Abstract
Background
The high similarity in anatomical and neurophysiological processes between pigs and humans make pigs an excellent model for metabolic diseases and neurological disorders. Lipids are essential for brain structure and function, and the polyunsaturated fatty acids (PUFA) have anti-inflammatory and positive effects against cognitive dysfunction in neurodegenerative diseases. Nutrigenomics studies involving pigs and fatty acids (FA) may help us in better understanding important biological processes. In this study, the main goal was to evaluate the effect of different levels of dietary soybean oil on the lipid profile and transcriptome in pigs’ brain tissue.
Results
Thirty-six male Large White pigs were used in a 98-day study using two experimental diets corn-soybean meal diet containing 1.5% soybean oil (SOY1.5) and corn-soybean meal diet containing 3.0% soybean oil (SOY3.0). No differences were found for the brain total lipid content and FA profile between the different levels of soybean oil. For differential expression analysis, using the DESeq2 statistical package, a total of 34 differentially expressed genes (DEG, FDR-corrected p-value < 0.05) were identified. Of these 34 DEG, 25 are known-genes, of which 11 were up-regulated (log2 fold change ranging from + 0.25 to + 2.93) and 14 were down-regulated (log2 fold change ranging from − 3.43 to -0.36) for the SOY1.5 group compared to SOY3.0. For the functional enrichment analysis performed using MetaCore with the 34 DEG, four pathway maps were identified (p-value < 0.05), related to the ALOX15B (log2 fold change − 1.489), CALB1 (log2 fold change − 3.431) and CAST (log2 fold change + 0.421) genes. A “calcium transport” network (p-value = 2.303e-2), related to the CAST and CALB1 genes, was also identified.
Conclusion
The results found in this study contribute to understanding the pathways and networks associated with processes involved in intracellular calcium, lipid metabolism, and oxidative processes in the brain tissue. Moreover, these results may help a better comprehension of the modulating effects of soybean oil and its FA composition on processes and diseases affecting the brain tissue.
Similar content being viewed by others
Background
The pigs (Sus scrofa) have global economic impact as it is the second most consumed meat-based protein source worldwide [1, 2]. Additionally, pigs are considered an animal model and have been used in research in the area of nutrigenomics and human metabolic diseases. Moreover, pigs can be used to understand neurodegenerative diseases due to similar of the brain anatomy, development, function, and neurophysiological process compared to the brains of small laboratory animals and humans [3,4,5,6].
The brain contains high lipid content, making up approximately 50% of the brain’s dry weight, only lower than the adipose tissue [7]. Lipids are essential for brain structure and function, and the central nervous system is fundamental for the regulation of metabolism and lipid balance [8, 9]. In addition, some regions of the brain are capable to detect nutrients and hormones that regulate energy balance and feeding [8, 9].
A noteworthy factor is that the diet fed to the pigs can alter the lipid and fatty acids (FA) profiles of the tissues [10, 11]. Thus, soybean oil has been commonly used as part of the feed composition for growing-finishing pigs because it results in improved growth performance and beneficial effects to consumers [12]. In addition, soybean oil is high in polyunsaturated fatty acids (PUFA), being rich in linolenic acid (LA, C18:2 n-6), which is associated with the reduction of cardiovascular diseases and serum cholesterol [13].
Dietary derived FA, such as LA and alpha-linolenic acid (ALA, C18:3 n-3), act as precursors of PUFA like docosahexaenoic acid (DHA, C22:6 n-3) and arachidonic acid (AA, C20:4 n-6). Dietary supplementation of DHA may have potential neuroprotection effects against chronic and acute inflammation in the central nervous system, as well as slowing cognitive decline in Alzheimer’s disease [14]. PUFA and their metabolites act in the brain by activating receptors and cell signaling pathways. Additionally, they are responsible for modulating the system related to signaling lipids, present in phospholipids of the neuronal cell membrane, and for regulating synaptic function [15, 16].
While the roles of specific classes of FA in brain function are being elucidated, the understanding of the genes involved in the dietary modulation of FA in the brain is unclear and limited. Thus, the objective of this work was to determine if different levels of dietary soybean oil fed to male pigs would modify the lipid and transcriptome profile of the brain.
Results
Total lipid content and FA profile
Table 1 shows the total lipid composition and FA profile of the brain tissue from pigs given diets with different levels of soybean oil (SOY1.5 vs. SOY3.0). No changes (p-value ≤ 0.05) were identified in the total lipid content and the FA profile between the treatments.
RNA-Seq data and differentially expressed genes
An average number of total reads per sample of 33.4 M and 32.9 M, was obtained for the SOY1.5 group, before and after quality control, respectively. For the SOY3.0 group, the average number of sequenced reads, before and after quality control, were 34.3 M and 33.9 M, respectively. Of the total reads obtained for both groups, after quality control, 95.02% of them reads were mapped against the reference genome SScrofa11.1 (Additional file 1, Table S1).
Differential analysis was performed comparing the level of gene expression between the groups, and a total of 22,931 genes were identified in the brain tissue. Of this 34 were DEG (FDR-corrected p-value < 0.05). Within the 34 DEG, 25 were known-genes, 11 being up-regulated (log2 fold change ranging from + 0.25 to + 2.93) and 14 being down-regulated (log2 fold change ranging from − 3.43 to -0.36) in the SOY1.5 compared to the SOY3.0. The genes with the most altered expression were CALB1 (log2 fold change − 3.43; FDR = 0.03) and VMO1 (log2 fold change + 2.93; FDR < 0.01). The list of expressed genes and DEG are demonstrated in Table S2.
Functional enrichment analysis
The MetaCore software was used to identify pathway maps from the list of 34 DEG (FDR < 0.05). Four pathway maps were identified (p-value < 0.05), related to the following genes: arachidonate 15-lipoxygenase type B (ALOX15B), calbidin-1 (CALB1), and calpastatin (CAST), as shown in Table 2.
The ALOX15B DEG, showing a down-regulation in the SOY1.5 group compared to SOY3.0 (log2 fold change − 1.489). The ALOX15B, participate in two of the four significant enriched pathway maps identified: “Linoleic acid metabolism” (p-value = 1.970e-2, Fig. 1), and “Prostaglandin-1 biosynthesis and metabolism” (p-value = 3.597e-2, Fig. 2).
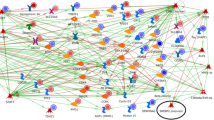
Linoleic acid metabolism in brain tissue of pigs fed diets containing different levels of soybean oil (SOY1.51 vs. SOY3.02). 1SOY1.5: corn-soybean meal diet containing 1.5% soybean oil. 2SOY1.5: corn-soybean meal diet containing 3.0% soybean oil. The experimental data is represented by the thermometer-like figure on the map. The downward thermometer (blue) indicates down-regulation of the ALOX15B DEG (log2 fold change − 1.489) in the SOY1.5 group compared to SOY3.0. Network objects are represented by individual symbols. The green “T” icon shows which object is associated with the brain tissue. Interactions between objects are represented by arrows, mechanisms, and logical relationships. Further explanations are provided at https://portal.genego.com/legends/MetaCoreQuickReferenceGuide.pdf.
