Abstract
Background
Red-tail catfish (Hemibagrus wyckioides) is an important commercially farmed catfish in southern China. Males of red-tail catfish grow faster than females, suggesting that all-male catfish will produce more significant economic benefits in aquaculture practice. However, little research has been reported on sex determination and gonadal development in red-tail catfish.
Results
In this study, we performed the first transcriptomic analysis of male and female gonads at four developmental stages at 10, 18, 30, and 48 days post hatching (dph) using RNA-seq technology. A total of 23,588 genes were screened in 24 sequenced samples, of which 28, 213, 636, and 1381 differentially expressed genes (DEGs) were detected at four developmental stages, respectively. Seven candidate genes of sex determination and differentiation were further identified. Real-time quantitative PCR (RT-qPCR) further confirmed that anti-Mullerian hormone (amh), growth differentiation factor 6a (gdf6a), testis-specific gene antigen 10 (tsga10), and cytochrome P450 family 17 subfamily A (cyp17a) were highly expressed mainly in the male, while cytochrome P450 family 19 subfamily A polypeptide 1b (cyp19a1b), forkhead box L2 (foxl2), and hydroxysteroid 17-beta dehydrogenase 1 (hsd17b1) were highly expressed in the female. The KEGG pathway enrichment data showed that these identified DEGs were mainly involved in steroid hormone biosynthesis and TGF-β signaling pathways.
Conclusions
Based on RNA-seq data of gonads at the early developmental stages, seven DEGs shared by the four developmental stages were identified, among which amh and gdf6a may be the male-biased expression genes, while foxl2, cyp19a1b and hsd17b1 may be the female-biased expression genes in red-tail catfish. Our study will provide crucial genetic information for the research on sex control in red-tail catfish, as well as for exploring the evolutionary processes of sex determination mechanisms in fish.
Similar content being viewed by others
Background
Red-tail catfish (Hemibagrus wyckioides), a freshwater species of the family Hemibagrus, Bagridae, Siluriformes, is mainly distributed in multiple Southeast Asian countries and the Lancang River of the Yunnan Province, China [1, 2]. Owing to its high protein content, excellent production, strong disease resistance, and absence of intermuscular bones, red-tail catfish has shown a sustained increase in its culture in southern China and has become a momentous economic aquaculture species in China in recent years [3, 4]. Like other catfish species, such as yellow catfish (Pelteobagrus fulvidraco) [5, 6], channel catfish (Ictalurus punctatus) [7], Lanzhou catfish (Silurus lanzhouensis) [8], and Ussuri catfish (Pseudobagrus ussuriensis) [9, 10], red-tail catfish shows sexual dimorphism in growth and sex-specific markers has been identified for gender identification [11]. Sexual dimorphism in growth refers to the difference between males and females of the same species, which is very common in fish, such as Nile tilapia (Oreochromis niloticus) [12], half-smooth tongue sole (Cynoglossus semilaevis) [13], and blotched snakehead (Channa maculate) [14]. In aquaculture practice, it is of great economic significance to breed monosexual fish populations based on the regulation mechanism of sex determination and differentiation of fish [15,16,17,18]. Therefore, the identification of sex-specific markers or sex-related genes is necessary to reveal the mechanism of sex determination and differentiation in addition to the identification of physiological sex [19, 20].
Fish exhibit all known forms of vertebrate sex determination to adapt to the variable habitats own to its extreme diversity, that is, fish sex determination patterns can be classified as genotypic sex determination (GSD), environmental sex determination (ESD), and genotypic sex determination with environmental effect (GSD + ESD) [19,20,21,22,23]. The expression of sex determination genes regulates the signal pathways of sex determination and sex differentiation, inducing the development of primordial gonads into ovaries or testes [24]. Therefore, whether genes involved in sex determination are conserved throughout evolution has raised great research interest. Several genes have been confirmed as master genes of sex determination in some fish species, such as cyp19a1a in the Nile tilapia (Oreochromis niloticus) [25], dmy and gsdfY gene in the medaka fish [26, 27], amhr2 in fugu (Takifugu rubripes) [28], sdy in rainbow trout (Oncorhynchus mykiss) [29], pfpdz1 in yellow catfish (Pelteobagrus fulvidraco) [30], and bcar1 in channel catfish (Ictalurus punctatus) [31]. These results indicate that genes involved in sex determination and differentiation in fish vary significantly among genus, which has brought great difficulties to the deep revealing of the mechanism of fish sex determination and differentiation. Moreover, the sex chromosomes are generally poorly differentiated in Siluriformes [32], which makes it more difficult to screen the sex determining genes of red-tail catfish. However, no genes related to sex determination and differentiation have yet been identified in red-tail catfish, suggesting that more or novel sex-related genes should be identified to adequately explain the complex mechanism of sex determination.
Transcriptome sequencing is a cost-effective and time-effective method to screen sex determination-related genes and other causal genes [33,34,35]. Identification of pathways involved in gonadal development could further illuminate the gene regulatory network controlling sex determination and subsequent maintenance of phenotypic sex [36, 37]. Sex-biased genes are expressed either in one sex or at significantly different levels between two sexes and give rise to different phenotypic sexes, which has provided a mechanism for organisms to produce different adaptive phenotypes on the same genetic background [38, 39]. Therefore, in this study, we performed RNA-seq using testes and ovaries at undifferentiated and differentiated four stages in red-tail catfish. Furthermore, the expression patterns of sex-biased DEGs shared by the four developmental stages were analyzed to investigate the mechanism of sex determination and differentiation, which will help us to better reveal the evolution of sex chromosomes and the mechanism of sex determination in higher vertebrates.
Results
Sample collection according to the gonadal differentiation time
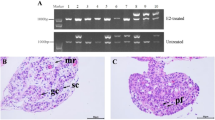
The time of gonadal differentiation was determined by histological analysis and it showed that there was no significant difference between female and male gonads before 10dph. At 18dph, female gonads gradually began to form ovarian cavities, but cavity structures did not appear in male gonads. From 30 to 48dph, the ovarian cavity gradually closed in the female gonads, whereas the male gonads still never developed a cavity structure (Fig. 1). It indicated that the gonads of two sexes began to show significantly morphological differences at 18dph or so, suggesting that the initial time of gonadal differentiation is around 18dph in red-tail catfish. Therefore, we sampled testis and ovary tissues at four stages before and after gonadal differentiation, namely, before differentiation (10dph), the initial time of gonadal differentiation (18dph), ovarian cavity fully formed (30dph), after differentiation (48dph). Therefore, a total of 24 cDNA libraries derived from male and female gonads at 10, 18, 30, and 48dph were constructed for transcriptome sequencing.
Summary statistics of RNA-seq data
The raw data of RNA-seq was deposited in the NCBI database Sequence Read Archive (PRJNA898908). A total of 1,959.69 M raw sequencing reads were generated with 48.49% GC content and Q30 bases distributed between 91.77 and 94.26%. After removing ambiguous nucleotides, 1922.16 M clean reads totaling 284.16 G bases were obtained for the following analysis. Then all the clean reads of each sample were mapped to the reference genome of red-tail catfish with an average total mapping ratio of 94.12 ± 1.23%, ranging from 91.69 to 95.77% (Table 1). In total, 23,588 genes were screened from the 24 sequencing samples for the following DEGs identification.
