Abstract
Background
Single-cell RNA sequencing is a state-of-the-art technology to understand gene expression in complex tissues. With the growing amount of data being generated, the standardization and automation of data analysis are critical to generating hypotheses and discovering biological insights.
Results
Here, we present scRNASequest, a semi-automated single-cell RNA-seq (scRNA-seq) data analysis workflow which allows (1) preprocessing from raw UMI count data, (2) harmonization by one or multiple methods, (3) reference-dataset-based cell type label transfer and embedding projection, (4) multi-sample, multi-condition single-cell level differential gene expression analysis, and (5) seamless integration with cellxgene VIP for visualization and with CellDepot for data hosting and sharing by generating compatible h5ad files.
Conclusions
We developed scRNASequest, an end-to-end pipeline for single-cell RNA-seq data analysis, visualization, and publishing. The source code under MIT open-source license is provided at https://github.com/interactivereport/scRNASequest. We also prepared a bookdown tutorial for the installation and detailed usage of the pipeline: https://interactivereport.github.io/scRNAsequest/tutorial/docs/. Users have the option to run it on a local computer with a Linux/Unix system including MacOS, or interact with SGE/Slurm schedulers on high-performance computing (HPC) clusters.
Similar content being viewed by others
Background
With the development of next-generation sequencing (NGS) technologies, researchers have started to focus more on the characterization at the single cell level [1, 2]. The single-cell RNA sequencing (scRNA-seq) technique, with the generation of expression profiles on individual cells, preserves both the representative biological patterns and heterogeneity, especially for some rare but informative subtypes [3,4,5]. As such, it has provided many novel insights into diversified complex biological systems, such as the brain [6, 7], lung [8], immune system [9,10,11], reproductive system [12,13,14], and multiple cancer-related tissues [15,16,17,18,19,20].
A general workflow for scRNA-seq analysis, with the goal of data exploration and interpretation, starts with read alignment and quality control (QC). These can give a quantitative estimation of genome mapping rate, unique molecular identifier (UMI) counts, mitochondria read percentage, and overall data quality. Following that, data composed of multiple samples and conditions requires batch effect correction and normalization [3, 21, 22]. Next, to achieve a basic understanding of the main trends and patterns in the dataset, some processing steps, including dimensionality reduction, feature selection, and clustering, are implemented [21, 23,24,25,26]. Furthermore, the diversified downstream analysis allows the researchers to uncover gene expression differences between different groups, investigate changes in gene regulatory networks, and infer trajectories of distinct cell lineages [3, 27,28,29].
To make the best use of scRNA-seq data, multiple analysis tools have been developed to optimize the individual steps [30]. For quality control, there are Cell Ranger (10 × Genomics) and Kallisto/bustools [31]. For preprocessing and harmonization, there are Seurat [32, 33], Scanpy [34], Harmony [35], and LIGER [36, 37]. The term, harmonization, was used for scRNA-seq data to emphasize that the data come from different sources [38]. Harmonization also differs from batch correction because it usually corrects the data on a 2-dimensional UMAP or t-SNE space rather than adjusting the UMI counts directly. Furthermore, Seurat and Scanpy can also be used to perform further data processing and downstream analysis [39]. However, having to choose different methods for the different steps, users are faced with the daunting tasks of tool comparisons, individual parameter optimizations, and the integration of multiple packages while assuring the reproducibility of the results. Thus, an automatic workflow with the combination of state-of-the-art methods, equipped with metrics evaluations and visualizations to help make decisions, will significantly benefit scRNA-seq data analyses by making them simpler and faster.
A similar R-based workflow, scFlow, has been developed to standardize the whole framework, which allows QC, integration, clustering, cell type annotation, differential expression (DE) analysis, and pathway analysis, but it does not provide downstream data visualization and sharing options [40]. Additionally, when the number of cells is over one million, R has a limitation in reading in such a large (one million by 20 k gene) matrix and creating a sparse matrix. To overcome these limitations, we developed scRNASequest, an end-to-end pipeline for single-cell RNA-seq analysis. Our implementation using both Python and R allows users to handle more than one million cells [34, 41]. Moreover, the pipeline is also customizable for parameter adjustment and diverse output formats, including a bookdown report [42], a slide deck presentation, and an integrated h5ad file, which can be visualized in cellxgene VIP [43, 44] and integrated into the CellDepot data repository [45]. Detailed comparisons of scRNASequest with similar tools have been summarized, highlighting unique advantages of our pipeline (Table S1). Overall, scRNASequest simplifies and generalizes the single-cell RNA-seq workflow, which offers the opportunity to analyze large datasets in a standard and time-efficient manner.
Implementation
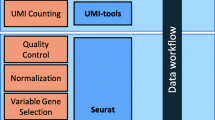
The scRNAsequest is a generalized pipeline including the critical steps necessary to perform an end-to-end scRNA-seq analysis (Fig. 1, Table 1). It is fully compatible with SGE and Slurm high-performance computing (HPC) clusters, and users can specify the HPC type and CPU number in the configuration file (config.yml). We offer flexible installation options using Conda or Docker, with the latter ensuring better distribution of the pipeline across different operating systems, including Linux and MacOS. Moreover, detailed instruction has been included in both the GitHub webpage and online tutorial, coupled with a demonstration dataset. Before initiating scRNAsequest, Cell Ranger has to be run on the raw sequencing data to generate basic quality metrics and the UMI count matrix [46]. Since our pipeline does not use gene annotation or any species-specific information, it requires the users to choose the correct species when running Cell Ranger.
