Abstract
Background
Cerasus sachalinensis is widely used in cool regions as a sweet cherry rootstock and is known for its sensitivity to soil waterlogging and waterlogging stress. However, the limited availability of Cerasus genomic resources has considerably restricted the exploration of its waterlogging response mechanism. To understand its reaction to short-term waterlogging, we analyzed the physiology and transcriptomes of C. sachalinensis roots in response to different waterlogging durations.
Results
In this study, 12,487 differentially expressed genes (DEGs) were identified from Cerasus sachalinensis roots under different waterlogging durations. Carbon metabolism and energy maintenance formed the first coping mechanism stage of C. sachalinensis in response to low oxygen conditions. Root energy processes, including root respiration and activities of the fermentation enzymes alcohol dehydrogenase, pyruvate decarboxylase, and lactate dehydrogenase, showed unique changes after 0 h, 3 h, 6 h, and 24 h of waterlogging exposure. Ribonucleic acid sequencing was used to analyze transcriptome changes in C. sachalinensis roots treated with 3 h, 6 h, and 24 h of waterlogging stress. After de novo assembly, 597,474 unigenes were recognized, of which 355,350 (59.47%) were annotated. To identify the most important pathways represented by DEGs, Gene Ontology and Kyoto Encyclopedia of Genes and Genomes databases were used to compare these genes. The first stage of root reaction to waterlogging stress was activation of carbohydrate metabolism to produce more glucose and maintain energy levels. At 3 h, the glycolytic and fermentation pathways were activated to maintain adenosine triphosphate production. At 24 h, pathways involved in the translation of proteins were activated to further assist the plant in tolerating waterlogging stress. These findings will facilitate a further understanding of the potential mechanisms of plant responses to waterlogging at physiological and transcriptome levels.
Conclusions
Carbon metabolism and energy maintenance formed the first coping mechanism C. sachalinensis in response to low oxygen conditions, and they may be responsible for its short-term waterlogging response. Our study not only provides the assessment of genomic resources of Cerasus but also paves the way for probing the metabolic and molecular mechanisms underlying the short-term waterlogging response in C. sachalinensis.
Similar content being viewed by others
Background
Soils with high clay content and poor drainage can be waterlogged or flooded by inappropriate irrigation practices or heavy rains. Under these conditions, excess water saturates the rhizosphere, and the remaining oxygen is quickly consumed by plant roots and soil microorganisms, resulting in hypoxic conditions [1]. In general, sweet cherry trees are grafted onto rootstocks that in turn determine the tolerance of the cherry tree to abiotic stress [2]. However, species of Prunus used as rootstocks are classified as sensitive to root hypoxia, although there are reported differences among genotypes regarding their ability to tolerate this stress [3, 4].
Cerasus sachalinensis (F. Schmidt) Kom. is native to northeastern China and northern Korea. This species is widely used as a sweet cherry rootstock in cool regions such as Dalian and Qinhuangdao because of its high propagation rate, cold resistance, and adaptability [5]. However, it is vulnerable to waterlogging after heavy rains, especially during the rainy season. Proper drainage systems are often lacking, and therefore waterlogging is very common for 3–24 h following rainfall. One study demonstrated that C. sachalinensis rootstocks are particularly sensitive to waterlogging [6]; the mechanism and responsive gene expression patterns, however, are not yet understood.
The diffusion rate of oxygen in water is much lower than in air, and therefore waterlogging inhibits plant growth and development, making the plants vulnerable to hypoxia or anoxia [7]. When roots are subjected to waterlogging, the oxygen-dependent energy-generating pathways (e.g., aerobic respiration) are suspended, which leads to a rapid reduction in cellular adenosine triphosphate (ATP). When the oxygen supply is limited, various adaptive responses are activated to address this energy depletion [8]. Some plants cope by relying on glycolysis and fermentation to supply essential ATP [9]. In other cases, plants that can survive under low oxygen conditions shift their energy metabolism from aerobic to anaerobic [10]. Plants that rely on fermentation pathways to regenerate nicotinamide adenine dinucleotide (NAD+) maintain the glycolysis pathway during these periods [11].
Cellular respiration both generates ATP, which is needed for cell maintenance and growth, and releases energy [12]. Waterlogging limits the oxygen supply, inhibits respiration, and greatly reduces the energy status of the roots. Respiration status, which reflects root physiological metabolic capacity, is often affected by biotic and abiotic factors, including waterlogging.
Changes in plant respiration pathways have been extensively studied. Approximately 20 anaerobically induced polypeptides (ANPs) have been identified [13]. ANPs are essential components for low oxygen tolerance in various plant species. Other studies have shown that ANPs were involved in the glycolytic and fermentation pathways that are necessary to maintain energy production under waterlogging conditions [14]. Subsequently, microarray studies have been conducted to assess responses to low oxygen [15, 16].
Ribonucleic acid sequencing (RNA-seq) provides a powerful tool for profiling the complete gene space of any organism owing to its high throughput, accuracy, and reproducibility. In plants, which often have large and complex genomes [17], RNA-seq has accelerated the discovery of novel genes, tissue-specific expression patterns, and functional analysis [18]. The RNA-seq approach has a higher sensitivity for gene expressions than microarrays. RNA-seq has been successfully used for waterlogging responses in rice [19], cucumber [20], maize [21], and rape [22]. However, only a few woody plants have been studied. To better understand the molecular mechanisms of the response of C. sachalinensis to waterlogging, the gene transcription changes from plants subjected to different durations of waterlogging were examined using the Illumina HiSeq™ 4000 sequencing platform (Illumina Inc., San Diego, CA, USA). The early stages of the response to waterlogging stress were focused on because they determine the switch from normal to low-oxygen metabolism and play an essential role in plant survival. Our results will facilitate understanding the response of waterlogging-intolerant woody plants to short-term waterlogging stress.
Methods
Plant growth and water treatments
Cerasus sachalinensis plants were obtained from Lianshanguang, Benxi, Liaoning Province, China (41°24′N, 124°17′E). Plants were grown in plastic pots (16 × 16 cm) under a transparent rain shelter at the experimental field (Shenyang, China, 41°N, 123°48′24″E) in April 2014. Over the course of the experiment, the average air temperature varied between 15 °C and 25 °C (mean = 20 °C ± 5 °C). Two weeks later, after seedlings had produced 10–12 leaves, 36 plants that were similar in height and free from disease were selected for the treatments. Plants were divided evenly into a control group (CK) and a treatment where pots were kept in tap water 3 cm deep (waterlogged, WL). WL roots were sampled at 3 h, 6 h, and 24 h after waterlogging was introduced, and CK roots were sampled at 0 h. The primary roots with some lateral roots were collected from each individual plant, frozen separately in liquid nitrogen, and stored at −80 °C. Roots were pooled prior to RNA extraction to prepare four samples: CK at 0 h, WL at 3 h, WL at 6 h, and WL at 24 h. All plants in the treatment (each treatment have nine plants) group were sampled at each time point.