Identification and functional annotation of DEGs
At four developmental stages, 28, 213, 636, and 1381 DEGs were obtained, respectively (Fig. 2). The number of DEGs continued to increase with gonadal development. In M10dph-vs-F10dph, there were a total of 28 DEGs, of which 20 were up-regulated and 8 were down-regulated. In M18dph-vs-F18dph, there were 213 DEGs in total, of which 84 were up-regulated and 129 were down-regulated. In M30dph-vs-F30dph, there was a total of 636 DEGs, of which 284 were up-regulated and 352 were down-regulated. In M48dph-vs-F48dph, there was a total of 1381 DEGs, of which 544 were up-regulated and 837 were down-regulated. The list of DEGs at four developmental stages was shown in Tables S1-S4.
The GO annotations of the DEGs were classified as molecular function, cellular component and biological process. The DEGs identified from four stages were enriched to 37, 195, 326, and 502 GO terms, respectively. The top 30 enriched GO terms in each period were illustrated in Fig. 3.
Histogram of top 30 GO terms enriched by DEGs at four developmental stages. M10dph-vs-F10dph (a), M18dph-vs-F18dph (b), M30dph-vs-F30dph (c), and M48dph-vs-F48dph (d). The x-axis shows the GO terms and the y-axis indicates negative log of the p value of significance. M_dph: male gonad samples at _ days post hatching; F_dph: female gonad samples at _ days post hatching
The DEGs identified from M10dph-vs-F10dph, M18dph-vs-F18dph, M30dph-vs-F30dph, and M48dph-vs-F48dph were further annotated to 13, 51, 92, and 116 KEGG pathways, respectively. As shown in the top 20 KEGG enrichments, only steroid hormone biosynthesis KEGG pathway was enriched at 10dph (Fig. 4a). The top 3 of 11 significantly enriched pathways were neuroactive ligand-receptor interaction, steroid hormone biosynthesis and cell adhesion molecules (CAMs) at 18dph (Fig. 4b). The top 5 significantly enriched pathways for the DEGs were neuroactive ligand-receptor interaction, steroid biosynthesis, steroid hormone biosynthesis, TGF-β signaling pathway and glycosaminoglycan biosynthesis-heparan sulfate/heparin at 30dph (Fig. 4c). The top 5 significantly enriched pathways for the DEGs at 48dph were essentially identical to those at 18dph except that cell adhesion molecules (CAMs) replaced glycosaminoglycan biosynthesis-heparan sulfate/heparin (Fig. 4d). Among the top 20 KEGG pathways at four developmental stages, steroid hormone biosynthesis pathway was commonly enriched. The DEGs at four developmental stages involved in the steroid hormone biosynthesis pathway were shown in Figs. S1, S2, S3 and S4. In addition, well-known sex-related pathways, such as TGF-β signaling pathway besides steroid hormone biosynthesis, were also identified.
The top 20 KEGG pathways enriched by DEGs at four developmental stages. M10dph-vs-F10dph (a), M18dph-vs-F18dph (b), M30dph-vs-F30dph (c), and M48dph-vs-F48dph (d). The x-axis indicates the enrichment score of each pathway and the y-axis shows the pathway. The color and size of dots indicate the p-value and number of differentially expressed genes (N ≥ 3) assigned to the corresponding pathway, respectively
Identification of sex-biased expression genes
In order to identify male- and female-biased expression genes, the above screened DEGs at four developmental stages were further compared and the DEGs shared by them were considered as sex-biased expression genes. For example, amh and tsga10 were up-regulated at four developmental stages, whereas cyp19a1b, foxl2, and hsd17b1 were down-regulated at four developmental stages, suggesting that these genes may be sex-biased expression genes and involved in gonadal differentiation.
To reveal main biological functions and identify biological pathways of the eight shared DEGs of four developmental stages, GO annotation and KEEG enrichment analysis were summarized in Table 2. As mucin-22-like was not annotated to the exact sex-related gene, no further analysis of this gene was performed. These seven genes were annotated to eighteen GO terms, such as gonad development, estrogen biosynthetic process, estradiol 17-beta-dehydrogenase activity and spermatogenesis. These genes were enriched in three KEGG pathways, including steroid hormone biosynthesis, TGF-β signaling pathway and cytokine-cytokine receptor interaction.
Verification of the expression patterns of sex-biased genes by RT-qPCR
In total, seven candidate sex-biased expression genes, namely amh, gdf6a, tsga10, cyp19a1b, foxl2, hsd17b1, and cyp17a were identified from the DEGs for RT-qPCR to verify the expression patterns in red-tail catfish. The expression levels of the selected seven genes were significantly different between two sexes at each developmental stage. Among these, amh and tsga10 were up-regulated at four developmental stages. The cyp19a1b, foxl2, and hsd17b1 were down-regulated at four developmental stages. The expressions of gdf6a and cyp17a were down-regulated in M10dph-vs-F10dph, but up-regulated in the other three developmental stages (Fig. 5). The gene expression patterns revealed by RT-qPCR results were consistent with the RNA-sequencing data, which indicated that the transcriptome data were reliable and useful for differential expression analysis.
Validation of sex-biased expression of seven DEGs by RT-qPCR. The y-axis indicates relative expression level. Error bars mean standard error. Asterisks represent the level of significant difference between sexes, * (p < 0.05), ** (p < 0.01), *** (p < 0.001). The real p-values were shown in Table S5
Discussion
The red-tail catfish shows the characteristics of sex dimorphism in growth, and males show obvious growth advantages. Although sex-specific markers have been developed for genotypic sex identification [11], less research has been focused on the sex determination and differentiation in red-tail catfish. Studies on the mechanism of sex determination provide paramount theoretical value for promoting the development of sex-control breeding. The identification of DEGs at different developmental stages by the gonadal transcriptome is an important way to investigate the molecular differences that regulate sex determination and sexual dimorphism [40]. A total of 28, 213, 636 and 1381 DEGs were identified between two sexes at the four developmental stages through the gonadal transcriptome of the red-tail catfish. There was an increase in the number of DEGs from 10 to 48dph, suggesting that there were more sex-related genes in the later stages of gonadal development compared to the early stages. This trend was similar to that revealed in the closely related fish, such as yellow catfish (Pelteobagrus fulvidraco) and channel catfish (Ictalurus punctatus) [40, 41].
As genes differentially expressed between two sexes at different developmental stages were involved in gonadal differentiation and development, these sex-related genes can be used to further identify sex determination and differentiation genes [40]. In red-tail catfish, seven candidate genes (amh, gdf6a, tsga10, cyp17a, foxl2, cyp19a1b and hsd17b1) were obtained by taking intersections of DEGs at four developmental stages. Two genes (cyp17a and cyp19a1b) shared during gonadal development in yellow catfish (Pelteobagrus fulvidraco) and two genes (amh and foxl2) at someone stage in half-smooth tongue sole (Cynoglossus semilaevis) were the same with those in red-tail catfish [40, 42]. Besides, the expression of these seven genes also showed different patterns before and after gonadal differentiation (18dph). For example, the expression of hsd17b1 in the female was higher after gonadal differentiation (48dph) than before gonadal differentiation (10dph), and the expression level was the highest at the initial time of gonadal differentiation (18dph). However, the expression of gdf6a in the male was higher after gonadal differentiation (48dph) than before gonadal differentiation (10dph), and its expression level increased with gonadal differentiation and development (from 10 to 48dph).