Ambient RNA removal
Ambient RNAs are contaminating RNAs in the cell suspension during scRNA-seq sample preparation [47]. Single-nucleus RNA-seq, with cell lysed during the nuclei extraction step, is particularly prone to ambient RNA contamination. In a recent paper [48], CellBender [49] outperforms other tools including DecontX [50] and SoupX [51]. Thus, before running the full workflow, we incorporated an optional step called scRMambient to remove ambient RNAs using CellBender.
Quality control and filtering of single-cell RNA-seq data
The pipeline starts with setting several parameters used for assessing data quality control defined in the configuration file (config.yml, Table 2). Multiple plots are generated to visually aid this QC assessment, indicating the number of genes detected, total UMI counts, and percentage of mitochondrial reads at a single-cell level. If the Cell Ranger-generated summary of sequencing metrics is available (metrics_summary.csv), the pipeline will also generate figures based on that. This step was implemented using Scanpy [34] and employs multiple adjustable parameters to filter out low-quality data (Table 3). Users can remove low-expression genes detected only in a small number of cells (min.cells = 3), low-quality cells with a limited number of genes expressed (min.features = 50), or potential doublets with too many counts (highCount.cutoff = 10,000) and an unusually high number of genes detected (highGene.cutoff = 3,000). The numbers in parentheses are the empirically established default values of some of the adjustable parameters used at this step.
Data harmonization and result evaluation
The scRNASequest pipeline takes filtered UMI count matrices from different samples or batches to create an integrated data object through predefined state-of-the-art methods [52, 53], including Seurat [32], Seurat RPCA [33], Harmony [35], and LIGER [36]. Log normalization or SCtransform [54] can be applied to the UMI matrix of each sample to obtain the normalized expression levels per cell and per gene. In addition, we implemented the kBET [55] (Fig. S1A) and silhouette [55,56,57] (Fig. S1B) metrics to evaluate the performance of different harmonization methods against samples or batches.
Reference-based cell type annotation
If a reference dataset is provided, the cell type annotation can be transferred by the Seurat reference mapping method to facilitate result interpretation [32, 33]. We also developed a separate tool, scRef, to easily convert a previously analyzed dataset to reference data, which ensures the flexibility and reproducibility of cell type annotation. This reference-building step converts the scRNA-seq data into an R object, following the standardized format of Azimuth reference (https://github.com/satijalab/azimuth-references) [32].
Differential expression analysis
The differentially expressed genes between different phenotypically labeled within a cell group defined by either cell types or cluster annotations can be calculated using the following methods: NEBULA [58], glmmTMB [59], and MAST [60]. If needed, scRNASequest also offers pseudo-bulk-based differential expression (DE) analysis using DESeq2 [61], limma [62], and edgeR [63]. However, as the benchmarking study reported previously, NEBULA outperforms other methods in general [64]. Thus, the pipeline uses NEBULA to perform DE analysis by default, and the parameters for running this step are defined in the configuration file (Table 2). A second input file listing the pairwise comparisons is also required. A configuration file with default parameters and an empty comparison file containing only the header line are automatically generated at the first call of the pipeline with the path to the data folder as an argument. The user has to populate the comparison file, otherwise no DE analysis will be performed. The output of DE analysis includes a table summary of gene name, fold change, p-value and adjusted p-value, and related figures such as volcano plots. To facilitate DE analysis on previously analyzed data, we also incorporated a standalone DE function called scDEG.
Standardized output
The pipeline generates h5ad files to store the final results. This file type is compatible with both Python and R interfaces and can be easily visualized through the cellxgene VIP platform [43, 44]. Furthermore, if the data meets the quality requirements, the analyst can publish the analysis result to the CellDepot [45] to facilitate data exploration and sharing, as well as long-term data management.
User provides gene expression UMI count matrix files (h5 or MEX) from Cell Ranger and sample metadata to the semi-automated workflow, scRNASequest. It generates basic quality control (QC) reports and allows users to choose from popular data harmonization tools such as Harmony [35], LIGER [36], and Seurat [32, 33] to remove batch effects. Azimuth [32] reference-based cell label transfer is enabled as optional to perform cell type identification. Cluster- or cell-type-specific multi-sample multi-condition single cell level DE analysis is by default performed through NEBULA [58]. Finally, an h5ad file will be generated to be loaded into the cellxgene VIP [43, 44] framework or CellDepot [45] single-cell data management system for interactive visualization and analysis. The main script for the analysis is scAnalyzer. Five additional scripts are also included in the scRNASequest pipeline suite: scRMambient, scTool, scRef, sc2celldepot, and scDEG.
Results
Highlights of scRNASequest
The main program of the scRNASequest pipeline is scAnalyzer, which performs a variety of single-cell/single-nucleus data processing and analysis functions. Key strengths of this pipeline include:
-
1) Semi-automated with a minimal number of input files.
