Abstract
Background
Rodents form the largest order among mammals in terms of species diversity, and home range is the area where an individual normally moves during its normal daily activities. Information about rodent home ranges is paramount in the development of effective conservation and management strategies. This is because rodent home range varies within species and different habitats. In Uganda, tropical high altitude forests such as the Mabira Central Forest Reserve are experiencing continuous disturbance. However, information on rodent home range is lacking. Therefore, a two year Capture-Mark-Release (CMR) of rodents was conducted in the intact forest habitat: Wakisi, regenerating forest habitat: Namananga, and the depleted forest habitat: Namawanyi of Mabira Central Forest Reserve in order to determine the dominant rodent species, their home ranges, and factors affecting these home ranges. The home ranges were determined by calculating a minimum convex polygon with an added boundary strip of 5 m.
Results
Overall, the most dominant rodent species were: Lophuromys stanleyi, Hylomyscus stella, Praomys jacksoni Mastomys natalensis, Lophuromys ansorgei, and Lemniscomys striatus. H. stella dominated the intact forest habitat, while L. stanleyi was the most dominant both in the regenerating and the depleted forest habitats. L. stanleyi had a larger home range in the depleted forest, and the regenerating forest habitats, respectively. In the regenerating forest habitat, M. natalensis had a larger home range size, followed by L. stanleyi, and L. striatus. While in the intact forest habitat, H. stella had the largest home range followed by P. jacksoni. H. stella, L. striatus, L. stanleyi, M. natalensis, and P. jacksoni were most dominant during the wet season while L. ansorgei was relatively more dominant during the dry season. L. ansorgei, and P. jacksoni had a larger home range in the dry season, and a lower home range in the wet season. H. stella, L. stanleyi, M. natalansis and L.striatus had larger home ranges in the wet season, and lower home ranges in the dry season.
The home ranges of the dominant rodent species varied across the three habitats in Mabira central forest reserve (\({F}_{(2, 15)}= 6.41\), \(p = 0.000\)).
Conclusion
The significant variation in home ranges of the dominant rodent species in Mabira Central Forest Reserve depending on the type of habitat presupposes that the rodent management strategies in disturbed forest reserves should focus on the type of habitat.
Similar content being viewed by others
Introduction
Rodents form the largest order among mammals in terms of species diversity. A home range is the area where an individual normally moves during its normal daily activities. It varies depending on various factors such as sex and breeding period. In small mammals, males typically have home ranges that can be twice as large as those of females [1]. In rodents, home range size of males is always larger than that of females [2]. Reproductively active males maintain larger home ranges than females because they have to eat more food to acquire more energy for mating success [3, 4]. Home range size can also vary within species due to differences in the quality of habitat, distribution, and abundance of food, or population density. Increase of population density during the breeding period affects the degree of intersexual overlap of home range, and factors affecting resource availability and distribution (such as nature of habitat, season, etc) directly affect home ranges [2].
Natural forests always form conducive habitats for many small mammals [5]. Natural forests are forests always composed of mainly indigenous trees, and are conducive for small mammals because they form quality habitats. Changing environmental factors may affect the different forest habitats. Thus, impacting on the population dynamics of the different small mammal species [6]. Many Ugandan forests are undergoing threatening levels of destruction due to increasing human activities [7, 8]. This has continuously impacted on the forest ecosystems [9].
Mabira forest is a natural forest reserve (protected tropical high forest) with a diversified rodent community structure. Due to increased settlements in the forest and encroachment by the neighboring community from the surrounding big cities and towns (Kampala, Jinja, Mukono, Kayunga), the forest has been transformed into three distinct habitats, namely; the depleted forest, regenerating forest (young and colonizing forest), and intact forest (mature mixed forest with very limited disturbance) [10, 11]. Specifically, Namananga forest reserve represents the section of Mabira that is under natural regeneration with an average tree height less than 15 m., with one part of the reserve in a swamp dominated by Learsia hexandra and the forested expanse dominated by Brousonetia papyrifera, and small fields of cultivation and areas of human settlement. Namawanyi forest reserve is dominated by Brosonetia papyrifera with very few indigenous trees and is fringed by fields of cultivation. It is the reserve with very high levels of destruction with many sections completely depleted and turning into bushed grasslands/ bushed fallows, and always experience seasonal bush burning. Wakisi forest section represents the intact part with relatively limited levels of disturbance.