Most of the identified sex-determining genes and candidate genes are from the TGF-β signaling pathway [43]. In this study, two genes belonging to the TGF-β signaling pathway, amh and gdf6a were identified. In zebrafish, amh mutants showed a female-biased sex ratio, and the proliferation and differentiation of male germ cells were disordered [44]. It was revealed that overexpression of amh in undifferentiated orange grouper (Epinephelus coioides) induced testis development [45]. In Japanese eel (Anguilla japonica), amh mainly expressed in the testis, and the expression level increased significantly with the differentiation of the testis [46]. Male-biased expression of amh was associated with the differentiation of the male gonads in yellow catfish (Pelteobagrus fulvidraco) [30]. In red-tail catfish, amh showed significantly different expression at four developmental stages. Furthermore, as in the southern catfish (Silurus meridionalis) [47], amh showed significantly higher expression in the male than in the female in red-tail catfish. As for gdf6a, most of studies have shown that it is related to the photoreceptor phylogeny of zebrafish (Danio rerio) [48,49,50]. It has been also reported that gdf6 on the Y chromosome is a master sex-determining gene in the turquoise killifish (Nothobranchius furzeri) [51]. In red-tail catfish, the expression of gdf6a was down-regulated at the undifferentiated stage (10dph), but up-regulated at the initial time of gonadal differentiation stage (18dph). In conclusion, gdf6a and amh showed different expression patterns at the undifferentiated stage (10dph), but showed similar expression patterns after gonadal differentiation (18dph) in red-tail catfish. Furthermore, both amh and gdf6a belong to the TGF-β signaling pathway genes [52]. These results suggest that amh may be involved in the molecular sex differentiation, however, gdf6a may play a main role in the morphological sex differentiation in red-tail catfish.
Besides TGF-β signaling pathway genes, DM domain containing genes and Sox family genes are also the main source genes of sex-determining genes or candidate genes [19, 43, 53], such as dmrt1 (a DM domain containing gene) in medaka fish (Oryzias latipes) and half-smooth tongue sole (Cynoglossus semilaevis) [26, 54, 55], sox3 (a sox family gene) in Indian ricefish (Oryzias dancena) [56], and sox9 (a sox family gene) in medaka fish (Oryzias latipes) and orange-spotted grouper (Epinephelus coioides) [57,58,59]. However, these genes were not differentially expressed in the critical period of gonadal differentiation in red-tail catfish. For example, dmrt1 was not differentially expressed between two sexes at 10dph and 18dph. Although the expression of dmrt1 were up-regulated at 30dph and 48dph, its expression was kept at a very low level. Similarly, sox9 was not differentially expressed at a low level at the four stages. Therefore, it is inferred that dmrt1 and sox9 may not be sex-determining genes, but play a role in the maintenance of gonadal development in red-tail catfish.
Genes involved in steroid hormone biosynthesis also were key physiological factors in regulating sex differentiation in fish [60]. Fortunately, cyp17a, cyp19a1b, and hsd17b1, belonging to the steroid hormone biosynthesis pathway, were screened from the DEGs in red-tail catfish. Cyp17a controls the synthesis of 17β-estradiol, and cyp17a (-/-) XX leads to male sex reversal in Nile tilapia (Oreochromis niloticus) [61]. The expression of cyp17a in red-tail catfish was similar to that in rice field eels (Monopterus albus), but different from that in Nile tilapia (Oreochromis niloticus) and fathead minnows (Pimephales promelas) [62,63,64], suggesting that the role for cyp17a during gonadal differentiation and development may vary with species or developmental stage. Cyp19 converts androgens to estrogens, and the expression of this gene determines the ratio of androgens to estrogens, which is vital for sex differentiation in most vertebrates [65]. During gonadal development and sex differentiation, treatment with the CYP19 inhibitor fadrozole resulted in gonad differentiation into testes in all individuals of zebrafish (Danio rerio) and masculinization in 97.1% of common carp (Cyprinus carpio), respectively [66, 67]. In the process of sex differentiation, cyp19a1 was highly expressed in the female gonad or specifically expressed in the early differentiated females [68, 69]. Similarly, the expression of cyp19a1b showed significant sexual dimorphism before and after gonadal differentiation in red-tail catfish. A role for cyp19a1b in gonadal differentiation has been revealed in north African catfish [70]. As for hsd17b1, a key enzyme in steroid hormone biosynthesis, may play an essential role in estrogen synthesis in the ovary [71,72,73,74,75,76]. The expression of cyp19a1 may be regulated by foxl2 [43, 77], and the expression pattern of hsd17b1 was similar to that of cyp19a1b in red-tail catfish. So foxl2 may regulate the expression of both hsd17b1 and cyp19a1b. Cyp19a1 and foxl2 were upregulated in sex-reversed females compared to wild-type males, suggesting that cyp19a1 and foxl2 were associated with ovarian development in yellow catfish (Pelteobagrus fulvidraco) [30]. Foxl2 was involved in gonadal differentiation and the maintenance of ovarian function, and its expression was upregulated in the female in the early stage of ovarian differentiation [78, 79]. The expression of foxl2 in red-tail catfish was consistent with the above findings in other fish. Knockdown of foxl2 can lead to complete sexual reversal in zebrafish, gibel carp (Carassius gibelio) and Nile tilapia (Oreochromis niloticus) [25, 80,81,82]. Therefore, we speculate that foxl2, cyp19a1b, and hsd17b1 may be female-biased genes and may be involved in the sex determination process in red-tail catfish.
Besides above candidate sex determination genes, tsga10 was identified from DEGs. Originally isolated from adult testis, tsga10 is over 80 kb in length and consists of 19 exons, and it may be involved in the maintenance of normal sperm structure and spermatogenesis [10, 83,84,85,86]. The expression of tsga10 was up-regulated at four developmental stages, but the overall expression level of the gene was very low in both sexes. This result was similar to that found in zebrafish (Danio rerio) [87]. What’s more, tsga10 knockout could cause infertility in male mice, resulting in disordered mitochondrial sheath formation and significantly reduced sperm motility [88]. There are no reports on tsga10 in relation to sex determination or differentiation in fish. Therefore, whether tsga10 is involved in sex determination or gonadal development in red-tail catfish needs to be further explored in future.
Conclusions
RNA-seq was used to identify candidate genes involved in sex determination and to reveal their expression levels at four different stages of gonadal development. Seven DEGs shared by the four developmental stages were identified, of which amh and gdf6a may be the male-biased expression genes, while foxl2, cyp19a1b and hsd17b1 may be the female-biased expression genes, which were involved in steroid hormone biosynthesis and TGF-β signaling pathways. Our results will provide insight into evolutionary processes of sex determination mechanisms in fish, as well as a useful genetic basis for sex-control breeding to produce monosexual populations.