-
2) Fast and efficient data processing powered by Python.
-
3) Allowing immediate visualization of the QC report for users to fine-tune the filtering parameters.
-
4) Providing harmonization results in a single run: Seurat, Harmony, and LIGER, coupled with kBET and Silhouette metrics to evaluate the results.
-
5) Ensuring seamless connections with other tools such as cellxgene VIP and CellDepot.
Performing QC and cell filtering on a sample dataset
We used a previously published dataset [65] to illustrate the QC step of scRNASequest. This mouse brain single-nucleus RNA-seq data contains six samples in total, three of them were from one mouse brain, and the other three were collected from another mouse. First, the pipeline generated the scatter plot of the number of genes detected versus the total counts, where n_genes_by_counts refers to the number of genes with at least one count in a cell) (Fig. 2A). It applied the default filtering criteria (min.cells = 3, min.features = 50, highCount.cutoff = 10,000, highGene.cutoff = 3,000) to generate the corresponding post-filtering plot (Fig. 2B). It also produced violin plots to provide an intuitive view of the pre- and post-filtering total count per cell distributions across samples (Fig. 2C, D). In addition, this QC step generated a variety of other plots, including the expression of the top 20 highly expressed genes, the percentage of mitochondrial reads, the percentage of reads mapped to various genomic regions (genome, gene, exonic regions, intronic regions, intergenic regions, antisense to gene regions, and transcriptome), and the percentage of Q30 bases in different read regions (barcode, RNA read, sample index and UMI). Finally, a summary was compiled with the number of cells and genes before and after each filtering step (Table 3). As expected, we observed decreasing cell counts and gene numbers due to the filtering steps, keeping only the cells of good quality. In this demo dataset, we eventually acquired 29,511 cells (in this case, nuclei) covering 25,115 genes for the downstream analysis.
QC plots of the number of genes with greater than 0 counts per cell and total counts, before and after the filtering step. Scatter plot of total counts (X-axis, using the total_counts parameter from Scanpy QC metrics) and the number of genes (Y-axis, using the n_genes_by_counts parameter from Scanpy QC metrics) detected before (A) and after (B) filtering out low-quality cells and lowly expressed genes. Violin plots for each individual sample before (C) and after (D) filtering. The blue lines in C and D show the median counts of all data, with the exact number on top of the plots
Delivering QC metrics through a Bookdown report
Besides the h5ad and RDS files with the processed data, the pipeline generates a report to convey the complex concepts of the scRNA-seq analysis and provide immediate access to the results. This document is generated using the bookdown R package and includes the key tables and figures generated by the scRNASequest workflow. Bookdown originated from R Markdown and is dynamically generated, with code and figures embedded together in a book layout [42]. The bookdown document generated by our pipeline is an interactive webpage in HTML format, allowing the user to explore the results in a web browser without installing any specific software. It contains a high-level summary of the project, such as the data quality, mapping metrics, cell distribution, and top gene expression values before and after filtering. A snapshot of the bookdown report is shown in Fig. 3A. Further, it can be hosted on GitHub, as exemplified here (https://tinyurl.com/bdebvdz4), for broad sharing, especially in publications. In this report, the figures were organized into three different sections: 1) QC plots, 2) Pre-filtering plots, and 3) Post-filtering plots. It offers the flexibility to switch to and visualize different plots by selecting the items of interest from the left menu bar (Fig. 3B-C).
Bookdown report. A Bookdown document generated from the mouse brain snRNA-seq data, showing the preface. Each individual item on the left leads to related plots or tables. Clicking the items on the navigation bar will open corresponding pages. Two highlighted examples are in panels B and C. B An example of the ‘Number of Genes’ plot layout. C An example of the ‘Top Genes’ plot layout
Presenting the results to collaborators with the slide deck
Powered by reveal.js, an R markdown template is utilized by scRNASequest to generate an interactive slide deck with an emphasis on the graphical representation of QC and basic analysis plots. This makes the primary analysis presentation-ready for engaging biologists to discuss the initial results immediately after the pipeline run is finished. This slide deck can be opened using a web browser without the need for installing other software. It also allows the users to add notes to the slides or navigate to any other pages easily using the buttons on the corners. A full example of the slide deck can be found at the following link: https://tinyurl.com/bdepwy69.
Data harmonization
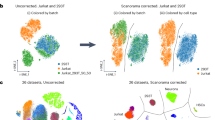
Due to the high prevalence of batch effects in scRNA-seq datasets, data harmonization is critical when integrating multiple datasets and enables the identification of biological differences instead of technical ones. Through running scRNASequest, we can retrieve the results from different harmonization tools easily using the output h5ad file. To compare the outputs of these methods side-by-side, UMAP embedding plots were used to visualize the cells before and after harmonization (Fig. 4A-E) [66]. The pipeline also generated kBET and silhouette metrics (Fig. S1A-B) to quantify the performance of each harmonization strategy [55]. Lower kBET scores and higher silhouette coefficients are better. Based on the demo dataset, LIGER turned out to be the best tool to harmonize multiple data together (Fig. S1A-B).