Information on home range size of different rodent species could be paramount in development of the effective conservation and management strategies of rodents in different habitats. However, for a number of natural forests in Uganda undergoing severe destruction, such information is scanty and lacking. We conducted this study to provide details on the home range of rodents in a tropical high forest located in central Uganda. The information generated may improve our understanding of the ecology of rodents in natural habitats, and provide a basis for developing effective management strategies for some of the selected species. Thus, in this study, it was hypothesized that home range sizes of dominant rodent species in Mabira Central Forest Reserve (MCFR) do not differ depending on rodent habitat type, and season.
Materials and methods
Study area and sampling sites
This study was carried out in Mabira central forest reserve. This reserve is found 54 km away from Kampala capital city at an altitude of 1070–1340 m a.s.l, average temperature of 26 °C, and covers three different districts: Mukono, Buikwe, and Kayunga districts. The area receives two main rain seasons: March to May (MAM) and September to December (SOND) [12, 13]. Due to continued forest disturbance, the forest has been reduced to three distinct habitats: the Intact part, regenerating, and the depleted part/bushed grassland. Specifically in this study, 3 habitats, were selected subjectively from the forest (Fig. 1): Wakisi forest section representing the intact part, Namananga forest representing the regenerating forest habitat, and Namawanyi forest, representing the depleted habitat [13]. The distance in between the intact and the regenerating forest habitat is approximately 8 km, while the distance between the regenerating and the depleted forest habitats is approximately 3 km, and the distance between the depleted and the intact forest habitat is approximately 5 km.
Rodent trapping by capture mark release
Six grids were laid for live-trapping of rodents using CMR method for a period of 24 months (September, 2018 to August, 2020). Grids 1, and 2 were located in the regenerating forest habitat fields, spaced at a distance of 2 km away from the other grids, Grids 3, and 4 were set in the intact forest habitat fields with approximately 1.5 km between them, while grids 5 and 6 were laid in the depleted forest habitat fields with 2 km in between them (Fig. 1). 49 Sherman Live traps were set in each of the six grids of size 70 m by 70 m (with a 5 m boundary strip at each of the corners) each containing 7 parallel lines spaced 10 m apart, and 10 m between traps, each parallel line having 7 trapping stations. Trapping of rodents was done using Sherman live traps, each baited with a mixture of local ghee, peanut butter, ripe bananas, and maize grains. The traps were set for 3 consecutive nights on a monthly basis. Trap inspection was done early morning on each day of trapping [13, 14].
Data processing and analysis
All captured rodents were carefully removed from the traps using a cloth bag, weighed using a Pesola balance, identified using morphometric measurements and recent literature [15], and thereafter given a unique identifier by toe clipping using a sterilized scissor and released at the same point of capture. In order to confirm the identified species, further analysis using deoxyribonucleic acid (DNA) was done at the Institute of Vertebrate Biology, the Czech Academy of Sciences.
Variables of interest for each animal trapped were: grid location and grid number, date, toe clipping code, species, sex, and body weight, maturity and breeding status. Composition of species over the study period was done using a statistical package StataIC12.0, and upon this, the dominant rodent species were identified as those with relatively higher species frequency.
Using PAST Statistics software, the Simpson Diversity Index (SDI) was estimated a measure of diversity in rodent species because it gives more weight to dominant species in a sample. The SDI was computed using the formula:
where; n is the number of individuals of different species, N is total number of individuals of all the species.
The home ranges for the dominant rodent species were then determined by calculating a minimum convex polygon (MCP) with an added boundary strip of 5 m (half the distance between neighboring traps [1, 16, 17] using Ad habitat package in R software version 4.3. For all the analyses, all locations where an individual was captured were used for MCP estimation. To ascertain whether home ranges varied across habitats, and seasons, Analysis of variance (ANOVA) and the interaction plot were used. The level of significance was 5%.
Results
Species composition
A total of 1537 rodent captures were made in 24 months. Out of the number of rodents captured, 562, 632, and 343 rodent individuals were trapped in the intact, regenerating, and depleted forest habitats, respectively. These comprised of 13 rodent species: Aethomys hindei (Thomas, 1902), Deomys ferrugineus (Thomas, 1888), Grammomys skuru (Thomas & Wroughton, 1907), Hybomys univittatus (Peters, 1876), Hylomyscus stella (Thomas,1911), Lophuromys stanleyi (Verheyen, et al., 2007), Lophuromys ansorgei (de Winton, 1896), Lemniscomys striatus Linnaeus, 1758), Mastomys natalensis (Smith, 1834), Mus bufo, Praomys jacksoni (de Winton, 1897), Rattus rattus Linnaeus, 1758, and Gerbilliscus kempii (Wroughton, 1906). H. stella and P. Jacksoni dominated the intact forest (IF), L. stanleyi and M. natalansis dominated the regenerating forest (RF), while in the depleted (DF), L. stanleyi, L. ansorgei, and L. striatus were most dominant. Overall, L. stanleyi was the most dominant natalansis rodent specie with 315 individuals and the least dominant were G. skuru, H. univittatus, R. rattus, and G. kempi (Table 1).