Root respiration
Roots were gently washed with deionized water and dried carefully with paper towels. Root samples of 0.05 g were used to measure respiration status as described by Zhou [5]. Root respiratory rate was measured as the oxygen consumption rate using an Oxytherm oxygen electrode (Hansatech, King’s Lynn, Norfolk, England) as reported by Bouma [23]. Root respiratory pathways were measured as described by Yu and Pan [24]. The contribution of each respiratory pathway was calculated with the following equation:
ATP content
The intracellular ATP content was measured using ATP determination kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu province, China). The results were expressed as μmol ATP/g fresh weight.
Analysis of total soluble sugars and starch
The concentrations of total soluble sugars and starch were analyzed by the anthrone method as described by Yemm and Willis [25]. Finely ground fresh tissue (approximately 150 mg) was homogenized in 3 mL of 80% ethanol and incubated in an ultrasonic bath for 30 min at 80 °C. After centrifugation (6000×g, 25 °C, 10 min), the supernatant was collected. The pellet was extracted again as described above, and the supernatant was obtained and combined with the previous aliquot. After adding 2 mL of anthrone reagent to the supernatant, the mixture was heated in boiling water for 10 min. After the mixture cooled to room temperature, the absorbance was measured at 620 nm. A standard curve was established using a series of diluted glucose solutions.
The pellet obtained after the extraction of the soluble sugars was further extracted with perchloric acid for starch determination. Subsequently, the starch (expressed as glucose equivalent) in the supernatant was determined with the same spectroscopic methods outlined above.
Glycolytic and fermentative enzyme assays
Root samples (0.5 g per replicate) were immediately frozen in liquid nitrogen and stored at −80 °C for enzyme activity measurements as described previously [26]. Hexokinase (HK, EC 2.7.1.1), pyruvate kinase (PK, EC 2.7.1.40), pyruvate decarboxylase (PDC, EC 4.1.1.1), alcohol dehydrogenase (ADH, EC 1.1.1.1), and lactate dehydrogenase (LDH, EC 1.1.1.17) activities were measured at 340 nm using an ultraviolet spectrophotometer (Purkinje TU-1900, Beijing, China), as described previously [27]. The LDH assay was conducted in 1.5 mL of reaction mix that contained 50 mM potassium phosphate buffer (pH 7.0), 0.2 mM NADH, 3 μM potassium cyanide, 4 mM 4-methylpyrazole, 0.4 ml of sample, and 10 mM sodium pyruvate to initiate the reaction [28]. Protein concentration was measured according to the methods of Bradford [29].
RNA extraction
RNA was extracted using the method of Chang [30], and then isolated and purified using a plant RNA extraction kit (R6827, Omega Bio-Tek, Norcross, GA, USA).
RNA quantification and qualification
RNA was checked for contamination and degradation by running samples on a 1% agarose gel for 20 min at 150 mV. RNA purity was checked using a NanoPhotometer® spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, Waltham, MA, USA). RNA concentration was measured using a Qubit® RNA Assay Kit with the Qubit® 2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA). RNA integrity was assessed using the RNA Nano 6000 Assay Kit for the Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA).
Library preparation for transcriptome sequencing
Complementary deoxyribonucleic acid (cDNA) library construction, sequencing, and assembly were performed following the methods of Su [31], Ma [32] and Wang [33]. Sequencing libraries were generated using a NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (New England Biolabs, Ipswich, MA, USA), and index codes were added to attribute sequences to each sample. Messenger RNA (mRNA) was purified using poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under elevated temperature in NEBNext First Strand Synthesis Reaction Buffer (5×). A random hexamer primer and M-MuLV Reverse Transcriptase (RNase H−) and DNA Polymerase I and RNase H were used to synthesize the first strand cDNA and second strand cDNA. After adenylation of 3′ ends of DNA fragments, a NEBNext Adaptor with a hairpin loop structure was ligated to prepare for hybridization. The library fragments were purified with the AMPure XP system (Beckman Coulter, Beverly, MA, USA). Then 3 μl USER Enzyme (New England Biolabs) was used with size-selected, adaptor-ligated cDNA at 37 °C for 15 min followed by 5 min at 95 °C. before the polymerase chain reaction (PCR), which was performed with Phusion High-Fidelity DNA polymerase, Universal PCR primers and Index (X) Primer. Finally, PCR products were purified (AMPure XP system) and library quality was assessed on the Agilent Bioanalyzer 2100 system. Illumina sequencing was performed at Novogene Bioinformatics Technology Co., Ltd., Beijing, China (www.novogene.com). The raw reads were deposited in the NCBI Sequence Read Archive (SRA, http://www.ncbi.nlm.nih.gov/Traces/sra).
Gene annotation
The unigenes of the transcriptomes were annotated through comparison with public databases, including the National Center for Biotechnology Information (NCBI) nonredundant (NR) protein sequence database, the NCBI nucleotide (NT) sequence database, the eukaryotic ortholog group (KOG) database, the Kyoto Encyclopedia of Genes and Genomes (KEGG) ortholog (KO) database, the Swiss-Prot protein database, the Gene Ontology (GO) database, and the protein family (Pfam) database, using NCBI’s basic local alignment search tool (BLAST) with a cutoff E-value of 10−5.
