Abstract
Background
Hyaline fibromatosis syndrome (HFS) is a recently introduced alternative term for two disorders that were previously known as juvenile hyaline fibromatosis (JHF) and infantile systemic hyalinosis (ISH). These two variants are secondary to mutations in the anthrax toxin receptor 2 gene (ANTXR2) located on chromosome 4q21. The main clinical features of both entities include papular and/or nodular skin lesions, gingival hyperplasia, joint contractures and osteolytic bone lesions that appear in the first few years of life, and the syndrome typically progresses with the appearance of new lesions.
Methods
We describe five Lebanese patients from one family, aged between 28 and 58 years, and presenting with nodular and papular skin lesions, gingival hyperplasia, joint contractures and bone lesions. Because of the particular clinical features and the absence of a clinical diagnosis, Whole Genome Sequencing (WGS) was carried out on DNA samples from the proband and his parents.
Results
A mutation in ANTXR2 (p. Gly116Val) that yielded a diagnosis of HFS was noted.
Conclusions
The main goal of this paper is to add to the knowledge related to the clinical and radiographic aspects of HFS in adulthood and to show the importance of Next-Generation Sequencing (NGS) techniques in resolving such puzzling cases.
Similar content being viewed by others
Background
Juvenile hyaline fibromatosis (JHF, OMIM # 228600) is a rare inherited autosomal recessive disorder [1] that was first described by McMurray as Molluscom fibrosum [2]. Clinically, it is characterized by skin lesions (nodules and/or pearly papules); gingival hyperplasia; joint contracture; abnormal growth of hyalinized fibrous tissues of the head, neck and extremities; and bone lesions [3]. Affected individuals are usually asymptomatic at birth, the onset of clinical signs occurs between 3 months and 4 years of age [4, 5], and these signs increase in severity with age [6, 7]. Most people with JHF survive until the fourth decade of life [8].
Infantile systemic hyalinosis (ISH, OMIM # 236490), another rarer disorder, shares many similarities with JHF [9, 10]. It is characterized by a more severe presentation than JHF and has an early onset (first weeks or months of life) and symptoms that include failure to thrive, short stature, diffuse thickening of the skin, hyperpigmented plaques over the joints, visceral involvement, persistent diarrhea and recurrent infections, and death usually occurs within the first 2 years of life [11–13].
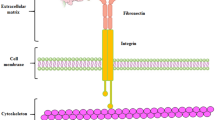
Deleterious mutations of Anthrax toxin receptor 2 gene, (ANTXR2; OMIM # 608041) have been shown to cause both JHF and ISH [14–16]. The presence of a significant overlap at the molecular, histological and clinical levels between JHF and ISH have led to the adoption by Nofal et al. of an unifying taxonomy of “hyaline fibromatosis syndrome or HFS”, signifying that both entities represent the same disorder but with different degrees of severity [10, 17]. ANTXR2 encodes a 55 kDa type I transmembrane (TM) protein which comprises an extracellular N-terminal von Willebrand A (vWA) domain, followed by an immunoglobulin-like domain (Ig-like), a TM domain and a cytosolic tail [18, 19]. This protein is responsible for binding laminin and collagen IV via the vWA domain and the consequent plays a role in basement membrane matrix assembly and endothelial cell morphogenesis [15]. The Ig-like domain contains two disulfide bonds that are essential for proper ANTXR2 localization in the endoplasmic reticulum [18]. The cytosolic tail contains multiple sites for posttranslational modifications such as palmitoylation [20], phosphorylation and ubiquitination [21].
Genotype-phenotype correlation studies have suggested that the mutational spectrum might explain the wide phenotypic variability. Milder phenotypes are associated with in-frame and missense mutations within the cytoplasmic domain, whereas the more severe forms are caused by missense and truncating mutations in the vWA domain and at least one insertion/deletion mutation causing a translational frameshift. However, this correlation is not always constant, thus indicating that modifier genes and/or environmental elements can be involved [15, 22].
Approximately 150 cases of HFS have been reported in the literature [23]. Most of them were diagnosed in early childhood [24], but only a few cases were investigated in adults; the oldest patient was 51 years old [25].
In this paper, we report a large Lebanese family with five HFS patients aged between 28 and 58 years. The oldest patient (58 years) is described here. The aim of this report is to augment the findings related to the clinical, radiographic and differential diagnosis of HFS.
Methods
Clinical report
We identified one Lebanese Shiite family with three branches from a small village in North Lebanon (Fig. 1). All five patients were born to healthy consanguineous couples. The pregnancies were not followed medically but were reported to be without complications. For all patients, the skin eruptions and gingival enlargement were first noticed at the age of 6 months, and the nodules continued to gradually increase in number and size.
At the time of physical examination, patient VI-3 was 42 years old, patient VI-4 was 36 years old, patient VI-5 was 30 years old, patient VI-10 was 28 years old and patient V-15 was 58 years old. Patients VI-3, VI-4, VI-10 and V-15 presented with an important postural deformity, and had been in wheelchairs since they were 10 years old, whereas patient VI-5 had a severe retardation of physical growth and development that caused movement difficulty.
The patients were thin with underdeveloped musculatures. Cognitive development, hearing and eyesight were noted to be normal in all patients.
