Abstract
Background
Guangdedendron micrum is the Late Devonian tree lycopsid that made up Xinhang fossil forest in Anhui, China, showing the earliest stigmarian rooting system. Based on new specimens of this lycopsid, the roots bearing rootlets, terminal parts of stems, vegetative leaves and monosporangiate strobili containing megaspores are researched in detail.
Results
The roots with four robust rhizomorphs are largely expanded and approach the size of those of the Late Carboniferous giant tree lycopsids in swampy forests. The rootlets along rhizomorphic axis leave oval to circular scars after abscission. Narrow-fusiform leaf cushions display a leaf scar, vascular bundle and ligule pit. Cylindrical megasporangiate strobili are borne singly, in pairs, or occasionally once-dichotomized. Of each megasporophyll, the pedicel consists of a keel and possibly undeveloped alations, and the long-triangular lamina presents a heel. Megasporangium is sessile and contains multiple Lagenicula megaspores with distinct spines and a large gula.
Conclusions
G. micrum displays large terminal monosporangiate strobili probably adapted to turbulent condition, and its megasporophylls together with multiple Lagenicula-type megaspores hint a possible primitive evolutionary status. These characteristics provide new insights into the evolution of fertile traits of early lycopsids.
Similar content being viewed by others
Introduction
The evolutionary radiation of vascular plants in the Devonian is one of the major events in the history of life, leading to the establishment of the terrestrial ecosystems [1, 2]. During the Devonian, trees originated and evolved independently in three major taxa, including pseudosporochnaleans (a kind of fern-like plants or possible stem ferns), archaeopteridaleans and lycopsids [3,4,5,6]. The Late Devonian is an important period for lycopsids, during which they rapidly evolved or diversified crucial traits such as bipolar growth, heterospory and arborescence [7, 8]. Arborescent lycopsids then dominated Carboniferous swamp forests ecosystem [9] and occupied the majority of biomass that turned into coals [10, 11]. However, Devonian forests preserved in-situ are rarely known and mostly limited to Euramerica [1, 5, 6, 12, 13], and only the Late Devonian (Frasnian) Svalbard forest was reported before 2019 to consist of in-situ fossil lycopsids [1].
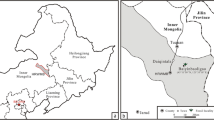
South China is regarded as a diversity hotspot of Late Devonian lycopsids as well as a potential research region for arborescent lycopsid evolution and Devonian lycopsid forests [5, 14]. Recently, Xinhang forest, a Famennian in-situ forest, was reported from Xinhang Town, Guangde City, Anhui Province of China [15], which primarily consists of a species of lycopsid, Guangdedendron micrum, that bears the earliest stigmarian rooting system. With the progress of clay excavation by local company, we keep on collecting plant specimens in Xinhang area. Based on new fossils, this article further studies the vegetative and reproductive characteristics of G. micrum, and provides information about its rhizomorphs, rootlets, vegetative stems bearing leaves, and monosporangiate strobili with megaspores.
Systematics
Class Lycopsida
Order Isoëtales sensu lato Meyen, 1987
Suborder Dichostrobiles DiMichele and Bateman, 1996
Genus: Guangdedendron Wang et al. 2019 emend
Emended generic diagnosis (with emended and additional diagnoses in brackets):
Small tree lycopsid, dioecious and monocarpic. Stigmaria-type rhizomorph with four axes, each of which equally divides at least once and bears helical rootlets nearly undivided. Stems dichotomous into a simple crown with terminal and pendulous megasporangiate strobili. Vegetative leaves linear with entire margin. [Leaf cushions and] leaf bases narrow-fusiform in shape, helically arranged in parastichies. [Leaf cushion or leaf base bearing ligule pit and vascular bundle scar. Leaf scars oval.] Megasporangiate strobili single, paired [or occasionally once-dichotomized], cylindrical with closely helically arranged megasporophylls. [Each megasporophyll comprised of a keeled pedicel and an upturned lamina with a downturned heel. Megasporangium long ellipsoidal, sessile and borne singly on adaxial surface of pedicel. Each megasporangium containing multiple Lagenicula-type] megaspores with trilete rays [and a gula].
Type species: Guangdedendron micrum Wang et al. 2019 emend.
Holotype: PKUB16001a (designated by Wang et al. 2019, housed in Department of Geology, Peking University, Beijing, China).
Specimens examined here:
PKUB16001a, 16001b, 16020a, 16035, 16047, 16049, 16052a, 16052b, 16053, 16058, 16064, 16065, 16067, 16097, 16,099, 16141, 16144, 16151, 21000, 21001, 21002a, 21002b, 21004–21011, 21013–21015, 21016a, 21016b, 21017a, 21017b, 21018; YC101–105 (Figs. 1–5, 7, Additional file 1: Fig. S1).
In-situ rooting systems of Guangdedendron micrum from Yongchuan mine. A Stem and connected rhizomorph axes. B Rooting system with branched rhizomorph axes. PKUB21015. C Stems with connected rhizomorph axes bearing rootlets. D Top view of the specimen shown in A, after removing the stem and surrounding rocks partially peeling off. A rooting system with four once-dichotomized rhizomorph axes. Arrows indicating 8 second-order branches. E Stem base and connected two rhizomorphic axes. F, G Rhizomorphic axes connected to moulds of stem bases and bearing rootlets. Scale bars: B, D, G (5 cm); diameter of the coin for scale: 2 cm (A, C, E, F)
Locality and horizon:
Jianchuan village, Xinhang Town, Guangde City, Anhui Province, China, Leigutai Member (Famennian) of Upper Devonian Wutong Formation.
Repository: Specimens numbered with PKUB and YC are separately housed at the Department of Geology, Peking University, Beijing, China and Anhui Geological Museum, Hefei, China, respectively.