Quantification of gene expression levels and differential expression analysis
Gene expression levels were estimated using the software package RSEM for each sample as described previously [7]. The DEGseq (2010) R package was used to identify differential expression genes, with a q-value <0.05 and |log2 (fold change)| > 1 as the threshold for significant differential expression [34]. The GO seq R packages based on Wallenius’ noncentral hypergeometric distribution was used for GO enrichment analysis [35]. KOBAS software was used to test the statistical enrichment of differentially expressed genes (DEGs) in KEGG pathways [36].
qPCR analysis
The expression patterns of twelve genes that encode enzymes involved in the glycolysis/gluconeogenesis pathway, such as c271205_g2 (phosphofructokinase), c275115_g1, c275115_g3, and c249838_g1 (pyruvate decarboxylase), c261927_g6 (glucose-6-phosphate isomerase), c251063_g2 (LDH), c253776_g1 and c272347_g1 (ADH), c268143_g3 (PK), c249865_g1 (superoxide dismutase), and c240800_g1 (L-ascorbate peroxidase), were analyzed using quantitative PCR (qPCR). New plant material was used for the RNA extraction for the qPCR assays. Three biological replicates were analyzed. Gene-specific primers were designed with Primer Premier 5.0 software. The reaction mixture included 5 μl SYBR Green Premix Ex Taq II (DRR820A, Takara, Dalian, China), 0.8 μl primes, 2.4 μl ddH2O, 1 μl cDNA and was run in an ABI StepOne™ Plus system. The 2-ΔΔT method was used to analyze the relative expression levels of genes.
Statistical analyses
All statistical analyses were performed with PASW software (version 18.0, SPSS, IBM, Armonk, NY, USA). The mean values of enzyme activities and root respiration rates between treatments were analyzed by one-way analysis of variance (ANOVA) and compared for statistically significant differences as determined by Duncan’s multiple range test (p < 0.05).
Results
Energy status of waterlogged roots
To characterize energetic responses to waterlogging in C. sachalinensis, we monitored the root respiration rate, contribution from root respiration pathways, and ATP content. In our study, the respiration rates decreased by 21% and 56% after 3 h and 24 h of waterlogging treatment, respectively, but no changes were observed at 6 h (Fig. 1). After 3 h, the basic respiration pathways changed from Embden-Meyerhof-Parnas (EMP)-tricarboxylic acid (TCA)-pentose phosphate pathway (PPP) to EMP-PPP-TCA. As the duration of waterlogging increased, the contribution rate of EMP decreased by 6.90% at 3 h and increased by 0.30% and 6.60% at 6 h and 24 h, respectively, compared to the control. The contribution rate of TCA was the lowest, ranging from 29.39%–14.21%; the contribution rate of PPP increased by 12.54%, 9.54%, and 7.89% at 3 h, 6 h, and 24 h, respectively (Fig. 2).
ATP is used as an energy source in root cells, and therefore its content can reflect the energy status of roots. There were no significant differences in treated roots at 3 h and 6 h, but at 24 h, ATP content had significantly decreased (p < 0.05) (Fig. 3).
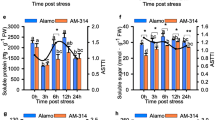
Concentrations of total soluble sugars and starch were examined during waterlogging (Fig. 4). In general, the high mean concentrations of total soluble sugars showed no significant differences at 0 h, 3 h, and 6 h, but significantly decreased at 24 h. Starch concentrations increased at 3 h and 6 h, but decreased at 24 h.
Carbohydrate concentration in Cerasus sachalinensis roots at different waterlogging durations. Note: Data indicate means (n = 9) ± SD. Different letters denote significant differences among means judged by a one-way ANOVA in relation to the control (Duncan’s multiple range test, p < 0.05). a soluble sugar content; b soluble starch content
Glycolytic and fermentative enzymes
To investigate the role of glycolysis and fermentation in mitigating energy deficits, we measured the activities of five enzymes: PK, HK, LDH, ADH, and PDC (Fig. 5). In C. sachalinensis roots, the activity of PK, one key enzyme of the EMP pathway, was not affected by waterlogging, but the activity of HK, another important enzyme, increased significantly at 3 h and 6 h, and then decreased at 24 h. The activity of LDH, the key enzyme for lactic acid fermentation, increased greatly at 3 h and 6 h, and then decreased at 24 h. At 3 h and 24 h, the activity of ADH, a key enzyme in ethanol fermentation, was not significantly different from the control, but at 6 h, it increased significantly (p < 0.05). Compared to the control, the activity of PDC, another central enzyme for ethanol fermentation, was significantly higher after 3 h, 6 h, and 24 h of waterlogging, and increased by 1.59-, 1.82-, and 1.42-fold, respectively.
Changes in glycolytic and fermentative enzymes at different waterlogging times in Cerasus sachalinensis roots. Note: Data indicate means (n = 9) ± SD. Different letters denote significant differences among means judged by a one-way ANOVA in relation to the control (Duncan’s multiple range test, p < 0.05). a–e activity of hexokinase, pyruvate kinase, alcohol dehydrogenase, pyruvate decarboxylase, and lactate dehydrogenase in control and waterlogging treatments
Sequence annotation and coding sequence prediction
Transcriptome sequences and the Illumina assembly data from C. sachalinensis roots were deposited in the NCBI Sequence Read Archive database under accession number SRP108195. In total, 617,203,902 paired-end raw reads were generated (Table 1). After adaptor sequences, ambiguous nucleotides, and low-quality sequences were removed, there were 588,477,556 clean reads remaining. Clean reads were assembled into 597,474 unigenes of 201–16,708 bp with an N50 length of 601 bp (Additional file 1: Figure S1). In total, 462,668 unigenes (77.43%) were between 200 and 500 bp; 79,121 unigenes (13.24%) were between 501 and 1000 bp; 37,855 unigenes (6.34%) were between 1001 and 2000 bp; and 17,830 unigenes (2.98%) were longer than 2000 bp (Additional file 2: Figure S2).
To understand the function of the assembled transcripts, the unigenes were annotated through comparison with entries in seven public databases (Table 2). Analyses showed that 228,046 unigenes (38.2%) had significant matches in the NR database, 149,145 (25.0%) in the NT database, and 226,826 (38.0%) in the Swiss-Prot database. In total, 355,350 unigenes (59.47%) were successfully annotated in at least one of the seven databases, with 35,167 unigenes (5.88%) in all databases.
For GO analysis, 241,843 unigenes were divided into three ontologies (Fig. 6). In the biological process category, genes involved in metabolic (128,475), cellular (127,807), and single-organism (102,113) processes were well represented. The cellular component category was mainly comprised of proteins involved in the cell (67,949), cell parts (67,898), and organelles (45,395). Within the molecular function category, binding (119,125), catalytic activity (108,077), and transporter activity (16,235) were highly represented. In addition, assembled unigene functions were evaluated through a search against the KOG database for functional prediction and classification.
In all, 142,876 unigenes were assigned to a KOG classification and divided into 26 specific categories (Fig. 7). The largest group was post-translational modification, protein turnover, and chaperones (19,668); followed by general function prediction only (18,401); translation, ribosomal structure, and biogenesis (18,046); signal transduction mechanisms (12,367); and energy production and conversion (11,258). Only a few unigenes were assigned to the extracellular structures (271) and cell motility (114) categories.