Each patient was found to have recurrent, painless, variable-sized nodules over the scalp, ear, lobules, postauricular folds, forehead, nose, upper lip, shoulder, elbows, thorax, chest, back, fingers, perianal area, knee and feet. Small, pearly papules were limited to the chin and paranasal folds. The nose and ears were deformed and bulbous, secondary to numerous tumors. All patients had severe gingival hypertrophy covering the teeth completely. Patients VI-10 and V-15 had flexion contractures of the elbows, and fingers and hips and knees, which resulted in a frog leg position (Fig. 2). Swellings and deformities in the feet, especially in the terminal phalanges of the toes, were also noted. The toenails were thickened.
Hematological and biochemical investigations were within normal limits. Only, patient VI-3 reported having persistent diarrhea since the age of 2 years. The clinical features of the present cases and of ISH and JHF, on the basis of the work of Urbina et al.[10] are shown in Table 1.
A skeletal X-ray of patient VI-10 showed subcutaneous soft tissue calcifications in the pinna of both ears and in the parietal region of the scalp, radial bone bowing, thoraco-lumbar scoliosis with paravertebral calcifications at T10, T11 and T12 levels, deformity of the iliac bones, thinned pubic rami, severe narrowing of the hip joints, acetabular protrusion, erosion of joint spaces, coxo-femoral ankylosis, thinned fibula, amyotrophy and cutaneous calcifications (Fig. 3).
X-rays of patient VI-10 showing (a) radial bone bowing and thin diaphyses, (b) deformity of the iliac bones, thinned pubic rami, severe narrowing of the hip joints, acetabular protrusion, erosion of joint spaces, coxo-femoral ankylosis, thinned fibula, amyotrophy and cutaneous calcifications, (c) thoraco-lumbar scoliosis with paravertebral calcifications at T10, T11 and T12 levels and (d) subcutaneous soft tissue calcifications in the pinna of both ears and in the parietal region of the scalp
The patients refused biopsies of their lesions.
DNA extraction and Whole Genome Sequencing (WGS)
Genomic DNA was extracted from peripheral blood samples by standard salt-precipitation methods [26]. Whole genome sequencing was carried out on the DNA of patient VI-4 and his parents with a HiSeq 2500 sequencer (Illumina, San Diego, CA, USA) at Sidra Medical and Research Center - Qatar. Genomic libraries were generated from 1 μg of genomic DNA using an Illumina TruSeq DNA PCR-Free Sample Preparation Kit. Genomic DNA was sheared using a Covaris system (Woburn, MA, USA). Isolated DNA fragment ends were blunted, A-tailed and ligated with sequencing adaptors with index sequences. Excess adapters and enzymes were removed using AMPure beads (Beckman Coulter Genomics, Danvers, MA, USA). Indexed libraries were size-selected to the 350 bp range using bead-based capture, and the concentration of amplifiable fragments was determined by qPCR, relative to sequencing libraries with a known concentration. Normalized libraries were clustered on a c-BOT machine, and 125 bp paired-end sequencing was performed on the HiSeq 2500 system.
WGS data analyses
Raw data were mapped to the human genome reference, build 19 (http://www.broadinstitute.org/ftp/pub/seq/references/Homo_sapiens_assembly19.fasta), using BWA aligner [27] version 0.7.7-r441, and variant calling was performed using GATK [28] version 3.3.2. Rare variant analysis was performed using the xbrowse tool (https://xbrowse.broadinstitute.org/). For the trio, the model of inheritance “autosomal recessive” was selected, with the severity of the variant effect set to ‘moderate to high impact’ (Nonsense, essential splice sites, missense frameshift and in frame), call quality as high (genotype quality > 20 and allele balance ratio > 25%) and allele frequency < 1% in 1000 genomes and The Exome Aggregation Consortium (ExAC) v0.3 datasets. The functional consequences of amino acid substitutions were predicted using various tools [29–32].
Sanger sequencing
Genomic sequence of ANTXR2 (NM_058172.5) was obtained from UCSC Genomic Browser on Human. Primers used for PCR amplification were designed using Primer3 software (http://frodo.wi.mit.edu) to amplify the region surrounding the mutation detected by WGS in exon 4. PCR reactions were performed using Taq DNA polymerase (Invitrogen Life Technologies, Carlsbad, CA, USA). PCR fragments were run on 1% agarose gel. The fragments were purified using « SIGMA-ALDRICH TM » kit and then sequenced using the Big Dye_ Terminator v 1.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). Sequence reaction was purified on Sephadex G50 (Amersham Pharmacia Biotech, Foster City, CA) and then loaded into an ABI 3100 system after the addition of Hidi formamide. Electropherograms were analyzed using Sequence Analysis Software version 5.2 (Applied Biosystems) and then aligned with the reference sequences using ChromasPro v1.7.6.1 (Technelysium, Queensland, Australia).
Results
WGS identified 2 mutations in ANTXR2 (NM_058172.5: c.347G > T) and in zinc finger protein 618 (ZNF618) (NM_133374.2: c.832G > T). The ANTXR2 mutation results in a substitution of glycine by valine, and the zinc finger protein 618 (ZNF618) mutation leads to a premature stop codon. Both had damaging effects, according to the majority of the effect predictors tested (Table 2).
Sanger sequencing confirmed the segregation of the c.347G > T mutation in ANTXR2 with the disease within the family (Fig. 1). The mutation was homozygous in the affected patients, heterozygous in the parents and heterozygous or not found in the unaffected siblings in this family.
