Abstract
Background
Tuberculosis is a serious infectious disease affecting millions of people. In spite of efforts to reduce the disease, increasing antibiotic resistance has contributed to persist in the top 10 causes of death worldwide. In fact, the increased cases of multi (MDR) and extreme drug resistance (XDR) worldwide remains the main challenge for tuberculosis control. Whole genome sequencing is a powerful tool for predicting drug resistance-related variants, studying lineages, tracking transmission, and defining outbreaks. This study presents the identification and characterization of resistant clinical isolates of Mycobacterium tuberculosis including a phylogenetic and molecular resistance profile study by sequencing the complete genome of 24 strains from different provinces of Ecuador.
Results
Genomic sequencing was used to identify the variants causing resistance. A total of 15/21 isolates were identified as MDR, 4/21 as pre-XDR and 2/21 as XDR, with three isolates discarded due to low quality; the main sub-lineage was LAM (61.9%) and Haarlem (19%) but clades X, T and S were identified. Of the six pre-XDR and XDR strains, it is noteworthy that five come from females; four come from the LAM sub-lineage and two correspond to the X-class sub-lineage. A core genome of 3,750 genes, distributed in 295 subsystems, was determined. Among these, 64 proteins related to virulence and implicated in the pathogenicity of M. tuberculosis and 66 possible pharmacological targets stand out.
Most variants result in nonsynonymous amino acid changes and the most frequent genotypes were identified as conferring resistance to rifampicin, isoniazid, ethambutol, para-aminosalicylic acid and streptomycin. However, an increase in the resistance to fluoroquinolones was detected.
Conclusion
This work shows for the first time the variability of circulating resistant strains between men and women in Ecuador, highlighting the usefulness of genomic sequencing for the identification of emerging resistance. In this regard, we found an increase in fluoroquinolone resistance. Further sampling effort is needed to determine the total variability and associations with the metadata obtained to generate better health policies.
Similar content being viewed by others
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), is one of the top 10 causes of death in 2020. The World Health Organization estimated that 9.9 million people were infected, and 1.5 million died [1]. Despite constant efforts over the years to lessen the impact of this disease, several challenges, such as patient adherence, long duration of treatment, and late diagnosis, have reduced the effectiveness of TB therapy [2]. In addition, the frequent emergence of drug-resistant strains is a serious global threat and poses significant challenges to public health, especially in low- and middle-income countries.
Drug resistance in bacteria, particularly in M. tuberculosis, negatively affects health programs worldwide due to the increase of resistant strains that cannot be treated with existing anti-TB drug therapy. Indeed, tuberculosis is considered to be one of the "Big three" infectious diseases worldwide [3]. Patients with resistant TB may develop it due to clinical factors such as late or inappropriate diagnosis, ineffective treatment, poor compliance to the regimen, and exposure to circulating multidrug-resistant and extensively drug-resistant tuberculosis (MDR/XDR-TB) strains [2]. The slow growth of Mtb slows diagnosis and consequently limits the timely detection of resistance to anti-TB drugs. This contributes to the rising incidence of MDR and XDR TB worldwide. Conversely, the accumulation of point mutations in coding regions for drug targets or drug-converting enzymes is an essential mechanism for acquiring resistance in Mtb [4].
The Mycobacterium tuberculosis complex is composed of the human-adapted members, the Mycobacterium tuberculosis sensu stricto (lineages 1, 2, 3, 4, and 7) and M. tuberculosis var. africanum (lineages 5 and 6; M. africanum), distinct phylogenetic lineages that have evolved over millennia [5,6,7] and lineage 8 and 9 are new lineages recently discovered in Africa [8]. Research on the distribution of lineages of Mtb in South America has shown that the Euro-American lineage (lineage 4) has a variable distribution of lineages/sub-lineages between and within these countries, establishing that the predominant strains in their America have evolved from the Euro-American lineage [9]. This lineage has been described as more transmissible than others [10]. From 10 sub-lineages, including in Lineage 4, LAM (L4.3), Haarlem (L4.1.2), X-type and T families are the most observed members of the Euro-American lineage in South and Central America and the Caribbean, a distribution profile shared with Europe and Middle Africa [11,12,13,14].
In 2015, due to drug resistance rising worldwide, the WHO proposed expanding rapid testing and detection of cases as one of the five high-priority actions to tackle the global DR-TB crisis [15]. As part of these actions, some molecular biology-based diagnostic methods have been applied in a clinical setting for their versatility and speed, including variants of Xpert MTB/RIF and GenoType MTBDRplus [16,17,18,19]. Although these methods are rapid and straightforward, Mtb continuously evolves through the genomic acquisition of single nucleotide polymorphisms (SNPs), insertions, and deletions (indels), impacting the ability to discriminate strains without the classic regions used for resistance detection [20]. Whole-genome sequencing (WGS), as a molecular diagnostic tool, has been greatly developed in TB research and is clinically useful for predicting drug resistance, lineage, tracing transmission, and defining outbreaks [21,22,23,24,25,26]. Furthermore, WGS has the potential to determine drug resistance much faster than traditional phenotypic drug susceptibility testing (DST) [27] and does not require biological safety infrastructure [28, 29]. These technologies are used differentially, with the highest concentration of sequenced strains found in 16 countries [30,31,32]. However, for some countries, particularly in South America, WGS information on the variability and distribution of circulating strains is unavailable.
In Latin America, are extensive information on Mycobacterium tuberculosis, highlighting its genetic diversity analyzed by molecular methods; despite this, very few studies have been carried out in Ecuador, in which the LAM and Haarlem lineages are described as the more prevalent, and a little for the Beijing family [33,34,35]. Recently Garzon-Chavez et al. [36] determined that the main circulating lineages in Ecuador were LAM, Haarlem, and S and only applied WGS for resolving the classification in 8 isolates with an ambiguous result from 373 strains assessed by 24 loci-MIRU-VNTR and DR analysis, previously. In this sense, it is essential to know the genetic composition of M. tuberculosis, especially the variations associated with drug resistance distributed within its genome. This study aims to assess the lineage and molecular resistance profile characterization in clinical isolates of M. tuberculosis through whole-genome sequencing.
Results
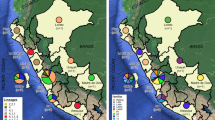
In order to assess the genetic variability of resistant M. tuberculosis strains, twenty-four culture-confirmed clinical isolates of M. tuberculosis with at least one antibiotic resistance were selected for whole genome sequencing. From them, the bacillary load test in the microscopy smear was greater than two crosses, and 66,7% of patients relapsed. The isolates come from four regions of western Ecuador (20, 83.3%, Guayaquil; 2, 8.3%, El Empalme, 1, 4.2% Babahoyo and Chone, respectively). The majority of the patients were female (64,5%); the mean age was 42.9 ± 12.9 years; comorbidity like HIV was present in three patients (Table 1). All isolates were only tested for phenotypic resistance to four first-line drugs: rifampicin (R), isoniazid (H), ethambutol (E), and pyrazinamide (Z); and one second-line drug: streptomycin (S). Thus, a wide range of drug resistance profiles were covered: 85.7% are polydrug resistant (50% resistant to all first-line drugs plus streptomycin), and 14.3% are MDR (Table 1 and Supplementary table 1).
The 24 strains isolated were sequenced using the Illumina platform with paired-end reads (2 × 150 bp). The sequences were filtered by the quality and trimmed when necessary. The depth obtained ranged from 22.7X to 53.6X (Table 1). The strains were mapped against the reference M. tuberculosis H37Rv (NC_000962.3) (4.4 million base pairs and 4008 protein-coding genes) to verify that they were free of contamination. The sequencing and mapping statistics are presented in Table 1. Most strains have an outstanding quality, depth, and percentage mapping against the reference; however, three strains presented an average depth below 20 and were discarded for further analysis. The 21 remaining strains were the novo assembled, and the assemblies obtained were checked for contamination. On average, we recovered draft genomes of 4.3 Mb distributed in 140 contigs per strain with at least an N50 of 56 Kb (Table 1 and Supplementary table 1). Although a complete genome was obtained for all strains, the fragmentation into several contigs is due primarily to regions of extreme base composition and low complexity. This can be seen in Fig. 1, which shows the reference M. tuberculosis H37Rv, the percentage of GC and GC-Skew, and the coverage plot of 21 genomes assembled and plotted according to drug resistance profile. Here, common breaks in coverage correspond to peaks in GC or GC-skew.