The unigene metabolic pathway analysis was conducted using the KEGG annotation system. This process predicted 132 pathways with 135,260 unigenes (Fig. 8). The pathways involving the highest numbers of unique transcripts were translation (16,208), carbohydrate metabolism (12,958), amino acid metabolism (9637), folding, sorting, and degradation (8774), and energy metabolism (7096).
Differential expression analysis of assembled C. sachalinensis transcripts from waterlogging stress
Using our de novo assembled transcriptome as a reference, we identified transcriptional responses to waterlogging. Reads from samples that had been exposed to waterlogging stress (3 h, 6 h, and 24 h) and from the control (0 h) were mapped to the obtained nonredundant unigenes from four libraries. The mapped reads were then used to estimate transcription levels according to fragment per kilobase of transcript per million reads (FPKM) values. On average, 74.54% of the clean reads were mapped (Table 1).
The genes with a q-value <0.05 and |log2 (foldchange)| > 1 were identified as significantly enriched or depleted. In total, 12,487 out of 597,474 unigenes (2.1%) were identified as DEGs among the treatments. The program DEGseq was used to identify the DEGs between the waterlogged and control samples (3 h/0 h, 6 h/0 h, and 24 h/0 h, respectively). DEGs with higher expression levels were considered upregulated (16,261), whereas those with lower expression levels were downregulated (19,101) (Fig. 9), that means some genes were up or down at different time points.
Compared with the control, 1217 genes were upregulated and 4160 were downregulated at 3 h. At 6 h, 9046 were upregulated and 7158 were downregulated, and at 24 h, 5998 were upregulated and 7783 were downregulated. Among these DEGs, 2447 were differentially expressed among all three treatments. In addition, 120 upregulated and 2327 downregulated genes were detected in all treatments (Fig. 10).
Venn diagrams of the differential expression transcripts under different treatment times. The number of DEGs exclusively up- or downregulated in Cerasus sachalinensis roots is shown. The number of DEGs with a common or opposite tendency of expression change between the two waterlogging times is shown in the overlapping regions. a Venn diagrams of the upregulated differential expression transcripts; b Venn diagrams of the downregulated differential expression transcripts
Functional classification of DEGs
To further analyze the possible functions of DEGs, we conducted a GO enrichment analysis for DEGs with the entire transcriptome as the background and compared each pair of samples.
GO enrichment analysis of DEGs at 3 h compared to the control indicated that certain genes related to processes such as gluconeogenesis were overexpressed. mRNAs that were highly enriched (q ≤ 0.05, after false discovery rate correction) at 3 h encoded proteins involved in organic substance biosynthetic, biosynthetic, primary metabolic, carbohydrate metabolic, and organic substance metabolic processes, suggesting that genes involved in these processes may play important roles in the initial response to waterlogging. GO enrichment analysis at 6 h indicated that the waterlogging treatment may have inhibited energy-consuming biosynthetic processes, with the top five overexpressed genes linked to the biological process category: regulation of cellular process, biological regulation, regulation of biological process, transcription, and DNA-templated and nucleic acid-templated transcription. All of these were linked to the process that controls ATP consumption. At 24 h, ribosome biogenesis, ribonucleoprotein complex biogenesis, translation, peptide biosynthetic process, and peptide metabolic process appeared to play important roles in waterlogging responses.
Compared with the control, KEGG pathway enrichment analysis for DEGs indicated that five pathways (carbon fixation in photosynthetic organisms, glycolysis/gluconeogenesis, nitrogen metabolism, plant-pathogen interaction, and starch and sucrose metabolism) were significantly enriched, and only proteasome was significantly depleted at 3 h (q ≤ 0.05) (Additional file 3: Table S1). At 6 h, the flavonoid biosynthesis pathway was significantly enriched, and the proteasome and spliceosome pathways were significantly depleted (q ≤ 0.05). At 24 h, the top four enriched pathways were ribosome, monoterpenoid biosynthesis, oxidative phosphorylation, and glycolysis/gluconeogenesis. Only the diterpenoid biosynthesis pathway was significantly depleted.
Response to waterlogging stress
We identified a relationship between gene expression and the activities of enzymes. We found that these pathways were significantly affected by waterlogging stress. The glycolysis/gluconeogenesis pathway was significantly enriched during the 24 h of waterlogging. There were 233 unigenes annotated as encoding enzymes involved in the glycolysis/gluconeogenesis pathway, and most of them were upregulated (Additional file 4: Table S2).
Most genes associated with sucrose metabolism were upregulated under waterlogging stress. Genes related to sucrose synthase were upregulated compared to the control; However, the expression of invertase genes were downregulated, which might explain the progressive increase in sucrose (Additional file 5: Figure S3)
The regulation of glycolysis indicates that ATP production may occur during different phases of waterlogging stress. Each glucose-1-P molecule is oxidized to L-lactate or ethanol, producing two ATP molecules. Most genes associated with fermentation were upregulated under waterlogging stress.
Two DEGs annotated as encoding PDC, c275115_g3 and c275115_g1, had 3.21- and 2.77-fold increased expression, respectively, at 3 h compared to the control. c253776_g1, which encodes ADH, was downregulated 8.93-fold, which may have led to a lower ATP level in the root at 3 h (Fig. 11). At 6 h, three DEGs annotated as encoding PDC, c275115_g3, c275115_g1, and c249838_g1, had 3.8-, 3.13-, and 6.83-fold increased expression, respectively. c251063_g2, which encodes LDH, was increased 2.90-fold, and c272347_g1, which encodes ADH, increased 10.20-fold, indicating that the fermentation pathway was activated to maintain ATP production in waterlogged C. sachalinensis roots under hypoxic conditions. At 24 h, c275115_g3 and c275115_g1, which encode PDC, were increased 4.15- and 4.51-fold.
Unigenes predicted to be involved in the glycolysis pathway. Red indicates significantly increased expression compared with the control (CK); green indicates significantly decreased expression; yellow indicates proteins encoded by both up- and downregulated genes. a 3 h/CK; b 6 h/CK; c 24 h/CK; purple indicates no significantly changed
qPCR validation
To verify the credibility of the RNA-seq data, the transcriptional levels of 12 unigenes, most of them not only associated with glycolysis and fermentation but also with different expression levels, were examined by real-time quantitative PCR (Additional file 6: Table S3). The results supported the RNA-seq data (Additional file 7: Figure S4, Additional file 8: Figure S5).