Discussion
Here, we report five adult patients from a consanguineous Lebanese family, who presented with nodular skin lesions, gingival hyperplasia, joint contractures and bone lesions. By WGS, we identified 2 mutations: a mutation in ZNF618 (c.832G > T) and a mutation in ANTXR2 (c.347G > T).
ZNF618, also known as KIAA1952 or NEDD10, is a protein-coding gene located on chromosome 9q32 and is implicated in transcriptional regulation. Association studies have demonstrated that ZNF618 may be involved in the occurrence of cleft lip [33], high blood pressure [34], kidney diseases [35] and, in women, in brachial-ankle pulse wave velocity and arterial stiffness [36, 37]. On the basis of these clinical characteristics, we excluded this gene as a candidate gene.
ANTXR2, also called the capillary morphogenesis protein gene-2 (CMG2) is located on chromosome 4q21 and is implicated in basement membrane matrix and cell morphogenesis [15]. Mutations of this gene have been found to be responsible for HFS. After reviewing the clinical features of our patients, we considered ANTXR2 to be a candidate gene responsible for the phenotype of the patients studied here.
A clinical diagnosis of HFS was missed because of the advanced age and status of the patients, the stage of the disease, the severity of the clinical manifestations, and incomplete knowledge of the syndrome’s pathogenesis. The patients reported here had undergone multiple surgeries in infancy for the resection of cutaneous nodules, but long-term regression was unlikely, and the tumors continued to increase in size and number. Their parents stopped treating the lesions, and no follow-up was performed for economic reasons. Biopsies were refused by the patients for many reasons, including pain and the absence of treatment. WGS allowed us to diagnose the disease, assess the genotype-phenotype correlations and offer genetic counseling and prenatal diagnosis to the people of the village.
Classification of HFS
HFS and inherited systemic hyalinosis represent the same disorder, comprising two variants with severe (ISH) and mild (JHF) forms of the disease.
Gilaberte et al. [6] have proposed 2 major and 3 minor diagnostic criteria for JHF. The major criteria are cutaneous lesions (including nodules, tumors and plaques) and gingival enlargement. The minor criteria include joint contractures, osteolytic lesions and/or cortical erosions, and a family history of JHF. In fact, the presence of persistent diarrhea, hyperpigmented plaques, growth retardation and death within the first 2 years of life are more consistent with ISH. The severity and progression of HFS vary among patients, and hence it is difficult to classify a patient into a single class because many cases of JHF are incorrectly identified as ISH, or vice versa and mutations in the same gene underlie both syndromes. Indeed, Bedford et al. [38] have described a severe form of JHF, with persistent or repeated episodes of diarrhea and death occurring in early infancy after several infections yet with no subcutaneous nodules. Hatamochi et al. [39] have reported a 6-year-old girl who was diagnosed with a severe form of JHF and presented with confluent papules and nodules, recurrent respiratory tract infections and chronic diarrhea since birth. Dhingra et al. [40] have reported a 3-year-old girl who presented with recurrent episodes of diarrhea and was diagnosed as having a case of JHF. ISH patients with atypical prolonged survival have also been reported [41]. Kawasaki et al. [42] have reported an elderly woman with JHF who died from aspiration pneumonia. For these reasons, we prefer to classify our patients as having HFS, which includes both disorders, as proposed by Nofal et al. [17].
In contrast, Nofal et al. [17] have classified HFS into three grades according to the severity of organ involvement: G1: mild, G2: moderate and G3: severe. On the basis of this gradation, the mild type presents with only skin involvement and gingival hypertrophy, the moderate type shows additional joint contractures and bone lesions, and the severe type has manifestations resulting from organ involvement, such as persistent diarrhea and recurrent pulmonary infections. Denadai et al. [22] have added a new lethal grade (G4) for patients with organ failure and/or septicemia. In the family studied here, patients VI-4, VI-5, VI-10 and V-15 can be classified as JHF grade 2 and patient VI-3 as ISH grade 3, thus demonstrating the difficulty of clearly differentiating these subclasses.
Prevalence
HFS is a rare genetic disorder, but it has been documented in families of different ethnic backgrounds on several continents [11]. The life expectancy of patients with HFS syndrome varies from early death in childhood to normal survivorship. The oldest known patient (58 years) is reported here.
Diagnosis
The diagnosis of this syndrome is based on the clinical features and/or the presence of a molecular diagnosis.
Clinical diagnosis
The clinical features associated with HFS syndrome consist of multiple subcutaneous skin nodules/papules, gingival hypertrophy and joint contractures and may be accompanied by systemic symptoms.
The specific pathogenesis of HFS also remains unclear, but some authors have suggested that it results from an abnormality in type IV or VI (α1, α2 and α3 chains) collagen [3, 43] or defective glycosaminoglycan formation [44, 45].
Skeletal radiographs in adults
To our knowledge, 33 cases of HFS have been reported in adults, of which 14 with X-ray findings. In 13 of these 14 cases, joint contractures, osteolytic destruction of the skull, of the large joints, of the long bones and of the extremities, triangular carpal bones and an isolated cortical erosion of mandibular bone and calcifications in the subcutaneous tumors were noted [4, 8, 16, 22, 25, 42, 46–52]. In one patient, no calcifications or bone involvement were noted on radiography [53].