Specific diagnosis (based on descriptions of Wang et al. [15] and this study): As for generic diagnosis. Rhizomorphs over 27.0 cm in depth. Rhizomorphic axes 8.3–31.0 cm long and 0.3–7.8 cm wide, forming angles of 19°–60° to ground surface. Rootlets up to 27.2 cm long and 7.0 mm wide. Rootlet scars circular and 2.5–3.9 mm in diameter. Preserved parts of in-situ stems reaching 88.0 cm in height and up to 18.7 cm in diameter, with rare dichotomy. Vegetative leaves 2.0–9.2 cm long and 1.2–9.0 mm wide, each with a single vein and an entire margin. The length–width ratios of leaf bases or leaf cushions ca. 6:1. Fertile axes with persistent leaves, terminated by strobili. Megasporangiate strobili cylindrical, with maximum length and width (excluding sporophyll laminae) of 23.4 cm and 3.0 cm, respectively. Strobilar axes up to 3.0 mm in width. Sporophyll laminae long-triangular in shape with the maximum length and width of 18.0 mm and 5.8 mm, showing entire margins. Basal portion of each lamina forming a downturned, inverted triangular heel. Sporophyll pedicels 6.0–8.0 mm in length, approximately perpendicular to strobilar axis. Pedicel displaying an abaxial keel 0.5–0.8 mm in height. Megasporangium horizontally elongated, 4.0–7.4 mm long. Megaspore ca. 1.5 mm in polar axis length, consisting of a smooth gula and a spherical body with spiny ornamentations.
Results
Description
The description in this article is based on a new fossil collection of Guangdedendron micrum as well as several specimens introduced in Wang et al. [15]. Plant organs described here include rooting system (Fig. 1, 2), stems and vegetative axes bearing leaves and leaf cushions or leaf bases (Figs. 3, 4), and strobili with the 3-D reconstruction (Figs. 5, 6) displaying megaspores (Figs. 7, 8).
Rooting systems of Guangdedendron micrum from Yongchuan (A, C, D) and Jianchuan (B, E–L) mines. A An in-situ stem with two rhizomorph axes. Arrow indicating portion enlarged in (G). B, C In-situ stems with expanded bases and rooting systems. D Two rhizomorph axes with rootlets and rootlet scars. PKUB16151. E, F Part and counterpart of a rhizomorph axis with helical rootlets and circular rootlet scars. PKUB21016a, 21016b. G Enlargement of arrowed portion in (A), showing a rhizomorph axis with rootlets and helically arranged rootlet scars. H Part of the rooting system showing rootlets. PKUB21008. I, J Rhizomorphic axes bearing rootlets and circular rootlet scars in helix. Arrows in I and J indicating portions enlarged in K and L, respectively. PKUB21009, 21010. K, L Enlargement of arrowed portions in I and J, respectively. Circular rootlet scars in helix. Scale bars: C (10 cm), E–G, J (1 cm), H (2 cm), K (5 mm), L (2 mm); diameter of the coin for scale: 2 cm (A, B, D, I)
Stems and vegetative leaves of Guangdedendron micrum, from Yongchuan (A, B, D, G–M, O–Q, S, U, V) and Jianchuan (C, E, F, N, R, W) mines. A, B In-situ stems with expanded bases. PKUB21000, 21014. C Two adjacent in-situ stems with expanded bases. D An in-situ stem with an expanded base. E, F Two sides of a stem displaying basal expansion and helically arranged oval fissures of broken leaf cushions after leaf abscission. PKUB21004. G–J Stems perpendicular to the bedding plane. H YC-103. K An in-situ stem with leaf cushions. L A once-dichotomized stem. YC-101. M, N Twice-dichotomized stems. M YC-102. O A once-dichotomized leafy stem with leaf bases. P, Q Part and counterpart of helically arranged leaf cushions. YC-105, 104. R A stem with helically arranged leaf cushions. Arrow indicating portion enlarged in Additional file 1: Fig. S1I. PKUB16065. S Fusiform leaf cushions. PKUB21013. T Line illustration of a leaf cushion based on arrowed portion in Q. U Helically arranged leaf bases. Arrow indicating portion enlarged in V. PKUB21007. V Enlargement of arrowed portion in U, indicating fusiform leaf bases with middle vertical grooves in the lower part. W Helically arranged leaf bases, each showing a vertical groove in the middle. PKUB16052a. Scale bars: A, E, F (2 cm), D, G (5 cm), P–R, U, W (1 cm), S, T, V (5 mm); diameter of the coin for scale: 2 cm (B, C, H, J, K, M–O); length of the hammer for scale: I (28.6 cm), L (27.3 cm)
Vegetative leaves of Guangdedendron micrum from Jianchuan mine. A, B Terminal parts of vegetative axes bearing leaves. PKUB21001, 16067. C, D Part and counterpart of a terminal vegetative axis. PKUB21017a, 21017b. E, F Tapering vegetative axes with persistent linear leaves. Arrow indicating portion in F enlarged in G. PKUB21018, 16144. G Enlargement of arrowed portion in F, showing veins of vegetative leaves. Scale bars: A–E (2 cm), F (10 cm), G (1 cm)
Fertile axes and strobili of Guangdedendron micrum from Jianchuan (A–E, H–J, L, M) and Yongchuan (F, G, K) mines. A, B Part and counterpart of a once-dichotomized axis bearing linear leaves and a single strobilus. The arrow in A indicating a leafy stem enlarged in Fig. 3W. Arrow in B indicating portion enlarged in Fig. 7D. PKUB16052a, 16052b. C A strobilus without basal fertile axis preserved. Arrows 1 and 2 indicating portions enlarged in Fig. 7E, G, respectively. Middle arrow indicating portion where carbonaceous material was peeled for maceration and displayed in Fig. 8A. PKUB16053. D, E Part and counterpart of a strobilus terminating a fertile axis. PKUB16001a, 16001b. F, G Part and counterpart of terminal strobili in pairs, with sporophylls along central strobilar axis and persistent vegetative leaves on fertile axis. PKUB21002a, 21002b. H At least eight strobili (arrows 1–8) preserved in the same direction (1 and 2, 7 and 8 possible paired, respectively). PKUB16047. I A dichotomized strobilus. PKUB21011. J Terminal strobili in pairs. PKUB16035. K A single and a pair of strobili perpendicular to the layers. L A short strobilus may partially preserved. PKUB16065. M Strobilus displaying central strobilar axis. PKUB16020a. Scale bars: A, B (5 cm), C, J, L, M (1 cm), D–I (2 cm); diameter of the coin for scale: 2 cm (K)