Genome sequence comparison of 21 resistant M. tuberculosis genomes (15 MDR, four pre-XDR, and 2 XDR strains) shown by BRIG. Circles display (from the inside): 1. Reference genome of strain H37Rv; 2–21. Read mapping coverage of sequencing reads calculated from 21 genomes (2, 3. XDR strains (turquoise); 4–7. pre-XDR strains (rose) and 8–21 MDR strains (light green)); 23. GC content; 24. GC skew values positive (green) to negative (purple);. The coverage shown in rings 2 through 21 is from mapping the reads against the reference using BWA. The height of the plot in each ring is proportional to the number of reads mapped at each nucleotide position in the reference genome from 0 to 30 × coverage. The lighter area of the rings represents regions with less coverage. Regions with genome coverage greater than 30 × are shown as solid blue bands
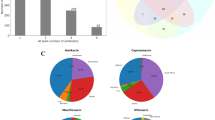
Prokka and RASTtk were used to determine the structure and function of genes. On average, the Prokka annotation showed the presence of ∼4,119 ± 26.28 genes; among them, 4,065 correspond to coding sequences (CDS), 53 are transfer RNAs, and 1 code for a tmRNAs (Table 1). Based on the annotations, the analysis of the pangenome was carried out with Roary [37]. Thus, the pangenome analysis of 21 genomes of M. tuberculosis revealed that there were 4735 gene families defined as the pangenome. The protein-coding genes identified for each strain have slight variation in number, ranging from a minimum of 4021 genes (strain S0036_Mtb_Ec) to a maximum of 4115 genes (strains S0017_Mtb_Ec and S0022_Mtb_Ec). A total of 3750 common genes (core genes) shared by all the genomes analyzed were identified. In addition, 99 soft-core genes (presented in 20 of the 21 strains), 353 accessory genes (shared between two and 19 strains), and 533 cloud genes (strain-specific genes) were identified (Fig. 2A). The core and pangenome size ratio were found to be 0.79; thus, the core forms 79% of the pangenome, representing a rather closed genome with less variability. Gene accumulation was calculated for the core and pangenome. It is observed that after including 21 strains, the increase in the total number of genes is significantly reduced, and on the other hand, the core genome increases minimally, indicating a pangenome close to closing. This can be observed in Figs. 2B and C; from this visualization, it can be suggested that genome characterization of approximately 21 strains provides the genetic repertoire of M. tuberculosis.
The pangenome of 21 M. tuberculosis isolates. a Flower plots showing the core, accessory, and strain-specific genes of the 21 Mtb strains. The flower plot shows the core gene number (in the center), the accessory gene number (in the annulus), and the strain-specific gene number (in the petals) for the 21 Mtb strains. b Gene accumulation curves of the pangenome (blue) and the core-genome (green). The blue and green boxes denote the Mtb pangenome and core size for each genomic comparison. c The curve (red) shows the number of new genes against the increase in the number of Mtb genomes
From the core genes and using Prokka and RASTtk, subsystems were annotated. Using COG annotations, a function was assigned for a mean of 1,731 genes for each strain analyzed. These annotated genes were classified into 21 metabolic pathways within four primary categories, including Metabolism (49.2%); Information, storage, and processing (17.5%); Cellular processes, and signaling (16.8%), and poorly characterized (16.5%). As for many other organisms, almost half of the genes identified in all strains analyzed (~ 2022 genes, ~ 53.9% of the total) could not be assigned function (Supplementary table 2 and Fig. 3a).
Functional annotation of 21 M. tuberculosis genomes. a COG categories and subcategories of predicted genes within the core genomes of 21 M. tuberculosis genomes by eggNOG Mapper v2.1.7. Each subcategory is graphed as an average percentage of the total number of genes in the core genomes. b Subsystem category distribution of 21 M. tuberculosis genomes based on RASTtk annotation. Genes analyzed 4,119 ± 26.28; genes in subsystems 1,521 ± 7.60; subsystems identified 295
In addition, using RASTtk [38], ∼1,521 genes were assigned to the subsystems (a group of proteins that implement a specific biological process or structural complex). It was observed that almost 18% of the annotated protein-coding genes were associated with amino acids and their derivatives (267 ± 3.04), followed by genes related to carbohydrates (13.1%, 199 ± 1.74), protein metabolism (10.9%, 166 ± 0.68), cofactors, vitamins, prosthetic groups, and pigments (9.5%, 144 ± 4.65 genes). About 60% of the genes identified in subsystems are involved in the Metabolism of proteins, amino acids, carbohydrates, lipids, and cofactors (Fig. 3b). Cell regulation and signaling (6.6%, 101 ± 1.7) and DNA metabolism (6.1%, 93 ± 1.32) also appear as subsystems with a relevant role. Partly due to the number of strains, a higher diversity in the percentage of genes in the subsystems was found for MDR strains than for pre-XDR and XDR strains (Supplementary table 3). Most of the genes involved in amino acid biosynthesis were conserved and proved to have an essential function for pathogenicity in bacteria, including M. tuberculosis [39].
Of particular interest are the strain-specific genes that may be responsible for the variability and particularities of this set. Therefore, based on the identification and classification of the genes, we performed a characterization analysis of the functional groups of strain-specific genes. By analyzing the distribution in the 21 strains, we found that most of the identified unique genes involved in metabolism and transport. In particular in the metabolism of lipids, secondary metabolites, coenzymes, amino acids, and inorganic compounds. A smaller proportion of genes are linked to replication, transcription, and translation, and only a few unique genes are related to cellular processes and signaling (Fig. 4).
Since our analysis is based on resistant strains, particular attention was devoted to specific genes, like those genes related to virulence. First, using the Virulence Factor Database (VFDB), a total of 64 virulence-related proteins implicated in the pathogenicity of M. tuberculosis were identified, classified as follows: 58.96% related to Secretion system; 11.36% to iron uptake, Siderophore; 4.26% to Regulation and Cellular Metabolism; 4.12% to the Cell wall and 1.42% to Heat-shock protein and Magnesium uptake (Supplementary table 4). Second, using DrugBank, 66 proteins were identified as potential drug targets (Supplementary table 5). Similarly, 38 families of Transporter proteins were identified by the Transporter Classification Database (TCDB), the most representative being the ATP-binding cassette (abc) superfamily (25.17%), followed by the type vii or esx protein secretion system (t7ss) family (23.65%), the general secretory pathway (sec) family (8.38%), the major facilitator superfamily (mfs) (5.86%) and the resistance-nodulation-cell division (rnd) superfamily (5.03%) (Supplementary table 6). Finally, the presence of CRISPR genes was analyzed using CRISPRfinder, and between 2 and 3 CRISPRs were detected in each genome (Supplementary table 1).
From the genomes, in silico lineage inference was performed using different web tools: TB-Profiler v2.8.13, PhyReSse v1.0, SNP-IT, Mykrobe v0.10.0, CASTB v1.5, TB-Lineage, PhyTB, GenTB and a comparison of their performance was made. Moreover, in silico spoligotyping was determined by KvarQ v0.12. All genomes in this study belonged to Euro-American Lineage (Lineage 4), being the sub-lineages LAM (61.9%, 13/21) and Haarlem (19.0%, 4/21) the most representative, and clades X, S, and T founded in lowest percentage (Supplementary table 7 and Fig. 5). Among all lineages characterized by complete genomic sequencing, isolates classified as MDR accounted for 71.4% (15/21) and resistant (pre-XDR and XDR) for 28.6% (6/21). Notably, most of the latter belong to the LAM sub-lineage, and within the LAM sub-lineage, 30.8% (4/13) are resistant with great epidemiological importance (Fig. 6). TB-profiler, Mykrobe, PhyTB, and GenTB were the most informative web tools for lineages characterization. These tools detailed more genotypes with 100% concordance into M. tuberculosis sub-lineages. However, isolates from the T sub-lineage, including genotypes 4.8 to 4.10, could not be effectively resolved. PhyReSse, on the other hand, showed a lower ability to differentiate sub-genotypes within genotypes 4.1.2 and 4.3.4. TB-Lineage, SNP-IT, and CASTB could not effectively differentiate sub-lineages with a wide variety of genotypes and subgenotypes, especially the LAM sublineage (Table 2).