Magnetic resonance imaging (MRI) of HFS lesions has rarely been described in adults and shows a hypointense, central, radiating scar and heterogeneous signal intensity. After the administration of a gadolinium contrast medium, the lesion showed diffuse enhancement, with the exception of the central scar and discrete enhancement of subcutaneous masses in contrasted phases [8, 51, 53].
Computed tomography (CT) of the head has demonstrated a normal aspect [51] or an abnormal bucco-lingual expansion with lingual cortical erosion [46], calcifications within the subcutaneous tumors, and a soft tissue mass extending from the hard palate into the nasal cavity and maxillary sinus [42]. Enhanced CT has revealed dye uptake in the submandibular and cervical lymph nodes bilaterally [42]. Brain CT has shown small ischemic regions in the right periventricular aspect, mild brain atrophy and extracranial tumor masses in the soft tissues of the right peritemporal and occipital aspects [47].
Histopathology
The histopathologic features of this disease include a normal epidermis with few inflammatory cells in the dermis and minor pigmentary incontinence. Deposits of an amorphous, homogeneous and eosinophilic, hyaline substance (periodic acid–Schiff positive), can be found in the papillary and reticular dermis, accompanied by a proliferation of spindle cells without atypia [10, 22].
Electron microscopic studies have shown stromal deposits of a fibrillogranular material focally displaying a banding pattern similar to that of type VI collagen and fibroblasts with prominent Golgi complexes, dilated endoplasmic reticulum, multi vesicular bodies and vesicles filled with a fibrillogranular material [3, 10, 43]. Calcospherules, defined as calcium-containing lamellar body have been described in JHF by Ko and Barr in 2003 [54].
Intestinal biopsy and imaging
The results of biopsy cases from patients with gastrointestinal signs include villous atrophy, edema, lymphangiectasia and hyalinosis. Rapid transit time has been described in real-time upper-gastrointestinal imaging investigations [55].
Immune system deficit
Deficits of the humoral and cellular branches of the immune system have been observed [56].
Laboratory studies
Laboratory examination may demonstrate a normal [22, 42, 57, 58] or abnormal aspect, such as an elevation of the Erythrocyte Sedimentation Rate (ESR) [47, 51, 59], thrombocytosis [60], mild anemia [4, 47, 51], or an elevation of serum albumin [61] or alkaline phosphatase [62].
Molecular diagnosis
ANTXR2 is the only gene in which pathogenic variants are known to cause HFS. Mutations of this gene disrupt the formation of basement membranes. This disruption may allow the hyaline material to leak from plasma components through the basement membrane into the perivascular space, thus explaining the histological features of HFS [15].
As shown in Table 3, 41 different ANTXR2 mutations have been described. Yan et al. [63] have reported that three frameshift mutations (c.1073-1074insC, c.1073-1074insCC and c.1074delT) represent approximately 60% of all pathogenic alleles. The incidence of insertions and deletions at positions 1073–1074 is probably due to its proximity to a low-complexity, GC-rich region encoding a stretch of proline residues that may constitute a vulnerable site for errors during DNA replication. The mutation p. Gly116Val identified in all patients in this study has previously been reported by Tümer et al. [64] in an 11-month-old Turkish girl with HFS. This mutation is located in the vWA domain and may damage ligand binding, not plasma membrane targeting, thus causing a severe manifestation of HFS. A comparison between the clinical signs of the patients in this study and the girl with the same mutation shows some differences: the girl presented with short stature and gingival hypertrophy and developed recurrent infections. She did not present with any visceral involvement. X-ray images did not show any osteolytic lesions [64]. These differences may be explained by the differences in age, and/or environmental factors.
Mode of inheritance and genetic counseling
An autosomal recessive mode of inheritance has been established for HFS. Therefore, the risk for a parental carrier to have an affected offspring is 25%.
Treatment and follow-up
Currently, only symptomatic treatments for HFS are available. Early surgical excision of the lesions is recommended for functional and cosmetic improvement [9, 52]. However, the lesions may recur and new lesions may appear [4, 52, 65, 66]. Intralesional steroid injections have been suggested because they can reduce the size of early lesions [9]. Capsulotomy, physiotherapy, treatment with cortisone and adrenocorticotropin (ACTH) have found modest success in the treatment of joint contractures [67]. Radiotherapy is not effective [10, 68]. Oral D-penicillamine may improve joint mobility and flexibility [65, 69]. Nonsteroidal anti-inflammatory drugs and opiates may be used to control pain and improve the quality of life [9, 70]. Gingival hyperplasia requires special dental care and many dental consultations to promote strict oral hygiene [71]. Gingivectomy may improve the quality of oral hygiene and nourishment by improving mastication and preventing gingival blood loss [72, 73]. Ablative laser surgery may be a reasonable choice and may be useful, at least as an adjunctive treatment [74]. In context of genetic therapies for this debilitating disorder, Deuquet et al. [18] have revealed that proteasome inhibitors may be potential therapeutic drugs for HFS patients with mutations in the ectodomain of ANTXR2.
Conclusions
We report a Lebanese family with five adult patients with HFS. WGS showed an ability to establish a diagnosis in such puzzling cases in which the clinical signs are atypical or very severe for the classical phenotype. HFS is still a poorly understood disease with a severity that ranges from being lethal during early childhood to chronic at a later age. More studies to find an effective treatment are essential. An accurate diagnosis of the disease requires an exhaustive analysis of the radiologic and histopathological clinical findings, and genetic studies are required for family planning and counseling.
