data-sharing agreement has been reached, this can be arranged through the corresponding author.
References
Holmes P, Mclean I. Australian Beef Report 2017. In. Toowoomba; 2017.
Chilcott C, Ash A, Lehnert SA, Stokes C, Charmley E, Collins K, et al. Northern Australia beef situation analysis. In: Hermit Park, Queensland: Cooperative Research Centre for Developing Northern Australia; 2020.
Greenwood PL, Gardner GE, Ferguson DM. Current situation and future prospects for the australian beef industry - A review. Asian-Australas J Anim Sci. 2018;31(7):992–1006.
Hayes BJ, Corbet NJ, Allen JM, Laing AR, Fordyce G, Lyons R, et al. Towards multi-breed genomic evaluations for female fertility of tropical beef cattle 1. J Anim Sci. 2018;97(1):55–62.
Fordyce G, Entwistle K, Norman S, Perry V, Gardiner B, Fordyce P. Standardising bull breeding soundness evaluations and reporting in Australia. Theriogenology. 2006;66(5):1140–8.
Barth AD. Review: the use of bull breeding soundness evaluation to identify subfertile and infertile bulls. animal. 2018;12(s1):s158–64.
Butler ML, Bormann JM, Weaber RL, Grieger DM, Rolf MM. Selection for bull fertility: a review. Translational Anim Sci. 2019;4(1):423–41.
Fortes MRS, Porto-Neto LR, Satake N, Nguyen LT, Freitas AC, Melo TP, et al. X chromosome variants are associated with male fertility traits in two bovine populations. Genet Sel Evol. 2020;52(1):46.
Fortes MRS, Reverter A, Hawken RJ, Bolormaa S, Lehnert SA. Candidate genes associated with testicular development, sperm quality, and hormone levels of inhibin, luteinizing hormone, and insulin-like growth factor 1 in Brahman bulls. Biol Reprod. 2012;87(3):58.
Sweett H, Fonseca PAS, Suárez-Vega A, Livernois A, Miglior F, Cánovas A. Genome-wide association study to identify genomic regions and positional candidate genes associated with male fertility in beef cattle. Sci Rep. 2020;10(1):20102–2.
Fortes MRS, Reverter A, Kelly M, McCulloch R, Lehnert SA. Genome-wide association study for inhibin, luteinizing hormone, insulin-like growth factor 1, testicular size and semen traits in bovine species. Andrology. 2013;1(4):644–50.
Abo-Ismail MK, Brito LF, Miller SP, Sargolzaei M, Grossi DA, Moore SS, et al. Genome-wide association studies and genomic prediction of breeding values for calving performance and body conformation traits in Holstein cattle. Genet Sel Evol. 2017;49(1):82.
Tenghe AMM, Bouwman AC, Berglund B, Strandberg E, de Koning DJ, Veerkamp RF. Genome-wide association study for endocrine fertility traits using single nucleotide polymorphism arrays and sequence variants in dairy cattle. J Dairy Sci. 2016;99(7):5470–85.
Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48(10):1284–7.
Browning BL, Browning SR. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am J Hum Genet. 2009;84(2):210–23.
Burns BM, Corbet NJ, Corbet DH, Crisp JM, Venus BK, Johnston DJ, et al. Male traits and herd reproductive capability in tropical beef cattle. 1. Experimental design and animal measures. Anim Prod Sci. 2013;53(2):87–100.
Porto-Neto LR, McWilliam SM, Alexandre PA, Reverter A, McGowan M, Fortes MRS, et al. Bull fertility update: historical data, new cohort and advanced genomics. Meat and Livestock Australia Limited; 2021.
Porto-Neto LR, Alexandre PA, Hudson NJ, Bertram J, McWilliam SM, Tan AWL, et al. Multi-breed genomic predictions and functional variants for fertility of tropical bulls. PLoS ONE. 2023;18(1):e0279398.
Loh P-R, Danecek P, Palamara PF, Fuchsberger C, A Reshef Y, Finucane K. Reference-based phasing using the Haplotype Reference Consortium panel. Nat Genet. 2016;48(11):1443–8.
Hayes BJ, Daetwyler HD. 1000 Bull Genomes Project to Map simple and complex genetic traits in cattle: applications and outcomes. Annu Rev Anim Biosci. 2019;7(1):89–102.
Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82.
Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 2015;4(1).
Listgarten J, Lippert C, Kadie CM, Davidson RI, Eskin E, Heckerman D. Improved linear mixed models for genome-wide association studies. Nat Methods. 2012;9(6):525–6.
Yang J, Zaitlen NA, Goddard ME, Visscher PM, Price AL. Advantages and pitfalls in the application of mixed-model association methods. Nat Genet. 2014;46(2):100–6.
R Core Team. R. A language and environment for statistical computing. In: Vienna, Austria: R Foundation for Statistical Computing; 2021.