Cell type label transfer
Automated cell type label transfer provides a fast and reproducible way to interpret the scRNA-seq results. We employed Seurat reference-based label transfer to annotate the cell types in our data. As a result, the algorithm identified major cell types in the mouse brain, including astrocytes, microglia, neurons, oligodendrocytes, and oligodendrocyte progenitor cells (OPCs). We used the UMAP after LIGER harmonization to present the label transfer results (Fig. 5A), with five major cell types separated clearly. We validated the correctness of the label transfer by identifying the most highly expressed genes in each cell type. We computed the top three genes within each cell type using the Scanpy rank_genes_groups function and presented the results in dotplots, comparing both reference dataset and query dataset (Fig. 5B). Many of these genes have been used as cell type marker genes in PanglaoDB [67]. For example, Mog is a marker gene for Oligodendrocytes, Csf1r has been used for microglia, Cspg4 and Vcan are markers for OPC. Similarly, Slc1a2 is a commonly used marker for astrocytes, while Syt1 is a neuronal cell marker. Due to the heterogeneity of neurons, we did not expect to see any neuron subtype specific markers showing up as top marker genes in this analysis.
Differential expression (DE) analysis
Differential expression analysis is critical for identifying molecular differences underlying different conditions. Based on a recent benchmarking analysis, NEBULA has been identified as an ideal DE analysis method in general [64]. In scRNASequest, we incorporated NEBULA as the default method for the DE analysis step [58]. However, users can also choose from a number of DE methods we have implemented in the pipeline, including glmmTMB [59] and MAST [60]. In our demo dataset, we performed DE analysis on each cell type by comparing male versus female samples. Xist, the X-inactive specific transcript gene on the X chromosome, was identified as the most significantly decreased gene in males, which provides a proof-of-concept example of the DE analysis (Fig. 6A, Table S2) [68]. In addition to the DE gene table and the volcano plot, the pipeline also provides QC figures to display the total UMI counts and the number of expressed genes in the cells included in the comparison (Fig. 6B-C, Fig. S2-3).
Differential expression analysis of male versus female in astrocytes. A Volcano plot of all genes with up- and down-regulated genes highlighted, respectively. Up-regulated genes were defined as genes with FDR < 0.05 and log2 FC > 1, and down-regulated genes were defined as genes with FDR < 0.05 and log2 FC < -1. B Histogram of total UMI counts. C Histogram of the number of expressed genes in each cell
Reference building using scRef
Label transfer extracts the cell-type-specific gene expression signatures from previously analyzed datasets and applies the identified patterns to the new data. This step is critical for scRNA-seq data analysis, since cell-type labeling facilitates functional dissection and biological interpretation. However, the lack of proper references with matching cell type composition to the analyzed dataset can pose a challenge. Here, we provide scRef, a standalone functionality to process previously analyzed public datasets and embed their labeling information into a formatted reference file. This reference can then be used as input to the scRNASequest pipeline and guide the label transfer using pre-existing knowledge. scRef enables the user to bypass the laborious and limited cell-type annotation process of using only a small set of marker genes and enhances the efficiency and reproducibility of the analysis.
Data visualization and exploration using Cellxgene VIP
Cellxgene VIP is a plug-in tool to generate various figures for the processed dataset [43]. It was developed based on the cellxgene [44] single-cell visualization platform that displays categorical and numeric metadata information, as well as UMAP and PCA embedding plots. To facilitate smooth navigation of the dataset for scientists without programming backgrounds, scRNASequest generates an h5ad file that can be easily loaded into cellxgene VIP [43]. While cellxgene only provides a limited set of functions to explore the data, cellxgene VIP provides an expanded array of features: violin plots, volcano plots, dot plots, heatmaps, and complex figures in this all-in-one plug-in. As an example, we show that the Xist gene indeed has lower expression in the male compared to the female (Fig. 7). Detailed tutorial of the cellxgene VIP functionalities can be found on the tutorial page (https://interactivereport.github.io/cellxgene_VIP/tutorial/docs/).
Integrating public datasets using sc2celldepot
We developed an API to load the scRNASequest result into a web-based application, CellDepot [45], designed to manage datasets from multiple projects and enable cross-dataset queries to create gene expression profiles in violin or dot plot formats. CellDepot also offers advanced search and filtering functions to locate datasets of interest quickly. To allow seamless export of scRNASequest outputs to CellDepot, the pipeline generates h5ad files containing all the necessary project and data information. However, it would be unnecessary and tedious work to run the entire pipeline on a previously analyzed public dataset (e.g., with cell type already labeled). Thus, we developed a functionality called sc2celldepot to easily convert public datasets into the required format and publish them into CellDepot.
Discussion
With the nearly exponentially growing amount of scRNA-seq data generated, having a standardized and automated analysis pipeline is critical for efficiently processing and interpreting such data. To fulfill this need, we developed scRNASequest by incorporating multiple state-of-the-art tools into a scRNA-seq analysis pipeline with full integration between its component functionalities. Compared to other scRNA-seq analytic workflows, including scFlow [40], nf-core/scrnaseq [69], single-cell-rna-seq (https://github.com/snakemake-workflows/single-cell-rna-seq), scRNAseq_KNIME [70] and ASAP (https://github.com/DeplanckeLab/ASAP), scRNASequest offers several advantages (Table S1). First, scRNASequest uses clearly defined configuration files to set up analysis, allowing the users to fine-tune the parameters for specific steps. Also, its output results are compatible with multiple downstream tools and platforms, saving time for cross-platform data conversion. Moreover, we not only offer analysis pipelines but also provide cellxgene VIP and CellDepot as data visualization and management tools, making scRNASequest an ecosystem for scRNA-seq data analysis.