Prostaglandin-1 biosynthesis and metabolism in brain tissue of pigs fed diets containing different levels of soybean oil (SOY1.51 vs. SOY3.02). 1SOY1.5: corn-soybean meal diet containing 1.5% soybean oil. 2SOY1.5: corn-soybean meal diet containing 3.0% soybean oil. The experimental data is represented by the thermometer-like figure on the map. The downward thermometer (blue) indicates down-regulation of the ALOX15B DEG (log2 fold change − 1.489) in the SOY1.5 group compared to SOY3.0. Network objects are represented by individual symbols. The green “T” icon shows which object is associated with the brain tissue. Interactions between objects are represented by arrows, mechanisms, and logical relationships. Further explanations are provided at https://portal.genego.com/legends/MetaCoreQuickReferenceGuide.pdf.
The CALB1 DEG, showing a down-regulation in the SOY1.5 group compared to SOY3.0 (log2 fold change − 3.431). The CALB1, participates in the enriched pathway map “Renal secretion of inorganic electrolytes” (p-value = 3.721e-2, Fig. 3).
Renal secretion of inorganic electrolytes in brain tissue of pigs fed diets containing different levels of soybean oil (SOY1.51 vs. SOY3.02). 1SOY1.5: corn-soybean meal diet containing 1.5% soybean oil. 2SOY1.5: corn-soybean meal diet containing 3.0% soybean oil. The experimental data is represented by the thermometer-like figure on the map. The downward thermometer (blue) indicates down-regulation of the CALB1 DEG (log2 fold change − 3.431) in the SOY1.5 group compared to SOY3.0. Network objects are represented by individual symbols. The green “T” icon shows which object is associated with the brain tissue. Interactions between objects are represented by arrows, mechanisms, and logical relationships. Further explanations are provided at https://portal.genego.com/legends/MetaCoreQuickReferenceGuide.pdf.
The CAST DEG, showing an up-regulation in the SOY1.5 group compared to SOY3.0 (log2 fold change + 0.421). The CAST participates in the enriched pathway map “Immune response IL-5 signaling via PI3K, MAPK, and NF-kB” (p-value = 4.770e-2, Fig. 4).
Immune response_IL-5_signaling in brain tissue of pigs fed diets containing different levels of soybean oil (SOY1.51 vs. SOY3.02). 1SOY1.5: corn-soybean meal diet containing 1.5% soybean oil. 2SOY1.5: corn-soybean meal diet containing 3.0% soybean oil. The experimental data is represented by the thermometer-like figure on the map. The upward thermometer (red) indicates up-regulation of the CAST DEG (log2 fold change + 0.421) in the SOY1.5 group compared to SOY3.0. Network objects are represented by individual symbols. The green “T” icon shows which object is associated with the brain tissue. Interactions between objects are represented by arrows, mechanisms, and logical relationships. Further explanations are provided at https://portal.genego.com/legends/MetaCoreQuickReferenceGuide.pdf.
To better understand the behavior of the genes and their interactions, process networks were additionally generated by using the MetaCore software. The “Calcium transport” process network (p-value = 2.303e-2), was the only network detected herein, containing the DEG CALB1 (log2 fold change − 3.431) and CAST (log2 fold change + 0.421) (Fig. 5).
Calcium transport in brain tissue of pigs fed diets containing different levels of soybean oil (SOY1.51 vs. SOY3.02). 1SOY1.5: corn-soybean meal diet containing 1.5% soybean oil. 2SOY1.5: corn-soybean meal diet containing 3.0% soybean oil. The experimental data are represented by the intensity of the blue and red circles on the network. The blue circle indicates down-regulation of the CALB1 DEG, and the red circle indicates up-regulation of the CAST DEG SOY1.5 group compared to SOY3.0. Green arrows indicate positive interactions, red arrows indicate negative interactions, and gray arrows indicate unspecified interactions. Further explanations are provided at https://portal.genego.com/legends/MetaCoreQuickReferenceGuide.pdf.
Discussion
No changes were identified in the total lipid content and the FA profile between the treatments. The results found in the functional enrichment analysis, demonstrated that the use of different levels of soybean oil alters the transcriptomic profile of pig brain, affecting key processes for the well-functioning of this tissue. For the enriched pathways illustrated in Figs. 1 and 2, the ALOX15B participates in lipid oxidation and peroxidation reactions. According to Stelzer et al. [18], among the pathways associated with this gene there were “eicosanoid synthesis” and “arachidonic acid metabolism” and the related Gene Ontology (GO) annotations include “calcium ion binding” and “lipid binding”. Fanalli et al. [19] demonstrated that the addition of soybean oil in the diet of pigs in different proportions acted on the modulation of genes, pathway maps and networks associated with inflammation, immune response, oxidative stress, and neurodegenerative diseases, in muscle and liver.
Lipoxygenases (LOX) are a family of enzymes responsible for the oxidation of lipids and the generation of a range of metabolites such as eicosanoids and PUFA-related compounds. These metabolites play diverse physiological and pathological roles in inflammatory, neurodegenerative, and cardiovascular diseases, as well as, in defence mechanisms [20, 21]. Lipoxygenases have also been reported in cell differentiation [22, 23], apoptosis [24], and play an important role in the immune response by helping to regulate cytokine secretion [25].
Among the LOX reported in mammals, the ALOX15 isoform may oxygenate complex lipid-protein assemblies found in biomembranes and lipoproteins [26]. The ALOX15 also binds to membranes, with intracellular calcium as a main cofactor for this interaction [27, 28]. It has been reported that ALOX15 is expressed at higher levels in human airway epithelial cells, in eosinophils and immature red blood cells [29]. Furthermore, according to van Leyen et al. [30] and Han et al. [31], expression and regulation of ALOX15 transcription also occurs in various areas of the brain, but at lower levels. In the study of Shalini et al. [32], a higher expression of ALOX15 mRNA was found in the prefrontal cortex.
The main product of AA oxygenation by ALOX15/15B is 15-hydroxyeicosatetraenoic (15-HETE) [33]. The 15-HETE is considered an important precursor of specialized pro-resolving lipid mediators and is associated with pro- and anti-inflammatory effects [34, 35]. It has also been reported that 15-HETE is a ligand and activator of the peroxisome proliferator-activated receptor gamma (PPAR-γ), which at high concentrations may generate reactive oxygen species in cells [36, 37], and may induce the production of the pro-inflammatory cytokine Interleukin-12 (IL-12) [35, 38]. Zhan et al. [39] demonstrated that the application of flaxseed-enriched diet (rich in n-3 PUFA, similar to soybean oil), showed decreases in the expression of pro-inflammatory cytokine genes through activation of PPAR-γ in muscle, adipose tissue and spleen of growing-finishing barrows.