Abbreviations
- ACTH:
-
Adrenocorticotropin
- ANTXR2 :
-
Anthrax toxin receptor 2 gene
- CMG2 :
-
Capillary morphogenesis protein gene-2
- CT:
-
Computed tomography
- ESR:
-
Erythrocyte Sedimentation Rate
- ExAC:
-
Exome Aggregation Consortium
- HFS:
-
Hyaline fibromatosis syndrome
- Ig-like:
-
Immunoglobulin–like domain
- ISH:
-
Infantile systemic hyalinosis
- JHF:
-
Juvenile hyaline fibromatosis
- MRI:
-
Magnetic resonance imaging
- NGS:
-
Next-Generation-Sequencing
- TM:
-
Transmembrane
- vWA:
-
von Willebrand A
- WGS:
-
Whole Genome Sequencing
- ZNF618 :
-
Zinc finger protein 618
References
Landing BH, Nadorra R. Infantile systemic hyalinosis: report of four cases of a disease, fatal in infancy, apparently different from juvenile systemic hyalinosis. Pediatr Pathol Affil Int Paediatr Pathol Assoc. 1986;6:55–79.
Murray J. On three peculiar cases of Molluscum Fibrosum in Children in which one or more of the following conditions were observed: hypertrophy of the gums, enlargement of the ends of the fingers and toes, numerous connecive-tissue tumours on the scalp, & c. Med Chir Trans. 1873;56:235–254.1.
Larralde M, Santos-Muñoz A, Calb I, Magariños C. Juvenile hyaline fibromatosis. Pediatr Dermatol. 2001;18:400–2.
Muniz ML, Lobo AZC, da MR MMC, Valente NYS, Kim CA, Lourenço SV, et al. Exuberant juvenile hyaline fibromatosis in two patients. Pediatr Dermatol. 2006;23:458–64.
Yesudian P, Janaki VR, Thambiah AS. Juvenile hyaline fibromatosis. Int J Dermatol. 1984;23:619–20.
Gilaberte Y, González-Mediero I, López Barrantes V, Zambrano A. Juvenile hyaline fibromatosis with skull-encephalic anomalies: a case report and review of the literature. Dermatology. 1993;187:144–8.
Rashmi MV, Geetha JP, Srinivas Arava NM, Kodandaswamy CR. Juvenile Hyaline Fibromatosis (JHF): A Rare Case with Recurrence. J Clin Diagn Res. 2014;8:161–2.
Slimani S, Haddouche A, Haid S, Ladjouze-Rezig A. Juvenile hyaline fibromatosis: focus on radiographic features in adulthood. Rheumatol Int. 2011;31:273–6.
Jaouad IC, Guaoua S, Hajjioui A, Sefiani A. Hyaline fibromatosis syndrome with mutation c.1074delT of the CMG2 gene: a case report. J Med Case Reports. 2014;8:291.
Urbina F, Sazunic I, Murray G. Infantile systemic hyalinosis or juvenile hyaline fibromatosis? Pediatr Dermatol. 2004;21:154–9.
Félix TM, Puga ACS, Cestari T, Cartell A, Cerski M. Infantile Systemic Hyalinosis: report of three unrelated Brazilian children and review of the literature. Clin Dysmorphol. 2004;13:231–6.
Mancini GM, Stojanov L, Willemsen R, Kleijer WJ, Huijmans JG, van Diggelen OP, et al. Juvenile hyaline fibromatosis: clinical heterogeneity in three patients. Dermatol Basel Switz. 1999;198:18–25.
Mendonça JA, Marini R, Schincariol NB, Laurindo IMM, Appenzeller S. Ultrasound findings in infantile systemic hyalinosis. Rheumatol Int. 2011;31:1393–5.
Dowling O, Difeo A, Ramirez MC, Tukel T, Narla G, Bonafe L, et al. Mutations in capillary morphogenesis gene-2 result in the allelic disorders juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet. 2003;73:957–66.
Hanks S, Adams S, Douglas J, Arbour L, Atherton DJ, Balci S, et al. Mutations in the gene encoding capillary morphogenesis protein 2 cause juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet. 2003;73:791–800.
Rahman N, Dunstan M, Teare MD, Hanks S, Edkins SJ, Hughes J, et al. The gene for juvenile hyaline fibromatosis maps to chromosome 4q21. Am J Hum Genet. 2002;71:975–80.
Nofal A, Sanad M, Assaf M, Nofal E, Nassar A, Almokadem S, et al. Juvenile hyaline fibromatosis and infantile systemic hyalinosis: a unifying term and a proposed grading system. J Am Acad Dermatol. 2009;61:695–700.
Deuquet J, Lausch E, Guex N, Abrami L, Salvi S, Lakkaraju A, et al. Hyaline fibromatosis syndrome inducing mutations in the ectodomain of anthrax toxin receptor 2 can be rescued by proteasome inhibitors. EMBO Mol Med. 2011;3:208–21.
Deuquet J, Lausch E, Superti-Furga A, van der Goot FG. The dark sides of capillary morphogenesis gene 2. EMBO J. 2012;31:3–13.
Abrami L, Leppla SH, van der Goot FG. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J Cell Biol. 2006;172:309–20.