Interactive visualization has been a crucial component for interpreting scRNA-seq results, and several tools and platforms have been developed to meet this need, such as iSEE [71], scSVA [72], SCope (https://github.com/aertslab/Scope), etc. [73]. We performed a systematic comparison demonstrating the diverse features of cellxgene VIP and existing tools (Table S3). Overall, cellxgene VIP shows advantages in interactive data analysis and flexibility in web sharing.
Given the rapidly evolving nature of single-cell omics technologies, we are devoted to implementing new features of scRNASequest in the future. We plan to: 1) support single-cell multimodal omics data analysis such as scATAC-seq and spatial transcriptomics, 2) incorporate trajectory analysis using Monocle3 and RNA Velocity, 3) implement cell–cell communication inference tools, and 4) allow more quality control features such as doublet/multiplet removal.
Conclusion
scRNASequest offers a one-stop-shop for analysts to process scRNA-seq data starting from UMI counts in either h5 or MEX format, perform harmonization of data from multiple samples, annotate cell types based on reference data, identify differentially expressed genes, and generate a suite of interactive reports in easy-to-access formats to enable biologists without advanced computational skills to explore the data interactively. With several user-friendly features such as the bookdown report, slide deck presentation, and cellxgene VIP exploration, users have the flexibility to analyze and share the results in multiple ways. The seamless integration with CellDepot makes data management and sharing possible for a collection of such datasets.
In summary, scRNASequest provides a user-friendly end-to-end pipeline for single-cell RNA-seq data analysis, visualization, and publishing to empower biologists to gain insights from high-volume sequencing data in digestible forms.
Availability of data and materials
Project name: scRNASequest.
Project home page: https://github.com/interactivereport/scRNASequest
Operating system(s): Linux system, MacOS.
Software prerequisite(s): Conda or Docker.
Programming language: Bash, Python, R
License: MIT license.
Any restrictions to use by non-academics: MIT license.
Single-nucleus RNA-seq data: E-MTAB-11115 (ArrayExpression accession) and GSE185538 (GEO accession).
Abbreviations
- scRNA-seq:
-
Single-cell RNA-seq
- HPC:
-
High-performance computing
- NGS:
-
Next-generation sequencing
- QC:
-
Quality control
- DE:
-
Differential expression
- UMI:
-
Unique molecular identifier
- FDR:
-
False discovery rate
- PCA:
-
Principal component analysis
- RPCA:
-
Reciprocal principal component analysis
- UMAP:
-
Uniform Manifold Approximation and Projection
- kBET:
-
K-nearest neighbor batch effect test
- OPC:
-
Oligodendrocyte progenitor cells
- Cellxgene VIP:
-
Cellxgene visualization in plug-in
References
Saliba AE, Westermann AJ, Gorski SA, Vogel J. Single-cell RNA-seq: advances and future challenges. Nucleic Acids Res. 2014;42(14):8845–60.
Kolodziejczyk AA, Kim JK, Svensson V, Marioni JC, Teichmann SA. The technology and biology of single-cell RNA sequencing. Mol Cell. 2015;58(4):610–20.
Chen G, Ning B, Shi T. Single-Cell RNA-Seq Technologies and Related Computational Data Analysis. Front Genet. 2019;10:317.
Hwang B, Lee JH, Bang D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med. 2018;50(8):1–14.
Kulkarni A, Anderson AG, Merullo DP, Konopka G. Beyond bulk: a review of single cell transcriptomics methodologies and applications. Curr Opin Biotechnol. 2019;58:129–36.
Marsh SE, Walker AJ, Kamath T, Dissing-Olesen L, Hammond TR, de Soysa TY, Young AMH, Murphy S, Abdulraouf A, Nadaf N, et al. Dissection of artifactual and confounding glial signatures by single-cell sequencing of mouse and human brain. Nat Neurosci. 2022;25(3):306–16.
Bocchi VD, Conforti P, Vezzoli E, Besusso D, Cappadona C, Lischetti T, Galimberti M, Ranzani V, Bonnal RJP, De Simone M, et al. The coding and long noncoding single-cell atlas of the developing human fetal striatum. Science. 2021;372(6542):eabf5759.
Melms JC, Biermann J, Huang H, Wang Y, Nair A, Tagore S, Katsyv I, Rendeiro AF, Amin AD, Schapiro D, et al. A molecular single-cell lung atlas of lethal COVID-19. Nature. 2021;595(7865):114–9.
Stephenson E, Reynolds G, Botting RA, Calero-Nieto FJ, Morgan MD, Tuong ZK, Bach K, Sungnak W, Worlock KB, Yoshida M, et al. Single-cell multi-omics analysis of the immune response in COVID-19. Nat Med. 2021;27(5):904–16.