Methods
Fish culture and ethics statement
Red-tail catfish were reared to be sexually mature in the Xishuangbanna Indigenous Fish Breeding Center and one full-sib family was established. After hatching there, fries were transferred to the National Aquatic Biological Resource Center (NABRC, Wuhan, China) and cultured in re-circulating aerated freshwater tanks at 26℃ prior to sample collection. Fries were fed three times daily with hatched artemia nauplii for the first two weeks, mixtures of artemia nauplii and frozen bloodworms for the next week, both frozen bloodworms and pellet feed for two weeks, then pellet feed for the following culture. All experiments and animal treatments were carried out according to the principles of Animal Care and Use Committee of Institute of Hydrobiology, Chinese Academy of Sciences.
Gonadal histology and sample collection
All sampled individuals were firstly euthanized using an overdose of MS222 before gonad sample collection at different developmental stages of red-tail catfish. Gonads were fixed with paraformaldehyde (PFA) for more than 24 h at 4℃, then dehydrated, embedded in paraffin for section. After the slices were deparaffinized and rehydrated, they were stained with hematoxylin and eosin solutions. Microphotography was performed using a microscope from Carl Zeiss (Axio Imager M2). Significant stages of the early gonad development were determined based on histological analysis of gonads at different developmental stages. Sexual phenotypes of all sampled individuals were determined based on the sex-specific genetic markers of red-tail catfish [11], and the results of the sex identification were shown in Fig. S5 (with the samples of 48dph as an example). In this study, four developmental stages (10dph, 18dph, 30dph, and 48dph) were set up with a total of 24 samples. The first developmental stage (10dph) included the male gonadal tissue sample “M10dph” and the female gonadal tissue sample “F10dph”, and 27 gonads pooled into one sequencing sample for each sex. At the second developmental stage (18dph), the male gonadal tissue sample “M18dph” and the female gonadal tissue sample “F18dph” were sampled, and 15 gonads were pooled into one sample for each sex. The male gonadal tissue sample “M30dph” and the female gonadal tissue sample “F30dph” consisted of 12 testes and ovaries at the third developmental stage (30dph), respectively. At the fourth developmental stage (48dph), 12 testes and ovaries were pooled into the male gonadal tissue sample “M48dph” and the female gonadal tissue sample “F48dph”, respectively. Each of sequencing samples at different stages was performed with three replicates. Gonad samples were carefully collected and immediately placed in RNAprotect Tissue Reagent, kept at 4 ℃ overnight, and then store at -20 ℃ until RNA extraction.
RNA extraction, library construction and sequencing
Total RNA was extracted using the mirVana miRNA Isolation Kit (Ambion, USA) following the manufacturer’s protocol. RNA purity and quantification were evaluated using the NanoDrop 2000 spectrophotometer (Thermo Scientific, USA). RNA integrity was evaluated using the Agilent 2100 Bioanalyzer (Agilent Technologies, USA). The samples with RNA Integrity Number (RIN) ≥ 7 were subjected to the subsequent analysis. The cDNA libraries were constructed using TruSeq Stranded mRNA LTSample Prep Kit (Illumina, USA) according to the manufacturer’s instructions. In brief, after the total RNA was extracted and digested by DNase, the mRNA was enriched by magnetic beads with Oligo (dT). Adding Fragmentation Buffer to break the mRNA into short fragments, which was used as template to synthesize first-strand cDNA with random hexamer primer. Then, a two-strand synthesis reaction system was prepared to synthesize second-strand cDNA, and second-strand cDNAs were purified by beads from kit. The purified double-stranded cDNA was then subjected to terminal repair, poly(A) addition and sequencing splicing, fragment size selection and PCR amplification. These RNA-Seq libraries were sequenced on the BGI DNBSEQ-T7 sequencing platform (Shanghai OE Biomedical Technology Company Limited, China) and 150 bp paired-end reads were generated.
Gonad transcriptome assembly and annotation
Raw data (raw reads) of fastq format were firstly processed using Trimmomatic (v 0.36) [89] and the reads containing ploy-N and the low quality reads were removed to obtain the clean reads (LEADING:3 TRAILING:3 ILLUMINACLIP:TruSeq3-PE-2.fa:2:30:10:8:true SLIDINGWINDOW:4:15 MINLEN:50). Then clean reads were mapped to the reference genome of red-tail catfish (PRJNA842523 and PRJNA841381) using Hisat2 (v 2.2.1.0) [90]. The reads with a perfect match were further used for subsequent annotation analysis based on the reference genome.
Differential expression analysis and DEGs identification
Relative gene expression levels of each gene were characterized by fragments per kilobase of transcript per million mapped reads (FPKM) [91]. FPKM of each gene was calculated and the read counts of each gene were obtained by HTSeq-count (v 0.9.1) [92]. Differential expression analysis between two sexes at four developmental stages was performed using the DESeq2 (v 1.20.0) (DESeqDataSetFromMatrix(countData = countData, colData = colData, design = ~ condition)) [93, 94]. Genes with p-value < 0.05 and fold change > 2 or fold change < 0.5 were assigned as the threshold for DEGs. Gene ontology (GO) enrichment and Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analysis of DEGs were performed to screen the significantly enriched term using R (v 3.2.0) based on the hypergeometric distribution [95,96,97,98]. In this study, the expression patterns of four developmental stages were revealed, and up-regulated/down-regulated was determined by comparing the male with the female. Unless otherwise stated, up-regulated refers to the expression in the male was higher than in the female, while down-regulated refers to the expression in the male was lower than in the female.
RT-qPCR verification of expression patterns
Seven candidate DEGs were selected from the four developmental stages to verify the reliability of RNA-seq data by RT-qPCR. The primers for these genes (Table 3) were designed using Primer Premier (v 5.0) [99]. The PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa) was used for cDNA synthesis following the manufacturer’s instructions. RT-qPCR was performed on a Step One real-time PCR System (Roche, USA) with iTaq Universal SYBR Green Supermix (BIO RAD). The β-actin gene was used as an endogenous reference gene and three biological replicates were performed for each reaction. The relative expression level was measured in terms of threshold cycle value and normalized using the 2−∆∆Ct method [100]. For statistical analysis, SPSS (v 19.0) was used for significance test (p < 0.05).
Availability of data and materials
The data that support the results of this present study are available from the corresponding author upon reasonable request.
The reference genome of red-tail catfish is available in the NCBI SRA database under the BioProject accession number PRJNA842523 (https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA842523) and PRJNA841381 (https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA841381).
Abbreviations
- amh :
-
Anti-Mullerian hormone
- cyp17a :
-
Cytochrome P450 family 17 subfamily A
- cyp19a1b :
-
Cytochrome P450 family 19 subfamily A polypeptide 1b
- CAMs:
-
Cell adhesion molecules
- dph:
-
Days post hatching
- DEGs:
-
Differentially expressed genes
- ESD:
-
Environmental sex determination
- F_dph:
-
Female gonad samples at _ days post hatching
- foxl2 :
-
Forkhead box L2
- FPKM:
-
Fragments per kilobase of transcript per million mapped reads
- gdf6a :
-
Growth differentiation factor 6a
- GSD:
-
Genotypic sex determination
- GO:
-
Gene ontology
- hsd17b1 :
-
Hydroxysteroid 17-beta dehydrogenase 1
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- M_dph:
-
Male gonad samples at _ days post hatching
- PFA:
-
Paraformaldehyde
- RIN:
-
RNA integrity number
- RT-qPCR:
-
Real-time quantitative PCR
- tsga10 :
-
Testis-specific gene antigen 10
References
Hee NH, Rainboth WJ. The Bagrid catfish Genus Hemibagrus (Teleostei : Siluriformes) in central Indochina with a new species from the Mekong River. Raffles B Zool. 1999;47(2):555–76.