Among the results of DHA oxidation by ALOX15, are the specialized pro-resolving lipid mediator D5, a mediator that may be associated in the resolution of inflammation and in the regulation of immune response [40]. Another important mediator related to the resolution of inflammation, reduction of leukocyte trafficking, and negative regulation of cytokine expression is neuroprotectin D1 (NPD1) [41, 42]. NPD1 is reported as an anti-inflammatory molecule, which acts in neuroplasticity and brain signaling, and when in altered conditions, may be found in neuroinflammatory disorders and chronic neurodegeneration [32].
In the study of Chaung et al. [43] dietary supplementation of phosphatidylserine and DHA improved antioxidant activity and cognitive function (spatial memory) in rat pups during brain development. Richter et al. [44] demonstrated that daily intake of soy-derived phosphatidylserine, had positive effects on cognitive function (learning and memory) in elderly people with impaired memory function.
The ALOX15 was found to have increased expression in the brains of Alzheimer’s patients [26, 45, 46]. Praticò et al. [45], reported higher levels of 12/15-LOX and its metabolites 12/15(S)-HETE in the temporal and frontal brain regions of Alzheimer’s patients. It was further found in in vitro studies using neuronal cells with Alzheimer’s mutation, that 12/15-LOX is associated with regulation of tau phosphorylation and Aβ plaque production. In addition, regulates synaptic pathology associated with behavioral deficiencies [47, 48].
Additionally, studies have shown that 12/15-LOX is crucial in Parkinson’s disease [49,50,51]. According to the research of Li et al. [49] and Canals et al. [50], activation of these isoforms was associated with a decrease in glutathione concentration (a marker of Parkinson’s disease) in neurons, which may induce nitric oxide neurotoxicity and damage to dopaminergic neurons. The mechanism of action of 12/15-LOX is still unclear. For example, inhibiting 12/15-LOX has been shown to reduce reactive oxygen species-induced neuronal cell death [51]. Other studies found that 12/15-LOX and its metabolites have both pro- and anti-inflammatory effects. This controversial nature of 12/15-LOX has been reported to be dependent on the metabolites produced, the site of inflammation, and the levels of these metabolites produced [35]. The brain is a tissue that contains a wide range of metabolites and in distinct concentrations. Thus, due to the controversial nature of ALOX15B in metabolic and oxidative processes, further investigations are needed to understand the influence of the downregulation of this DEG in the SOY1.5 group that was found in our study. Further research is required to confirm the action of ALOX15B in the progression of neurodegenerative and inflammatory diseases.
For the enriched pathway in Fig. 3, the CALB1 gene binds to intracellular calcium transported via the epithelial calcium channel and transports it across the cytosol toward the basolateral membrane [52]. As a protein-encoding gene that participates in calcium transport, GO annotations for the CALB1 are found to be related to “calcium ion binding” and “vitamin D binding” [18]. It is a highly conserved calcium-binding protein that belongs to a family of high-affinity calcium-binding proteins [53, 54]. Furthermore, studies have shown that the CALB1 gene is highly expressed in brain tissue and found in the majority of neuronal cells and that it is not vitamin D dependent [53,54,55].
Calcium is one of the most important signaling factors and acts to regulate several important cellular functions such as growth, differentiation, proliferation, cell survival and apoptosis, membrane excitability, and gene transcription. Calcium is also essential for maintaining normal brain function [56]. Thus, the dysregulation of calcium homeostasis and endoplasmic reticulum stress is associated with several pathological conditions such as Parkinson’s, Huntington’s, and Alzheimer’s diseases, and affects numerous signaling pathways [56, 57]. This pathogenic event may also cause amyloidogenesis, energy deficits in neurons, protein aggregation and oxidative stress, and changes in mitochondrial dysfunction, plasticity, and synaptic transmission [58].
Disturbed mitochondrial calcium regulation may also be associated with the link between neuronal dysfunction and disruption of the mitochondria-associated membrane (MAM) contact site of the endoplasmic reticulum and mitochondria, since calcium acts to modulate neurotransmitter release during the synapse [59]. This dysregulation of the MAM-mitochondria linkage dysfunction may also be associated with neurodegenerative diseases such as Alzheimer’s disease [59]. The MAMs are regions of the endoplasmic reticulum that mediate communication between the reticulum and the mitochondria [59, 60]. They are regions that are involved in calcium transport, are responsible for several lipid biosynthetic enzymatic activities, and are also a strategic site for lipid metabolism [59, 61, 62]. According to Vance [59], defects associated with these regions have been identified in neurodegenerative diseases and insulin resistance/type 2 diabetes.
The CALB1 helps maintain calcium homeostasis, regulate intracellular calcium responses to physiological stimuli, and modulating synaptic transmission [54]. Another important role of CALB1, is its action in the prevention of neuronal death [54, 63]. The CALB1 also plays an important role in buffering cytosolic calcium and helps prevent lipid peroxidation, through its expression in pancreatic-β cells, by eliminating the production of lipid hydroperoxide, which is induced by proinflammatory cytokines [64]. There is evidence that CALB1 acts to protect neurons against calcium-mediated neurotoxicity and may be considered a cytochemical marker for neuronal plasticity [55].
Decreases in CALB1 expression/concentration in brain tissue has been associated with neurodegeneration in Alzheimer’s, Parkinson’s, and Huntington’s diseases [18, 65] and in ischemic injury studies [66, 67]. Lower CALB1 expression has also been associated with a higher rate of neuronal death [68]. Increased expression of CALB1, on the other hand, has been reported to induce neurite growth in dopaminergic neuronal cells, demonstrating its protective role, especially in neurological diseases, such as Parkinson’s disease [63, 69].
For Alzheimer’s disease, it has been reported that CALB1 has protective effects against the pro-apoptotic action of mutant presenilin 1 (PS-1), attenuating the increase in intracellular calcium and aiding in the prevention of impaired mitochondrial function [70]. PS-1 acts by sensitizing cells to apoptosis induced by Aβ peptide, which damages neurons through a mechanism involving disruption of calcium homeostasis and generation of oxidative stress [70]. Thus, regarding CALB1 down-expression in the SOY1.5 group, we observed that a lower percentage of soybean oil CALB1 gene is less expressed indicating a negative relationship with this diet and a positive relationship with the neurodegenerative processes.
For the enriched pathway in Fig. 4, IL-5 activates and elevates the expression of CAST. The CAST binds to and inhibits calpain 1 (mu) in the presence of calcium, which activates and cleaves the apoptosis regulatory protein Bax. The Bax will act by preventing or reducing the frequency, rate, or extent of cell death by apoptotic process [71, 72]. The protein encoded by CAST is an endogenous calpain inhibitor and is also related to the proteolysis of amyloid precursor protein. Furthermore, this protein is thought to influence the expression levels of genes that encode structural or regulatory proteins “Neuroscience” and “neurodegenerative diseases” are two related pathways associated with this gene. Related GO annotations of CAST include “RNA binding” and “cysteine-type endopeptidase inhibitor activity” [18, 73].