UPGMA tree for 21 Mtb genome analyzed in this study. The un-rooted tree was constructed based on 2891 core genes Multi Locus Sequence Typing (cgMLST) extracted from the 21 samples and 297 reference genomes using Ridom SeqSphere v8.3.1. Drug resistance of 21 Ecuadorian isolates are represented: XDR (Strong pink); pre-XDR (Very soft pink); MDR (Moderate green). Lineages were identified in all samples: L1 (Strong red); L2 (Bright red); L3 (Soft orange): L4 (Very soft orange); L5 (Light grayish cyan); L6 (Very soft blue); L7 (Slightly desaturated blue); L8 (Moderate blue). UPGMA = unweighted pair group method with arithmetic mean
ML phylogenetic tree showing the relationship of 21 M. tuberculosis drug resistance pattern and Lineages. Abbreviations: rifampicin (R), isoniazid (H), pyrazinamide (Z), and ethambutol (E), fluoroquinolone (FQ), streptomycin (S), ethionamide (Eth), and aminoglycosides (Amk, Km, Cm). Color strips represent the sub-lineages. ML: Maximum Likelihood
To determine drug-resistance single nucleotide polymorphism (SNPs) and small insertions and deletion (indels), we performed a single nucleotide variant calling (SNV) comparing the samples with the H37Rv genome. From the 21 Mtb clinical isolates analyzed in this study, 21,596 high-quality variants were discovered (19,547 SNPs; 1154 insertions and 892 deletions) (Supplementary table 8). Overall, on average 1028 SNPs were found per sample (range 682–1152 SNPs), corresponding to a median SNP density of 1 SNP per 4.2 kb. Few variants were present in all samples, only 1.32% of SNPs (n = 289) and 0.8% of indels (n = 5). Most SNPs were found in coding regions (88%, 17,214) and the remaining 2,333 in intergenic regions. Of the SNPs in coding regions, the majority of the variants lead to non-synonymous (NS) changes in amino acids (n = 10,685), including modification of the coding gene (97.5%), loss of start codon (0.1%); gaining of stop codon (1.82%); lost of stop codon (0.6%). The amino acid changes more frequently were Thr25Ala, Ile245Thr, Lys212Asn, Gly13Asp, Ala182Val, Asn140Ser, Gln20Leu, Gly33Ser, Thr136Ala, and Val259Ala observed 27, 25, 24 23 and 22 each time, respectively. Among synonymous changes (6529) including 99.35% of synonymous, 0.4% of missense, 0.2% of stop retained variants.
Applying the web-based tools TB-Profiler v2.8.13, PhyReSse v1.0 Mykrobe v0.10.0, CASTB, KvarQ v0.12, PhyTB, GenTB, SAM-TB for genomic analysis of 21 strains phenotypic characterized MDR and polydrug-resistance according to WHO guidelines we identified genotypically 71.43% of strains MDR (15/21), 19.05% pre-XDR (4/21) and 9.52% XDR (2/21). A total of 55 SNVs were identified and distributed into 18 genes known to confer resistance to first- and second-line drugs. Among these, 67.27% (37/55) SNVs were related to resistance to first-line drugs, and genetic regions associated with pyrazinamide resistance presented more frequent variation regions (30.9%, 17/55). Among all genomes, the most frequently genotype identified were Ser315Thr (76.2%,16/21), Ser450Leu (57.1%,12/21), and Met306Ile (33.3%, 7/21), encoding a substitution in katG, rpoB and embB gene that confers resistance to rifampicin, isoniazid, and ethambutol, respectively; whereas for second-line drugs were Thr202Ala in thyA which is associated with para-aminosalicylic acid resistance in 61.9% (13/21) and Lys43Arg in rpsL related with streptomycin resistance (28.5%,6/21) (Fig. 7).
We also evaluated the prevalence of resistance in the fluoroquinolone among isolates sequenced by looking at gyrA and gyrB gene mutation frequencies. In the gyrA gene, the SNV Asp94Asn, known to confer fluoroquinolone resistance, was found in all pre-XDR isolates, while SNV Arg90Val was found in XDR isolate and one pre-XDR. Additionally, the known SNV Ser95Thr in the gyrA gene was found in 20 isolates. For resistance characterization in tuberculosis, the web-based tools SAM-TB (76.4%) and Mykrobe (72.7%) approached the most frequently identified mutations, complemented by PhyReSse (50.9%), to determine genetic resistance by whole-genome sequencing in surveillance programs (Supplementary table 9 and 10).
Discussion
The present study describes for the first time the use of whole genome sequencing in the screening of TB drug susceptibility in Ecuador and provides information on the phylogenetic characteristics and the identification of the predominant mutations in circulating isolates. Despite its limited number of samples including in this study (24 isolates), it represents the initial effort to determine genetic diversity using WGS. Most isolates in our study were from Guayaquil (79.17%), one of the most economically important cities in Ecuador; the high number of tuberculosis cases detected in this city is possibly due to the high mobility from other provinces for trade or work in addition to the location of the leading health centers for the monitoring of this pathogen.
The potential of WGS to provide a quick and comprehensive view of the genotype and reliable prediction of the lineages has been extensively reported [5, 7, 14, 40]; thus, its application in surveillance programs is necessary. The Euro-American lineage (lineage 4) presents a variable distribution in Argentina with a predominance of M and Ra strains [41], for Brazil, lineage 4 varies between 25–99% [13, 42, 43], for Colombia, between 85–99% [44,45,46], for Mexico between 69–98% [47, 48] and for Peru around 90% [12, 49, 50]. Ecuador is not an exception, in fact, all the samples analyzed by us belong to the Euro-American lineage. This lineage in South and Central America and the Caribbean is represented mainly by the LAM, Haarlem, and T families sub-lineages [11, 41, 51]. In our study, most of the cases analyzed also corresponded to LAM (61.9%), followed by the Haarlem lineage (19.0%). This result provides genomic information from Ecuadorian isolates of Mycobacterium tuberculosis that contribute to the knowledge of the distribution of Mtb lineages in South America and confirms the European origin of the circulating strains in Ecuador [9, 52, 53]. Similar results have been described in some, but not all, previous studies using genotyping by 24-loci MIRU-VNTR, Spoligotyping, and SNP-PCR strategies in Ecuador [36, 54].
The movement or conservation of genes in bacteria is a fundamental factor related to virulence, survival, and host adaptability, allowing them to co-evolve together [55]. Several studies in M. tuberculosis have shown that the ability of transmission within populations, latency periods, and drug resistance are due to genetic differences within the members of the M. tuberculosis complex and that many of its genes have been under positive selection at different periods [56]. Furthermore, the composition within its genome remains relatively stable due to the absence of horizontal gene transfer, and this superficial level of genetic variation is probably because these pathogens have a strongly clonal nature [57]. The highly conserved genetic content and extreme clonal nature suggest that M. tuberculosis should have a relatively high percentage of core genome, however, analysis of core and pangenome have shown more variability than expected [58,59,60,61]. A pangenome is a union of the entire genetic pool of several strains of a species under comparison, essentially consisting of a core genome containing genes and sequences shared in all strains and an accessory genome consisting of genes and sequences which may be absent from one or more strains and genes that are unique to each strain remaining. In our study, the genetic composition of 21 drug-resistant isolates was analyzed, and 3750 genes (79.2%) were identified in the core genome, considering it a relatively large core genome in line with previous studies in which few genomes were evaluated. Therefore, it is necessary to expand the number of isolates to determine exactly which are the essential genes in M. tuberculosis within those circulating in Ecuador.
Characterizing the protein's functionality in microorganisms of importance in public health is essential to understanding their pathogenicity, resistance to antibiotics, and virulence, which allow them to adapt or survive in their host. Our results showed that annotations of protein-encoding genes are mainly associated with metabolisms being Amino acids and their derivatives, Energy production and conversion, and lipid metabolism, the most representative. Similar studies in other M. tuberculosis populations also showed a high presence of genes related to energy production and conversion (C), amino acid transport and metabolism (E), and lipid transport and metabolism (I) [72,73,74]. This suggests that these genes are widely conserved to ensure the interactions of M. tuberculosis with its human host, especially in mycobacterial persistence, host pathogens struggle for nutrients and immune recognition. On the other hand, we were able to identify mainly virulence factors associated with the secretion system (58.96%), the most important being the transporter proteins of the ATP-binding cassette (abc) superfamily (25.17%) and the type vii or esx protein secretion system (t7ss) family (23.65%). It has been suggested that high levels of transporters are involved in cell detoxification, nutrient recycling, and antibiotics and drug efflux, significantly affecting the survival and development of multiple drug-resistant strains in Mtb [58, 59, 62, 63]. It should be noted that the unique genes identified 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase and Arylamine N-acetyltransferase were previously characterized as high-confidence drug targets [64,65,66].