Xue CJ, Tian SK, Li YM, Li XS, Liu YT, Leng Y, Zhang JS, He WP. Preliminary report on the domestication and artificial propagation of Mystus wyckioides (in Chinese). J Hydroecol. 2010;31(4):142–5.
Zhang YJ, Liu ZY, Song WH, Wang X. The biology of Mystus wyckioides and its status of introduction and culture (in Chinese). J Hydroecol. 2007;27(5):41–2.
Zhou YL, Wu JJ, Wang ZW, Li GH, Zhou L, Gui JF. Microsatellite polymorphism and genetic differentiation of different populations screened from genome survey sequencing in red-tail catfish (Hemibagrus wyckioides). Aquacult Rep. 2021;19:100614.
Wang D, Mao HL, Chen HX, Liu HQ, Gui JF. Isolation of Y- and X-linked SCAR markers in yellow catfish and application in the production of all-male populations. Anim Genet. 2009;40(6):978–81.
Dan C, Mei J, Wang D, Gui JF. Genetic differentiation and efficient sex-specific marker development of a pair of Y- and X-linked markers in yellow catfish. Int J Biol Sci. 2013;9(10):1043–9.
Wen M, Pan QW, Larson W, Eche C, Guiguen Y. Characterization of the sex determining region of channel catfish (Ictalurus punctatus) and development of a sex-genotyping test. Gene. 2023;850:146933.
Wang T, Li Z, Yu ZX, Wang ZW, Lian ZQ, Du WX, Zhao X, Wang MT, Miao C, Ding M, Wang Y, Zhou L, Zhang XJ, Li XY, Gui JF. Production of YY males through self-fertilization of an occasional hermaphrodite in Lanzhou catfish (Silurus lanzhouensis). Aquaculture. 2021;539:736622.
Pan ZJ, Li XY, Zhou FJ, Qiang XG, Gui JF. Identification of sex-specific markers reveals male heterogametic sex determination in Pseudobagrus ussuriensis. Mar Biotechnol. 2015;17(4):441–51.
Pan ZJ, Zhu CK, Chang GL, Wu N, Ding HY, Wang H. Differential expression analysis and identification of sex-related genes by gonad transcriptome sequencing in estradiol-treated and non-treated Ussuri catfish Pseudobagrus ussuriensis. Fish Physiol Biochem. 2021;47(2):565–81.
Zhou YL, Wu JJ, Wang ZW, Li GH, Mei J, Zhou L, Gui JF. Identification of sex-specific markers and heterogametic XX/XY sex determination system by 2b-RAD sequencing in redtail catfish (Mystus wyckioides). Aquac Res. 2019;50(8):2251–66.
Sun YL, Jiang DN, Zeng S, Hu CJ, Ye K, Yang C, Yang SJ, Li MH, Wang DS. Screening and characterization of sex-linked DNA markers and marker-assisted selection in the Nile tilapia (Oreochromis niloticus). Aquaculture. 2014;433:19–27.
Chen SL, Li J, Deng SP, Tian YS, Wang QY, Zhuang ZM, Sha ZX, Xu JY. Isolation of female-specific AFLP markers and molecular identification of genetic sex in half-smooth tongue sole (Cynoglossus semilaevis). Mar Biotechnol. 2007;9(2):273–80.
Zhao J, Ou M, Wang YP, Liu HY, Zhu XP, Chen BX, Chen KC. Breeding of YY super-male of blotched snakehead (Channa maculata) and production of all-male hybrid (Channa argus ♀×C. maculate ♂). Aquaculture. 2021;538:736450.
Mei J, Gui JF. Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish. Sci China Life Sci. 2015;58(2):124–36.
Zhou L, Gui JF. Applications of genetic breeding biotechnologies in Chinese aquaculture. In: Gui JF, Tang QS, Li ZJ, editors. Aquaculture in China: success stories and modern trends. Oxford: John Wiley & Sons Ltd; 2018. p. 465–96.
Lu M, Li XY, Li Z, Du WX, Zhou L, Wang Y, Zhang XJ, Wang ZW, Gui JF. Regain of sex determination system and sexual reproduction ability in a synthetic octoploid male fish. Sci China Life Sci. 2021;64(1):77–87.
Lu M, Li Z, Peng F, Wang Y, Li XY, Wang ZW, Zhang XJ, Zhou L, Gui JF. Changes in ploidy drive reproduction transition and genomic diversity in a polyploid fish complex. Mol Biol Evol. 2022;39(9):msac188.
Li XY, Gui JF. Diverse and variable sex determination mechanisms in vertebrates. Sci China Life Sci. 2018;61(12):1503–14.
Gui JF, Zhou L, Li XY. Rethinking fish biology and biotechnologies in the challenge era for burgeoning genome resources and strengthening food security. Water Biol Secur. 2022;1:100002.
Luckenbach JA, Borski RJ, Daniels HV, Godwin J. Sex determination in flatfishes: mechanisms and environmental influences. Semin Cell Dev Biol. 2009;20(3):256–63.
Valenzuela N, Adams DC, Janzen FJ. Pattern does not equal process: Exactly when is sex environmentally determined? Am Nat. 2003;161(4):676–83.
Ospina-Alvarez N, Piferrer F. Temperature-dependent sex determination in fish revisited: prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS ONE. 2008;3(7):e2837.
Lin XH, Zhou DY, Zhang XM, Li GL, Zhang YL, Huang CL, Zhang ZX, Tian CX. A first insight into the gonad transcriptome of Hong Kong catfish (Clarias fuscus). Animals. 2021;11(4):1131.
Zhang XB, Li MR, Ma H, Liu XY, Shi HJ, Li MH, Wang DS. Mutation of foxl2 or cyp19a1a Results in female to male sex reversal in XX Nile tilapia. Endocrinology. 2017;158(8):2634–47.
Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, Hori H, Hamaguchi S, Sakaizumi M. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417(6888):559–63.
Myosho T, Otake H, Masuyama H, Matsuda M, Kuroki Y, Fujiyama A, Naruse K, Hamaguchi S, Sakaizumi M. Tracing the emergence of a novel sex-determining gene in medaka. Oryzias luzonensis Genetics. 2012;191(1):163–70.
Kamiya T, Kai W, Tasumi S, Oka A, Matsunaga T, Mizuno N, Fujita M, Suetake H, Suzuki S, Hosoya S, Tohari S, Brenner S, Miyadai T, Venkatesh B, Suzuki Y, Kikuchi K. A trans-species missense SNP in amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (Fugu). Plos Genet. 2012;8(7):e1002798.