The CAST is a cell-permeable peptide that acts as an endogenous inhibitor of calpain in the central nervous system [73, 74]. Calpains are cysteine proteases that are activated by calcium, that is, they are positively regulated by calcium and negatively regulated by CAST [75, 76]. These proteases, when in dysregulation of calcium homeostasis, have been implicated in neuronal cell dysfunction and death [76], as well as neurodegenerative diseases [77,78,79].
Calpains have several important roles such as differentiation, cell attachment motility, signal transduction covering cell signaling pathways, regulation of gene expression and membrane fusion [73, 75]. Furthermore, calpains are reported to play important roles in neuronal functions, implying that the activation of this protease needs to be under a rigid control, which is performed by CAST. Thus, the well-known calpain-calpastatin system may be an important target for therapeutic approaches related to neurodegenerative diseases [76].
According to Goll et al. [75], CAST is also involved in the regulation of kinases, receptors, and transcription factors. CAST expression has been shown to have a neuroprotective effect on cerebral ischemia [80]. In the study of Rao et al. [81], higher expression of CAST in JNPL3 (mutant tau P301L) mouse models was used to attenuate calpain expression, which has been linked to the development of tauopathy (neurotoxicity caused by tau protein) and neurodegeneration in Alzheimer’s disease. In an Amyotrophic Lateral Sclerosis mouse model, higher CAST expression was associated with neuroprotective effects. According to Rao et al. [82], the CAST gene reduces calpain activation, decreases abnormal cytoskeletal protein breakdown, increases survival time, inhibits tau production and CDK5 activation, and decreases SOD1.
The calpain-calpastatin system is also reported in excitotoxicity, a pathological or neurodegenerative process that is initiated by overactivation of neurotransmitters such as glutamate. Excitotoxicity leads to increased cellular calcium levels, which causes activation of various proteases, including calpains [83]. Furthermore, missing CAST may impair early stages of neurogenesis [84]. Thus, we observed a higher expression of CAST in the SOY1.5 group, that suggests a positive relationship between the gene and the metabolic and oxidative processes found for this group.
The identified network, along with the illustrated genes, corroborate the results found in the pathway maps, indicating that varying the amount of soybean oil in the diet of immunocastrated male pigs influences gene expression in brain tissue. Furthermore, the significance of the detected DEG and their association with intracellular calcium is noteworthy. This processes network (Calcium transport) and the genes enriched in this network corroborate the results found in the pathway maps, indicating that changing the level of soybean oil in pigs’ diet has an effect on gene expression. Therefore, the findings of our study point in a promising direction for furthering our understanding of the pathways and networks associated with calcium-dependent metabolic processes involved in lipid metabolism and oxidative processes. More research is needed to better understand the mechanisms by which dietary factors like FA may influence important physiological processes and gene expression in brain tissue. Understanding the mechanisms involved in calcium homeostasis and energy metabolism in the initiation and progression of neurodegenerative diseases and oxidative/inflammatory processes is extremely important.
Conclusion
This study showed that different levels of soybean oil in pig diets affect the transcriptomic profile but not the total lipid content or FA profile of brain tissue. The genes, pathways, and networks identified herein play important roles in lipid metabolism, immune response, and calcium transport. Furthermore, because pigs are model animals for human metabolic diseases, the DEG identified, as well as their action in brain tissue, demonstrate the importance of FA in metabolic and oxidative processes. Thus, the current study may help future research in the field of nutrigenomics and help to better understand how the diet, with the inclusion of soybean oil, may influence and modulate biological processes important for brain tissue. Further investigation is required to define what proportion of soybean oil helps in directing and modulating neuroprotection and reducing inflammation in brain tissue.
Methods
Ethics Statement
The procedures involving animals were evaluated and approved by the Ethics Committee for the Use of Animals (CEUA, number 2018-28, and protocol 2018.5.1787.11.6) of the Luiz de Queiroz College of Agriculture (ESALQ) – University of São Paulo (USP). All procedures followed the guidelines by the Brazilian Council of Animal Experimentation and the ethical principles in animal research, according to FASS [85], the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. This study was carried out in compliance with the ARRIVE guidelines.
Animals, experimental design, and diets
Thirty-six immunocastrated male pigs, the offspring of three sires and thirty-two females of the Large White breed, were used for this study. Pigs were genotyped for the halothane mutation (RYR1 gene) and only homozygous halothane-negative (NN) were used [86]. The pigs had an average body weight of 28.44 ± 2.95 kg and an average age of 71 ± 1.8 days, and were randomly distributed to the treatments during the experimental period of 98 days. Two treatments were used, with six replicate pens per treatment, and three pigs per pen, totalizing 18 pigs per treatment. The pigs ad libitum access to feed and water throughout the experimental period, and each pen was equipped with a dry feeder and a nipple drinker. The immunocastration was performed by administering two doses of 2 ml of Vivax® (Pfizer Animal Health, Parkville, Australia) on day 56 (127 days of age) and day 70 (141 days of age), according to the manufacturer’s recommendations.
The experimental diet consisted of a six-phase diet: Grower I - day 0 to 21; Grower II - day 21 to 42; Finisher I - day 42 to 56; Finisher II - day 56 to 63; Finisher III - day 63 to 70; and, Finisher IV - day 70 to 98. Dietary treatments consisted of corn-soybean meal diets either containing 1.5% soybean oil (SOY1.5), a standard diet used in pig production, or containing 3.0% soybean oil (SOY3.0). The diets were formulated to meet or exceed the nutritional requirements according to Rostagno et al. [87], and were provided as a meal form, without antibiotic growth promoters. The diets were formulated to have a similar metabolizable energy content (3.36 Mcal/kg). Details of the diets in this study are adapted and described in Tables S4–S6 [19, 88, 89].
The pigs were slaughtered with a final body weight of 133.9 ± 9.4 kg on day 98 of the experiment. Whole brains of the animals were collected and immediately frozen in liquid nitrogen, transported, and stored in a -80 °C freezer until total RNA extraction. The same portion of the middle region of the frontal lobe was delimited in all brain samples in order to obtain a sample as uniform as possible with the same proportion of white and gray matter and the layers. Complete procedures were described in Almeida et al. [88] and Silva et al. [90].