The regenerating forest habitat had the highest species richness, followed by the depleted and intact forest, respectively. The Simpson diversity index indicated that species diversity was highest in the regenerating forest habitats followed by depleted forest habitats and lowest in intact forest habitats (Table 2).
Home range of the dominant rodent species across habitats
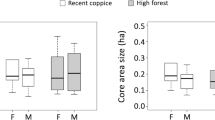
Results in Table 3 show the home ranges of the dominant rodent species in Mabira central forest reserve. L. ansorgei had the largest home range size in the depleted forest habitat and a smaller home range in the intact forest. H. stella had a larger home range in the intact forest habitat as compared to the regenerating forest. Besides, L. stanleyi had a relatively larger home range in the regenerating forest habitat as compared to the depleted forest habitat. M. natalansis had a higher home range in the regenerating forest as compared to the depleted forest habitat. L. striatus had a larger home range in the depleted forest habitat as compared to the regenerating forest habitat. P. jacksoni had a larger home range in the intact forest habitat as compared to regenerating forest habitat (Table 3).
Results in Fig. 2 indicate that L. ansorgei had the highest home range size in the depleted forest. This was closely followed by L. stanleyi, and L. striatus, respectively in the same habitat. In the regenerating forest habitat, L. stanleyi had a relatively larger home range size, followed by M. natalensis, H. stella and L. striatus. While in the intact forest habitat, H. stella had the largest home range followed by P. jacksoni, and L. striatus, respectively.
Further analysis indicated that home ranges of the dominant rodent species significantly varied across the three habitats (\({F}_{2, 15}= 6.41\), \(p = 0.000\)) (Table 4).
Distribution of dominant species by season
H. stella, L. striatus, L. stanleyi, M. natalansis, and P. jacksoni were most dominant during the wet season while L. ansorgei was relatively more dominant during the dry season (Table 5).
Dominant species home ranges across seasons
L. ansorgei had a larger home range in the dry season, and a lower home range in the wet season. This was followed by P. jacksoni with a larger home range in the dry season and lower home range in the wet season. In contrast, H. stella had a larger home range in the wet season and a lower home range in the dry season \(.\) L. stanleyi had a larger home range size in the wet season and lower home range in the wet season. M. natalensis had larger home range size in wet season and lower home ranges in the dry season (Table 6).
Further analysis indicated that there was no significant difference in home ranges of the dominant rodent species across seasons (\({F}_{1, 10}= 0.252, p = 0.422)\) (Table 7).
Discussion
In total, 13 species were recorded from the three study sites. The regenerating forest habitat had the highest species diversity, and this could be attributed to the evident plant diversity therein. This is because habitats with high plant cover and diversity tend to have many food alternatives, thus attracting more rodent species. This observation is in agreement with the findings in many other studies done previously [13, 18]. Across those studies, it is clearly pointed out that regenerating forest habitats (Secondary forests) tend to have high diversity of plants, and they dominate most gaps created as a result of habitat destruction. This attracts more rodents due to increased food alternatives. Besides, such differences could further be explained by the ever changing human activities which modify different habitat attributes, thus impacting on rodent communities. Due to the loss of plant cover, forest and bush encroachment, changes in the small mammal community were most likely caused by the loss of food resources, disruption of habitat structures, cover and shelter and by increased predation risk due to exposure [19, 20]. The low species diversity in the depleted forest habitat, could be attributed to the frequent bush and charcoal burning within the area.