Reconstruction of the longest strobilus terminating the fertile axis after bifurcation, based on Fig. 5A, B. PKUB16052a, 16052b. Scale bar = 2 cm
Sporophylls and spores of Guangdedendron micrum from Jianchuan mine. A A partially preserved strobilus, showing sporangia on the adaxial surface of sporophyll pedicels. PKUB16049. B A partially preserved strobilus, showing megaspores and sporophylls. PKUB16141. C Mid-upper part of a strobilus with helically arranged sporophylls in face and lateral view. Arrow indicating portion enlarged in H. PKUB16058. D Enlargement of portion in Fig. 5B (arrow, 180° rotated), showing sporophyll laminae and pedicel in lateral view. E Enlargement of portion in Fig. 5C (arrow 1, 180° rotated), respectively. Showing sporophyll laminae and pedicel in lateral view and partially preserved sporangia, arrows indicating megaspores. F Enlargement of arrowed portion in (A), showing sporangia. G Enlargement of portion in Fig. 5C (arrow 2, 180° rotated), showing sporophyll laminae in face view. H Enlargement of portion in (C) (arrow), showing sporophyll laminae in face view with downturned heels. I Part of a strobilus showing megaspores, the arrow indicating portion enlarged in (K). PKUB16020a. J, K Enlargement of arrowed portions in Additional file 1: Fig. S1Q and 7I, respectively. Showing megaspores and spiny ornamentations. L Megaspores with spiny ornamentations. Scale bars: A, C, D (1 cm), B, F (5 mm), E, G–I (2 mm), J (1 mm), K, L (500 μm)
Megaspores of Guangdedendron micrum displaying ornamentations under LM after maceration (B–I) or under SEM (J–T), respectively. A A piece of carbonaceous fragment of strobilus peeled from Fig. 5C (middle arrow indicating portions, nearby the apex of strobilus) before maceration. B, C Megaspores after maceration of fragment in (A). Arrows in (B) and (C) indicating portions enlarged in (D–I), respectively. D, E Enlargement of portions in (B) (arrow 1 and 2), respectively, displaying megaspore clusters probably borne in tetrads. F–I Enlargement of portions in (B) (arrow 3) and C (arrow 1–3), respectively. Megaspore consisting of a body with spiny ornamentations and a prominent gula. J, K Megaspore clusters under SEM, probably borne in tetrads. L, M Megaspore in lateral view, each consisting of a body and a gula. Arrows in (L) and (M) indicating portions enlarged in (P) and (Q), respectively. N, O Megaspores in roughly proximal view. Arrow in (N) indicating portion enlarged in (R). P–R Enlargement of portions in (L) (arrow), M (arrow) and N (arrow), respectively. Showing spiny and tapered ornamentations. S Spiny and tapered ornamentations. T Enlargement of portion in Additional file 3: Fig. S3C (arrow 2), showing spiny ornamentations. Scale bars: A (1 mm), B, C (2 mm), D–J, L–O (200 μm), K (500 μm), P, S (20 μm), Q, R, T (50 μm)
The stigmarian rhizomorph has four evenly separated axes and then usually dichotomizes once (Fig. 1A–D). These rhizomorphic axes expand up to 41.1 cm in plane (Fig. 1D), over 27.0 cm in depth (Fig. 1C) and extend at 19°–51° to ground surface (Fig. 1C, E–G). Rhizomorph axes are up to 31.0 cm in length, and their first-order and second-order branches are 7.2–7.8 cm and 2.5–6.5 cm in diameter, respectively (Figs. 1A–D, 2A, C–G). Rootlets are helically arranged along rhizomorph axes, extend in different directions and taper gradually toward apices (Figs. 1C, F, G, 2A–H). Rootlets are over 12 cm in length, with a maximum width of 5.0 mm (Fig. 2A, C–H). When abscised, they leave oval to circular rootlet scars 2.5–3.9 mm in diameter on rhizomorphic axes (Fig. 2–I–L). Rootlets appear unbranched in most cases. No root hairs have been observed.
Stems are preserved as compressions or erect casts, up to 73.1 cm long and 1.1–12.2 cm in diameter except for the expanded bases (Fig. 3A–O; Additional file 1: Fig. S1A–F, H). The expanded bases are connected to rhizomorphs (Fig. 3B–D). Dichotomies are occasional, and at most continuously twice observed on stems and at angles of 13°–43° (Fig. 3L–O; Additional file 1: Fig. S1E, F). Stems or axes display leaf cushions (Fig. 3K) or leaf bases (Fig. 3O) when vegetative leaves have been abscised or not, respectively. Sometimes only oval or elongate fissures can be recognized along the stems due to poor preservation (Fig. 3E, F, Additional file 1: Fig. S1G, H). Leaf cushions are fusiform in shape, 15.6–22.7 mm long and 2.6–4.3 mm wide (Fig. 3P–S). Leaf scars are oval, 6.5–8.1 mm in length and 3.5–3.7 mm in width, appearing on the middle portion of leaf cushions (Fig. 3P, Q, T). The leaf scar shows a depressed ligule pit (Lp) on the top and a vascular bundle scar in the middle (Fig. 3P, Q, T; Additional file 1: Fig. S1I). Leaf bases are fusiform and somewhat narrower, 14.0–25.0 mm in length and 3.0–3.7 mm in width, with the ratio of length to width ca. 6:1 (Fig. 3U–W). Each leaf cushion or leaf base bears a longitudinal groove in the lower part (Fig. 3P, Q, V, W). Leaf cushions or leaf bases are helically arranged in parastichies, forming angles of 65°–80° with the horizontal line (Fig. 3P–S, U–W; Additional file 1: Fig. S1J).