Kratochvil M. Scattermore: Scatterplots with More Points. In: R package version 0.7. 2020.
Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26(6):841–2.
Fonseca PAS, Suárez-Vega A, Marras G, Cánovas Á. GALLO: an R package for genomic annotation and integration of multiple data sources in livestock for positional candidate loci. Gigascience. 2020;9(12).
Howe KL, Achuthan P, Allen J, Allen J, Alvarez-Jarreta J, Amode MR, et al. Ensembl 2021. Nucleic Acids Res. 2020;49(D1):D884–91.
Hu Z-L, Park CA, Reecy JM. Building a livestock genetic and genomic information knowledgebase through integrative developments of animal QTLdb and CorrDB. Nucleic Acids Res. 2018;47(D1):D701–10.
McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GRS, Thormann A, et al. The Ensembl variant effect predictor. Genome Biol. 2016;17(1):122.
Porto-Neto LR, Reverter A, Prayaga KC, Chan EKF, Johnston DJ, Hawken RJ, et al. The Genetic Architecture of climatic adaptation of tropical cattle. PLoS ONE. 2014;9(11):e113284.
Frizzas OG, Grossi DA, Buzanskas ME, Paz CCP, Bezerra LAF, Lôbo RB, et al. Heritability estimates and genetic correlations for body weight and scrotal circumference adjusted to 12 and 18 months of age for male Nellore cattle. animal. 2009;3(3):347–51.
Corbet NJ, Burns BM, Johnston DJ, Wolcott ML, Corbet DH, Venus BK, et al. Male traits and herd reproductive capability in tropical beef cattle. 2. Genetic parameters of bull traits. Anim Prod Sci. 2013;53(2):101–13.
Karim L, Takeda H, Lin L, Druet T, Arias JA, Baurain D, et al. Variants modulating the expression of a chromosome domain encompassing PLAG1 influence bovine stature. Nat Genet. 2011;43(5):405–13.
Littlejohn M, Grala T, Sanders K, Walker C, Waghorn G, Macdonald K, et al. Genetic variation in PLAG1 associates with early life body weight and peripubertal weight and growth in Bos taurus. Anim Genet. 2012;43(5):591–4.
Fortes MR, Kemper K, Sasazaki S, Reverter A, Pryce JE, Barendse W, et al. Evidence for pleiotropism and recent selection in the PLAG1 region in australian beef cattle. Anim Genet. 2013;44(6):636–47.
Butler ML, Hartman AR, Bormann JM, Weaber RL, Grieger DM, Rolf MM. Genome-wide association study of beef bull semen attributes. BMC Genom. 2022;23(1):74.
Yang J, Ferreira T, Morris AP, Medland SE, Madden PA, Heath AC, et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44(4):369–75. s361-363.
Palmer C, Pe’er I. Statistical correction of the winner’s curse explains replication variability in quantitative trait genome-wide association studies. PLoS Genet. 2017;13(7):e1006916.
Buzanskas ME, Grossi DdA, Ventura RV, Schenkel FS, Chud TCS, Stafuzza NB, et al. Candidate genes for male and female reproductive traits in Canchim beef cattle. J Anim Sci Biotechnol. 2017;8(1):67.
Fortes MRS, Lehnert SA, Bolormaa S, Reich C, Fordyce G, Corbet NJ, et al. Finding genes for economically important traits: Brahman cattle puberty. Anim Prod Sci. 2012;52(3):143–50.
Phillips DJ. Activins, inhibins and follistatins in the large domestic species. Domest Anim Endocrinol. 2005;28(1):1–16.
Mapel XM, Hiltpold M, Kadri NK, Witschi U, Pausch H. Bull fertility and semen quality are not correlated with dairy and production traits in Brown Swiss cattle. JDS Commun. 2022;3(2):120–5.
Hiltpold M, Kadri NK, Janett F, Witschi U, Schmitz-Hsu F, Pausch H. Autosomal recessive loci contribute significantly to quantitative variation of male fertility in a dairy cattle population. BMC Genom. 2021;22(1):225.
Ramirez-Diaz J, Cenadelli S, Bornaghi V, Bongioni G, Montedoro SM, Achilli A, et al. Identification of genomic regions associated with total and progressive sperm motility in italian holstein bulls. J Dairy Sci. 2023;106(1):407–20.
Kim SJ, Ha JW, Kim H. Genome-wide identification of discriminative genetic variations in beef and dairy cattle via an Information-Theoretic Approach. Genes. 2020;11(6).
Mueller JL, Skaletsky H, Brown LG, Zaghlul S, Rock S, Graves T, et al. Independent specialization of the human and mouse X chromosomes for the male germ line. Nat Genet. 2013;45(9):1083–7.