Yano A, Guyomard R, Nicol B, Jouanno E, Quillet E, Klopp C, Cabau C, Bouchez O, Fostier A, Guiguen Y. An immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss. Curr Biol. 2012;22(15):1423–8.
Dan C, Lin QH, Gong GR, Yang TY, Xiong ST, Xiong Y, Huang PP, Gui JF, Mei J. A novel PDZ domain-containing gene is essential for male sex differentiation and maintenance in yellow catfish (Pelteobagrus fulvidraco). Sci Bull. 2018;63(21):1420–30.
Bao LS, Tian CX, Liu SK, Zhang Y, Elaswad A, Yuan ZH, Khalil K, Sun FY, Yang YJ, Zhou T, Li N, Tan SX, Zeng QF, Liu Y, Li YR, Li Y, Gao DY, Dunham R, Davis K, Waldbieser G, Liu ZJ. The Y chromosome sequence of the channel catfish suggests novel sex determination mechanisms in teleost fish. BMC Biol. 2019;17(1):6.
Gong GR, Xiong Y, Xiao SJ, Li XY, Huang PP, Liao Q, Han QQ, Lin QH, Dan C, Zhou L, Ren F, Zhou Q, Gui JF, Mei J. Origin and chromatin remodelling of young X/Y sex chromosomes in catfish with sexual plasticity. Natl Sci Rev. 2023;10:nwac239.
Bar I, Cummins S, Elizur A. Transcriptome analysis reveals differentially expressed genes associated with germ cell and gonad development in the Southern bluefin tuna (Thunnus maccoyii). BMC Genomics. 2016;17:217.
Yu P, Wang Y, Yang WT, Li Z, Zhang XJ, Zhou L, Gui JF. Upregulation of the PPAR signaling pathway and accumulation of lipids are related to the morphological and structural transformation of the dragon-eye goldfish eye. Sci China Life Sci. 2021;64(7):1031–49.
Yu P, Wang Y, Li Z, Jin H, Li LL, Han X, Wang ZW, Yang XL, Li XY, Zhang XJ, Zhou L, Gui JF. Causal gene identification and desirable trait recreation in goldfish. Sci China Life Sci. 2022;65(12):2341–53.
Fan ZF, You F, Wang LJ, Weng SD, Wu ZH, Hu JW, Zou YX, Tan XG, Zhang PJ. Gonadal transcriptome analysis of male and female olive flounder (Paralichthys olivaceus). Biomed Res Int. 2014;2014:291067.
Shen FF, Long Y, Li FY, Ge GD, Song GL, Li Q, Qiao ZG, Cui ZB. De novo transcriptome assembly and sex-biased gene expression in the gonads of Amur catfish (Silurus asotus). Genomics. 2020;112(3):2603–14.
Mank JE, Ellegren H. All dosage compensation is local: Gene-by-gene regulation of sex-biased expression on the chicken Z chromosome. Heredity. 2009;102(3):312–20.
Hale MC, McKinney GJ, Thrower FP, Nichols KM. Evidence of sex-bias in gene expression in the brain transcriptome of two populations of rainbow trout (Oncorhynchus mykiss) with divergent life histories. PLoS ONE. 2018;13(2):e0193009.
Lu JG, Luan PX, Zhang XF, Xue SQ, Peng LN, Mahbooband S, Sun XW. Gonadal transcriptomic analysis of yellow catfish (Pelteobagrus fulvidraco): identification of sex-related genes and genetic markers. Physiol Genomics. 2014;46(21):798–807.
Zeng QF, Liu SK, Yao J, Zhang Y, Yuan ZH, Jiang C, Chen AL, Fu Q, Su BF, Dunham R, Liu ZJ. Transcriptome display during testicular differentiation of channel catfish (Ictalurus Punctatus) as revealed by RNA-seq analysis. Biol Reprod. 2016;95(1):19.
Lin GM, Gao D, Lu JG, Sun XW. Transcriptome Profiling Reveals the Sexual Dimorphism of Gene Expression Patterns during Gonad Differentiation in the Half-Smooth Tongue Sole (Cynoglossus semilaevis). Mar Biotechnol. 2021;23(1):18–30.
Li XY, Mei J, Ge CT, Liu XL, Gui JF. Sex determination mechanisms and sex control approaches in aquaculture animals. Sci China Life Sci. 2022;65(6):1091–122.
Lin QH, Mei J, Li Z, Zhang XM, Zhou L, Gui JF. Distinct and cooperative roles of amh and dmrt1 in self-renewal and differentiation of male germ cells in zebrafish. Genetics. 2017;207(3):1007–22.
Han YL, Zhao M, Wang L, Yu ZS, Wang J, Yu Q, Xiao L, Lu MW, Li SS, Zhang Y, Lin HR. Overexpression of anti-mullerian hormone gene in vivo affects gonad sex differentiation in undifferentiated orange-spotted groupers (Epinephelus coioides). Front Endocrinol. 2019;10:210.
Lin CJ, Jeng SR, Lei ZY, Yueh WS, Dufour S, Wu GC, Chang CF. Involvement of transforming growth factor beta family genes in gonadal differentiation in Japanese eel, Anguilla japonica, according to sex-related gene expressions. Cells. 2021;10(11):3007.
Zheng SQ, Tao WJ, Yang HW, Kocher TD, Wang ZJ, Peng ZG, Jin L, Pu DY, Zhang YG, Wang DS. Identification of sex chromosome and sex-determining gene of southern catfish (Silurus meridionalis) based on XX, XY and YY genome sequencing. Proc Biol Sci. 1971;2022(289):20212645.
French CR, Erickson T, French DV, Pilgrim DB, Waskiewicz AJ. Gdf6a is required for the initiation of dorsal-ventral retinal patterning and lens development. Dev Biol. 2009;333(1):37–47.
Gosse NJ, Baier H. An essential role for radar (gdf6a) in inducing dorsal fate in the zebrafish retina. P Natl Acad Sci USA. 2009;106(7):2236–41.
Nadolski NJ, Balay SD, Wong CXL, Waskiewicz AJ, Hocking JC. Abnormal cone and rod photoreceptor morphogenesis in gdf6a mutant zebrafish. Invest Ophth Vis Sci. 2020;61(4):9.
Reichwald K, Petzold A, Koch P, Downie BR, Hartmann N, Pietsch S, Baumgart M, Chalopin D, Felder M, Bens M, Sahm A, Szafranski K, Taudien S, Arisi I, Weise A, Bhatt SS, Sharma V, Kraus JM, Schmid F, Priebe S, Liehr T, Goerlach M, Than ME, Hiller M, Kestler HA, Volff JN, Schartl M, Cellerino A, Englert C, Platzer M. Insights into sex chromosome evolution and aging from the genome of a short-lived fish. Cell. 2015;163(6):1527–38.
Pan QW, Kay T, Depince A, Adolfi M, Schartl M, Guiguen Y, Herpin A. Evolution of master sex determiners: TGF-beta signalling pathways at regulatory crossroads. Philos T R Soc B. 1832;2021(376):20200091.