Discussion
Dynamic changes in mRNAs and metabolites in response to low oxygen conditions have been evaluated in a wide range of species, including Arabidopsis thaliana (L.) Heynh. [37], rice [19], mung bean [38], Chlamydomonas reinhardtii P. A. dang. [39], Jatropha curcas L. [40], and kiwifruits [41]. The metabolic changes found in our study were similar to those found in these species. Sasidharan [38] demonstrated that waterlogging caused elevated levels of mRNAs that encode the enzymes LDH, PDC, and ADH. Similarly, we found that mRNAs encoding enzymes involved in fermentation were also changed in C. sachalinensis roots under waterlogging conditions.
Comparisons of metabolism and transcriptomes among different waterlogging durations
Cerasus sachalinensis is one of the most popular rootstocks in northeastern China and is known for its sensitivity to waterlogging [6]. At present, little genomic information is available for Cerasus, and the response of Cerasus to waterlogging stress has not been investigated through transcriptome analysis.
Metabolic responses to waterlogging varied during different periods of the experiment. At 3 h, compared to the control, the root respiration rate, especially the rate of the TCA cycle, decreased in response to waterlogging, whereas the glycolytic flux and relatived enzyme activities maintained a relatively high level. By 6 h, the roots enacted metabolic changes to cope with waterlogging, and normal metabolic activities under these adverse conditions were ensured by a high soluble carbohydrate concentration and glycolysis-related enzyme activities. With persistent waterlogging conditions at 24 h, soluble carbohydrates were nearly depleted and ATP generation was inhibited, resulting in an energy crisis in the waterlogged roots.
The transcriptome differences were compared among different waterlogging times and indicated 12,487 DEGs. Among them, 5377 were found at 3 h, 16,204 were found at 6 h, and 13,780 were found at 24 h. Thus, we concluded that 6 h as a peak of transcriptional changes in four times. These findings are similar to those of Liu, who studied Arabidopsis roots [42]. In contrast, the research on Prunus avium (L.) L. Mazzard F12/1, a rootstock that is sensitive to hypoxia, showed that after 6 h of hypoxic root conditions, only 764 DEGs changed their expression and 65% of DEGs were upregulated; the greatest difference in the number of DEGs was observed at 72 h of hypoxia [43].
To identify pathway changes among varying waterlogging durations, we compared the enriched KEGG pathways at different times (Additional file 9: Figure S6). Compared with that of the control, the response at 3 h showed that carbohydrate-related and energy-related mRNAs were abundant, presumably to maintain glycolytic flux in the root. After 6 h of waterlogging, biosynthetic process pathways, and in particular flavonoid biosynthesis, were significantly enriched (q < 0.05), likely because flavonoid accumulation plays a role in the removal of radical oxygen species (ROS) [44]. After 24 h, translation, metabolism of terpenoids and polyketides, energy metabolism, and carbohydrate metabolism were enriched. The responses of plant roots to waterlogging stress differed according to the duration of waterlogging. Initially, carbohydrate-related and energy-related DEGs were upregulated to provide energy generation at 3 h. Next, because ROS accumulated as the byproducts of metabolic processes, the flavonoid biosynthesis pathway was enriched to remove ROS at 6 h. Finally, as the waterlogging time lengthened and energy depletion became a greater concern, the pathways that benefited from the maintenance of cellular energy generation were enriched.
Effects of waterlogging on carbohydrate metabolism
Carbohydrates are the primary energy resource under waterlogging conditions, and they are an important nutrient for hypoxic roots. Soluble sugars and starch are important carbohydrates; previous studies have shown that sufficient soluble sugar reserves were essential for plant survival during waterlogging. Research on mung bean [38] found that waterlogging-tolerant genotypes retained higher contents of sugar than intolerant genotype. In addition, research on two species of Rorippa found that 1 d of submergence significantly reduced the sugar concentration [45]. Short-term waterlogging in Lotus corniculatus L. var. japonicus Regel resulted in increased starch concentrations, whereas the total soluble sugar concentrations were not significantly changed [46]. Research on cucumber found that starch metabolism was activated after 8 h of waterlogging [20], which was similar to our study in which after 3 h of waterlogging, not only the carbohydrate concentration but also the genes involved in carbohydrate metabolism, including the glycolysis/gluconeogenesis pathway and starch metabolism, were affected in the roots. However, after 24 h of waterlogging, C. sachalinensis showed a rapid decrease in starch and soluble sugar concentrations, which distinguished the reaction in this species from that of other plants.
Effect of waterlogging on fermentation pathways
Under low oxygen waterlogging conditions, oxygen-dependent root respiration is greatly limited. Glycolysis is one of the important pathways for energy production under hypoxic conditions, and maintaining enhanced glycolysis may be crucial for tree survival [47]. In our research, glycolysis was significantly enriched at 3 h and 24 h.
The glycolysis and fermentative processes were enhanced under waterlogging conditions. Fermentative processes included ethanol fermentation (catalyzed by PDC and ADH) and lactate fermentation (catalyzed by LDH). ADH accelerates ethanol fermentation and allows for glycolysis to supply the plants with ATP during waterlogging, which improves the ability of plants to acclimate to stress [48]. ADH and LDH are primarily involved in anaerobic metabolism, whereas PDC is involved in both anaerobic and aerobic metabolism.
Research on Dendranthema spp. [49], Arabidopsis thaliana [50], and cucumber [51] showed that waterlogging enhanced both the transcript abundance of ADH, LDH, and PDC and the activity of the three enzymes. Some studies have shown that under waterlogging conditions, lactic acid fermentation was activated first, followed by alcoholic fermentation [7, 47]. In gray poplar, LDH transcripts rapidly increased in abundance after 5 h of hypoxic treatment but dropped after 24 h of hypoxia because of low cytosolic pH [52]. Research has shown that ethanol fermentation was the dominant energy conversion process [53, 54], and therefore most studies focused on ADH and PDC. In Arabidopsis thaliana, whereas four genes encode PDC, only PDC1 and PDC2 were upregulated under anoxia [55]. Komatsu [56] reported at least six ADH genes, but only the ADH2 gene was specifically expressed under waterlogging. However, other research found that ADH1 was induced shortly after the onset of waterlogging [57, 58].