Abrami L, Kunz B, van der Goot FG. Anthrax toxin triggers the activation of src-like kinases to mediate its own uptake. Proc Natl Acad Sci U S A. 2010;107:1420–4.
Denadai R, Raposo-Amaral CE, Bertola D, Kim C, Alonso N, Hart T, et al. Identification of 2 novel ANTXR2 mutations in patients with hyaline fibromatosis syndrome and proposal of a modified grading system. Am J Med Genet A. 2012;158A:732–42.
Rangel Rivera DA, Mendoza Rojas VC, Uribe Pérez CJ, Contreras-García GA. Hyaline fibromatosis syndrome: case report of two siblings. Arch Argent Pediatría. 2015;113:e264–267.
Bernárdez C, Martinez Barba E, Kutzner H, Requena L. A mild case of hyaline fibromatosis syndrome, presenting in an adult. J Eur Acad Dermatol Venereol. 2015;30:902–4.
Camarasa JG, Moreno A. Juvenile hyaline fibromatosis. J Am Acad Dermatol. 1987;16:881–3.
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60.
DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–8.
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9.
Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–81.
Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575–6.
Shihab HA, Gough J, Cooper DN, Day INM, Gaunt TR. Predicting the functional consequences of cancer-associated amino acid substitutions. Bioinforma Oxf Engl. 2013;29:1504–10.
Letra A, Menezes R, Govil M, Fonseca RF, McHenry T, Granjeiro JM, et al. Follow-up association studies of chromosome region 9q and nonsyndromic cleft lip/palate. Am J Med Genet A. 2010;152A:1701–10.
Levy D, Larson MG, Benjamin EJ, Newton-Cheh C, Wang TJ, Hwang S-J, et al. Framingham Heart Study 100K Project: genome-wide associations for blood pressure and arterial stiffness. BMC Med Genet. 2007;8 Suppl 1:S3.
Yoshida T, Kato K, Yokoi K, Oguri M, Watanabe S, Metoki N, et al. Association of gene polymorphisms with chronic kidney disease in Japanese individuals. Int J Mol Med. 2009;24:539–47.
Choi S, Jung S, Kim MK, Shin J, Shin M-H, Shin DH, et al. Gene and dietary calcium interaction effects on brachial-ankle pulse wave velocity. Clin Nutr Edinb Scotl. 2015;29:1504–10.
Park CY, Jung S, Kim MK, Choi BY, Shin M-H, Shin DH, et al. Habitual dietary intake of β-carotene, vitamin C, folate, or vitamin E may interact with single nucleotide polymorphisms on brachial-ankle pulse wave velocity in healthy adults. Eur J Nutr. 2015;55:855–66.
Bedford CD, Sills JA, Sommelet-Olive D, Boman F, Beltramo F, Cornu G. Juvenile hyaline fibromatosis: a report of two severe cases. J Pediatr. 1991;119:404–10.
Hatamochi A, Sasaki T, Kawaguchi T, Suzuki H, Yamazaki S. A novel point mutation in the gene encoding capillary morphogenesis protein 2 in a Japanese patient with juvenile hyaline fibromatosis. Br J Dermatol. 2007;157:1037–9.
Dhingra M, Amladi S, Savant S, Nayak C. Juvenile hyaline fibromatosis and infantile systemic hyalinosis: divergent expressions of the same genetic defect? Indian J Dermatol Venereol Leprol. 2008;74:371–4.
Thauvin-Robinet C, Faivre L, Beer F, Justrabo E, Nivelon-Chevallier A, Huer F. Infantile systemic hyalinosis: a case with atypical prolonged survival. Acta Paediatr. 2001;90:705–6.
Kawasaki G, Yanamoto S, Mizuno A, Fujita S. Juvenile hyaline fibromatosis complicated with oral squamous cell carcinoma: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:200–4.
Glover MT, Lake BD, Atherton DJ. Infantile systemic hyalinosis: newly recognized disorder of collagen? Pediatrics. 1991;87:228–34.
Kitano Y, Horiki M, Aoki T, Sagami S. Two cases of juvenile hyalin fibromatosis. Some histological, electron microscopic, and tissue culture observations. Arch Dermatol. 1972;106:877–83.
Malathi BG, Prabha CV, Padma SR, Muley PR, Jeyachandran P. Juvenile hyaline fibromatosis--a rare case report. Indian J Pathol Microbiol. 2006;49:257–9.
Altuğ HA, Günal A, Günhan O, Sençimen M. Juvenile hyaline fibromatosis of the mandible with bone involvement: report of a rare case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e59–63.
Fetisovova Z, Adamicova K, Pec M, Mellova Y, Chromej I, Pec J. Dermatological findings in an adult patient with juvenile hyaline fibromatosis. J Eur Acad Dermatol Venereol. 2003;17:473–6.
Hallock GG. Juvenile hyaline fibromatosis of the hand in an adult. J Hand Surg. 1993;18:614–7.
Hallock GG. An adult with juvenile hyaline fibromatosis of the foot. Foot Ankle Int. 1994;15:634–7.
Horiuchi Y, Mukosaka K, Fujimaki T. Systemic hyalinosis of delayed onset. Dermatology. 1999;198:83–5.
Keser G, Karabulut B, Oksel F, Calli C, Ustün EE, Akalin T, et al. Two siblings with juvenile hyaline fibromatosis: case reports and review of the literature. Clin Rheumatol. 1999;18:248–52.