All resistant isolates analyzed showed any variations in genes related to confer resistance, classified them in 71.43% of strains MDR (15/21), 19.05% pre-XDR (4/21), and 9.52% XDR (2/21). Both XDR strains presented similar mutations profile (8 non-synonyms and one deletion SNV in the coding region, 2 SNV in the intergenic region), but differing in additional Ser315Gly mutation in the katG gene arise to isolate S0013_Mtb_Ec, for the four pre-XDR isolates the S0202_Mtb_Ec was that more mutations were identified (9 genomic regions). It is brought up that of these six isolates of concern, five belong to women within the same age range.
The study of the M. tuberculosis resistance is an important factor within its surveillance plan in all countries, for which the timely detection of strains resistant to treatment drugs allows the application of an efficient drug strategy, reducing or limiting the incidence of new cases, especially when applying culture-based methods to detect it. In this study, we found high concordance between WGS and conventional culture-based DST in predicting phenotypic drug resistance to anti-TB drugs; In addition, WGS was able to determine resistance patterns that DST does not evaluate. These findings are in agreement with previous studies [40, 67, 68]. Drug resistance is mediated through mutations in specific gene targets. The key, therefore, is to identify single nucleotide polymorphisms (SNPs) that are responsible for or strongly associated with resistance. In the case of the first-line drug in Mtb treatment, mutations in the 81-bp rifampicin resistance determining region (RRDR) of the rpoB gene, also known as a hotspot region, have been accurate predictors of rifampicin resistance in many studies [69,70,71]. On the other hand, it has been established that INH resistance, predominantly mediated through loss of catalase-peroxidase activity via mutations in katG, produces high-level resistant strains [18, 72, 73]. Finally, in ethambutol, most resistance-related genes are located on the embB, embC, and upstream of the embA [74, 75]. Meanwhile, previous studies have agreed that the genes rrs, rpsL, and gid are related to different levels of streptomycin resistance [76, 77], and the chromosomal mutations in the quinolone resistance determining region of gyrA or gyrB are the main mechanism of resistance to fluoroquinolones in M. tuberculosis [78, 79]. In our study, we found 57.1%, 70.0%, 47.6%, and 28.6% and 28.45% of mutations S450L (rpoB), S315T/G (katG), M306V/I (embB), K43R (rpsL) and c492t, a514c (rrs locus) related to rifampicin, isoniazid, ethambutol, streptomycin and fluoroquinolones resistance respectively. The increased resistance to fluoroquinolones, also recently reported in other studies [80], is of concern, probably caused by the increased administration of this drug without prescription [81]. It is therefore imperative to monitor the increase in these resistances in tuberculosis surveillance programs [82, 83].
Whole-genome sequencing (WGS) is a powerful method for detecting drug resistance, genetic diversity, and transmission dynamics of M. tuberculosis. Despite their advantages, the analysis of genomic sequencing data remains an obstacle to the routine use of WGS technology in clinical tuberculosis because it requires bioinformatics expertise, high-performance computing, funding, and training that are not readily available in most clinical laboratories [84, 85], which represents a significant challenge for TB control efforts. Over the last few years, many TB-specific genome browsers and WGS analysis tools such as TBProfiler, KvarQ, Mykrobe Predictor TB, CASTB, PhyTB, GenTB, PhyResSE, and others have been developed for genotyping and drug resistance identification, however, despite their use have not been widely used within antimicrobial resistance (AMR) surveillance program on low- and middle-income countries (LMICs) [86]. In our results, web-based tools enabled an effective and user-friendly identification of resistance-associated mutations in M. tuberculosis. The recently developed SAM-TB platform [21] is the most capable of determining the mutations present in the isolates of our study, which, when used in conjunction with web-based tools Mykrobe and PhyReSse, would allow rapid screening of M tuberculosis isolates. These tools could easily be implemented in surveillance programs based on microbiological procedures to obtain results efficiently and cheaply [84, 87, 88].
Given that the sample size used is small and could be underestimating the real frequencies of mutations in Mycobacterium tuberculosis present in this region, it becomes imperative to make greater efforts -and use the advantage of WGS- to analyze a larger number of samples to identify and study the mutations present in patients with tuberculosis including other regions of Ecuador.
Conclusion
The findings of this study demonstrate the usefulness of applying next-generation sequencing tools such as WGS to characterize mutations and describe the existing variability in tuberculosis strains that allow adequate monitoring to generate health policies. We identified the variability of resistant strains circulating among men and women in Ecuador and showed that mainly strains of the American-European lineage 4 circulate, with a higher proportion of the LAM sub-lineage. XDR/MDR strains are not associated with a specific lineage, region or other metacharacter analyzed. However, we found an association between sex and resistance, which should be verified with further sampling. Within the observed resistances, an increase in fluoroquinolone resistance is evident, which should be monitored. Additional sampling is needed to determine the total variability and associations with the metadata obtained to generate better health policies.
Although M. tuberculosis is considered a highly conserved clonal species, among the 21 strains analyzed, we found unique genes specialized in lipid metabolism that are particularly interesting for identifying potential new drug targets.
Methods
Twenty-four clinical isolates were sampled and collected from private laboratories between March to December 2020; these isolates had been previously characterized as multidrug-resistant through drug susceptibility testing. The resistance pattern were performed for first-line anti-tuberculosis drugs, isoniazid (H), rifampicin (R), pyrazinamide (Z), ethambutol (E), and one second-line drug (streptomycin (S)), the proportional method by [89] on Lowenstein-Jensen medium and the resistance profile was defined according to WHO recommendations. The critical concentrations of anti-TB drugs evaluated were as follows: rifampicin, 40.0 μg/ml; isoniazid, 0.2 μg/ml; ethambutol, 0.4 μg/ml; streptomycin, 4.0 μg/ml, and 200 µg/mL for pyrazinamide.
DNA extraction and sequencing
Genomic DNA was extracted from 24 isolates of Mycobacterium tuberculosis grown in a Lowenstein-Jensen medium using the CTAB method [90]. The quantity of isolated DNA was measured using a Qubit 4.0 fluorometer (Invitrogen, Carlsbad, CA, USA.). DNA samples that fulfilled the integrity, purity, and quantity standards were sequenced. Genomic DNA libraries were prepared for whole genome sequencing using the Tagmentation-based library prep kit according to the manufacturer's instructions (Illumina Inc., San Diego, CA) and sequenced on an Illumina MiniSeq platform with High Output Reagent Kit, producing 150 bp paired-end reads.
Bioinformatic analyses
Using the Galaxy platform (https://usegalaxy.org/), reads were classified by Kraken version 2 [91] to detect any contamination or the presence of other mycobacteria; In addition, FastQC version 0.11.9 [92] and Trimmomatic version 0.38 [93] were used to control the quality and trim the low-quality ends of the reads, respectively. In particular, a sliding window was used to trim sequences with an average quality value lower than 20.
Genome assembly
The final high-quality reads were assembled using Megahit version 1.1.3 [94]. The assembly was carried out with a kmer range from 29 to 141 at intervals of 20 and 800 bp as minimum contig size. To perform contig taxonomic assignment, we used Kaiju version 1.9.0 [95, 96]. For generating metrics and evaluating the quality of the assemblies using M. tuberculosis strain HR37v (NC_000962) as a reference genome, Quast version 5.0.2 was used [97].
Gene prediction and functional annotation
The structural and functional annotation was performed with Prokka version 1.12 [98] and the "Rapid Annotation using Subsystem Technology (RASTtk server)" pipeline online [38], and the predicted proteins were also annotated for the identification with the Clusters of Orthologous Groups (COG) by eggNOG Mapper v2.1.7 (http://eggnog-mapper.embl.de/). For the identification of CRISPRs, we used the webserver of CRISPRFinder (https://crispr.i2bc.paris-saclay.fr/Server/). Analysis of virulence-related proteins, potential drug targets, and Transporter proteins by Virulence Factor Database (VFDB), DrugBank, and Transporter Classification Database (TCDB) was performed in the Pathosystems Resource Integration Center (PATRIC) online (https://www.patricbrc.org/).