Linear vegetative leaves along stems possess entire margins, 3.3–8.5 cm long and 2.9–9.0 mm wide, and depart at angles of 59°–98° from stems (Figs. 3U, W, 4E, F, 5A, B, D, F). Leaves of similar shape are densely arranged along slender terminal twigs (Fig. 4A–D), indicating terminal portion of a possible juvenile plant. Each leaf has an obvious single vein extending from base to apex (Fig. 4B, G).
Fertile axes terminated by strobili are up to 6.4 cm long and 2.1–5.5 mm wide (Fig. 5A, B, D–F). An axis, 17.6 cm long and 3.5–7.8 mm wide, produces two daughter axes with half the width of the parent and at angles of ca. 60° (Fig. 5A, B). The vegetative leaves depart at angles of 70°–85° from fertile axes and curve distally (Fig. 5A, B, D, F; Additional file 1: Fig. S1L, O).
Pendulous strobili are borne singly (Fig. 5A, B, D, E, H, K; Additional file 1: Fig. S1K, L, O), in pairs (Fig. 5F–H, J, K; Additional file 1: Fig. S1M, N) or occasionally once-dichotomized (Fig. 5I; Additional file 1: Fig. S1Q). Strobili are cylindrical and slightly curved, up to 23.4 cm in length and 0.9–2.4 cm in width (excluding sporophyll laminae) (Fig. 5A–L; Additional file 1: Fig. S1K–Q). The longest strobilus terminating the fertile axis (Fig. 5A, B) is reconstructed in Fig. 6. Sporophylls are helically and compactly arranged along strobilar axes that are 1.2–3.0 mm wide (Figs. 5M, 7A, D). Each sporophyll in lateral view shows a horizontal pedicel, from which a lamina arises at an angle of ca. 110° (Fig. 7D–F). The lamina is long-triangular in front view and tapers acropetally, 13.0–18.0 mm long and 2.4–5.8 mm at the widest part (Fig. 7G, H; Additional file 2: Fig. S2). The lamina forms a downturned heel at the base, which is 0.8–1.0 mm high and 2.3–2.5 mm wide (Fig. 7H). The pedicel is 6.0–8.0 mm in length (Fig. 7D–F), and shows an abaxial keel 0.5–0.8 mm high (Fig. 7D, E).
All the strobili in our collection only exhibit megaspores and are thus megasporangiate. Each megasporangium is sessile and long-ellipsoidal, 4.0–7.4 mm long and over 1.3–1.5 mm high (partially preserved or covered in height), and attached to the adaxial surface of sporophyll pedicel (Fig. 7E, F). The upper portions of megasporangia are usually incompleted because of overlap by the adjacent sporophyll(s). In-situ megaspores are sometimes observed within strobili, displaying circular shapes (Fig. 7I, J; Additional file 1: Fig. S1Q) and spiny ornamentations (Fig. 7J–L). A megasporangium contains multiple megaspores (Fig. 7B, E, arrows) considering their sizes.
Lagenicula megaspores are observed under LM after maceration (Fig. 8A–I) or under SEM (Fig. 8J–T; Additional file 3: Fig. S3), and are probably born in tetrads (Fig. 8D, J, K; Additional file 3: Fig. S3A). Each megaspore is composed of a spherical body and a prominent gula in lateral view (Fig. 8F–I, L, M; Additional file 3: Fig. S3B–D) and is somewhat conical in proximal view (Fig. 8N, O). Megaspores are ca. 1.5 mm in the length of polar axis, while the body and gula are 765–1256 μm and 400–817 μm in maximum length, respectively (Fig. 8F–N; Additional file 3: Fig. S3B–D). The gula has no visible ornamentation on the surface (Fig. 8E–J, L–N; Additional file 1: Fig. S3B, C), while the body displays ornamentations of densely and evenly distributed spines (Fig. 8E–I, P–R; Additional file 3: Fig. S3E–H). The spines are often curved, ca. 85 μm in diameter at bases and taper towards apices (Fig. 8P–S; Additional file 3: Fig. S3E–H). Some parts of ornamentations could display a verrucate appearance after the spines are truncated (Fig. 8T; Additional file 3: Fig. S3I).
Comparison with Guangdedendron micrum described by Wang et al. [15]
Fossils in this study are collected from the same sections and morphologically consistent with Guangdedendron micrum, which is considered as a possible monocarpic and dioecious tree lycopsid bearing stigmarian rhizomorphs [15]. This study supports the former conclusions and adds new traits of rootlet scars, stems and axes bearing vegetative leaves, terminal megasporangiate strobili and megaspores. The length and width of rhizomorph axis are expanded, approaching in the size of Stigmaria ficoides from the Middle Pennsylvanian [16]. Stems of 8.0–10.0 cm width (Fig. 1A, C, E, 3A–K) and axes with dichotomy (Fig. 3L–O) are found again, but they are still unusual, thus supporting G. micrum as a small tree with an advanced rooting system type and a simple crown [15]. The lower portions of stems lack vegetative leaves but show leaf cushions indicating leaf abscission. Vegetative terminal twigs in Fig. 4A–C with leaves gradually shorten upwards may indicate apex of juvenile plants of G. micrum, as reconstructed in Fig. 6A in Wang et al. [15]. The strobili of G. micrum are large and often borne in pairs, and occasionally dichotomize once.
tituted different populations and lived far from the fossil locality, or the microsporangiate strobili are difficult to be preserved or identified.