Quintal D, Jackson R. Juvenile hyaline fibromatosis. A 15-year follow-up. Arch Dermatol. 1985;121:1062–3.
Van Raak SM, Meuffels DE, Van Leenders GJLH, Oei EHG. Hyaline fibromatosis of Hoffa’s fat pad in a patient with a mild type of hyaline fibromatosis syndrome. Skeletal Radiol. 2014;43:531–4.
Ko CJ, Barr RJ. Calcospherules associated with juvenile hyaline fibromatosis. Am J Dermatopathol. 2003;25:53–6.
Shieh JT, Hoyme HE, Arbour LT, et al. Hyalinosis, Inherited Systemic. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJ, et al., editors. GeneReviews (®). Seattle: University of Washington, Seattle; 1993. [cited 2016 Mar 12]. Available from:http://www.ncbi.nlm.nih.gov/books/NBK1525/.
Klebanova Y, Schwindt C. Infantile Systemic Hyalinosis. A Case Report of Compromised Cellular and Humoral Branches of the Immune System Leading to Infections. Pediatr Asthma Allergy Immunol. 2009;22:127–30.
Lim AAT, Kozakewich HPW, Feingold M, Padwa BL. Juvenile hyaline fibromatosis: report of a case and comparison with infantile systemic hyalinosis. J Oral Maxillofac Surg. 2005;63:271–4.
Narayanan DL, Phadke SR. Infantile Systemic Hyalinosis with Mutation in ANTXR2. Indian J Pediatr. 2016. doi: 10.1007/s12098-015-1990-1.
Ribeiro SL, Guedes EL, Botan V, Barbosa A, Freitas EJ. Juvenile hyaline fibromatosis: a case report and review of literature. Acta Reumatol Port. 2009;34:128–33.
Lee JY-Y, Tsai Y-M, Chao S-C, Tu Y-F. Capillary morphogenesis gene-2 mutation in infantile systemic hyalinosis: ultrastructural study and mutation analysis in a Taiwanese infant. Clin Exp Dermatol. 2005;30:176–9.
Mohamed S, Ahmed W, Al-Jurayyan N, Faqeih E, Al-Nemri A, Al-Ghamdi M. Infantile Systemic Hyalinosis Complicated with Right Atrial Thrombus and Pericardial Effusion in an Infant. Pediatr Neonatol. 2014. doi: 10.1016/j.pedneo.2014.01.008.
Uslu H, Bal N, Guzeldemir E, Pektas ZO. Three siblings with juvenile hyaline fibromatosis. J Oral Pathol Med. 2007;36:123–5.
Yan SE, Lemmin T, Salvi S, Lausch E, Superti-Furga A, Rokicki D, et al. In-depth analysis of hyaline fibromatosis syndrome frameshift mutations at the same site reveal the necessity of personalized therapy. Hum Mutat. 2013;34:1005–17.
Tümer L, Kasapkara C, Fong K, Serdaroğlu A, McGrath JA. Hyaline fibromatosis syndrome resulting from a new homozygous missense mutation, p.Gly116Val, in ANTXR2. J Dermatol. 2013;40:677–8.
El-Maaytah M, Jerjes W, Shah P, Upile T, Murphy C, Ayliffe P. Gingival hyperplasia associated with juvenile hyaline fibromatosis: a case report and review of the literature. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 2010;68:2604–8.
Finlay AY, Ferguson SD, Holt PJ. Juvenile hyaline fibromatosis. Br J Dermatol. 1983;108:609–16.
Thomas JE, Moossavi M, Mehregan DR, McFalda WL, Mahon MJ. Juvenile hyaline fibromatosis: a case report and review of the literature. Int J Dermatol. 2004;43:785–9.
Puretic S, Puretic B, Fiser-Herman M, Adamcic M. A unique form of mesenchymal dysplasia. Br J Dermatol. 1962;74:8–19.
Glover MT, Lake BD, Atherton DJ. Clinical, histologic, and ultrastructural findings in two cases of infantile systemic hyalinosis. Pediatr Dermatol. 1992;9:255–8.
Anadolu RY, Oskay T, Ozsoy N, Erdem C. Juvenile non-hyaline fibromatosis: juvenile hyaline fibromatosis without prominent hyaline changes. J Cutan Pathol. 2005;32:235–9.
Çam B, Kurkcu M, Ozturan S, Haytac C, Uguz A, Ogden G. Juvenile hyaline fibromatosis: a case report follow-up after 3 years and a review of the literature. Int J Dermatol. 2015;54:217–21.
Hakki SS, Ataoglu T, Avunduk MC, Erdemli E, Gunhan O, Rahman N. Periodontal treatment of two siblings with juvenile hyaline fibromatosis. J Clin Periodontol. 2005;32:1016–21.
Krasuska-Sławińska E, Polnik D, Rokicki D, Koeber B. Treatment of Massive Labial and Gingival Hypertrophy in a Patient With Infantile Systemic Hyalinosis-A Case Report. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 1962;2015(73):e1–5.
Çalişkan E, Şençimen M. Comment on “Treatment of Massive Labial and Gingival Hypertrophy in a Patient With Infantile Systemic Hyalinosis-A Case Report.”. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 2015;74:426.
Antaya RJ, Cajaiba MM, Madri J, Lopez MA, Ramirez MCM, Martignetti JA, et al. Juvenile hyaline fibromatosis and infantile systemic hyalinosis overlap associated with a novel mutation in capillary morphogenesis protein-2 gene. Am J Dermatopathol. 2007;29:99–103.