In our research, LDH activity increased significantly after 6 h of waterlogging and then decreased after 24 h (p < 0.05). PDC was the key enzyme that linked glycolysis and fermentation, and its levels increased rapidly after 3 h of waterlogging, whereas ADH and LDH activities were significantly increased in C. sachalinensis roots (p < 0.05). ADH was encoded by at least two genes that showed different expressions at different times. One ADH gene (c253776_g1) was significantly downregulated at 3 h, another ADH gene (c272347_g1) was significantly upregulated at 6 h (p < 0.05), and both PDC genes were also significantly upregulated. These results indicated that although the two ADH genes (c253776_g1 and c272347_g1) were strongly induced, they played different roles during waterlogging. Lactate and alcohol fermentation were activated in C. sachalinensis roots at 6 h, thereby marking 6 h as a peak of metabolic and transcriptional changes.
Conclusion
In this study, transcriptomes of C. sachalinensis roots were sequenced using the Illumina platform. In total, 588,332,548 high-quality reads with 88.3 Gb sequence coverage were obtained, 597,474 unigenes (≥ 200 bp) were assembled, and 59.47% were annotated.
Transcripts involved in carbon metabolism changed under different waterlogging durations. In C. sachalinensis, these changes were mediated by a shortage of ATP. The glycolysis and fermentation pathways were stimulated to maintain ATP, and as a result, anaerobic respiration was high at 6 h. Thus, transcript patterns revealed that energy maintenance was the primary coping mechanism that C. sachalinensis adopted to survive under low oxygen conditions, which may be responsible for its remarkable waterlogging sensitivity.
We compared transcriptome differences among roots that were waterlogged for varying durations. In total, 12,487 DEGs were identified. Additionally, through comparisons to the control (3 h/0 h, 6 h/0 h, and 24 h/0 h), genes responsive to waterlogging were determined. There were 120 transcripts that showed upregulation and 2315 that were downregulated in all three pairwise comparisons between the waterlogged and control samples. In this transcriptome analysis, we found that energy maintenance was the primary coping mechanism under short-term waterlogging conditions. Six hours was an important time at which there was a peak in metabolic and transcriptional changes. These results will contribute to elucidating the metabolic and molecular mechanisms for short-term waterlogging in species sensitive to such adverse conditions.
Abbreviations
- ADH:
-
Alcohol dehydrogenase
- ANOVA:
-
Analysis of variance
- ANPs:
-
Anaerobically induced polypeptides
- ATP:
-
Adenosine triphosphate
- cDNA:
-
Complementary deoxyribonucleic acid
- CK:
-
Control
- DEG, DEGs:
-
Differentially expressed gene(s)
- FPKM:
-
Fragments per kilobases per million mapped reads
- GO:
-
Gene ontology
- HK:
-
Hexokinase
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- KO:
-
Kyoto Encyclopedia of Genes and Genomes ortholog database
- KOG:
-
Eukaryotic ortholog group
- LDH:
-
Lactate dehydrogenase
- mRNA:
-
Messenger RNA
- NAD:
-
Nicotinamide adenine dinucleotide
- NCBI:
-
National Center for Biotechnology Information
- NR:
-
NCBI nonredundant protein sequences
- NT:
-
NCBI nonredundant nucleotide sequences
- PCR:
-
Polymerase chain reaction
- PDC:
-
Pyruvate decarboxylase
- Pfam:
-
Protein family
- qPCR:
-
Quantitative PCR
- RNA:
-
Ribonucleic acid
- RNA-seq:
-
High-throughput RNA-sequencing
- WL:
-
Waterlogged treatment
References
Pimentel P, Almada RD, Salvatierra A, Toro G, Arismendi MJ, Pino MT, Sagredo B, Pinto M. Physiological and morphological responses of Prunus species with different degree of tolerance to long-term root hypoxia. Sci Hortic. 2014;180:14–23.
Iacona C, Cirilli M, Zega A, Frioni E, Silvestri C, Muleo R. A somaclonal myrobalan rootstock increases waterlogging tolerance to peach cultivar in controlled conditions. Sci Hortic. 2013;156:1–8.
Rubio-Cabetas MJ, Amador ML, Gómez-Aparisi J, Jaime J, Sancho S. Physiological and biochemical parameters involved in Waterlogging stress in Prunus. ISHS Acta Horticulturae. 2011;903:1215–23.
Pinochet J. ‘Replantpac’ (Rootpac® R), a plum–almond hybrid rootstock for replant situations. Hortscience. 2010;45(2):299–301.
Zhou W, Qin S, Lyu D, Zhang P. Soil sterilisation and plant growth-promoting rhizobacteria promote root respiration and growth of sweet cherry rootstocks. Arch Agron Soil Sci. 2015;61(3):361–70.
Qin SJ, Lü DG, Li ZX, Ma HY, Liu LZ, Liu GC. Effects of water stress on respiration and other physiological metabolisms of Cerasus sachalinensis Kom. Seedlings. Sci Agric Sin. 2011;1(44):201–9.
Qi B, Yang Y, Yin Y, Xu M, Li H. De novo sequencing, assembly, and analysis of the Taxodium 'Zhongshansa' roots and shoots transcriptome in response to short-term waterlogging. BMC Plant Biol. 2014;14:201.
Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LACJ, Perata P, van Dongen JT. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature. 2011;479(7373):419–22.
Caruso P, Baldoni E, Mattana M, Pietro Paolo D, Genga A, Coraggio I, Russo G, Picchi V, Reforgiato Recupero G, Locatelli F. Ectopic expression of a rice transcription factor, Mybleu, enhances tolerance of transgenic plants of Carrizo citrange to low oxygen stress. Plant Cell, Tissue and Organ Culture (PCTOC). 2012;109(2):327–39.
Edwards JM, Roberts TH, Atwell BJ. Quantifying ATP turnover in anoxic coleoptiles of rice (Oryza sativa) demonstrates preferential allocation of energy to protein synthesis. J Exp Bot. 2012;63(12):4389–402.
Kumutha D, Sairam RK, Ezhilmathi K, Chinnusamy V, Meena RC. Effect of waterlogging on carbohydrate metabolism in pigeon pea (Cajanus cajan L.): Upregulation of sucrose synthase and alcohol dehydrogenase. Plant Sci. 2008;175(5):706–16.
Millar AH, Whelan J, Soole KL, Day DA. Organization and regulation of mitochondrial respiration in plants. Annu Rev Plant Biol. 2011;62:79–104.
Christianson JA, Llewellyn DJ, Dennis ES, Wilson IW. Comparisons of early transcriptome responses to low-oxygen environments in three dicotyledonous plant species. Plant Signal Behav. 2010;5(8):1006–9.