Variant calling
Processed reads were mapped with Bowtie2 [99] using H37Rv (NCBI ID: NC_000962.3) as a reference genome. Sequence alignment files were sorted and indexed with Samtools v0.1.19 [100]. Bcftools [101] were used for calling variants, and vcftools [102] were used to filter the raw variants (minimum quality score of 30, minimum depth 10). From high-quality variants annotated for 21 genomes, the effect and impact were determined by SnpEff [103].
Pangenome analyses
The pangenome was analyzed with Roary [37] and organized as follows: core, soft-core, shell, and cloud genes if the genes were presented in all, 20, 3—19, and 1—2 samples, respectively; the characteristic curves of the Mtb pangenome, the core-genome, and the new genes were depicted using the pangenome Profile Analyze Tool (PanGP) [58, 104, 105]. Alignments for the core genes Multi Locus Sequence Typing (cgMLST) were used for generating a phylogenetic tree using Ridom SeqSphere v8.3.1 [106]. The output tree was visualized and annotated using the online tool iTOL [107].
Phylogenetic analysis
From high-quality reads the M. tuberculosis complex (MTBC) lineages/sublineages were determined and compared using the user-friendly web tools TB-Profiler v2.8.13 (https://tbdr.lshtm.ac.uk/), PhyReSse v1.0 (The Phylo-Resistance-Search-Engine) (https://bioinf.fz-borstel.de/mchips/phyresse/), SNP-IT [108], Mykrobe v0.10.0 (https://www.mykrobe.com/), CASTB v1.5 (The Comprehensive Analysis Server for the Mycobacterium tuberculosis complex) (http://castb.ri.ncgm.go.jp/CASTB/), TB-Lineage (https://tbinsight.cs.rpi.edu/run_tb_lineage.html), PhyTB (http://pathogenseq.lshtm.ac.uk/phytblive/index.php), GenTB (https://gentb.hms.harvard.edu/) and in silico spoligotyping were determined by KvarQ v0.12 (https://gap.tbportals.niaid.nih.gov/#/dashboard/home), the different web-programs were working on default parameters in our on-line pipeline.
Predicting susceptibility and drug resistance
Genes related to resistance to rifampicin (R), isoniazid (H), pyrazinamide (Z), and ethambutol (E) [first-line drug], and fluoroquinolone (FQ), streptomycin (S), ethionamide (Eth), aminoglycosides (Amk, Km, Cm), para-aminosalicylic acid (PAS) [second-line drugs] were considered for the analysis. After variant calling and annotation, variants on each resistance gene were determined and compared from the variant call format (VCF) files using the web-based tools TB-Profiler v2.8.13, PhyReSse v1.0 Mykrobe v0.10.0, CASTB, KvarQ v0.12, PhyTB, GenTB, SAM-TB (https://samtb.szmbzx.com/index).
Fundings
This work was funded by grant FCI-016–2017 from University of Guayaquil. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. GM is a doctoral student in the PEDECIBA program. LB is a member of PEDECIBA and the Sistema Nacional de Investigadores (SNI) of ANII.
Availability of data and materials
The Mycobacterium tuberculosis whole-genome sequencing data will be deposited in the public archive of NCBI under the BioProject ID PRJNA827129, BioSamples SAMN29877831—SAMN29877851.
Abbreviations
- TB:
-
Tuberculosis
- Mtb:
-
Mycobacterium tuberculosis
- MTBC:
-
M. tuberculosis Complex
- R:
-
Rifampicin
- H:
-
Isoniazid
- Z:
-
Pyrazinamide
- E:
-
Ethambutol
- FQ:
-
Fluoroquinolone
- S:
-
Streptomycin
- Eth:
-
Ethionamide
- Amk, Km, Cm:
-
Aminoglycosides
- PAS:
-
Para-aminosalicylic acid
- MDR:
-
Multi-drug resistance
- XDR:
-
Extreme-drug resistance
- DST:
-
Drug susceptibility testing
- SNPs:
-
Single nucleotide polymorphisms
- Indels:
-
Insertions, and deletions
- WGS:
-
Whole-genome sequencing
- CDS:
-
Coding sequences
- VFDB:
-
Virulence Factor Database
- TCDB:
-
Transporter Classification Database
- cgMLST:
-
core-genome Multi Locus Sequence Typing
- COG:
-
Clusters of Orthologous Groups
- PATRIC:
-
Pathosystems Resource Integration Center
References
Factsheet Global TB report 2021 [Internet]. [cited 2022 Jun 18]. Available from: https://www.who.int/publications/m/item/factsheet-global-tb-report-2021.
Singh R, Dwivedi SP, Gaharwar US, Meena R, Rajamani P, Prasad T. Recent updates on drug resistance in Mycobacterium tuberculosis. J Appl Microbiol. 2020;128(6):1547–67.
Makam P, Matsa R. “Big Three” Infectious Diseases: Tuberculosis, Malaria and HIV/AIDS. Curr Top Med Chem. 2021;21(31):2779–99.
Alame Emane AK, Guo X, Takiff HE, Liu S. Drug resistance, fitness and compensatory mutations in Mycobacterium tuberculosis. Tuberculosis. 2021;1(129):102091.
Napier G, Campino S, Merid Y, Abebe M, Woldeamanuel Y, Aseffa A, et al. Robust barcoding and identification of Mycobacterium tuberculosis lineages for epidemiological and clinical studies. Genome Med. 2020;12(1):114.
Coll F, McNerney R, Guerra-Assunção JA, Glynn JR, Perdigão J, Viveiros M, et al. A robust SNP barcode for typing Mycobacterium tuberculosis complex strains. Nat Commun. 2014;5(1):4812.
Senghore M, Diarra B, Gehre F, Otu J, Worwui A, Muhammad AK, et al. Evolution of Mycobacterium tuberculosis complex lineages and their role in an emerging threat of multidrug resistant tuberculosis in Bamako, Mali. Sci Rep. 2020;10(1):327.
Ngabonziza JCS, Loiseau C, Marceau M, Jouet A, Menardo F, Tzfadia O, et al. A sister lineage of the Mycobacterium tuberculosis complex discovered in the African Great Lakes region. Nat Commun. 2020;11(1):2917.
Woodman M, Haeusler IL, Grandjean L. Tuberculosis Genetic Epidemiology: A Latin American Perspective. Genes. 2019;10(1):53.
Coscolla M, Gagneux S. Consequences of genomic diversity in Mycobacterium tuberculosis. Semin Immunol. 2014;26(6):431–44.
Demay C, Liens B, Burguière T, Hill V, Couvin D, Millet J, et al. SITVITWEB – A publicly available international multimarker database for studying Mycobacterium tuberculosis genetic diversity and molecular epidemiology. Infect Genet Evol. 2012;12(4):755–66.
Santos-Lazaro D, Gavilan RG, Solari L, Vigo AN, Puyen ZM. Whole genome analysis of extensively drug resistant Mycobacterium tuberculosis strains in Peru. Sci Rep. 2021;11(1):9493.
Tatara MB, Perdigão J, Viveiros M, Kritski A, da Silva KE, Sacchi FPC, et al. Genetic Diversity and Molecular Epidemiology of Mycobacterium tuberculosis in Roraima State. Brazil Am J Trop Med Hyg. 2019;101(4):774–9.
Lagos J, Couvin D, Arata L, Tognarelli J, Aguayo C, Leiva T, et al. Analysis of Mycobacterium tuberculosis Genotypic Lineage Distribution in Chile and Neighboring Countries. Manganelli R, editor. PLOS ONE. 2016 Aug 12;11(8):e0160434.
World Health Organization. Global tuberculosis report 2015 [Internet]. World Health Organization; 2015 [cited 2022 Jun 20]. 192 p. Available from: https://apps.who.int/iris/handle/10665/191102.
Thosani P, Agarwal A, Karade S, Sen S, Katoch CS, Jindamwar P, et al. Assessment of resistance to second line anti-tubercular drugs by line probe assay at a tertiary care hospital. 2022 Jun 19 [cited 2022 Jun 18]; Available from: https://www.mjdrdypv.org/preprintarticle.asp?id=336821;type=0.
Iem V, Chittamany P, Suthepmany S, Siphanthong S, Somphavong S, Kontogianni K, et al. Pooling sputum for Xpert MTB/RIF and Xpert Ultra testing during the Covid-19 pandemic in Lao People’s Democratic Republic. PLOS Glob Public Health. 2022;2(4):e0000116.
Rahman SMM, Ather MdF, Nasrin R, Hoque MA, Khatun R, Rahman T, et al. Performance of WHO-Endorsed Rapid Tests for Detection of Susceptibility to First-Line Drugs in Patients with Pulmonary Tuberculosis in Bangladesh. Diagnostics. 2022;12(2):410.