The most dominant rodent species were; L. stanleyi, H.stella, P.jacksoni, M. natalensis, L. ansorgei, and L. striatus. L. stanleyi was the most captured rodent specie and prefers inhabiting areas with thick vegetation (disturbed forest habitats) [21]. The dominance of L. stanleyi in the regenerating and depleted forest habitats is a sign that there might have been forests before human invasions and settlements in such areas. This finding further confirms that Lophuromys species are highly flexible and tend to take advantage of habitats under regeneration/changing environments or undergoing any form of transformation. H. stella and P. jacksoni were reported second, and third, respectively in numbers. The two species occurred mainly in the undisturbed part of the forest reserve or areas with limited levels of disturbance. This finding was not a surprise because the duo are known as forest dwellers, and tend to prefer intact forests with very limited disturbance [15]. The current result is in agreement with previous studies done in Uganda and Tanzania [22, 23]. The high number of M. natalansis, and L. ansorgei, especially in the regenerating forest habitats, confirms that the two have a relatively wider distribution compared to many African rodents, and their distributions gradually increase with increase in habitat disturbance [15]. Besides, the two species prefer thick vegetation with relatively cool environments, these where evident in the regenerating forest in form of bushed grass lands and abandoned bushed garden patches.
L. ansorgei and L. striatus were observed to have larger home ranges in the depleted forest and a lower home ranges in the intact and regenerating forest habitats, respectively. L. stanleyi, and M. natalensis had larger home ranges in the regenerating forest as compared to other habitats. In the intact forest habitat, H. stella and P. jacksoni had the larger home ranges, and relatively smaller home ranges in the regenerating forest. Across all habitats, it was noted that the higher the number of a particular specie in a given habitat, the larger its home range could be. This could be due to the fact that competition for scarce resources (food, nesting places, among others) always increases with increase in rodent abundance. Further analysis indicated that home ranges of the dominant rodent species significantly varied across the three habitats. This finding is consistent with the study done by [2], which noted that factors affecting resource availability and distribution directly affect home range size. Besides, home range can also vary amongst different species due to differences in the quality of habitat, distribution, and abundance of food [24].
Majority of the dominant species (H. stella, L. striatus, L. stanleyi, M. natalensis) were most dominant during the wet season. This could be attributed to the availability food alternatives, and the conducive conditions for breeding. The home ranges of the dominant rodent species did not vary depending on seasons. This could be attributed to the fact in Uganda we always have two main rainy seasons (MAM & SOND), however rains were received throughout the study period, and therefore no clear distinction among the two seasons. Thus, it was not possible to ascertain any significant variations in home ranges across seasons.
L. ansorgei and P. jacksoni had a high home range in dry and lower in wet seasons. H. stella, L. stanleyi, M. natalensis, and L. striatus had higher home ranges in the wet season and lower home ranges in the dry season. This could be premised on the fact that resources were sufficiently available throughout the time of trapping in the study area [25]. These findings concur with the findings of other scholars, including [16].
Conclusion
Thirteen species were captured throughout the study period with high numbers of rodents captured in the regenerating forest and low numbers in the depleted forest habitat. L. stanleyi, H.stella, P. jacksoni, M. natalensis, L. ansorgei, and L. striatus were the most dominant rodent species. Overall, L. stanleyi was the most dominant specie and tends to dominate habitats with thick vegetation (the regenerating and depleted forest habitats). L. ansorgei had a larger home range size while M. natalensis had the lowest home range size. L. ansorgei had the highest home range size in the depleted forest, closely followed by L. stanleyi, and L. striatus, respectively in the same habitat. In the regenerating forest habitat, M. natalensis had a relatively larger home range size, followed by L. stanleyi, and L. striatus, while in the intact forest habitat, H. stella had the biggest home range followed by P. jacksoni. Further analysis indicated that home ranges of the dominant rodent species in Mabira Central Forest Reserve significantly varied across the three habitats. To the contrary, the home ranges of the dominant rodent species did not significantly vary across seasons. Thus, the rodent management strategies in disturbed forest reserves should focus most on the type of the habitat.
Availability of data and materials
The authors hereby confirm that the data generated and analyzed during the current study are available at a free cost from the corresponding author on request.
Abbreviations
- ACE:
-
African Centre of Excellence
- IRPM:
-
Innovative Rodent Pest Management
- BTD:
-
Biosensor Technology Development
- MCFR:
-
Mabira Central Forest Reserve
- MAM:
-
March to May
- SOND:
-
September to December
- DNA:
-
Deoxyribonucleic acid
- MCP:
-
Minimum Convex Polygon
- ANOVA:
-
Analysis of Variance
- UWA:
-
Uganda Wildlife Athority
- UNCST:
-
Uganda National Council for Science and Technology
- CMR:
-
Capture-Mark-Recapture
- SUA:
-
Sokoine University of Agriculture
References
Stickel LF. A Comparison of Certain Methods of Measuring Ranges of Small Mammals. J Mammal. 1954;35(1):1.