Gomathi R, Rao PNG, Chandran K, Selvi A. Adaptive responses of sugarcane to waterlogging stress: an over view. Sugar Tech. 2015;17(4):325–38.
Chang WWP, Huang L, Shen M, Webster C, Burlingame AL, Roberts JKM. Patterns of protein synthesis and tolerance of anoxia in root tips of maize seedlings acclimated to a low-oxygen environment, and identification of proteins by mass spectrometry. Plant Physiol. 2000;122(2):295–318.
Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, Peacock WJ, Dolferus R, Dennis ES. Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell. 2002;14(10):2481–94.
Jain M. Next-generation sequencing technologies for gene expression profiling in plants. Brief Funct Genomic. 2012;11(1):63–70.
Jain M, Srivastava PL, Verma M, Ghangal R, Garg R. De novo transcriptome assembly and comprehensive expression profiling in Crocus sativus to gain insights into apocarotenoid biosynthesis. Sci Rep. 2016;6:22456.
Narsai R, Rocha M, Geigenberger P, Whelan J, van Dongen JT. Comparative analysis between plant species of transcriptional and metabolic responses to hypoxia. New Phytol. 2011;190(2):472–87.
Qi XH, Xu XW, Lin XJ, Zhang WJ, Chen XH. Identification of differentially expressed genes in cucumber (Cucumis sativus L.) root under waterlogging stress by digital gene expression profile. Genomics. 2012;99(3):160–8.
Campbell MT, Proctor CA, Dou Y, Schmitz AJ, Phansak P, Kruger GR, Zhang C, Walia H. Genetic and molecular characterization of submergence response identifies Subtol6 as a major submergence tolerance locus in maize. PLoS One. 2015;10(3):e0120385.
Lee YH, Kim KS, Jang YS, Choi IH. Nitric oxide production and scavenging in waterlogged roots of rape seedlings. Genes & Genomics. 2014;36(6):691–9.
Bouma TJ, Yanai RD, Elkin AD, Hartmond U, Flores-Alva DE, Eissenstat DM. Estimating age-dependent costs and benefits of roots with contrasting life span: comparing apples and oranges. New Phytol. 2001;150(3):685–95.
Yu R, Pan R. Effect of blue light on the respiration of rice (Oryza sativa) seedlings. Chin J Rice Sci. 1996;10(3):159–62.
Yemm EW, Willis AJ. The respiration of barley plants. New Phytol. 1956;55(2):229–52.
Mustroph A, Stock J, Hess N, Aldous S, Dreilich A, Grimm B. Characterization of the Phosphofructokinase gene family in Rice and its expression under oxygen deficiency stress. Front Plant Sci. 2013;4(7):125.
Waters I, Morrell S, Greenway H, Colmer TD. Effects of anoxia on wheat seedlings: II. Influence of O2 supply prior to anoxia on tolerance to anoxia, alcoholic fermentation, and sugar levels. J Exp Bot. 1991;42(11):1437–47.
Hanson AD, Jacobsen JV. Control of lactate dehydrogenase, lactate glycolysis, and alpha -amylase by O2 deficit in barley aleurone layers. Plant Physiol. 1984;75(3):566–72.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1):248–54.
Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Report. 1993;11(2):113–6.
Su X, Li Q, Chen S, Dong C, Hu Y, Yin L, Yang J. Analysis of the transcriptome of Isodon rubescens and key enzymes involved in terpenoid biosynthesis. Biotechnol Biotechnol Equip. 2016;30(3):592–601.
Ma Z, Tan Y, Cui G, Feng Y, Cui Q, Song X. Transcriptome and gene expression analysis of DHA producer Aurantiochytrium under low temperature conditions. Sci Rep. 2015;5:14446.
Wang Y, Xue S, Liu X, Liu H, Hu T, Qiu X, Zhang J, Lei M. Analyses of long non-coding RNA and mRNA profiling using RNA sequencing during the pre-implantation phases in pig endometrium. Sci Rep. 2016;6:20238.
Hu L, Li H, Chen L, Lou Y, Amombo E, Fu J. RNA-seq for gene identification and transcript profiling in relation to root growth of bermudagrass (Cynodon dactylon) under salinity stress. BMC Genomics. 2015;16(1):1–12.
Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11(2):1–12.
Mao X, Cai T, Olyarchuk JG, Wei L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 2005;21(19):3787–93.
Mustroph A, Barding GA Jr, Kaiser KA, Larive CK, Bailey-Serres J. Characterization of distinct root and shoot responses to low-oxygen stress in Arabidopsis with a focus on primary C- and N-metabolism. Plant Cell Environ. 2014;37(10):2366–80.
Sairam RK, Dharmar K, Chinnusamy V, Meena RC. Waterlogging-induced increase in sugar mobilization, fermentation, and related gene expression in the roots of mung bean (Vigna radiata). J Plant Physiol. 2009;166(6):602–16.
Grossman AR, Catalanotti C, Yang W, Dubini A, Magneschi L, Subramanian V, Posewitz MC, Seibert M. Multiple facets of anoxic metabolism and hydrogen production in the unicellular green alga Chlamydomonas reinhardtii. New Phytol. 2011;190(2):279–88.
Juntawong P, Sirikhachornkit A, Pimjan R, Sontirod C, Sangsrakru D, Yoocha T, Tangphatsornruang S, Srinives P. Elucidation of the molecular responses to waterlogging in Jatropha roots by transcriptome profiling. Front Plant Sci. 2014;5:658.
Zhang JY, Huang SN, Mo ZH, Xuan JP, Jia XD, Wang G, Guo ZR. De novo transcriptome sequencing and comparative analysis of differentially expressed genes in kiwifruit under waterlogging stress. Mol Breed. 2015;35(11):1–12.
Liu F, VanToai T, Moy LP, Bock G, Linford LD, Quackenbush J. Global transcription profiling reveals comprehensive insights into hypoxic response in Arabidopsis. Plant Physiol. 2005;137(3):1115–29.
Arismendi MJ, Almada R, Pimentel P, Bastias A, Salvatierra A, Rojas P, Hinrichsen P, Pinto M, Di Genova A, Travisany D, et al. Transcriptome sequencing of Prunus sp. rootstocks roots to identify candidate genes involved in the response to root hypoxia. Tree Genet Genomes. 2015;11(1):1–16.
Brunetti C, Di Ferdinando M, Fini A, Pollastri S, Tattini M. Flavonoids as antioxidants and developmental regulators: relative significance in plants and humans. Int J Mol Sci. 2013;14(2):3540–55.