Total lipid content and FA profile analyses
For the analysis of total lipid content, 5 g of brain samples were used (in duplicate), which were ground, packed in plastic bags and stored at 4ºC. The ground samples were dried in an oven with air circulation at 105 °C for 12 h. After drying, the samples were packed in filter paper cartridges and placed in a Soxhlet type extraction system. The extraction was conducted with hexane and occurred during six hours, according to the method described by AOAC [91]. The percentage of total lipid in the samples was obtained by the difference between the weight of the flask containing the extracted lipid and the empty flask (previously weighed, the flask was left in an oven at 105 °C for 2 h before each weighing) multiplied by 100.
The FA profile was determined from the total lipid content using 10 g samples. The lipids were cold extracted using the method proposed by Bligh Dyer [92] and the methylation of the samples was performed according to Hartman e Lago [93], with adaptations based on AOCS [94] (method AM 5 − 04). The complete procedures were described by Silva et al. [90] and Almeida et al. [88].
Data were analyzed as a randomized complete block design using the MIXED procedure of SAS (SAS Inst. Inc., Cary, NC), with pen being considered as the experimental unit. The model included the random effects of pen and block and the fixed effects of soybean oil levels. Outliers were removed from the data sets and residuals were tested for a normal distribution using the Shapiro-Wilk test (UNIVARIATE procedure). Means were adjusted by using the LSMEANS statement. Differences were declared significant when p-value ≤ 0.05 based on the F-test.
RNA extraction, library preparation, sequencing and data analysis
For the total RNA extraction from the brain samples, we used the commercial kit for RNA extraction (RNeasy® Mini Kit, Qiagen) and the Trizol reagent (Invitrogen). The inclusion of first step using the Trizol, allowed for better phase separation and thus lipid removal as brain tissue has a large amount of lipids (~ 10%). Quality and concentration of total RNA was obtained by using the NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific) and Qubit® 2.0 Fluorometer. The RNA integrity was evaluated by using the Agilent 2100 Bioanalyzer (Agilent, Santa Clara-CA, USA). All samples presented an RNA Integrity Number (RIN) greater than or equal to 7.5 (Table S3).
For library preparation, 2 µL of total RNA from each sample was used, according to the protocol described in the TruSeq RNA Sample Preparation kit v2 manual (Illumina, San Diego, CA). The average library size was estimated using the Agilent Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA) and the libraries were quantified using quantitative PCR with the quantification kit, from the KAPA library (KAPA Biosystems, Foster City, CA, USA). TruSeq PE Cluster kit v3-cBot-HS (Illumina, San Diego, CA, USA) was used for the sequencing. The samples were pooled and sequenced by using the HiSeq 2500 equipment (Illumina, San Diego, CA, USA) with a TruSeq SBS Kit v3-HS (200 cycles), according to the manufacturer’s instructions. All sequencing steps were performed at the ESALQ-USP Animal Genomics Center, located in the Animal Biotechnology Laboratory of ESALQ-USP, Piracicaba, São Paulo, Brazil.
For the steps of quality control, low complexity reads and adapters were removed using Trim Galore software (v.0.6.5). The minimum length of reads after removal was 70 bases, with Phred Score lower than 33. Quality control was done by using FastQC software (v.0.11.8) [http://www.bioinformatics.bbsrc.ac.uk/projects/fastqc/]. The reference genome used was the Sus Scrofa 11.1, available from Ensembl [http://www.ensembl.org/Sus_scrofa/Info/Index]. Alignment, mapping, and abundance (read counts) of mRNAs for all known-genes was performed using STAR software (v.2.7.6a) [95], and the gene expression levels were normalized using the counts scaled by total number of reads or counts per million (CPM).
Identification of differentially expressed genes, and functional enrichment analysis
The differentially expressed genes (DEG) between the SOY1.5 and SOY3.0 groups were identified by using the DESeq2 statistical package (R/Bioconductor) [http://www.bioconductor.org/packages/release/bioc/html/DESeq2.html], using a multi-factor design [96]. Before statistical analysis, filtering criteria were used: (i) removal of genes with zero counts for all samples, that is, unexpressed genes, (ii) removal of genes with less than one read per sample on average were removed (very lowly expressed); (iii) removal of genes that were not present in at least 50% of the samples were removed (rarely expressed). The model used, included treatments as the variable of interest and father as a fixed effect. Correction for multiple testing was performed, according to the False Discovery Rate (FDR) method [97], and the threshold value used for significance was FDR < 0.05.
The enrichment analysis was performed using the MetaCore software (Clarivate Analytics, London, UK, v.21.4, build 70,700) [https://clarivate.com/products/metacore/]. The pathway maps were identified from the list of known-genes DEG obtained from SOY1.5 vs. SOY3.0 (FDR < 0.05) comparison. For annotation and functional enrichment, the Homo sapiens genome was used as background reference. Functional enrichment analysis to obtain comparative pathways and networks was performed, using the standard parameter. The filters for the metabolic maps of interest were used: energy metabolism, lipid metabolism, steroid metabolism, regulation of cellular processes (immune response, neurophysiological process, and oxidative stress), regulation of metabolism, mental disorders, nutritional and metabolic diseases, nervous system diseases, and tox processes. To understand the behavior of genes and their interactions, networks were created.
Data Availability
The dataset supporting the conclusions of this article is available in the European Nucleotide Archive (ENA) repository (EMBL-EBI), under accession PRJEB52665 [http://www.ebi.ac.uk/ena/data/view/PRJEB52665]. The original contributions presented in the study are included in the article and supplementary material, further inquiries can be directed to the corresponding author.
References
OECD-FAO. OECD-FAO Agricultural Outlook 2021–2030. OECD; 2021.
Pan Z, Yao Y, Yin H, Cai Z, Wang Y, Bai L, et al. Pig genome functional annotation enhances the biological interpretation of complex traits and human disease. Nat Commun. 2021;12:5848.
Lind NM, Moustgaard A, Jelsing J, Vajta G, Cumming P, Hansen AK. The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev. 2007;31:728–51.
Lunney JK. Advances in Swine Biomedical Model Genomics. Int J Biol Sci. 2007;3:179–84.
Dawson H, Chen C, Wang T, Urban J. Comparative Nutrigenomics Analysis of the Pig, Mouse and Human (P15-004-19).Curr Dev Nutr. 2019;3.
Hoffe B, Holahan MR. The Use of Pigs as a Translational Model for Studying Neurodegenerative Diseases.Front Physiol. 2019;10.
Hsu M-C, Huang Y-S, Ouyang W-C. Beneficial effects of omega-3 fatty acid supplementation in schizophrenia: possible mechanisms. Lipids Health Dis. 2020;19:159.
Hamilton JA, Hillard CJ, Spector AA, Watkins PA. Brain uptake and utilization of fatty acids, lipids and lipoproteins: application to Neurological Disorders. J Mol Neurosci. 2007;33:2–11.
Bruce KD, Zsombok A, Eckel RH. Lipid Processing in the Brain: A Key Regulator of Systemic Metabolism.Front Endocrinol (Lausanne). 2017;8 APR.