Priotto JW, Steinmann AR. Factors affecting home range size and overlap in Akodon azarae (Muridae : Sigmodontinae) in natural pasture of Argentina. Acta Theriol (Warsz). 1999;44(1):37–44.
Workneh Gebresilassie, Afework Bekele, Belay G, Balakrishnan M. Home Range and Reproduction of Rodents in Maynugus. SINET Ethiop J Sci. 2008;29(1):57–62.
Mlyashimbi ECM, Mariën J, Kimaro DN, Tarimo AJP, Machang’U RS, et al. Home ranges, sex ratio and recruitment of the multimammate rat (Mastomys natalensis) in semi-arid areas in Tanzania. Mammalia. 2020;84(4):336–43.
Ministry of Water and Environment. State of Uganda ’ S Forestry Report. Gov Print. 2016;2016:128.
Adler PB, Drake JM. Environmental variation, stochastic extinction, and competitive coexistence. Am Nat. 2008;172(5):1–10.
Obua J, Agea JG, Ogwal JJ. Status of forests in Uganda. Afr J Ecol. 2010;48(4):853–9.
Ministry of Water and Environment. Ecological baseline report for Mabira. Gov Print. 2017;00285(August 2016):107.
Bantihun G, Bekele A. Diversity and habitat association of small mammals in Aridtsy forest, Awi Zone. Ethiopia Zool Res. 2015;36(2):88–94.
Ministry of water and environment. Revised forest management plan for Mabira central forest reserves. Uganda Gov Print. 2017;2020(July 2010-June 2020):191.
Mitchell N. Rainforest change analysis in Eastern Africa: A new multi- sourced, semi-quantitative approach to investigating more than 100 years of forest cover disturbance Dissertation Mathematisch-Naturwissenschaftlichen Fakultät Rheinischen Friedrich-Wilhelms-Uni. Erscheinungsjahr: 2011;(September). https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=42eada7232ca9ccfcdfca00a6fe06c4a1945d8c0.
Fungo B, Eilu G, Tweheyo M, Baranga D. Forest disturbance and cropping mixtures influence crop raiding by red-tailed monkey and grey-cheeked mangabey around Mabira Forest Reserve. Uganda J Ecol Nat Environ. 2013;5(2):14–23.
Ssuuna J, Makundi RH, Isabirye M, Sabuni CA, Babyesiza WS, Mulungu LS. Rodent species composition, relative abundance, and habitat association in the Mabira Central Forest Reserve, Uganda. J Vertebr Biol. 2020;69(2):3–7.
Cutrera AP, Antinuchi CD, Mora MS, Vassallo AI. Home-range and activity patterns of the south American subterranean rodent Ctenomys talarum. J Mammal. 2006;87(6):1183–91.
Monadjem A, Taylor PJ, Denys C, Cotterill FPD. Ara Monadjem, Peter J. Taylor, Christiane Denys, Fenton P.D. Cotterill Rodents of Sub-Saharan Africa. 2015.
Borremans B, Hughes NK, Reijniers J, Sluydts V, Katakweba AAS, Mulungu LS, et al. Happily together forever: Temporal variation in spatial patterns and complete lack of territoriality in a promiscuous rodent. Popul Ecol. 2014;56(1):109–18.
Mulungu LS, Borremans B, Ngowo V, Mdangi ME, Katakweba AS, Tesha P, et al. Comparative study of movement patterns of Mastomys natalensis in irrigated rice and fallow fields in eastern Tanzania. Afr J Ecol. 2015;53(4):473–9.
Conde CFV y, Rocha CFD. Habitat disturbance and small mammal richness and diversity in an Atlantic rainforest area in southeastern Brazil / Distúrbio no habitat e riqueza e diversidade de pequenos mamíferos em uma área de Mata Atlântica no sudeste do Brasil. Brazilian J Biol. 2006;66(4):983–90.
Hoffmann A, Zeller U. Influence of variations in land use intensity on species diversity and abundance of small mammals in the Nama Karoo. Namibia Belgian J Zool. 2005;135(SUPPL.1):91–6.
NEMA. Harnessing our environment as infrastructure for sustainable livelihood development. Natl State Environ Rep Uganda. 2016;1–201. Available from: http://www.nemaug.org
Yalden DW. Small mammals of the Bale Mountains. Ethiopia Afr J Ecol. 1988;26(4):281–94.