Simieneh A, Tadesse M, Kebede W, Gashaw M, Abebe G. Combination of Xpert® MTB/RIF and DetermineTM TB-LAM Ag improves the diagnosis of extrapulmonary tuberculosis at Jimma University Medical Center, Oromia, Ethiopia. PLoS ONE. 2022;17(2):e0263172.
Tantivitayakul P, Ruangchai W, Juthayothin T, Smittipat N, Disratthakit A, Mahasirimongkol S, et al. Homoplastic single nucleotide polymorphisms contributed to phenotypic diversity in Mycobacterium tuberculosis. Sci Rep. 2020;10(1):8024.
Yang T, Gan M, Liu Q, Liang W, Tang Q, Luo G, et al. SAM-TB: a whole genome sequencing data analysis website for detection of Mycobacterium tuberculosis drug resistance and transmission. Brief Bioinform. 2022;23(2):bbac030.
Lam C, Martinez E, Crighton T, Furlong C, Donnan E, Marais BJ, et al. Value of routine whole genome sequencing for Mycobacterium tuberculosis drug resistance detection. Int J Infect Dis. 2021;113:S48-54.
Wang L, Yang J, Chen L, Wang W, Yu F, Xiong H. Whole-genome sequencing of Mycobacterium tuberculosis for prediction of drug resistance. Epidemiol Infect. 2022;150:e22.
Brown AC. Whole-Genome Sequencing of Mycobacterium tuberculosis Directly from Sputum Samples. In: Parish T, Kumar A, editors. Mycobacteria Protocols [Internet]. New York, NY: Springer US; 2021 [cited 2022 Jun 18]. p. 459–80. (Methods in Molecular Biology). Available from: https://doi.org/10.1007/978-1-0716-1460-0_20.
Torres Ortiz A, Coronel J, Vidal JR, Bonilla C, Moore DAJ, Gilman RH, et al. Genomic signatures of pre-resistance in Mycobacterium tuberculosis. Nat Commun. 2021;12(1):7312.
Freschi L, Vargas R, Husain A, Kamal SMM, Skrahina A, Tahseen S, et al. Population structure, biogeography and transmissibility of Mycobacterium tuberculosis. Nat Commun. 2021;12(1):6099.
Bogaerts B, Delcourt T, Soetaert K, Boarbi S, Ceyssens PJ, Winand R, et al. A Bioinformatics Whole-Genome Sequencing Workflow for Clinical Mycobacterium tuberculosis Complex Isolate Analysis, Validated Using a Reference Collection Extensively Characterized with Conventional Methods and In Silico Approaches. J Clin Microbiol. 59(6):e00202–21.
Shea J, Halse TA, Lapierre P, Shudt M, Kohlerschmidt D, Van Roey P, et al. Comprehensive Whole-Genome Sequencing and Reporting of Drug Resistance Profiles on Clinical Cases of Mycobacterium tuberculosis in New York State. J Clin Microbiol. 2017;55(6):1871–82.
Coll F, McNerney R, Preston MD, Guerra-Assunção JA, Warry A, Hill-Cawthorne G, et al. Rapid determination of anti-tuberculosis drug resistance from whole-genome sequences. Genome Med. 2015;7(1):51.
Cohen KA, Manson AL, Abeel T, Desjardins CA, Chapman SB, Hoffner S, et al. Extensive global movement of multidrug-resistant M. tuberculosis strains revealed by whole-genome analysis. Thorax. 2019;74(9):882–9.
Gygli SM, Keller PM, Ballif M, Blöchliger N, Hömke R, Reinhard M, et al. Whole-Genome Sequencing for Drug Resistance Profile Prediction in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2019;63(4):e02175-e2218.
The CRyPTIC Consortium and the 100,000 Genomes Project. Prediction of Susceptibility to First-Line Tuberculosis Drugs by DNA Sequencing. N Engl J Med. 2018 Oct 11;379(15):1403–15.
Arias APJ, Lahiguera MJ, Borrás R, Cardona CG, Silva MG, López MJV, et al. Comparative study of the genetic diversity of Mycobacterium tuberculosis Complex by Simplified Amplified Fragment Length Polymorphism and Mycobacterial Interspersed Repetitive Unit Variable Number Tandem Repeat Analysis. Rev Ecuat Med Cienc Biológicas [Internet]. 2018 May 21 [cited 2022 Jul 4];39(1). Available from: https://remcb-puce.edu.ec/remcb/article/view/568.
Zurita J, Espinel N, Barba P, Ortega-Paredes D, Zurita-Salinas C, Rojas Y, et al. Genetic diversity and drug resistance of Mycobacterium tuberculosis in Ecuador. Int J Tuberc Lung Dis. 2019;23(2):166–73.
Garzon-Chavez D, Zurita J, Mora-Pinargote C, Franco-Sotomayor G, Leon-Benitez M, Granda-Pardo JC, et al. Prevalence, Drug Resistance, and Genotypic Diversity of the Mycobacterium tuberculosis Beijing Family in Ecuador. Microb Drug Resist. 2019;25(6):931–7.
Garzon-Chavez D, Garcia-Bereguiain MA, Mora-Pinargote C, Granda-Pardo JC, Leon-Benitez M, Franco-Sotomayor G, et al. Population structure and genetic diversity of Mycobacterium tuberculosis in Ecuador. Sci Rep. 2020;10(1):6237.
Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MTG, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31(22):3691–3.
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, et al. The RAST Server: Rapid Annotations using Subsystems Technology. BMC Genomics. 2008;9(1):75.
Smith I. Mycobacterium tuberculosis Pathogenesis and Molecular Determinants of Virulence. Clin Microbiol Rev [Internet]. 2003 [cited 2022 Jul 9]; Available from: https://journals.asm.org/doi/epub/https://doi.org/10.1128/CMR.16.3.463-496.2003.
Chatterjee A, Nilgiriwala K, Saranath D, Rodrigues C, Mistry N. Whole genome sequencing of clinical strains of Mycobacterium tuberculosis from Mumbai, India: A potential tool for determining drug-resistance and strain lineage. Tuberculosis. 2017;1(107):63–72.
Díaz Acosta CC, Russomando G, Candia N, Ritacco V, Vasconcellos SEG, de Berrêdo Pinho Moreira M, et al. Exploring the “Latin American Mediterranean” family and the RDRio lineage in Mycobacterium tuberculosis isolates from Paraguay, Argentina and Venezuela. BMC Microbiol. 2019 Dec;19(1):131.
Hadi SA, Kolte IV, Brenner EP, Cunha E a. T, Simonsen V, Ferrazoli L, et al. Identification of a predominant genotype of Mycobacterium tuberculosis in Brazilian indigenous population. Sci Rep. 2021 Jan 13;11(1):1224.
Salvato RS, Reis AJ, Schiefelbein SH, Gómez MAA, Salvato SS, da Silva LV, et al. Genomic-based surveillance reveals high ongoing transmission of multi-drug-resistant Mycobacterium tuberculosis in Southern Brazil. Int J Antimicrob Agents. 2021;58(4):106401.
Sánchez-Corrales L, Tovar-Aguirre OL, Galeano-Vanegas NF, Jiménez PAC, Martínez-Vega RA, Maldonado-Londoño CE, et al. Phylogenomic analysis and Mycobacterium tuberculosis antibiotic resistance prediction by whole-genome sequencing from clinical isolates of Caldas, Colombia. PLoS ONE. 2021;16(10):e0258402.
Cerezo I, Jiménez Y, Hernandez J, Zozio T, Murcia MI, Rastogi N. A first insight on the population structure of Mycobacterium tuberculosis complex as studied by spoligotyping and MIRU-VNTRs in Bogotá, Colombia. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis. 2012;12(4):657–63.
Ferro BE, Nieto LM, Rozo JC, Forero L, van Soolingen D. Multidrug-Resistant Mycobacterium tuberculosis. Southwestern Colombia Emerg Infect Dis. 2011;17(7):1259–62.
Munro-Rojas D, Fernandez-Morales E, Zarrabal-Meza J, Martínez-Cazares MT, Parissi-Crivelli A, Fuentes-Domínguez J, et al. Genetic diversity of drug and multidrug-resistant Mycobacterium tuberculosis circulating in Veracruz, Mexico. PLoS ONE. 2018;13(3):e0193626.