Fong K, Rama Devi AR, Lai-Cheong JE, Chirla D, Panda SK, Liu L, et al. Infantile systemic hyalinosis associated with a putative splice-site mutation in the ANTXR2 gene. Clin Exp Dermatol. 2012;37:635–8.
Koonuru MK, Venugopal SP. Infantile systemic hyalinosis in identical twins. Intractable Rare Dis Res. 2015;4:210–13.
Aggarwal MLS, Chilakamarri V, Chennuri VS, Karra M. Identical Twins with Infantile Systemic Hyalinosis: Case study and review of literature. J Orthop Case Rep. 2016;6:69–71.
Huang Y-C, Xiao Y-Y, Zheng Y-H, Jang W, Yang Y-L, Zhu X-J. Infantile systemic hyalinosis: a case report and mutation analysis in a Chinese infant. Br J Dermatol. 2007;156:602–4.
Lindvall LE, Kormeili T, Chen E, Ramirez MCM, Grum-Tokars V, Glucksman MJ, et al. Infantile systemic hyalinosis: Case report and review of the literature. J Am Acad Dermatol. 2008;58:303–7.
Al Sinani S, Al Murshedy F, Abdwani R. Infantile systemic hyalinosis: a case report with a novel mutation. Oman Med J. 2013;28:53–5.
Youssefian L, Vahidnezhad H, Aghighi Y, Ziaee V, Zeinali S, Abiri M, et al. Hyaline Fibromatosis Syndrome: A Novel Mutation and Recurrent Founder Mutation in the CMG2/ANTXR2 Gene. Acta Derm Venereol. 2016. doi: 10.2340/00015555-2459.
Rahvar M, Teng J, Kim J. Systemic Hyalinosis With Heterozygous CMG2 Mutations: A Case Report and Review of Literature. Am J Dermatopathol. 2016;38:e60–3.
Shieh JTC, Swidler P, Martignetti JA, Ramirez MCM, Balboni I, Kaplan J, et al. Systemic hyalinosis: a distinctive early childhood-onset disorder characterized by mutations in the anthrax toxin receptor 2 gene (ANTRX2). Pediatrics. 2006;118:e1485–1492.
Sugiura K, Ohno A, Kono M, Kitoh H, Itomi K, Akiyama M. Hyperpigmentation over the metacarpophalangeal joints and the malleoli in a case of hyaline fibromatosis syndrome with ANTXR2 mutations. J Eur Acad Dermatol Venereol. 2015. doi:10.1111/jdv.13290.
Vahidnezhad H, Ziaee V, Youssefian L, Li Q, Sotoudeh S, Uitto J. Infantile systemic hyalinosis in an Iranian family with a mutation in the CMG2/ANTXR2 gene. Clin Exp Dermatol. 2015;40:636–9.
El-Kamah GY, Fong K, El-Ruby M, Afifi HH, Clements SE, Lai-Cheong JE, et al. Spectrum of mutations in the ANTXR2 (CMG2) gene in infantile systemic hyalinosis and juvenile hyaline fibromatosis. Br J Dermatol. 2010;163:213–5.
Mallet S, Boye T, Hesse S, Fournier B, Guennoc B, Carsuzaa F. Juvenile hyaline fibromatosis. Ann Dermatol Vénéréologie. 2010;137:364–8.
Wang Y-Y, Wen C-Q, Wei Z, Jin X. A novel splice site mutation in ANTXR2 (CMG2) gene results in systemic hyalinosis. J Pediatr Hematol Oncol. 2011;33:e355–357.
Acknowledgements
We express our deepest gratitude and sympathy to the family for their full cooperation throughout the study.
Funding
This study was supported by the “Conseil de Recherche de l’Université Saint-Joseph.” The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
Data from this study are freely available and can be obtained by contacting the corresponding author.
Author’s contributions
ZH wrote the manuscript. AM, ZH, EC and NJ carried out the clinical genetic diagnosis of the patients. WH and AM executed the clinical diagnosis of the patients. WL and EW performed WGS. FM made substantial contribution to conception, design and analysis of data. RA, RT and PJ performed bioinformatics data analysis and validation. AM, EC and LC supervised the study and reviewed the paper. SH performed the clinical radiologic diagnoses. All the authors made intellectual contributions and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from legally authorized representatives of the patient (parental consent) to participate in this study and its publication and any accompanying images. The patients were not able to sign by themselves due to the presence of variable-sized nodules over the fingers. Only patient V-15 signed the consent using a stick.
Ethics approval and consent to participate
This study was granted approval from the Saint Joseph University of Beirut Committee on Clinical Investigation and conformed to the tenets of the Declaration of Helsinki. Approval for the study, publication of photographs and informed written consent were obtained from legally authorized patient representatives.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article has been updated to correct the spelling of one of the authors names. Puthen Jitesh has been changed to Puthen Jithesh.
An erratum to this article is available at http://dx.doi.org/10.1186/s12863-017-0480-z.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Haidar, Z., Temanni, R., Chouery, E. et al. Diagnosis implications of the whole genome sequencing in a large Lebanese family with hyaline fibromatosis syndrome. BMC Genet 18, 3 (2017). https://doi.org/10.1186/s12863-017-0471-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12863-017-0471-0