Wimmers F, Donato M, Kuo A, Ashuach T, Gupta S, Li C, Dvorak M, Foecke MH, Chang SE, Hagan T, et al. The single-cell epigenomic and transcriptional landscape of immunity to influenza vaccination. Cell. 2021;184(15):3915-3935 e3921.
Nieto P, Elosua-Bayes M, Trincado JL, Marchese D, Massoni-Badosa R, Salvany M, Henriques A, Nieto J, Aguilar-Fernandez S, Mereu E, et al. A single-cell tumor immune atlas for precision oncology. Genome Res. 2021;31(10):1913–26.
Garcia-Alonso L, Lorenzi V, Mazzeo CI, Alves-Lopes JP, Roberts K, Sancho-Serra C, Engelbert J, Mareckova M, Gruhn WH, Botting RA, et al. Single-cell roadmap of human gonadal development. Nature. 2022;607(7919):540–7.
Sohni A, Tan K, Song HW, Burow D, de Rooij DG, Laurent L, Hsieh TC, Rabah R, Hammoud SS, Vicini E, et al. The Neonatal and Adult Human Testis Defined at the Single-Cell Level. Cell Rep. 2019;26(6):1501-1517 e1504.
Wagner M, Yoshihara M, Douagi I, Damdimopoulos A, Panula S, Petropoulos S, Lu H, Pettersson K, Palm K, Katayama S, et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun. 2020;11(1):1147.
Wu SZ, Al-Eryani G, Roden DL, Junankar S, Harvey K, Andersson A, Thennavan A, Wang C, Torpy JR, Bartonicek N, et al. A single-cell and spatially resolved atlas of human breast cancers. Nat Genet. 2021;53(9):1334–47.
Ma L, Wang L, Khatib SA, Chang CW, Heinrich S, Dominguez DA, Forgues M, Candia J, Hernandez MO, Kelly M, et al. Single-cell atlas of tumor cell evolution in response to therapy in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Hepatol. 2021;75(6):1397–408.
Smalley I, Chen Z, Phadke M, Li J, Yu X, Wyatt C, Evernden B, Messina JL, Sarnaik A, Sondak VK, et al. Single-Cell Characterization of the Immune Microenvironment of Melanoma Brain and Leptomeningeal Metastases. Clin Cancer Res. 2021;27(14):4109–25.
Bollen Y, Stelloo E, van Leenen P, van den Bos M, Ponsioen B, Lu B, van Roosmalen MJ, Bolhaqueiro ACF, Kimberley C, Mossner M, et al. Reconstructing single-cell karyotype alterations in colorectal cancer identifies punctuated and gradual diversification patterns. Nat Genet. 2021;53(8):1187–95.
Ho DW, Tsui YM, Chan LK, Sze KM, Zhang X, Cheu JW, Chiu YT, Lee JM, Chan AC, Cheung ET, et al. Single-cell RNA sequencing shows the immunosuppressive landscape and tumor heterogeneity of HBV-associated hepatocellular carcinoma. Nat Commun. 2021;12(1):3684.
Yao J, Cui Q, Fan W, Ma Y, Chen Y, Liu T, Zhang X, Xi Y, Wang C, Peng L, et al. Single-cell transcriptomic analysis in a mouse model deciphers cell transition states in the multistep development of esophageal cancer. Nat Commun. 2020;11(1):3715.
Luecken MD, Theis FJ. Current best practices in single-cell RNA-seq analysis: a tutorial. Mol Syst Biol. 2019;15(6):e8746.
Vallejos CA, Risso D, Scialdone A, Dudoit S, Marioni JC. Normalizing single-cell RNA sequencing data: challenges and opportunities. Nat Methods. 2017;14(6):565–71.
Sun S, Zhu J, Ma Y, Zhou X. Accuracy, robustness and scalability of dimensionality reduction methods for single-cell RNA-seq analysis. Genome Biol. 2019;20(1):269.
Feng C, Liu S, Zhang H, Guan R, Li D, Zhou F, Liang Y, Feng X. Dimension reduction and clustering models for single-Cell RNA sequencing data: a comparative study. Int J Mol Sci. 2020;21(6):2181.
Qi R, Ma A, Ma Q, Zou Q. Clustering and classification methods for single-cell RNA-sequencing data. Brief Bioinform. 2020;21(4):1196–208.
Su K, Yu T, Wu H. Accurate feature selection improves single-cell RNA-seq cell clustering. Brief Bioinform. 2021;22(5):bbab034.
Andrews TS, Kiselev VY, McCarthy D, Hemberg M. Tutorial: guidelines for the computational analysis of single-cell RNA sequencing data. Nat Protoc. 2021;16(1):1–9.
Lahnemann D, Koster J, Szczurek E, McCarthy DJ, Hicks SC, Robinson MD, Vallejos CA, Campbell KR, Beerenwinkel N, Mahfouz A, et al. Eleven grand challenges in single-cell data science. Genome Biol. 2020;21(1):31.
Jackson CA, Castro DM, Saldi GA, Bonneau R, Gresham D. Gene regulatory network reconstruction using single-cell RNA sequencing of barcoded genotypes in diverse environments. Elife. 2020;9:e51254.