Sasidharan R, Mustroph A, Boonman A, Akman M, Ammerlaan AM, Breit T, Schranz ME, Voesenek LA, van Tienderen PH. Root transcript profiling of two Rorippa species reveals gene clusters associated with extreme submergence tolerance. Plant Physiol. 2013;163(3):1277–92.
Rocha M, Licausi F, Araujo WL, Nunes-Nesi A, Sodek L, Fernie AR, van Dongen JT. Glycolysis and the Tricarboxylic acid cycle are linked by Alanine Aminotransferase during hypoxia induced by Waterlogging of Lotus japonicus. Plant Physiol. 2010;152(3):1501–13.
Kreuzwieser J, Rennenberg H. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014;37(10):2245–59.
Ismond KP, Dolferus R, De Pauw M, Dennis ES, Good AG. Enhanced low oxygen survival in Arabidopsis through increased metabolic flux in the fermentative pathway. Plant Physiol. 2003;132(3):1292–302.
Kürsteiner O, Dupuis I, Kuhlemeier C. The pyruvate decarboxylase1 gene of arabidopsis is required during anoxia but not other environmental stresses. Plant Physiol. 2003;132(2):968–78.
Baxter Burrell A, Chang R, Springer P, Bailey Serres J. Gene and enhancer trap transposable elements reveal oxygen deprivation-regulated genes and their complex patterns of expression in Arabidopsis. Ann Bot. 2003;91(2):129–41.
Xuewen X, Huihui W, Xiaohua Q, Qiang X, Xuehao C. Waterlogging-induced increase in fermentation and related gene expression in the root of cucumber (Cucumis sativus L.). Sci Hortic. 2014;179:388–95.
Kreuzwieser J, Hauberg J, Howell KA, Carroll A, Rennenberg H, Millar AH, Whelan J. Differential response of gray poplar leaves and roots underpins stress adaptation during hypoxia. Plant Physiol. 2009;149(1):461–73.
Kato-Noguchi H. Pyruvate metabolism in rice coleoptiles under anaerobiosis. Plant Growth Regul. 2006;50(1):41–6.
Takahashi H, Greenway H, Matsumura H, Tsutsumi N, Nakazono M. Rice alcohol dehydrogenase 1 promotes survival and has a major impact on carbohydrate metabolism in the embryo and endosperm when seeds are germinated in partially oxygenated water. Ann Bot. 2014;113(5):851–9.
Mithran M, Paparelli E, Novi G, Perata P, Loreti E. Analysis of the role of the pyruvate decarboxylase gene family in Arabidopsis thaliana under low-oxygen conditions. Plant Biol. 2014;16(1):28–34.
Komatsu S, Sugimoto T, Hoshino T, Nanjo Y, Furukawa K. Identification of flooding stress responsible cascades in root and hypocotyl of soybean using proteome analysis. Amino Acids. 2010;38(3):729–38.
Voesenek LACJ, Bailey-Serres J. Flood adaptive traits and processes: an overview. New Phytol. 2015;206(1):57–73.
Xie LJ, Yu LJ, Chen QF, Wang FZ, Huang L, Xia FN, Zhu TR, Wu JX, Yin J, Liao B, et al. Arabidopsis acyl-CoA-binding protein ACBP3 participates in plant response to hypoxia by modulating very-long-chain fatty acid metabolism. Plant J. 2015;81(1):53–67.
Acknowledgements
We are grateful to Professor Huiyuan Gao in Shandong Agricultural University for his guidance and comment on the manuscript. We also thanks Dr. Jinghong Xuan for kindly providing plant materials and Novogene Bioinformatics Technology Co., Ltd. for their skillful support in RNA-sequencing.
Funding
Funding was provided by The National Natural Science Foundation of China (grant 31,572,077), the Higher School Innovation Team of Liaoning Province (grant LT2014014), the Program for Higher Educational Excellent Talents of Liaoning Province (grant LJQ2014070), and The National Natural Science Foundation of Liaoning Province (grant 201,602,665).
The funding bodies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Availability of data and materials
The data sets supporting the results of this article are included within the manuscript and its additional files. The methods used in this study complied with local regulations as set forth by the Shenyang government. The RNA-seq data has been deposited in Sequence Read Archives Database (https://www.ncbi.nlm.nih.gov/sra/) under accession number SRP108195.
Author information
Authors and Affiliations
Contributions
Q.S.J., L.D.G, and Z.P. conceived and designed the study. Z.P. and J.L.T. performed the experiments. Z.P. wrote the current manuscript. Q.S.J., H.J.L., and Z.P. reviewed and edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Cerasus sachalinensis plants were obtained from Lianshanguang, Benxi, Liaoning Province, China (41°24′N, 124°17′E), with the permission of the assistant station master, Dr. Jinghong Xuan, of Fruit and Silkworm Administrative General Station of Liaoning Province.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1: Figure S1.
Length distribution of assembled unigenes. (TIFF 2478 kb)
Additional file 2: Figure S2.
Length distribution of unigenes and transcript in Cerasus sachalinensis roots. (PPT 109 kb)
Additional file 3: Table S1.
Significantly enriched metabolic pathways at different waterlogging durations. (XLSX 12 kb)
Additional file 4: Table S2.
Differentially expressed genes of glycolysis/gluconeogenesis pathway at different waterlogging durations. (XLSX 97 kb)
Additional file 5: Table S3.
Primers for RT-qPCR of all tested genes. (XLSX 12 kb)
Additional file 6: Figure S3.
Changes in the expression of genes in sucrose and fermentation pathways of C. sachalinensis under waterlogging stress. (PPT 540 kb)
Additional file 7: Figure S4.
Real-time PCR validation of the tested genes expression. (PPT 352 kb)
Additional file 8: Figure S5.
Correlation of gene expression results respectively obtained by two methods (RT-qPCR analysis and RNA-Seq). (TIFF 7672 kb)
Additional file 9: Figure S6.
Scatter plot of KEGG pathway enrichment statistics and the most enrichment pathway at different waterlogging durations. a-f: Top 20 statistics of up-regulated and down regulated pathway enrichment at different waterlogging durations. (PPTX 2794 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, P., Lyu, D., Jia, L. et al. Physiological and de novo transcriptome analysis of the fermentation mechanism of Cerasus sachalinensis roots in response to short-term waterlogging. BMC Genomics 18, 649 (2017). https://doi.org/10.1186/s12864-017-4055-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-017-4055-1