Bachtrog D, Mank JE, Peichel CL, Kirkpatrick M, Otto SP, Ashman TL, Hahn MW, Kitano J, Mayrose I, Ming R, Perrin N, Ross L, Valenzuela N, Vamosi JC. Sex determination: why so many ways of doing it? Plos Biol. 2014;12(7):e1001899.
Chen SL, Zhang GJ, Shao CW, Huang QF, Liu G, Zhang P, Song WT, An N, Chalopin D, Volff JN, Hong YH, Li QY, Sha ZX, Zhou HL, Xie MS, Yu QL, Liu Y, Xiang H, Wang N, Wu K, Yang CG, Zhou Q, Liao XL, Yang LF, Hu QM, Zhang JL, Meng L, Jin LJ, Tian YS, Lian JM, Yang JF, Miao GD, Liu SS, Liang Z, Yan F, Li YZ, Sun B, Zhang H, Zhang J, Zhu Y, Du M, Zhao YW, Schartl M, Tang QS, Wang J. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat Genet. 2014;46(3):253–60.
Cui ZK, Liu Y, Wang WW, Wang Q, Zhang N, Lin F, Wang N, Shao CW, Dong ZD, Li YZ, Yang YM, Hu MZ, Li HL, Gao FT, Wei ZF, Meng L, Liu Y, Wei M, Zhu Y, Guo H, Cheng CHK, Schartl M, Chen SL. Genome editing reveals dmrt1 as an essential male sex-determining gene in Chinese tongue sole (Cynoglossus semilaevis). Sci Rep. 2017;7:42213.
Takehana Y, Matsuda M, Myosho T, Suster ML, Kawakami K, Shin-I T, Kohara Y, Kuroki Y, Toyoda A, Fujiyama A, Hamaguchi S, Sakaizumi M, Naruse K. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nat Commun. 2014;5:4157.
Nakamoto M, Suzuki A, Matsuda M, Nagahama Y, Shibata N. Testicular type sox9 is not involved in sex determination but might be in the development of testicular structures in the medaka, Oryzias latipes. Biochem Biophys Res Commun. 2005;333(3):729–36.
Nakamura S, Watakabe I, Nishimura T, Toyoda A, Taniguchi Y, Tanaka M. Analysis of medaka sox9 orthologue reveals a conserved role in germ cell maintenance. PLoS ONE. 2012;7(1):e29982.
Luo YS, Hu W, Liu XC, Lin HR, Zhu ZY. Molecular cloning and mRNA expression pattern of sox9 during sex reversal in orange-spotted grouper (Epinephelus coioides). Aquaculture. 2010;306(1–4):322–8.
Baroiller JF, Guiguen Y, Fostier A. Endocrine and environmental aspects of sex differentiation in fish. Cell Mol Life Sci. 1999;55(6–7):910–31.
Yang LY, Zhang XF, Liu SJ, Zhao CH, Miao YY, Jin L, Wang DS, Zhou LY. Cyp17a1 is required for female sex determination and male fertility by regulating sex steroid biosynthesis in fish. Endocrinology. 2021;162(12):bqab205.
Yu HS, Cheng HH, Guo YQ, Xia LX, Zhou RJ. Alternative splicing and differential expression of P450c17 (CYP17) in gonads during sex transformation in the rice field eel. Biochem Biophys Res Commun. 2003;307(1):165–71.
Ijiri S, Kaneko H, Kobayashi T, Wang DS, Sakai F, Paul-Prasanth B, Nakamura M, Nagahama Y. Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile tilapia Oreochromis niloticus. Biol Reprod. 2008;78(2):333–41.
Leet JK, Lesteberg KE, Schoenfuss HL, Olmstead AW, Amberg JJ, Ankley GT, Sepulveda MS. Sex-specific gonadal and gene expression changes throughout development in fathead minnow. Sex Dev. 2013;7(6):303–7.
Trant JM, Gavasso S, Ackers J, Chung BC, Place AR. Developmental expression of cytochrome P450 aromatase genes (CYP19a and CYP19b) in zebrafish fry (Danio rerio). J Exp Zool. 2001;290(5):475–83.
Fenske M, Segner H. Aromatase modulation alters gonadal differentiation in developing zebrafish (Danio rerio). Aquat Toxicol. 2004;67(2):105–26.
Singh AK, Singh R. In vivo response of melatonin, gonadal activity and biochemical changes during CYP19 inhibited sex reversal in common carp Cyprinus carpio (L). Anim Reprod Sci. 2013;136(4):317–25.
Karube M, Fernandino JI, Strobl-Mazzulla P, Strussmann CA, Yoshizaki G, Somoza GM, Patino R. Characterization and expression profile of the ovarian cytochrome p-450 aromatase (cyp19A1) gene during thermolabile sex determination in pejerrey, Odontesthes bonariensis. J Exp Zool Part A. 2007;307A(11):625–36.
Inaba H, Hara S, Horiuchi M, Ijiri S, Kitano T. Gonadal expression profiles of sex-specific genes during early sexual differentiation in Japanese eel Anguilla japonica. Fish Sci. 2021;87(2):203–9.
Rasheeda MK, Sridevi P, Senthilkumaran B. Cytochrome P450 aromatases: Impact on gonadal development, recrudescence and effect of hCG in the catfish, Clarias gariepinus. Gen Comp Endocrinol. 2010;167(2):234–45.
Ge CM, Lu WQ, Chen A. Quantitative proteomic reveals the dynamic of protein profile during final oocyte maturation in zebrafish. Biochem Biophys Res Commun. 2017;490(3):657–63.
Koyama T, Nakamoto M, Morishima K, Yamashita R, Yamashita T, Sasaki K, Kuruma Y, Mizuno N, Suzuki M, Okada Y, Ieda R, Uchino T, Tasumi S, Hosoya S, Uno S, Koyama J, Toyoda A, Kikuchi K, Sakamoto T. A SNP in a steroidogenic enzyme is associated with phenotypic sex in Seriola fishes. Curr Biol. 2019;29(11):1901–9.
Zou CC, Wang LJ, Zou YX, Wu ZH, Wang WX, Liang SS, Wang L, You F. Characteristics and sex dimorphism of 17 beta-hydroxysteroid dehydrogenase family genes in the olive flounder Paralichthys olivaceus. J Steroid Biochem Mol Biol. 2020;199:105597.
Fan B, Xie DZ, Li YW, Wang XL, Qi X, Li SS, Meng ZN, Chen XH, Peng JY, Yang YJ, Li YY, Wang L. A single intronic single nucleotide polymorphism in splicing site of steroidogenic enzyme hsd17b1 is associated with phenotypic sex in oyster pompano, Trachinotus anak. P Roy Soc B-Biol Sci. 2021;288(1963):20212245.
Guo L, Yang JW, Liu BS, Zhang N, Zhu KC, Guo HY, Ma QW, Li YL, Jiang SG, Zhang DC. Colinearity based sex-specific marker development in the golden pompano (Trachinotus ovatus). Aquaculture. 2021;544:737044.
Zhang KX, Song FB, Liang YS, Zhang WW, Zhou KX, Chen YM, Zhen P, Luo J. Development and verification of sex-specific molecular marker for golden pompano (Trachinotus blochii). Aquac Res. 2022;53(10):3726–35.