Comparison with Late Devonian heterosporous lycopsids bearing monosporangiate strobili in China
Despite an even larger collection of Guangdedendron micrum, no strobili containing microspores have been found. Previous studies of coeval heterosporous lycopsids often report mega- and microsporangiate strobili [17,18,19]. One possible explanation is that G. micrum has few male individuals and parthenogenesis happened, while other interpretations include that the male individuals consSeveral Late Devonian members of the Isoëtales sensu lato have been reported in China, e.g. Sublepidodendron songziense [17, 20, 21], Sublepidodendron grabaui [19, 22], Minostrobus chaohuensis [18, 23], Changxingia longifolia [24] and Changxingia sp. [25]. These taxa, together with Guangdedendron micrum, could be assigned to the Suborder Dichostrobiles, i.e. isoetaleans that produce monosporangiate strobili [26]. Major morphological traits of these plants are compared in Table 1, while a more detailed comparison is given (see Additional file 4: Table S1). Among these plants, most of them are recognized as arborescent, while G. micrum and possibly S. songziense bear stigmarian rooting system. Changxingia longifolia and Changxingia sp. display the smallest sizes of stems and megasporangiate strobili. S. songziense and S. grabaui possess lateral branches, but G. micrum bears a crown with fewer bifurcations. G. micrum shows the leaf bases of similar shape with M. chaohuensis and S. grabaui, but the latter displays only leaf bases with false leaf scars and no typical leaf cushions. Strobili of G. micrum are often in pairs and occasionally dichotomous, and larger than those of other coeval taxa. Alations along sporophyll pedicels are distinct in M. chaohuensis but relatively undeveloped in G. micrum, C. longifolia and S. songziensis. Considering the number of megaspores in each megasporangium, G. micrum and S. songziense display multiple but C. longifolia and Changxingia sp. possibly four, and M. chaohuensis contains four megaspores with some of them aborted. All of these plants exhibit Lagenicula megaspores with distinct gula, which is however larger in G. micrum.
Comparison with Late Devonian heterosporous lycopsids outside of South China
Several Late Devonian heterosporous lycopsids outside South China are known for their bisporangiate strobili. Two such lycopsids were recently reported from Gondwana palaeocontinent. Cymastrobus irvingii is a 3-D preserved bisporangiate strobilus from Famennian in New South Wales, Australia [27]. Its megasporangia contain a large number of megaspores, up to 500 μm in diameter. Casts of the megaspores show numerous circular pores surrounding the trilete mark and in several rows but no gula, thus differing from the Lagenicula-type megaspores in Guangdedendron micrum. Kowieria alveoformis from the Famennian of South Africa produces up to four Lagenicula megaspores [28] similar with those of G. micrum, while they differ in the number of megaspores per sporangium. In addition, K. alveoformis bears sporophylls homomorphic to vegetative leaves, in contrast with those of G. micrum. Clevelandodendron ohioensis is a Famennian lycopsid from the USA [29]. It displays a straight and totally unbranched stem terminated by a single bisporangiate strobilus, and contains Triletes megaspores and microspores. Unlike species mentioned above, Jurinodendron (= Cyclostigma) kiltorkense bears monosporangiate strobili and was a widespread taxon during the Upper Devonian and Early Mississippian [30]. J. kiltorkense is similar with G. micrum in the shape of sporophylls and Lagenicula-type megaspores [31]. J. kiltorkense differs from G. micrum in its small circular leaf scars (about 1.5 mm in diameter) and lacking of leaf cushions.
Discussion
Most Devonian lycopsid fertile zones or strobili show no bifurcations [8, 32]. However, bifurcated strobili or fertile zones occurred in several taxa of the Middle-Late Devonian lycopsids, e.g. the Givetian Yuguangia ordinata [33], Frasnian Kossoviella timanica [34] and an undetermined “type C” [35], and Famennian Hefengstrobus bifucus [36] and Guangdedendron micrum (see details in Table 2). The evolutionary route of this trait is unclear. In most cases, the two resulted parts of strobili show nearly similar width as the strobilus before bifurcation. We suggest that the bifurcated strobili or fertile zones are produced by the dichotomy of an apical meristem after the shoot turns into reproductive growth. Alternatively, the last branching point on the terminal fertile axes may represent an earlier bifurcation than the transition to reproductive growth, i.e., the sequence of reproductive growth differentiation and apical meristem bifurcation controls the growth pattern of lycopsid strobili. In G. micrum, the strobili could be borne in pairs or bifurcated, which together indicate that the apical meristem has a relatively independent potential for bifurcation and sporophyll differentiation. Many tassel-fern species (especially Phlegmariurus) included in living Lycopodiales show similar dichotomized fertile zones indicating ready shift from vegetative branches to fertile branches (strobili) [37]. Large size and multi-dichotomized strobili may correspond to individual’s adaptation in this group to the epiphytic habit [37]. Strobili of G. micrum are various in growth patterns and sizes with lengths ranging from 5.0 [15] to 23.0 cm (9.6 cm on average), and individuals bearing large strobili may result in improved production of offsprings and surviving the turbulent condition near coastal area.
The earliest heterosporous lycopsids appeared in the Middle Devonian [33, 38, 39], and over ten heterosporous lycopsid genera have been reported so far from the Late Devonian [14]. Many of these early heterosporous lycopsids produce the gulate Lagenicula megaspores (see details in Table 3), while Guangdedendron micrum presents the relatively larger gula. Lagenicula appears not only in bisporangiate strobili of Givetian Mixostrobus, Famennian Kowieria as well as Carboniferous Flemingites [28, 38, 40, 41], but also in the megasporangiate strobili of all the members of Suborder Dichostrobiles reported from the Upper Devonian of South China [14, 25]. However, the taxa dominating the Carboniferous swamp, e.g. Sigillariostrobus, Lepidocarpon and Achlamydocarpon, bear megaspore types including Tuberculatisporites and Cystosporites [42,43,44]. We consider that there was a significant evolutionary change in the megaspore type between the early and late representatives of heterosporous lycopsids.