Lu P, Zhang LY, Yin JD, Everts AKR, Li DF. Effects of soybean oil and linseed oil on fatty acid compositions of muscle lipids and cooked pork flavour. Meat Sci. 2008;80:910–8.
Alencar SAdaS, Kiefer C, Nascimento KMR, de Viana S, Corassa LH, de Gomes A. Dietary soybean oil modulates fatty acid composition of pork. Trop Anim Health Prod. 2021;53:357.
Stein HH, Berger LL, Drackley JK, Fahey GC, Hernot DC, Parsons CM. Nutritional Properties and feeding values of soybeans and their coproducts. In: Soybeans. Elsevier; 2008. pp. 613–60.
Fan L, Eskin NAM. The use of antioxidants in the preservation of edible oils. Handbook of Antioxidants for Food Preservation.Elsevier; 2015.pp. 373–88.
Sun GY, Simonyi A, Fritsche KL, Chuang DY, Hannink M, Gu Z, et al. Docosahexaenoic acid (DHA): an essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot Essent Fatty Acids. 2018;136:3–13.
Rapoport SI. Translational studies on regulation of brain docosahexaenoic acid (DHA) metabolism in vivo. Prostaglandins Leukot Essent Fatty Acids. 2013;88:79–85.
Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–85.
Ulbricht TLV, Southgate DAT. Coronary heart disease: seven dietary factors. The Lancet. 1991;338:985–92.
Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, et al. The GeneCards suite: from Gene Data Mining to Disease Genome sequence analyses. Curr Protoc Bioinformatics. 2016;54:1–33.
Fanalli SL, da Silva BPM, Gomes JD, de Almeida VV, Freitas FAO, Moreira GCM, et al. Differential Gene expression Associated with soybean oil level in the Diet of Pigs. Animals. 2022;12:1632.
Gertow K, Nobili E, Folkersen L, Newman JW, Pedersen TL, Ekstrand J, et al. 12- and 15-lipoxygenases in human carotid atherosclerotic lesions: Associations with cerebrovascular symptoms. Atherosclerosis. 2011;215:411–6.
Karatas H, Cakir-Aktas C. 12/15 lipoxygenase as a therapeutic target in Brain Dosorders. Archives of Neuropsychiatry. 2019;56:288–91.
van Leyen K, Duvoisin RM, Engelhardt H, Wiedmann M. A function for lipoxygenase in programmed organelle degradation. Nature. 1998;395:392–5.
Adel S, Karst F, González-Lafont À, Pekárová M, Saura P, Masgrau L et al. Evolutionary alteration of ALOX15 specificity optimizes the biosynthesis of antiinflammatory and proresolving lipoxins. Proceedings of the National Academy of Sciences. 2016;113:E4266-75.
Claria J. Regulation of cell proliferation and apoptosis by bioactive lipid mediators. Recent Pat Anticancer Drug Discov. 2006;1:369–82.
Danielsson KN, Rydberg EK, Ingelsten M, Akyürek LM, Jirholt P, Ullström C, et al. 15-Lipoxygenase-2 expression in human macrophages induces chemokine secretion and T cell migration. Atherosclerosis. 2008;199:34–40.
Ivanov I, Kuhn H, Heydeck D. Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15). Gene. 2015;573:1–32.
Watson A, Doherty FJ. Calcium promotes membrane association of reticulocyte 15-lipoxygenase. Biochem J. 1994;298:377–83.
Brinckmann R, Schnurr K, Heydeck D, Rosenbach T, Kolde G, Kühn H. Membrane translocation of 15-Lipoxygenase in hematopoietic cells is calcium-dependent and activates the oxygenase activity of the enzyme. Blood. 1998;91:64–74.
Nadel JA, Conrad DJ, Ueki IF, Schuster A, Sigal E. Immunocytochemical localization of arachidonate 15-lipoxygenase in erythrocytes, leukocytes, and airway cells. J Clin Invest. 1991;87:1139–45.
van Leyen K, Kim HY, Lee S-R, Jin G, Arai K, Lo EH. Baicalein and 12/15-Lipoxygenase in the ischemic brain. Stroke. 2006;37:3014–8.
Han J, Sun L, Xu Y, Liang H, Cheng Y. Activation of PPARγ by 12/15-lipoxygenase during cerebral ischemia-reperfusion injury. Int J Mol Med. 2015;35:195–201.
Shalini S-M, Ho CF-Y, Ng Y-K, Tong J-X, Ong E-S, Herr DR, et al. Distribution of Alox15 in the rat brain and its role in prefrontal cortical resolvin D1 formation and spatial Working Memory. Mol Neurobiol. 2018;55:1537–50.
Snodgrass RG, Brüne B. Regulation and Functions of 15-Lipoxygenases in Human Macrophages.Front Pharmacol. 2019;10.
Kutzner L, Goloshchapova K, Heydeck D, Stehling S, Kuhn H, Schebb NH. Mammalian ALOX15 orthologs exhibit pronounced dual positional specificity with docosahexaenoic acid. Biochim Biophys Acta. 2017;1862:666–75.
Singh NK, Rao GN. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog Lipid Res. 2019;73:28–45.
Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, et al. Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–82.
Sun L, Xu Y-W, Han J, Liang H, Wang N, Cheng Y. 12/15-Lipoxygenase metabolites of arachidonic acid activate PPARγ: a possible neuroprotective effect in ischemic brain. J Lipid Res. 2015;56:502–14.
Li J, Rao J, Liu Y, Cao Y, Zhang Y, Zhang Q, et al. 15-Lipoxygenase promotes chronic Hypoxia–Induced Pulmonary artery inflammation via positive Interaction with Nuclear Factor-κB. Arterioscler Thromb Vasc Biol. 2013;33:971–9.
Zhan ZP, Huang FR, Luo J, Dai JJ, Yan XH, Peng J. Duration of feeding linseed diet influences expression of inflammation-related genes and growth performance of growing-finishing barrows. J Anim Sci. 2009;87:603–11.
Perry SC, Kalyanaraman C, Tourdot BE, Conrad WS, Akinkugbe O, Freedman JC, et al. 15-Lipoxygenase-1 biosynthesis of 7S,14S-diHDHA implicates 15-lipoxygenase-2 in biosynthesis of resolvin D5. J Lipid Res. 2020;61:1087–103.
Hong S, Gronert K, Devchand PR, Moussignac R-L, Serhan CN. Novel docosatrienes and 17S-Resolvins generated from Docosahexaenoic Acid in Murine Brain, Human Blood, and glial cells. J Biol Chem. 2003;278:14677–87.
Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol. 2009;158:960–71.
Chaung H-C, Chang C-D, Chen P-H, Chang C-J, Liu S-H, Chen C-C. Docosahexaenoic acid and phosphatidylserine improves the antioxidant activities in vitro and in vivo and cognitive functions of the developing brain. Food Chem. 2013;138:342–7.