Tang WQ, Mu Y, Valenzuela N, Du WG. Effects of incubation temperature on the expression of sex-related genes in the Chinese pond turtle, Mauremys reevesii. Sex Dev. 2017;11(5–6):307–19.
Baron D, Guiguen Y. Gene expression during gonadal sex differentiation in rainbow trout (Oncorhynchus mykiss): from candidate genes studies to high throughout genomic approach. Fish Physiol Biochem. 2003;28(1–4):119–23.
Wang DS, Kobayashi T, Zhou LY, Nagahama Y. Molecular cloning and gene expression of foxl2 in the Nile tilapia, Oreochromis niloticus. Biochem Biophys Res Commun. 2004;320(1):83–9.
Yang YJ, Wang Y, Li Z, Zhou L, Gui JF. Sequential, divergent, and cooperative requirements of foxl2a and foxl2b in ovary development and maintenance of zebrafish. Genetics. 2017;205(4):1551–72.
Gan RH, Wang Y, Li Z, Yu ZX, Li XY, Tong JF, Wang ZW, Zhang XJ, Zhou L, Gui JF. Functional divergence of multiple duplicated foxl2 homeologs and alleles in a recurrent polyploid fish. Mol Biol Evol. 2021;38(5):1995–2013.
Li MH, Yang HH, Li MR, Sun YL, Jiang XL, Xie QP, Wang TR, Shi HJ, Sun LN, Zho LY, Wang DS. Antagonistic roles of dmrt1 and foxl2 in sex differentiation via estrogen production in tilapia as demonstrated by TALENs. Endocrinology. 2013;154(12):4814–25.
Modarressi MH, Cameron J, Taylor KE, Wolfe J. Identification and characterisation of a novel gene, TSGA10, expressed in testis. Gene. 2001;262(1–2):249–55.
Modarressi MH, Behnam B, Cheng M, Taylor KE, Wolfe J, van der Hoorn FA. Tsga10 encodes a 65-kilodalton protein that is processed to the 27-kilodalton fibrous sheath protein. Biol Reprod. 2004;70(3):608–15.
Behnam B, Modarressi MH, Conti V, Taylor KE, Puliti A, Wolfe J. Expression of tsga10 sperm tail protein in embryogenesis and neural development: from cilium to cell division. Biochem Biophys Res Commun. 2006;344(4):1102–10.
Miryounesi M, Nayernia K, Mobasheri MB, Dianatpour M, Oko R, Savad S, Modarressi MH. Evaluation of in vitro spermatogenesis system effectiveness to study genes behavior: Monitoring the expression of the testis specific 10 (tsga10) gene as a model. Arch Iran Med. 2014;17(10):692–7.
Asghari-Givehchi S, Hossein-Modarressi M. Identification and expression analysis of zebrafish testis-specific gene 10 (tsga10). Int J Dev Biol. 2019;63(11–12):623–9.
Luo G, Hou MQ, Wang B, Liu ZX, Liu WQ, Han TT, Zhang DZ, Zhou XP, Jia WM, Tan Y, Wu YL, Wang JR, Zhang XQ. Tsga10 is essential for arrangement of mitochondrial sheath and male fertility in mice. Andrology. 2021;9(1):368–75.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357-U121.
Roberts A, Trapnell C, Donaghey J, Rinn JL, Pachter L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011;12(3):R22.
Anders S, Pyl PT, Huber W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–9.
Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25(1):25–9.
Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:D480–4.
Yu G, Wang LG, Han Y, He QY. ClusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–7.
Zhai ZH, Chen XN, Wang J. Primer design with Primer Premier 5.0 (in Chinese). Med Educ Res Pract. 2008;16(4):695–8.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25(4):402–8.
Acknowledgements
We would like to thank all the people who contributed to this work and the editor and reviewers for their valuable comments and suggestions.
Funding
This work was supported by National Natural Science Foundation of China (32172974) and the National Key R&D Program of China (2018YFD0901201).
Author information
Authors and Affiliations
Contributions
Wen-Yu Wei conducted the experiment and analysis and wrote the manuscript. Yi Gong, Xin-Fen Guo, Min Liu, Yu-Lin Zhou, Zhi Li and Li Zhou contributed to material preparation, data analysis and experiment. Zhong-Wei Wang and Jian-Fang Gui contributed to experimental design, supervision and manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments and animal treatments were approved by the Animal Care and Use Committee of Institute of Hydrobiology, Chinese Academy of Sciences. All experiments and animal treatments were carried out according to the principles of Animal Care and Use Committee of Institute of Hydrobiology, Chinese Academy of Sciences. This study complies with ARRIVE guidelines (https://arriveguidelines.org).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
The list of DEGs identified in M10dph-vs-F10dph.
Additional file 2: Table S2.
The list of DEGs identified in M18dph-vs-F18dph.
Additional file 3: Table S3.
The list of DEGs identified in M30dph-vs-F30dph.
Additional file 4: Table S4.
The list of DEGs identified in M48dph-vs-F48dph.
Additional file 5: Fig. S1.
The DEGs involved in the steroid hormone biosynthesis pathway in M10dph-vs-F10dph (ko00140, https://www.kegg.jp/pathway/map00140). The pink box in the figure represents up-regulated genes and the blue box represents down-regulated genes.
Additional file 6: Fig. S2.
The DEGs involved in the steroid hormone biosynthesis pathway in M18dph-vs-F18dph (ko00140, https://www.kegg.jp/pathway/map00140). The pink box in the figure represents up-regulated genes and the blue box represents down-regulated genes.
Additional file 7: Fig. S3.
The DEGs involved in the steroid hormone biosynthesis pathway in M30dph-vs-F30dph (ko00140, https://www.kegg.jp/pathway/map00140). The pink box in the figure represents up-regulated genes, the blue box represents down-regulated genes, and the olive box indicate that both up- and down-regulated genes are included.
Additional file 8: Fig. S4.
The DEGs involved in the steroid hormone biosynthesis pathway in M48dph-vs-F48dph (ko00140, https://www.kegg.jp/pathway/map00140). The pink box in the figure represents up-regulated genes, the blue box represents down-regulated genes, and the olive box indicate that both up- and down-regulated genes are included.
Additional file 9: Table S5.
The real p-values of RT-qPCR.
Additional file 10: Fig. S5.
The electrophoretic pattern of the sex identification in 36 males and 36 females. (a): 351 bp Y‐specific fragment amplified by the Y‐specific primer pair 18‐Fy and 18‐Ry only in male individuals; (b): 727 bp Y‐specific fragment amplified by Y‐specific primer pair 20‐Fy and 20‐Ry only in male individuals. M1-M12: sample “M48dph1”, M13-M24: sample “M48dph2”, M25-M36: sample “M48dph3”. F1-F12: sample “F48dph1”, F13-F24: sample “F48dph2”, F25-F36: sample “F48dph3”. M: DL 2000 DNA marker.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wei, WY., Gong, Y., Guo, XF. et al. Gonadal transcriptomes reveal sex-biased expression genes associated with sex determination and differentiation in red-tail catfish (Hemibagrus wyckioides). BMC Genomics 24, 183 (2023). https://doi.org/10.1186/s12864-023-09264-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09264-x