During the Middle-Late Devonian, the lycopsids evolved well-differentiated strobili and sporophylls [45]. Each specialized sporophyll is composed of a pedicel and an upturned or curved lamina. Reduction in the number of megaspores per sporangium together with alations widened and upturned to enclose the sporangium are considered as evolutionary trends within the Suborder Dichostrobiles [23, 46,47,48]. In one megasporangium, Minostrobus chaohuensis contains one functional megaspore and three aborted megaspores, which is regarded as the most derived taxon among the Famennian members of Dichostrobiles [23]. As for the other taxa, Changxingia longifolia and Changxingia sp. have probable four megaspores per sporangium, while Guangdedendron micrum and Sublepidodendron songziense [21] show multiple megaspores that are considered primitive. On the other hand, the alations of C. longifolia and S. songziensis resemble those of Achlamydocarpon, which leave the sporangium largely exposed and represent a primary status, while Minostrobus represents the intermediate [23] and Lepidocarpon the most derived [46]. Although carefully serial dégagement has been applied to the G. micrum sporophylls in lateral and face views, the existence of alations still cannot be confirmed, but suggests undeveloped alations probably comparable with C. longifolia and S. songziensis. The relatively primitive characteristic combination of G. micrum hints a basal position in the lineage of Suborder Dichostrobiles. In addition, as suggested by Philips and DiMichele [49], there are many megaspores exposed on the surfaces of compressed strobili, probably indicating the free sporing habit.
Materials and methods
Specimens of fossil plant Guangdedendron micrum in this study were further collected from the same localities and horizons illustrated by Wang et al. [15], i.e., the upper part (Leigutai Member) of the Upper Devonian (Famennian) Wutong Formation at Jianchuan and Yongchuan mines, Xinhang Town, Guangde City, Anhui Province, China. Several specimens observed in the previous study (including PKUB16001a, PKUB16099, PKUB16141, PKUB16144, PKUB16151 and PKUB21006, see Taxonomy subsection) are also reinvestigated. Specimens of G. micrum are preserved as casts, impressions and compressions. The plant morphology is exposed using steel needles. Carbonaceous fragments of the strobili with spores are either macerated with HCl and HF, or collected and fixed with conductive tape, for observation under the light microscope (LM) and scanning electron microscope (SEM), respectively. Photographs are taken with the digital camera, LM and SEM. Specimens numbered with PKUB and YC are separately housed at the Department of Geology, Peking University, Beijing, China, and Anhui Geological Museum, Hefei, China, respectively.
Availability of data and materials
All data generated or analyzed during this study are included in this manuscript, 8 figure files, 3 table files, its Additional file 4: Table S1 and 3 additional figure files.
References
Berry CM, Marshall JEA. Lycopsid forests in the early Late Devonian paleoequatorial zone of Svalbard. Geology. 2015;43:1043–6.
Xue JZ, Deng ZZ, Huang P, Huang KJ, Benton MJ, Cui Y, Wang DM, Liu JB, Shen B, Basinger JF, Hao SG. Belowground rhizomes in paleosols: the hidden half of an Early Devonian vascular plant. Proc Natl Acad Sci USA. 2016;113:9451–6.
Meyer-Berthaud B, Decombeix AL. L’évolution des premiers arbres: les stratégies dévoniennes. CR Palevol. 2009;8:155–65.
Meyer-Berthaud B, Soria A, Decombeix AL. The land plant cover in the Devonian: a reassessment of the evolution of the tree habit. Geol Soc Lond Spec Publ. 2010;339:59–70.
Berry CM. Palaeobotany: the rise of the Earth’s early forests. Curr Biol. 2019;29:R792–4.
Berry CM. The evolution of the first forests in the Devonian. Vestnik Institute of Geology, Komi Science Center Ural Branch of the Russian Academy of Sciences. 2019;11:20–4.
Pigg KB. Isoetalean lycopsid evolution: from the Devonian to the present. Am Fern J. 2001;91:99–114.
Taylor TN, Taylor EL, Krings M. Paleobotany: the biology and evolution of fossil plants. 2nd ed. Amsterdam: Academic Press; 2009.
Falcon-Lang HJ, Jud NA, Nelson WJ, DiMichele WA, Chaney DS, Lucas SG. Pennsylvanian coniferopsid forests in sabkha facies reveal the nature of seasonal tropical biome. Geology. 2011;39:371–4.
Greb SF, DiMichele WA, Gastaldo RA. Evolution and importance of wetlands in earth history. Special Paper of the Geological Society of America. 2006;399:1–40.
Berner RA. The long-term carbon cycle, fossil fuels and atmospheric composition. Nature. 2003;426:323–6.
Mintz JS, Driese SG, White JD. Environmental and ecological variability of Middle Devonian (Givetian) forests in Appalachian basin paleosols, New York, United States. Palaios. 2010;25:85–96.
Stein WE, Berry CM, Hernick LV, Mannolini F. Surprisingly complex community discovered in the mid-Devonian fossil forest at Gilboa. Nature. 2012;483:78–81.
Liu L, Wang DM, Meng MC, Xue JZ, Tang YG. New advances in studies of Devonian lycopsids. Acta Palaeontol Sin. 2018;57:344–55.
Wang DM, Qin M, Liu L, Liu L, Zhou Y, Zhang YY, Huang P, Xue JZ, Zhang SH, Meng MC. The most extensive Devonian fossil forest with small lycopsid trees bearing the earliest stigmarian roots. Curr Biol. 2019;29:2604–15.
Thomas BA, Seyfullah LJ. Stigmaria Brongniart: a new specimen from Duckmantian (Lower Pennsylvanian) Brymbo (Wrexham, North Wales) together with a review of known casts and how they were preserved. Geol Mag. 2015;152:858–70.