Richter Y, Herzog Y, Lifshitz Y, Hayun R, Zchut S. The effect of soybean-derived phosphatidylserine on cognitive performance in elderly with subjective memory complaints: a pilot study. Clin Interv Aging. 2013;8:557.
Praticò D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, et al. 12/15-Lipoxygenase is increased in Alzheimer’s Disease. Am J Pathol. 2004;164:1655–62.
Yang H, Zhuo J-M, Chu J, Chinnici C, Praticò D. Amelioration of the Alzheimer’s Disease phenotype by absence of 12/15-Lipoxygenase. Biol Psychiatry. 2010;68:922–9.
Succol F, Praticò D. A role for 12/15 lipoxygenase in the amyloid betha precursor protein metabolism. J Neurochem. 2007;103:380–7.
Joshi YB, Giannopoulos PF, Praticò D. The 12/15-lipoxygenase as an emerging therapeutic target for Alzheimer’s disease. Trends Pharmacol Sci. 2015;36:181–6.
Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–63.
Canals S, Casarejos MJ, de Bernardo S, Rodríguez-Martín E, Mena MA. Nitric oxide triggers the toxicity due to glutathione depletion in midbrain cultures through 12-Lipoxygenase. J Biol Chem. 2003;278:21542–9.
Zhang Y. Peroxynitrite-Induced neuronal apoptosis is mediated by Intracellular Zinc Release and 12-Lipoxygenase activation. J Neurosci. 2004;24:10616–27.
Lambers TT, Mahieu F, Oancea E, Hoofd L, de Lange F, Mensenkamp AR, et al. Calbindin-D28K dynamically controls TRPV5-mediated Ca2 + transport. EMBO J. 2006;25:2978–88.
Christakos S, Gabrielides C, Rhoten WB. Vitamin D-Dependent calcium binding proteins: Chemistry, distribution, functional considerations, and Molecular Biology. Endocr Rev. 1989;10:3–26.
Christakos S, Mady LJ, Dhawan P. Calbindin-D 28K and Calbindin-D 9K and the epithelial calcium channels TRPV5 and TRPV6. In: Vitamin D. Elsevier; 2018. pp. 343–59.
Ferrante RJ, Kowall NW, Richardson EP. Neuronal Plasticity: A Potential Role in Spiny Striatal Neuron Degeneration in Huntington’s Disease. Trophic Regulation of the Basal Ganglia.Elsevier; 1994.pp. 465–77.
Genovese I, Giamogante F, Barazzuol L, Battista T, Fiorillo A, Vicario M, et al. Sorcin is an early marker of neurodegeneration, Ca2 + dysregulation and endoplasmic reticulum stress associated to neurodegenerative diseases. Cell Death Dis. 2020;11:861.
Supnet C, Bezprozvanny I. Neuronal Calcium Signaling, mitochondrial dysfunction, and Alzheimer’s Disease. J Alzheimer’s Disease. 2010;20:487–98.
Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–92.
Vance JE. MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim Biophys Acta. 2014;1841:595–609.
Yang M, Li C, Yang S, Xiao Y, Xiong X, Chen W et al. Mitochondria-Associated ER Membranes – The Origin Site of Autophagy.Front Cell Dev Biol. 2020;8.
Arruda AP, Hotamisligil GS. Calcium homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metab. 2015;22:381–97.
Lee S, Min KT. The interface between ER and mitochondria: molecular compositions and functions. Mol Cells. 2018;41:1000–7.
Castrogiovanni P, Sanfilippo C, Imbesi R, Maugeri G, lo Furno D, Tibullo D, et al. Brain CHID1 expression correlates with NRGN and CALB1 in healthy subjects and AD patients. Cells. 2021;10:882.
Rabinovitch A, Suarez-Pinzon WL, Sooy K, Strynadka K, Christakos S. Expression of Calbindin-D 28k in a pancreatic islet β-Cell line protects against Cytokine-Induced apoptosis and necrosis. Endocrinology. 2001;142:3649–55.
Iacopino AM, Christakos S. Specific reduction of calcium-binding protein (28-kilodalton calbindin-D) gene expression in aging and neurodegenerative diseases. Proceedings of the National Academy of Sciences. 1990;87:4078–82.
Burke RE, Baimbridge KG. Relative loss of the striatal striosome compartment, defined by calbindin-D28k immunostaining, following developmental hypoxic-ischemic injury. Neuroscience. 1993;56:305–15.
Ahmadian SS, Rezvanian A, Peterson M, Weintraub S, Bigio EH, Mesulam M-M, et al. Loss of calbindin-D 28K is associated with the full range of tangle pathology within basal forebrain cholinergic neurons in Alzheimer’s disease. Neurobiol Aging. 2015;36:3163–70.
Kook S-Y, Jeong H, Kang MJ, Park R, Shin HJ, Han S-H, et al. Crucial role of calbindin-D28k in the pathogenesis of Alzheimer’s disease mouse model. Cell Death Differ. 2014;21:1575–87.
Yuan H-H, Chen R-J, Zhu Y-H, Peng C-L, Zhu X-R. The neuroprotective effect of overexpression of Calbindin-D28k in an animal model of Parkinson’s Disease. Mol Neurobiol. 2013;47:117–22.
Guo Q, Christakos S, Robinson N, Mattson MP. Calbindin D28k blocks the proapoptotic actions of mutant presenilin 1: Reduced oxidative stress and preserved mitochondrial function. Proceedings of the National Academy of Sciences. 1998;95:3227–32.
Shen Z-J, Esnault S, Schinzel A, Borner C, Malter JS. The peptidyl-prolyl isomerase Pin1 facilitates cytokine-induced survival of eosinophils by suppressing bax activation. Nat Immunol. 2009;10:257–65.
Ilmarinen P, Moilanen E, Kankaanranta H. Regulation of Spontaneous Eosinophil Apoptosis—A Neglected Area of Importance.J Cell Death. 2014;7:JCD.S13588.
Li Y, Bondada V, Joshi A, Geddes JW. Calpain 1 and calpastatin expression is developmentally regulated in rat brain. Exp Neurol. 2009;220:316–9.
Teng F, Yin Y, Guo J, Jiang M. Calpastatin peptide attenuates early brain injury following experimental subarachnoid hemorrhage. Exp Ther Med. 2020;19:2433–40.
Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801.
Stifanese R, Averna M, de Tullio R, Pedrazzi M, Beccaria F, Salamino F, et al. Adaptive modifications in the Calpain/Calpastatin system in Brain cells after persistent alteration in Ca2 + homeostasis. J Biol Chem. 2010;285:631–43.
Ray S. Currently evaluated calpain and caspase inhibitors for Neuroprotection in Experimental Brain Ischemia. Curr Med Chem. 2006;13:3425–40.
Vosler PS, Brennan CS, Chen J. Calpain-mediated signaling mechanisms in neuronal Injury and Neurodegeneration. Mol Neurobiol. 2008;38:78–100.