Sacher F, Möller C, Bone W, Gottwald U, Fritsch M. The expression of the testis-specific Dyrk4 kinase is highly restricted to step 8 spermatids but is not required for male fertility in mice. Mol Cell Endocrinol. 2007;267(1):80–8.
Park Y-J, Pang M-G. Mitochondrial functionality in male fertility: from spermatogenesis to fertilization. Antioxidants. 2021;10(1):98.
Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol. 2002;22(16):5923–37.
Lee WY, Park MH, Kim KW, Song H, Kim KB, Lee CS, et al. Identification of lactoferrin and glutamate receptor-interacting protein 1 in bovine cervical mucus: a putative marker for oestrous detection. Reprod. 2017;52(1):16–23.
Tang H, Pang P, Qin Z, Zhao Z, Wu Q, Song S et al. The CPNE Family and their role in cancers. Front Genet. 2021;12.
Maitra R, Grigoryev DN, Bera TK, Pastan IH, Lee B. Cloning, molecular characterization, and expression analysis of Copine 8. Biochem Biophys Res Commun. 2003;303(3):842–7.
Munkley J, Lafferty NP, Kalna G, Robson CN, Leung HY, Rajan P, et al. Androgen-regulation of the protein tyrosine phosphatase PTPRR activates ERK1/2 signalling in prostate cancer cells. BMC Cancer. 2015;15(1):1–11.
Nunes-Xavier CE, Mingo J, López JI, Pulido R. The role of protein tyrosine phosphatases in prostate cancer biology. Biochim Biophys Acta Mol Cell Res. 2019;1866(1):102–13.
González-Fernández L, Ortega-Ferrusola C, Macias-Garcia B, Salido GM, Peña FJ, Tapia JA. Identification of protein tyrosine phosphatases and dual-specificity phosphatases in mammalian spermatozoa and their role in sperm motility and protein tyrosine Phosphorylation1. Biol Reprod. 2009;80(6):1239–52.
de Lamirande E, O’Flaherty C. Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta. 2008;1784(1):106–15.
Tulsiani DR, Zeng HT, Abou-Haila A. Biology of sperm capacitation: evidence for multiple signalling pathways. Soc Reprod Fertil Suppl. 2007;63:257–72.
Naz RK, Rajesh PB. Role of tyrosine phosphorylation in sperm capacitation / acrosome reaction. Reprod Biol Endocrin. 2004;2(1):75.
Stöhr H, Marquardt A, Nanda I, Schmid M, Weber BHF. Three novel human VMD2-like genes are members of the evolutionary highly conserved RFP-TM family. Eur J Hum Genet. 2002;10(4):281–4.
O’Driscoll KE, Hatton WJ, Burkin HR, Leblanc N, Britton FC. Expression, localization, and functional properties of Bestrophin 3 channel isolated from mouse heart. Am J Physiol Cell Physiol. 2008;295(6):C1610–1624.
Jiang L, Liu Y, Ma MM, Tang YB, Zhou JG, Guan YY. Mitochondria dependent pathway is involved in the protective effect of bestrophin-3 on hydrogen peroxide-induced apoptosis in basilar artery smooth muscle cells. Apoptosis. 2013;18(5):556–65.
Song W, Yang Z, He B. Bestrophin 3 ameliorates TNFα-induced inflammation by inhibiting NF-κB activation in endothelial cells. PLoS ONE. 2014;9(10):e111093–3.
Salicioni AM, Platt MD, Wertheimer EV, Arcelay E, Allaire A, Sosnik J, et al. Signalling pathways involved in sperm capacitation. Soc Reprod Fertil Suppl. 2007;65:245–59.
Staub C, Johnson L, Review. Spermatogenesis in the bull. animal 2018;12:s27-s35.
Naval-Sánchez M, Porto-Neto LR, Cardoso DF, Hayes BJ, Daetwyler HD, Kijas J, et al. Selection signatures in tropical cattle are enriched for promoter and coding regions and reveal missense mutations in the damage response gene HELB. Genet Sel Evol. 2020;52(1):27.
Aguiar TS, Torrecilha RBP, Milanesi M, Utsunomiya ATH, Trigo BB, Tijjani A et al. Association of Copy Number Variation at Intron 3 of HMGA2 with navel length in Bos indicus. Front Genet. 2018;9.
Maiorano AM, Cardoso DF, Carvalheiro R, Júnior GAF, de Albuquerque LG, de Oliveira HN. Signatures of selection in Nelore cattle revealed by whole-genome sequencing data. Genomics. 2022;114(2):110304.
Mackay TF. Q&A: genetic analysis of quantitative traits. J Biol. 2009;8(3):23.
Nguyen TH, Turek I, Meehan-Andrews T, Zacharias A, Irving HR. A systematic review and meta-analyses of interleukin-1 receptor associated kinase 3 (IRAK3) action on inflammation in in vivo models for the study of sepsis. PLoS ONE. 2022;17(2):e0263968.