Wang Q, Hao SG, Wang DM, Wang Y, Denk T. A Late Devonian arborescent lycopsid Sublepidodendron songziense Chen emend. (Sublepidodendraceae Kräusel et Weyland 1949) from China, with a revision of the genus Sublepidodendron (Nathorst) Hirmer 1927. Rev Palaeobot Palynol. 1949;2003(127):269–305.
Meng MC, Wang DM, Yao JX. Vegetative characters, growth habit and microsporangiate strobilus of lycopsid Minostrobus chaohuensis. PLoS ONE. 2015;10(3): e0122167.
Meng MC, Liu L, Wang DM, Yao JX. Growth architecture and microsporangiate strobilus of Sublepidodendron grabaui (Lycopsida) from the Late Devonian of South China. Rev Palaeobot Palynol. 2016;224:83–93.
Wang Q, Hao SG, Wang DM, Dilcher DL. An anatomically preserved arborescent lycopsid, Sublepidodendron songziense (Sublepidodendraceae), from the Late Devonian of Hubei, China. Am J Bot. 2002;89:1468–77.
Meng MC, Wang DM, Tian T. New insights of the megasporangiate strobilus of Sublepidodendron songziense from the Upper Devonian of Hubei Province. Acta Palaeontol Sin. 2014;53:180–90.
Wang Y, Xu HH. Sublepidodendron grabaui comb. nov., a lycopsid from the Upper Devonian of China. Bot J Linn Soc. 2005;149:299–311.
Meng MC, Wang DM, Xue JZ, Zhu X. New insights and evolutionary significance of the megasporangiate strobilus of Minostrobus chaohuensis (Lycopsida) from the Upper Devonian of South China. Rev Palaeobot Palynol. 2013;190:20–40.
Wang DM, Meng MC, Xue JZ, Basinger JF, Guo Y, Liu L. Changxingia longifolia gen. et. sp. nov., a new lycopsid from the Late Devonian of Zhejiang Province, South China. Rev Palaeobot Palynol. 2014;203:35–47.
Wang DM, Qin M, Meng MC, Liu L, Ferguson DK. New insights into the heterosporous lycopsid Changxingia from the Upper Devonian Wutong Formation of Zhejiang Province, China. Plant Syst Evol. 2017;303:11–21.
DiMichele WA, Bateman RM. The rhizomorphic lycopsids: a case-study in paleobotanical classification. Syst Bot. 1996;21:535–52.
Evreïnoff M, Meyer-Berthaud B, Decombeix A, Lebrun R, Steemans P, Tafforeau P. A new Late Devonian isoetalean lycopsid from New South Wales, Australia: Cymastrobus irvingii gen. et sp. nov. Palaeontol Electron. 2017; 20. 3. 47A: 1–16.
Gess RW, Prestianni C. Kowieria alveoformis gen. nov. sp. nov., a new heterosporous lycophyte from the Latest Devonian of Southern Africa. Rev Palaeobot Palynol. 2018;249:1–8.
Chitaley S, Pigg KB. Clevelandodendron ohioensis gen. et. sp. nov., a slender upright lycopsid from the Late Devonian Cleveland Shale of Ohio. Am J Bot. 1996;83:781–9.
Doweld AB. Jurinodendron—a new replacement name of Cyclostigma S. Haughton ex O. Heer, 1872 (Lycopodiophyta). Paleontol J. 1872;2001(35):218–21.
Chaloner WG. The cone of Cyclostigma kiltorkense Haughton, from the Upper Devonian of Ireland. Bot J Linn Soc. 1968;61:25–36.
Gensel PG, Andrews HN. Plant life in the Devonian. New York: Praeger Publisher; 1986. p. 1–380.
Hao SG, Xue JZ, Wang Q, Liu ZF. Yuguangia ordinata gen. et. sp. nov., a new lycopsid from the Middle Devonian (Late Givetian) of Yunnan, China, and its phylogenetic implications. Int J Plant Sci. 2007;168:1161–75.
Orlova OA, Zavialova N, Snigirevsky S, Jurina A, Lidskaya A. Kossoviella timanica Petrosjan emend. from the Upper Devonian of North Timan: morphology and spore ultrastructure. Earth Environ Sci Trans R Soc Edinb. 2018;108:355–72.
Liu L, Wang DM, Xue JZ. New lycopsids strobilus with divergent morphologies from the Upper Devonian (Frasnian) of Hunan, China. Acta Palaeontol Sin. 2018;57:333–43.
Xu HH, Wang Y. A new lycopsid cone from the Upper Devonian of western Junggar Basin, Xinjiang, China. Acta Palaeontol Sin. 2002;41:251–8.
Field AR. Systematics and rarity of Australia’s tassel-ferns (Lycopodiaceae: Lycopodiophyta). PhD thesis, James Cook University. 2011.
Senkevitsch MA, Jurina AL, Arkhangelskaya AD. On fructifications, morphology and anatomy of Givetian lepidophytes in Kazakhstan (USSR). Palaeontogr Abt B Palaophytol. 1993;230:43–58.
Cai CY, Chen LZ. On a Chinese Givetian lycopod, Longostachys latisporophyllus Zhu, Hu and Feng, emend.: its morphology, anatomy and reconstruction. Palaeontogr Abt B Palaophytol. 1996;238:1–43.
Balme BE. Fossil in situ spores and pollen grains: an annotated catalogue. Rev Palaeobot Palynol. 1995;87:81–323.
Stevens LG, Hilton J, Rees AR, Rothwell GW, Bateman RM. Systematics, phylogenetics, and reproductive biology of Flemingites arcuatus sp. nov., an exceptionally preserved and partially reconstructed Carboniferous arborescent lycopsid. Int J Plant Sci. 2010;171:783–808.