Vieth B, Parekh S, Ziegenhain C, Enard W, Hellmann I. A systematic evaluation of single cell RNA-seq analysis pipelines. Nat Commun. 2019;10(1):4667.
Melsted P, Booeshaghi AS, Liu L, Gao F, Lu L, Min KHJ, da Veiga BE, Hjorleifsson KE, Gehring J, Pachter L. Modular, efficient and constant-memory single-cell RNA-seq preprocessing. Nat Biotechnol. 2021;39(7):813–8.
Hao Y, Hao S, Andersen-Nissen E, Mauck WM 3rd, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184(13):3573-3587 e3529.
Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, Hao Y, Stoeckius M, Smibert P, Satija R. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888-1902 e1821.
Wolf FA, Angerer P, Theis FJ. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19(1):15.
Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, Baglaenko Y, Brenner M, Loh PR, Raychaudhuri S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat Methods. 2019;16(12):1289–96.
Welch JD, Kozareva V, Ferreira A, Vanderburg C, Martin C, Macosko EZ. Single-cell multi-omic integration compares and contrasts features of brain cell identity. Cell. 2019;177(7):1873-1887 e1817.
Liu J, Gao C, Sodicoff J, Kozareva V, Macosko EZ, Welch JD. Jointly defining cell types from multiple single-cell datasets using LIGER. Nat Protoc. 2020;15(11):3632–62.
Xu C, Lopez R, Mehlman E, Regier J, Jordan MI, Yosef N. Probabilistic harmonization and annotation of single-cell transcriptomics data with deep generative models. Mol Syst Biol. 2021;17(1):e9620.
Kharchenko PV. The triumphs and limitations of computational methods for scRNA-seq. Nat Methods. 2021;18(7):723–32.
Khozoie C, Fancy N, Marjaneh MM, Murphy AE, Matthews PM, Skene N. scFlow: A Scalable and Reproducible Analysis Pipeline for Single-Cell RNA Sequencing Data. bioRxiv. 2021:2021-08. Preprint at: https://www.biorxiv.org/content/10.1101/2021.08.16.456499v2.abstract.
Amezquita RA, Lun ATL, Becht E, Carey VJ, Carpp LN, Geistlinger L, Marini F, Rue-Albrecht K, Risso D, Soneson C, et al. Orchestrating single-cell analysis with Bioconductor. Nat Methods. 2020;17(2):137–45.
Xie Y. Bookdown. 2016.
Li K, Ouyang Z, Chen Y, Gagnon J, Lin D, Mingueneau M, Chen W, Sexton D, Zhang B. Cellxgene VIP unleashes full power of interactive visualization and integrative analysis of scRNA-seq, spatial transcriptomics, and multiome data. bioRxiv. 2020:2020-08. Preprint at: https://www.biorxiv.org/content/10.1101/2020.08.28.270652v2.abstract.
Megill C, Martin B, Weaver C, Bell S, Prins L, Badajoz S, McCandless B, Pisco AO, Kinsella M, Griffin F, et al. cellxgene: a performant, scalable exploration platform for high dimensional sparse matrices. bioRxiv. 2021:2021-04. Preprint at: https://www.biorxiv.org/content/10.1101/2021.04.05.438318v1.abstract.
Lin D, Chen Y, Negi S, Cheng D, Ouyang Z, Sexton D, Li K, Zhang B. Cell Depot: A Unified Repository for scRNA-seq Data and Visual Exploration. J Mol Biol. 2022;434(11):167425.
Shainer I, Stemmer M. Choice of pre-processing pipeline influences clustering quality of scRNA-seq datasets. BMC Genomics. 2021;22(1):661.
Slovin S, Carissimo A, Panariello F, Grimaldi A, Bouche V, Gambardella G, Cacchiarelli D. Single-Cell RNA Sequencing Analysis: A Step-by-Step Overview. Methods Mol Biol. 2021;2284:343–65.
Caglayan E, Liu Y, Konopka G. Neuronal ambient RNA contamination causes misinterpreted and masked cell types in brain single-nuclei datasets. Neuron. 2022;110(24):4043-4056 e4045.
Fleming SJ, Marioni JC, Babadi M. CellBender remove-background: a deep generative model for unsupervised removal of background noise from scRNA-seq datasets. bioRxiv. 2019:791699. Preprint at: https://www.biorxiv.org/content/10.1101/791699v1.
Yang S, Corbett SE, Koga Y, Wang Z, Johnson WE, Yajima M, Campbell JD. Decontamination of ambient RNA in single-cell RNA-seq with DecontX. Genome Biol. 2020;21(1):57.
Young MD, Behjati S. SoupX removes ambient RNA contamination from droplet-based single-cell RNA sequencing data. Gigascience. 2020;9(12):151.
Tran HTN, Ang KS, Chevrier M, Zhang X, Lee NYS, Goh M, Chen J. A benchmark of batch-effect correction methods for single-cell RNA sequencing data. Genome Biol. 2020;21(1):12.
Luecken MD, Buttner M, Chaichoompu K, Danese A, Interlandi M, Mueller MF, Strobl DC, Zappia L, Dugas M, Colome-Tatche M, et al. Benchmarking atlas-level data integration in single-cell genomics. Nat Methods. 2022;19(1):41–50.