Fagerstone K a, Ramey C a. Rodents and lagomorphs. Rangel Wildl. 1996;(January 1983):83–132 ST-Rodents and lagomorphs. Available from: AZTNC Science Files
Mizerovská D, Nicolas V, Demos TC, Akaibe D, Colyn M, Denys C, et al. Genetic variation of the most abundant forest-dwelling rodents in Central Africa (Praomys jacksoni complex): Evidence for Pleistocene refugia in both montane and lowland forests. J Biogeogr. 2019;46(7):1466–78.
Wolff JO, Edge WD, Wang G. Effects of adult sex ratios on recruitment of juvenile gray-tailed voles. Microtus canicaudus J Mammal. 2002;83(4):947–56.
Mulungu LS, Mlyashimbi ECM, Ngowo V, Mdangi M, Katakweba AS, Tesha P, et al. Food preferences of the multi-mammate mouse, Mastomys natalensis, in irrigated rice habitats in Tanzania. Int J Pest Manag. 2014;60(1):1–8.
Acknowledgements
We are grateful for all the facilitation received from the ACE IRPM & BTD, SUA, Morogoro, Tanzania, Busitema University, Uganda, Supervisors, and the research assistants. We further acknowledge the support provided by Prof. Josef Bryja including DNA analysis.
Authors' information (optional)
1. James Ssuuna, PhD student under the Africa Centre of Excellence for Innovative Rodent Pest Management and Biosensor Technology Development, Sokoine University of Agriculture, Morogoro, Tanzania, Lecturer at the Department of Natural Resource Economics, Busitema University, Tororo, Uganda.
2. Rhodes H. Makundi , Professor at the Africa Centre of Excellence for Innovative Rodent Pest Management and Biosensor Technology Development, Sokoine University of Agriculture, Morogoro, Tanzania.
3. Simon J. Chidodo , student at the Department of Wildlife Management, Sokoine University of Agriculture, Morogoro, Tanzania.
4. Moses Isabirye, Professor at the Department of Natural Resource Economics, Busitema University, Tororo, Uganda.
5. Nsajigwa E. Mbije (PhD), Lecturer at the Department of Wildlife Management, Sokoine University of Agriculture, Morogoro, Tanzania. 6. Loth S. Mulungu, Professor at the Institute of Pest Management, Morogoro, Tanzania.
Funding
All field work was financially supported by the African Centre of Excellence for Innovative Rodent Pest Management and Biosensor Technology Development, Sokoine University of Agriculture, Morogoro, Tanzania. Specifically, the funder facilitated training in data collection, and analysis. Ssuuna James, the corresponding author, financially supported manuscript presentations (transport from Uganda to Tanzania, and buying data to facilitate online presentations). Species identification (DNA analysis) was done by Prof. Josef Bryja at the Institute of Vertebrate Biology, Czech Academy of Sciences at a free cost.
Author information
Authors and Affiliations
Contributions
Ssuuna J., Makundi. R.H, Mulungu. L. S., and Isabirye M. conceived the idea and designed the materials and methods, and wrote the original manuscript draft. Ssuuna J. was the major contributor, set the grids and collected the data. Ssuuna, Chidodo, Mbije, and Mulungu, did data analysis and interpretation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study did not involve human participants (human data), or live animals. However, We confirm that all methods used in this study were carried out in accordance with relevant guidelines and regulations for the reporting of animal experiments. Besides, the study followed all procedures for conducting research on wild animals and was approved by the Sokoine University of Agriculture research ethical committee: Ref.no: PFC/D/2017/0009. As a requirement in to conduct scientific research in Uganda, permission was got from the Uganda Wildlife Authority (UWA), an institution mandated to oversee all wildlife in Uganda (Ref.no: UWA/COD/96/05). Another permission in form of a license was secured from the National forest authority (NFA), an institution responsible to protect forests in Uganda, especially forest reserves (Ref.no: NFA/N/2.1/18, License no: 292). Finally, clearance was got from the Uganda National Council for Science and Technology (UNCST), a body responsible for assessing all scientific studies through reviewing proposals and thereafter issue a unique identifier to the researcher (Ref.no:NS73ES). Soft copies of all permits/clearances required before conducting any scientific research on wildlife are attached.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
^Loth S. Mulungu is Deceased
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ssuuna, J., Makundi, R.H., Chidodo, S.J. et al. Spatio-temporal home range of the dominant rodent species in Mabira central forest reserve, Uganda. BMC Ecol Evo 23, 40 (2023). https://doi.org/10.1186/s12862-023-02148-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12862-023-02148-4