Doyle TJ, Kaur G, Putrevu SM, Dyson EL, Dyson M, McCunniff WT, et al. Immunoprotective properties of primary sertoli cells in mice: potential functional pathways that confer immune privilege. Biol Reprod. 2012;86(1):6, 1–14.
Washburn RL, Hibler T, Kaur G, Dufour JM. Sertoli cell Immune Regulation: a double-edged Sword. Front Immuno. 2022;13.
Tung KS, Harakal J, Qiao H, Rival C, Li JC, Paul AG, et al. Egress of sperm autoantigen from seminiferous tubules maintains systemic tolerance. J Clin Investi. 2017;127(3):1046–60.
Gao R, Wang L, Cai H, Zhu J, Yu L. E3 ubiquitin ligase RLIM negatively regulates c-Myc transcriptional activity and restrains cell proliferation. PLoS ONE. 2016;11(9):e0164086.
Singh G, Roy J, Rout P, Mallick B. Genome-wide profiling of the PIWI-interacting RNA-mRNA regulatory networks in epithelial ovarian cancers. PLoS ONE. 2018;13(1):e0190485.
Caffrey JJ, Shears SB. Genetic rationale for microheterogeneity of human diphosphoinositol polyphosphate phosphohydrolase type 2. Gene. 2001;269(1–2):53–60.
Sevilla LM, Bayo P, Latorre V, Sanchis A, Pérez P. Glucocorticoid receptor regulates overlapping and differential gene subsets in developing and adult skin. Mol Endocrinol. 2010;24(11):2166–78.
Sánchez-Barrena MJ, Vallis Y, Clatworthy MR, Doherty GJ, Veprintsev DB, Evans PR, et al. Bin2 is a membrane sculpting N-BAR protein that influences leucocyte podosomes, motility and phagocytosis. PLoS ONE. 2012;7(12):e52401.
Peñagaricano F, Souza AH, Carvalho PD, Driver AM, Gambra R, Kropp J, et al. Effect of maternal methionine supplementation on the transcriptome of bovine preimplantation embryos. PLoS ONE. 2013;8(8):e72302.
Leal-Gutiérrez JD, Elzo MA, Johnson DD, Hamblen H, Mateescu RG. Genome wide association and gene enrichment analysis reveal membrane anchoring and structural proteins associated with meat quality in beef. BMC Genom. 2019;20(1):151.
Gontan C, Achame EM, Demmers J, Barakat TS, Rentmeester E, van IJcken W, et al. RNF12 initiates X-chromosome inactivation by targeting REX1 for degradation. Nature. 2012;485(7398):386–90.
Tan WLA, Porto-Neto LR, Reverter A, Fortes MRS. Multibreed sequence level genome-wide association study of semen traits in tropical australian cattle. World Congress on Genetics Applied to Livestock production: 2022; Rotterdam, Netherlands. Wageningen Academic Publishers. 2022.
Acknowledgements
The authors are very grateful to the beef producers that took part in this project providing access to archived biological samples and performance records, and the Cooperative Research Centre for Beef Genetic Technologies via its legacy database that also contributed data to this project. The authors also would like to acknowledge John Bertram for his contributions to the collection of phenotypic records throughout this project. The authors also recognise that a small subset of the GWAS Manhattan plots relating to sperm morphology traits has been submitted for publication in the proceeding of the World Congress Genetics Applied to Livestock Production [83].
Funding
This project was co-funded by CSIRO, the University of Queensland and Meat and Livestock Australia (L.GEN.1818).
Author information
Authors and Affiliations
Contributions
AT performed the analyses, wrote the main text, and prepared the figures and tables. MF, LPN and AR conceptualised and supervised the work and aided AT with statistical analysis and interpretation of results. MM was involved in the collection of phenotypic records used in this study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors confirm that all methods were performed in accordance with the relevant guidelines and regulations. The animal data used in this paper were obtained from two separate projects overseen by two different institutional review boards. In the first project, the authors confirm that the institutional review board JM Rendel Laboratory Animal Experimentation Ethics Committee (CSIRO, Queensland, Australia) approved the protocols involved in handling and sampling of the two research populations (TBC107 and RH225-06, 1999–2006 and 2006–2010). For the second project, the authors confirm that the institutional review board CSIRO Animal Care and Use Committee granted waiver of ethics approval on the four industry herds, as ethics approval was not required for archived historical samples of these animals obtained from producers. No animals were handled by the authors, only existing data was used in this project.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tan, W.L.A., Neto, L.R.P., Reverter, A. et al. Sequence level genome-wide associations for bull production and fertility traits in tropically adapted bulls. BMC Genomics 24, 365 (2023). https://doi.org/10.1186/s12864-023-09475-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09475-2