Hafemeister C, Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019;20(1):296.
Buttner M, Miao Z, Wolf FA, Teichmann SA, Theis FJ. A test metric for assessing single-cell RNA-seq batch correction. Nat Methods. 2019;16(1):43–9.
Shahapure KR, Nicholas C. Cluster quality analysis using silhouette score. In: 2020 IEEE 7th International Conference on Data Science and Advanced Analytics (DSAA). 2020. p. 747–8.
Aranganayagi S, Thangavel K. Clustering categorical data using silhouette coefficient as a relocating measure. In: International Conference on Computational Intelligence and Multimedia Applications (ICCIMA 2007). 2007. p. 13–7.
He L, Davila-Velderrain J, Sumida TS, Hafler DA, Kellis M, Kulminski AM. NEBULA is a fast negative binomial mixed model for differential or co-expression analysis of large-scale multi-subject single-cell data. Commun Biol. 2021;4(1):629.
Brooks ME, Kristensen K, Van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R journal. 2017;9(2):378–400.
Finak G, McDavid A, Yajima M, Deng J, Gersuk V, Shalek AK, Slichter CK, Miller HW, McElrath MJ, Prlic M, et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 2015;16:278.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–40.
Gagnon J, Pi L, Ryals M, Wan Q, Hu W, Ouyang Z, Zhang B, Li K. Recommendations of scRNA-seq differential gene expression analysis based on comprehensive benchmarking. Life (Basel). 2022;12(6):850.
Kleshchevnikov V, Shmatko A, Dann E, Aivazidis A, King HW, Li T, Elmentaite R, Lomakin A, Kedlian V, Gayoso A, et al. Cell 2location maps fine-grained cell types in spatial transcriptomics. Nat Biotechnol. 2022;40(5):661–71.
Becht E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG, Ginhoux F, Newell EW. Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol. 2019;37(1):38-44.
Franzen O, Gan LM, Bjorkegren JLM. PanglaoDB: a web server for exploration of mouse and human single-cell RNA sequencing data. Database (Oxford). 2019;2019:046.
Loda A, Heard E. Xist RNA in action: Past, present, and future. PLoS Genet. 2019;15(9):e1008333.
Ewels PA, Peltzer A, Fillinger S, Patel H, Alneberg J, Wilm A, Garcia MU, Di Tommaso P, Nahnsen S. The nf-core framework for community-curated bioinformatics pipelines. Nat Biotechnol. 2020;38(3):276–8.
Kausar S, Asif M, Baudot A. scRNAseq_KNIME workflow: A Customizable, Locally Executable, Interactive and Automated KNIME workflow for single-cell RNA seq. bioRxiv. 2023:2023-01. Preprint at: https://www.biorxiv.org/content/10.1101/2023.01.14.524084v1.abstract.
Rue-Albrecht K, Marini F, Soneson C, Lun ATL. iSEE: Interactive SummarizedExperiment Explorer. F1000Res. 2018;7:741.
Tabaka M, Gould J, Regev A. scSVA: an interactive tool for big data visualization and exploration in single-cell omics. bioRxiv. 2019:512582. Preprint at: https://www.biorxiv.org/content/10.1101/512582v1.
Cakir B, Prete M, Huang N, van Dongen S, Pir P, Kiselev VY. Comparison of visualization tools for single-cell RNAseq data. NAR Genom Bioinform. 2020;2(3):lqaa052.
Acknowledgements
We acknowledge scientists at Biogen who provided feedback on the pipeline.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
B.Z. supervised the study. K.L., Y.H.S., Z.O., W.H., and X.Z. developed the software. Y.H.S. and W.W. wrote the manuscript with editing from M.I.Z., F.C., and B.Z. Z.G., J.Z., Y.H.S., and Z.O. revised the manuscript. All authors tested the pipeline and reviewed the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
K.L., Y.H.S., S.N., Z.G., J.Z., Y.C., S.P., W.H., M.I.Z., H.Y., S.C., A.G., M.S., D.H., F.C., and B.Z. are employees of Biogen and hold stocks from the company. W.W. was a co-op at Biogen and does not hold Biogen’s stocks. Z.O. and X.Z. are employees of BioInfoRx, Inc.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table S1.
Detailed comparison of multiple single-cell RNA-seq data processing workflows.
Additional file 2: Supplementary Table S2.
An example of NEBULA differential expression analysis results. FDR: False discovery rate. The NEBULA-HL method was used.
Additional file 3: Supplementary Table S3.
Detailed comparison of multiple single-cell RNA-seq data visualization software.
Additional file 4: Fig. S1.
Evaluation metrics for harmonization. (A) Batch correction performance evaluation by plots of kBET metrics and (B). Silhouette coefficients. For our demo dataset, LIGER was identified as the best batch correction method. Fig. S2. Percentage of top 50 features. Fig. S3. Number of cells with UMI > 0 for each gene (left) and the percentage of cells with UMI > 0 for each gene (right).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, K., Sun, Y.H., Ouyang, Z. et al. scRNASequest: an ecosystem of scRNA-seq analysis, visualization, and publishing. BMC Genomics 24, 228 (2023). https://doi.org/10.1186/s12864-023-09332-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09332-2