Jourdi H, Hamo L, Oka T, Seegan A, Baudry M. BDNF mediates the neuroprotective effects of positive AMPA receptor modulators against MPP+-induced toxicity in cultured hippocampal and mesencephalic slices. Neuropharmacology. 2009;56:876–85.
Rami A, Volkmann T, Agarwal R, Schoninger S, Nürnberger F, Saido TC, et al. β2-Adrenergic receptor responsiveness of the calpain–calpastatin system and attenuation of neuronal death in rat hippocampus after transient global ischemia. Neurosci Res. 2003;47:373–82.
Rao M, McBrayer MK, Campbell J, Kumar A, Hashim A, Sershen H, et al. Specific calpain inhibition by calpastatin prevents Tauopathy and Neurodegeneration and restores normal lifespan in tau P301L mice. J Neurosci. 2014;34:9222–34.
Rao M, Campbell J, Palaniappan A, Kumar A, Nixon RA. Calpastatin inhibits motor neuron death and increases survival of hSOD1 G93A mice. J Neurochem. 2016;137:253–65.
Neumar RW, Meng FH, Mills AM, Xu YA, Zhang C, Welsh FA, et al. Calpain activity in the rat brain after transient forebrain ischemia. Exp Neurol. 2001;170:27–35.
Machado VM, Morte MI, Carreira BP, Azevedo MM, Takano J, Iwata N et al. Involvement of calpains in adult neurogenesis: implications for stroke. Front Cell Neurosci. 2015;9 FEB.
FASS. Guide for the care and use of agricultural animals in agricultural research and teaching. Champaingn, IL, US; 2010.
Fujii J, Otsu K, Zorzato F, de Leon S, Khanna VK, Weiler JE et al. Identification of a Mutation in Porcine Ryanodine Receptor Associated with Malignant Hyperthermia. Science (1979). 1991;253:448–51.
Rostagnolo HS, Albino LFT, Donzele JL, Gomes PC, de Oliveira RF, Lopes DC et al. Tabelas Brasileiras Para Aves e Suínos: Composição de Alimentos e Exigências Nutricionais. 3rd edition. Viçosa, MG: Universidade Federal de Viçosa; 2011.
Almeida VV, Silva JPM, Schinckel AP, Meira AN, Moreira GCM, Gomes JD, et al. Effects of increasing dietary oil inclusion from different sources on growth performance, carcass and meat quality traits, and fatty acid profile in genetically lean immunocastrated male pigs. Livest Sci. 2021;248:104515.
Fanalli SL, da Silva BPM, Gomes JD, Ciconello FN, de Almeida VV, Freitas FAO, et al. Effect of dietary soybean oil inclusion on liver-related transcription factors in a pig model for metabolic diseases. Sci Rep. 2022;12:10318.
da Silva JPM, Costa KA, Almeida VV, Coutinho LL, da Silva BPM, Cesar ASM. Fatty acid profile in brain and hepatic tissues from pigs supplemented with canola oil. Revista Brasileira de Agrotecnologia. 2021;11:414–20.
AOAC. Official methods of analysis of the Association os Official Analytical Chemists.Association of Official Analytical Chemists. 1995;2.
Bligh EG, Dyer WJ. A rapid method of total extration and purification. Can J Biochem Physiol. 1959;37:911–7.
Hartman L, Lago RC. Rapid preparation of fatty acid methyl esters from lipids. Lab Pract. 1973;22:475–81.
AOCS. Official approved procedure am 5 – 04, Rapid determination of oil/fat utilizing high temperature solvent extraction. American Oil Chemists’ Society; 2005.
Dobin A, Gingeras TR. Mapping RNA-seq Reads with STAR. Curr Protoc Bioinformatics. 2015;51:11.14.1-11.14.19.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Benjamini Y, Hochberg Y. Controlling the false Discovery rate: a practical and powerful Approach to multiple testing. J Roy Stat Soc: Ser B (Methodol). 1995;57:289–300.
Acknowledgements
The authors would like to thank the collaborative efforts between the University of São Paulo and Iowa State University. We also give thanks to DB Genética Suína for providing the animals, housing, feeds, and employees, who helped in carrying out this research.
Funding
This study was supported by the São Paulo Research Foundation (FAPESP, Grant numbers: 2017/25180-2, 2018/15653-3, 2018/26797-6, 2018/26816-0, 2020/10042-6, and 2021/01694-2, the Brazilian National Council for Scientific and Technological Development (CNPq) that provided a researcher fellowship to A. S. M. Cesar, L. L. Coutinho. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Author information
Authors and Affiliations
Contributions
All authors contributed to this study. Conceptualization, B.P.M.d.S., V.V.d.A., H.F., G.C.M.M., J.M.R., J.E.K., D.K., J.C.d.C.B., L.L.C., and A.S.M.C. Writing-original draft preparation, B.P.M.d.S., and A.S.M.C. Performed data analysis, B.P.M.d.S., V.V.d.A., and A.S.M.C. Interpretation and discussion of the results, B.P.M.d.S., H.F., V.V.d.A., L.L.C., B.S.-V., J.A., and A.S.M.C. Critically reviewed and editing, S.L.F., J.D.G., V.V.d.A., F.A.O.F., B.S.-V., J.M.R., J.E.K., D.K., S.M.d.A., J.P.M.d.S., J.A., L.C.d.A.R., G.B.M., A.L.F., and A.S.M.C. Supervision, A.S.M.C. Funding acquisition, A.S.M.C. Corresponding author: correspondence to Aline Silva Mello Cesar, alinecesar@usp.br.
All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures involving animals were evaluated and approved by the Ethics Committee for the Use of Animals (CEUA, number 2018-28, and protocol 2018.5.1787.11.6) of the Luiz de Queiroz College of Agriculture (ESALQ). All procedures followed the guidelines by the Brazilian Council of Animal Experimentation and the ethical principles in animal research, according to the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. This study was carried out in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1.
Table S1. Total reads in brain samples of pigs fed diets containing different levels of soybean oil.
Additional file 2
. Table S2. Differentially expressed genes in brain tissue of pigs fed diets containing different levels of soybean oil.
Additional file 3.
Table S3. Quality, concentrattion and RNA integrity number of brain samples of pigs fed diets containing different levels of soybean oil.
Additional file 4. Table S4.
Composition of the experimental diets (as-fed basis). Table S5. Analyzed fatty acid profile of grower diets (as-fed basis). Table S6. Analyzed fatty acid profile of finisher diets (as-fed basis).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
da Silva, B.P., Fanalli, S.L., Gomes, J.D. et al. Brain fatty acid and transcriptome profiles of pig fed diets with different levels of soybean oil. BMC Genomics 24, 91 (2023). https://doi.org/10.1186/s12864-023-09188-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09188-6