Phillips TL. Reproduction of heterosporous arborescent lycopods in the Mississippian-Pennsylvanian of Euramerica. Rev Palaeobot Palynol. 1979;27:239–89.
Calder JH, Gibling MR, Scott AC, Davies SJ, Hebert BL. A fossil lycopsid forest succession in the classic Joggins section of Nova Scotia: Paleoecology of a disturbance-prone Pennsylvanian wetland, in Greb SF and DiMichele WA, Wetlands through time. Geol Soc Am Spec Pap. 2006;399:169–95.
DiMichele WA. Diaphorodendron, gen. nov., a segregate from Lepidodendron (Pennsylvanian age). Syst Bot. 1985;10:453–8.
Bonacorsi NK, Leslie AB. Functional diversity and convergence in the evolution of plant reproductive structures. Ann Bot. 2019;123:145–52.
Abbott ML. Lycopod fructifications from the Upper Freeport (No. 7) coal in southeastern Ohio. Palaeontogr Abt B Palaophytol. 1963;112:93–118.
Thomas BA. Carboniferous Lepidodendraceae and Lepidocarpaceae. Bot Rev. 1978;44:321–64.
Bateman RM. Morphometric reconstruction, palaeobiology and phylogeny of Oxroadia gracilis Alvin emend and O. conferta sp. nov.: anatomically-preserved rhizomorphic lycopsids from the Dinantian of Oxroad Bay, SE Scotland. Palaeontogr Abt B Palaophytol. 1992;228:29–103.
Phillips TL, DiMichele WA. Comparative ecology and life-history of arborescent lycopsids in Late Carboniferous swamps of Euramerica. Ann Mo Bot Gard. 1992;79:560–88.
Wang Q, Li CS, Geng BY, Chitaley S. A new species of Lepidostrobus from the Upper Devonian of Xinjiang, China and its bearing on the phylogenetic significance of the order Isoëtales. Bot J Linn Soc. 2003;143:55–6.
Chitaley S, McGregor DC. Bisporangiostrobus harrisii gen. et sp. nov., an eligulate lycopsid cone with Duosporites megaspores and Geminospora microspores from the Upper Devonian of Pennsylvania, USA. Palaeontogr Abt B Palaophytol. 1988;210:127–49.
Acknowledgements
The authors thank Pu Huang (Nanjing Institute of Geology and Palaeontology, CAS), Zhi-Kun Gai (Institute of Vertebrate Paleontology and Paleoanthropology, CAS), Lu Liu (Beijing Museum of Natural History), Zhen-Zhen Deng, Ying-Ying Zhang, Jiang-Nan Yang (Peking University) and Shi-Hui Zhang for help in the field.
Funding
This work is supported by the National Natural Science Foundation of China and Public welfare geological project of Anhui Province (Nos. 42072016, 41802015 and 2021-g-1-4).
Author information
Authors and Affiliations
Contributions
All authors collected fossils; XG, LL and D-M W conceived the project and prepared the figures; XG, MQ and D-M W conducted the observations and took photographs of specimens; XG and LL wrote the manuscript reviewed by all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All field works were conducted in compliance with local, national and international guidelines and regulations. The collected specimens were deposited in the Jianchuan and Yongchuan quarry in Anhui Province, China and consented by these local quarry companies. Thus, no specific permits were required to take samples. This research did not involve experiments or materials on any live plants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Stems and strobili of Guangdedendron micrum from Jianchuan (A–G, I, L–O) and Yongchuan (H, K, P, Q) mines. (A, B) Two sides of a stem with expanded base and leaf cushions. PKUB21005. (C, D) Two sides of a stem. PKUB21006. (E, F) In-situ once-dichotomized stems. Two arrows in Fig. S1E indicating two daughter axes. (G) Oval fissures helically arranged along stem. (H) Oval fissures helically arranged along in-situ stem. (I) Enlargement of portion in Fig. 3R (arrow), showing a ligule pit (Lp) and a vascular bundle scar (Vs). (J) Interpretative line drawing of helically arranged leaf bases according to Fig. 3U, indicating outlines (black lines) and parastichies (red dotted lines) of leaf bases. PKUB16049. (K, L) Dichotomized fertile axes with terminal single strobilus. (M, N) Terminal strobili in pairs. PKUB16097, 16099. (O) Dichotomized fertile axis with partially preserved strobili. (P) Over ten strobili preserved in the same direction. (Q) A possibly once-dichotomized strobilus. Arrow indicating portion enlarged in Fig. 7J. PKUB16064. Scale bars: A–D (5 cm), F, L (2 cm), G, M–O, Q (1 cm), I (2 mm), J (5 mm); diameter of the coin for scale: 2 cm (E, H, K, P).
Additional file 2: Fig. S2.
Enlargement of Fig. 5C (arrow 2), 10 stages (A–J) of serial dégagement showing structure and arrangement of sporophylls, and interpretative line drawings (A’ –J’). Scale bars = 2 mm.
Additional file 3: Fig. S3.
SEM of megaspores of Guangdedendron micrum displaying body, gula and ornamentations. (A) Several megaspores. (B) Megaspore in lateral view. (C) Two megaspores. Arrows 1 and 2 indicating portions enlarged in Fig. S3E and 8 T, respectively. (D) Megaspore in lateral view. Arrow indicating portion enlarged in Fig. S3F. (E, F) Enlargement of portions in Fig. S3C (arrow 1) and S3D (arrow), respectively. Showing tapered and curved spiny ornamentations. (G, H) Tapered spiny ornamentations. (I) Persistent basal portions of spiny ornamentations with upper parts missing or abscised. Scale bars: A (500 μm), B–D (200 μm), E–H (100 μm), I (50 μm).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gao, X., Liu, L., Qin, M. et al. Re-study of Guangdedendron micrum from the Late Devonian Xinhang forest. BMC Ecol Evo 22, 69 (2022). https://doi.org/10.1186/s12862-022-02021-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12862-022-02021-w