Lefort B, Pérez-Martínez D, Viveros D, Zenteno-Cuevas R, Lefort B, Pérez-Martínez D, et al. Diversidad genética de Mycobacterium tuberculosis circulando en la jurisdicción V de Jalapa, Veracruz. México Rev Chil Infectol. 2021;38(5):639–46.
Sheen P, Couvin D, Grandjean L, Zimic M, Dominguez M, Luna G, et al. Genetic Diversity of Mycobacterium tuberculosis in Peru and Exploration of Phylogenetic Associations with Drug Resistance. PLoS ONE. 2013;8(6):e65873.
Taype CA, Agapito JC, Accinelli RA, Espinoza JR, Godreuil S, Goodman SJ, et al. Genetic diversity, population structure and drug resistance of Mycobacterium tuberculosis in Peru. Infect Genet Evol. 2012;12(3):577–85.
Realpe T, Correa N, Rozo JC, Ferro BE, Gomez V, Zapata E, et al. Population Structure among Mycobacterium tuberculosis Isolates from Pulmonary Tuberculosis Patients in Colombia. PLoS ONE. 2014;9(4):e93848.
Wiens KE, Woyczynski LP, Ledesma JR, Ross JM, Zenteno-Cuevas R, Goodridge A, et al. Global variation in bacterial strains that cause tuberculosis disease: a systematic review and meta-analysis. BMC Med. 2018;30(16):196.
Brynildsrud OB, Pepperell CS, Suffys P, Grandjean L, Monteserin J, Debech N, et al. Global expansion of Mycobacterium tuberculosis lineage 4 shaped by colonial migration and local adaptation. Sci Adv. 2018;4(10):eaat5869.
Jiménez P, Calvopiña K, Herrera D, Rojas C, Pérez L, Grijalva M, et al. Identification of the Mycobacterium tuberculosis Beijing lineage in Ecuador. Biomédica [Internet]. 2017 Jun 1 [cited 2022 Jun 18];37(2). Available from: http://www.revistabiomedica.org/index.php/biomedica/article/view/3450.
Brites D, Gagneux S. Co-evolution of Mycobacterium tuberculosis and Homo sapiens. Immunol Rev. 2015;264(1):6–24.
Chiner-Oms Á, López MG, Moreno-Molina M, Furió V, Comas I. Gene evolutionary trajectories in Mycobacterium tuberculosis reveal temporal signs of selection. Proc Natl Acad Sci. 2022;119(17):e2113600119.
Moreno-Molina M, Shubladze N, Khurtsilava I, Avaliani Z, Bablishvili N, Torres-Puente M, et al. Genomic analyses of Mycobacterium tuberculosis from human lung resections reveal a high frequency of polyclonal infections. Nat Commun. 2021;12(1):2716.
Yang T, Zhong J, Zhang J, Li C, Yu X, Xiao J, et al. Pan-Genomic Study of Mycobacterium tuberculosis Reflecting the Primary/Secondary Genes, Generality/Individuality, and the Interconversion Through Copy Number Variations. Front Microbiol [Internet]. 2018 [cited 2022 Jun 20];9. Available from: https://www.frontiersin.org/article/https://doi.org/10.3389/fmicb.2018.01886
Zakham F, Sironen T, Vapalahti O, Kant R. Pan and Core Genome Analysis of 183 Mycobacterium tuberculosis Strains Revealed a High Inter-Species Diversity among the Human Adapted Strains. Antibiotics. 2021;10(5):500.
Dar HA, Zaheer T, Ullah N, Bakhtiar SM, Zhang T, Yasir M, et al. Pangenome Analysis of Mycobacterium tuberculosis Reveals Core-Drug Targets and Screening of Promising Lead Compounds for Drug Discovery. Antibiotics. 2020;9(11):819.
Periwal V, Patowary A, Vellarikkal SK, Gupta A, Singh M, Mittal A, et al. Comparative Whole-Genome Analysis of Clinical Isolates Reveals Characteristic Architecture of Mycobacterium tuberculosis Pangenome. Hoshino Y, editor. PLOS ONE. 2015 Apr 8;10(4):e0122979.
Cassio Barreto de Oliveira M, Balan A. The ATP-Binding Cassette (ABC) Transport Systems in Mycobacterium tuberculosis: Structure, Function, and Possible Targets for Therapeutics. Biology. 2020;9(12):443.
Yang Z, Zeng X, Tsui SKW. Investigating function roles of hypothetical proteins encoded by the Mycobacterium tuberculosis H37Rv genome. BMC Genomics. 2019;20(1):394.
Diamanti E, Hamed MM, Lacour A, Bravo P, Illarionov B, Fischer M, et al. Targeting the IspD Enzyme in the MEP Pathway: Identification of a Novel Fragment Class. ChemMedChem. 2022;17(5):e202100679.
Wang X, Dowd CS. The MEP pathway: Promising drug targets in the fight against tuberculosis. ACS Infect Dis. 2018;4(3):278–90.
Mi J, Gong W, Wu X. Advances in Key Drug Target Identification and New Drug Development for Tuberculosis. BioMed Res Int. 2022;25(2022):5099312.
Quan TP, Bawa Z, Foster D, Walker T, del Ojo Elias C, Rathod P, et al. Evaluation of Whole-Genome Sequencing for Mycobacterial Species Identification and Drug Susceptibility Testing in a Clinical Setting: a Large-Scale Prospective Assessment of Performance against Line Probe Assays and Phenotyping. Diekema DJ, editor. J Clin Microbiol. 2018 Feb;56(2):e01480–17.
Katale BZ, Mbelele PM, Lema NA, Campino S, Mshana SE, Rweyemamu MM, et al. Whole genome sequencing of Mycobacterium tuberculosis isolates and clinical outcomes of patients treated for multidrug-resistant tuberculosis in Tanzania. BMC Genomics. 2020;21(1):174.
Guo Y, Cao X, Yang J, Wu X, Liu Y, Wan B, et al. Rifampin-resistance-associated mutations in the rifampin-resistance-determining region of the rpoB gene of Mycobacterium tuberculosis clinical isolates in Shanghai, PR China. J Med Microbiol. 2021 Mar;70(3).
Kardan-Yamchi J, Amini S, Hamzelou G, Rahimi Foroushani A, Ghodousi A, Cirillo DM, et al. Assessment of the GenoType MTBDRsl VER 2.0 compared to the phenotypic drug susceptibility testing and whole genome sequencing for the rapid detection of resistance to fluoroquinolone and second-line injectable drugs among rifampicin-resistant Mycobacterium tuberculosis isolates. Arch Microbiol. 2021 Sep 1;203(7):3989–96.
Cox H, Goig GA, Salaam-Dreyer Z, Dippenaar A, Reuter A, Mohr-Holland E, et al. Whole-Genome Sequencing Has the Potential To Improve Treatment for Rifampicin-Resistant Tuberculosis in High-Burden Settings: a Retrospective Cohort Study. J Clin Microbiol. 2022;60(3):e02362-e2421.
Pinhata JMW, Brandao AP, Mendes F de F, Rabello MC da S, Ferrazoli L, de Oliveira RS. Correlating genetic mutations with isoniazid phenotypic levels of resistance in Mycobacterium tuberculosis isolates from patients with drug-resistant tuberculosis in a high burden setting. Eur J Clin Microbiol Infect Dis. 2021 Dec 1;40(12):2551–61.
Bakhtiyariniya P, Khosravi AD, Hashemzadeh M, Savari M. Detection and characterization of mutations in genes related to isoniazid resistance in Mycobacterium tuberculosis clinical isolates from Iran. Mol Biol Rep [Internet]. 2022 Apr 2 [cited 2022 Jun 18]; Available from: https://link.springer.com/https://doi.org/10.1007/s11033-022-07404-2.
Xu Y, Jia H, Huang H, Sun Z, Zhang Z. Mutations Found in embCAB, embR, and ubiA Genes of Ethambutol-Sensitive and -Resistant Mycobacterium tuberculosis Clinical Isolates from China. BioMed Res Int. 2015;2015:951706.
Sun Q, Xiao T yang, Liu H can, Zhao X qin, Liu Z guang, Li Y nan, et al. Mutations within embCAB Are Associated with Variable Level of Ethambutol Resistance in Mycobacterium tuberculosis Isolates from China. Antimicrob Agents Chemother. 2017 Dec 21;62(1):e01279–17.
Rocha DMGC, Magalhães C, Cá B, Ramos A, Carvalho T, Comas I, et al. Heterogeneous Streptomycin Resistance Level Among Mycobacterium tuberculosis Strains From the Same Transmission Cluster. Front Microbiol [Internet]. 2021 [cited 2022 Jun 18];12. Available from: https://www.frontiersin.org/article/https://doi.org/10.3389/fmicb.2021.659545.
Cohen KA, Stott KE, Munsamy V, Manson AL, Earl AM, Pym AS. Evidence for Expanding the Role of Streptomycin in the Management of Drug-Resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2020;64(9):e00860-e920.
Chaiyachat P, Chaiprasert A, Nonghanphithak D, Smithtikarn S, Kamolwat P, Pungrassami P, et al. Whole-genome analysis of drug-resistant Mycobacterium tuberculosis reveals novel mutations associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2021;58(3):106385.
Kabir S, Tahir Z, Mukhtar N, Sohail M, Saqalein M, Rehman A. Fluoroquinolone resistance and mutational profile of gyrA in pulmonary MDR tuberculosis patients. BMC Pulm Med. 2020;11(20):138.
Mirzayev F, Viney K, Linh NN, Gonzalez-Angulo L, Gegia M, Jaramillo E, et al. World Health Organization recommendations on the treatment of drug-resistant tuberculosis, 2020 update. Eur Respir J. 2021;57(6):2003300.
Aydın Kayalı R, Özkan SA, Biçmen C, Erer OF. The Relation Between the Emergence of Fluoroquinolone Resistance and Fluoroquinolone Exposure in New Cases of Active Pulmonary Tuberculosis. Turk Thorac J. 2021;22(1):45–9.
Dalhoff A. Global Fluoroquinolone Resistance Epidemiology and Implictions for Clinical Use. Interdiscip Perspect Infect Dis. 2012;2012:1–37.
Xia H, Zheng Y, Liu D, Wang S, He W, Zhao B, Increase S, in Moxifloxacin Resistance Rate among Multidrug-Resistant Mycobacterium tuberculosis Isolates in China, et al. to 2013. Microbiol Spectr. 2007;9(3):e00409-e421.
McNerney R, Zignol M, Clark TG. Use of whole genome sequencing in surveillance of drug resistant tuberculosis. Expert Rev Anti Infect Ther. 2018;16(5):433–42.
Davedow T, Carleton H, Kubota K, Palm D, Schroeder M, Gerner-Smidt P, et al. PulseNet International Survey on the Implementation of Whole Genome Sequencing in Low and Middle-Income Countries for Foodborne Disease Surveillance. Foodborne Pathog Dis. 2022;19(5):332–40.
Waddington C, Carey ME, Boinett CJ, Higginson E, Veeraraghavan B, Baker S. Exploiting genomics to mitigate the public health impact of antimicrobial resistance. Genome Med. 2022;14(1):15.
Sanchini A, Jandrasits C, Tembrockhaus J, Kohl TA, Utpatel C, Maurer FP, et al. Improving tuberculosis surveillance by detecting international transmission using publicly available whole genome sequencing data. Eurosurveillance. 2021;26(2):1900677.
van der Werf MJ, Ködmön C. Whole-Genome Sequencing as Tool for Investigating International Tuberculosis Outbreaks: A Systematic Review. Front Public Health [Internet]. 2019 [cited 2022 Aug 4];7. Available from: https://www.frontiersin.org/articles/https://doi.org/10.3389/fpubh.2019.00087
Canetti G, Rist N, Grosset J. [Measurement of sensitivity of the tuberculous bacillus to antibacillary drugs by the method of proportions. Methodology, resistance criteria, results and interpretation]. Rev Tuberc Pneumol (Paris). 1963 Mar;27:217–72.
Van Soolingen D. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J Intern Med. 2001;249(1):1–26.
Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15(3):R46.
Andrews S. FastQC: a quality control tool for high throughput sequence data. 2010. 2017.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20.
Li D, Liu CM, Luo R, Sadakane K, Lam TW. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31(10):1674–6.
Menzel P, Ng KL, Krogh A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat Commun. 2016;7(1):11257.
Kaiju: Fast and sensitive taxonomic classification for metagenomics [Internet]. [cited 2022 Jun 20]. Available from: https://kaiju.binf.ku.dk/.
Gurevich A, Saveliev V, Vyahhi N, Tesler G. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29(8):1072–5.
Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–9.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–9.
Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60.
Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, et al. Twelve years of SAMtools and BCFtools. GigaScience. 2021 Feb 16;10(2):giab008.
Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, et al. The variant call format and VCFtools. Bioinformatics. 2011;27(15):2156–8.
Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w 1118; iso-2; iso-3. Fly (Austin). 2012;6(2):80–92.
Zhao Y, Jia X, Yang J, Ling Y, Zhang Z, Yu J, et al. PanGP: A tool for quickly analyzing bacterial pangenome profile. Bioinformatics. 2014;30(9):1297–9.
PanGP: pangenome Profile Tool Website [Internet]. [cited 2022 Jun 20]. Available from: https://pangp.zhaopage.com/.
Jünemann S, Sedlazeck FJ, Prior K, Albersmeier A, John U, Kalinowski J, et al. Updating benchtop sequencing performance comparison. Nat Biotechnol. 2013;31(4):294–6.
Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016 Jul 8;44(Web Server issue):W242–5.
Lipworth S, Jajou R, de Neeling A, Bradley P, van der Hoek W, Maphalala G, et al. SNP-IT Tool for Identifying Subspecies and Associated Lineages of Mycobacterium tuberculosis Complex. Emerg Infect Dis. 2019;25(3):482–8.
Acknowledgements
We acknowledge the FCI Research program of University of Guayaquil for the partial financial support of the procurement of the reagents to MiniSeq sequencing machine that were used to run the samples. We thank the private laboratories for supporting the isolate analyzed in this study. We also thank the Omics Lab of Universidad Espiritu Santo for permitting the use of the MiniSeq sequencing machine employed to run the samples. We also thank the Bioinformatics Unit of the Institute Pasteur de Montevideo for their assistance and support.
Author information
Authors and Affiliations
Contributions
GM conceived and designed the study, performed the collection of isolates, sample processing, data analysis (bioinformatic processing of the raw sequencing data) and wrote the main manuscript text, DAM and JCFC performed sample processing (library prep and sequencing), data analysis and review the draft manuscript, LB conceived and designed the study, performed a data analysis, validation, wrote the main manuscript text and review the manuscript; The funding acquisition was performed by GM. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the University San Francisco de Quito (code P2019-188E), certified by the Ministry of Public Health from Ecuador, following guidelines from the Declaration of Helsinki. The samples were processed and anonymized by the clinical laboratory, hence samples are for the researchers in the study de-identified. We obtained a laboratory's permission to use the positive samples.
Consent of publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary table 1. Sociodemographic, epidemiological and assembly quality data from 21 isolates of Mycobacterium tuberculosis.
Additional file 2: Supplementary table 2.
Distribution of COG annotations on 21 samples of Mycobacterium tuberculosis.
Additional file 3: Supplementary table 3.
Distribution of Subsystems on 21 samples of Mycobacterium tuberculosis by Rastk.
Additional file 4: Supplementary table 4.
Distribution of Virulence Factor on 21 samples of Mycobacterium tuberculosis.
Additional file 5: Supplementary table 5.
Distribution of potential drug target on 21 samples of Mycobacterium tuberculosis.
Additional file 6: Supplementary table 6.
Distribution of Transporters on 21 samples of Mycobacterium tuberculosis.
Additional file 7: Supplementary table 7.
Characterization of Mycobacterium tuberculosis sub-lineages in 21 Ecuadorian isolates characterized by web-tools and their correlation with phenotypic and genotypic drug resistance profile.
Additional file 8: Supplementary table 8.
Genetic variants characterized in 21 Ecuadorian isolates of Mycobacterium tuberculosis.
Additional file 9: Supplementary table 9.
Mutations identified in resistance related genes by web-tools on 21 samples of Mycobacterium tuberculosis.
Additional file 10: Supplementary table 10.
Distribution of mutations identified in resistance related genes for web-tools on 21 samples of Mycobacterium tuberculosis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Morey-León, G., Andrade-Molina, D., Fernández-Cadena, J.C. et al. Comparative genomics of drug-resistant strains of Mycobacterium tuberculosis in Ecuador. BMC Genomics 23, 844 (2022). https://doi.org/10.1186/s12864-022-09042-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-022-09042-1