Abstract
Background
mRNA interaction with other mRNAs and other signaling molecules determine different biological pathways and functions. Gene co-expression network analysis methods have been widely used to identify correlation patterns between genes in various biological contexts (e.g., cancer, mouse genetics, yeast genetics). A challenge remains to identify an optimal partition of the networks where the individual modules (clusters) are neither too small to make any general inferences, nor too large to be biologically interpretable. Clustering thresholds for identification of modules are not systematically determined and depend on user-settable parameters requiring optimization. The absence of systematic threshold determination may result in suboptimal module identification and a large number of unassigned features.
Results
In this study, we propose a new pipeline to perform gene co-expression network analysis. The proposed pipeline employs WGCNA, a software widely used to perform different aspects of gene co-expression network analysis, and Modularity Maximization algorithm, to analyze novel RNA-Seq data to understand the effects of low-dose 56Fe ion irradiation on the formation of hepatocellular carcinoma in mice. The network results, along with experimental validation, show that using WGCNA combined with Modularity Maximization, provides a more biologically interpretable network in our dataset, than that obtainable using WGCNA alone. The proposed pipeline showed better performance than the existing clustering algorithm in WGCNA, and identified a module that was biologically validated by a mitochondrial complex I assay.
Conclusions
We present a pipeline that can reduce the problem of parameter selection that occurs with the existing algorithm in WGCNA, for applicable RNA-Seq datasets. This may assist in the future discovery of novel mRNA interactions, and elucidation of their potential downstream molecular effects.
Similar content being viewed by others
Background
RNA-Seq, an approach to genome profiling that uses deep-sequencing technologies, has become an increasingly common technique to understand biological phenomena at the molecular level. This method generates quantitative count data on thousands of different mRNAs within each experiment. Comparing the expression of genes between different experimental conditions identifies hundreds of differentially expressed genes, but translating these lists into key functional distinctions between conditions has proved challenging. Since there are thousands of genes in each sample, many researchers filter their gene lists based on different criteria, in order to extract meaningful biological information. One such filtering criteria is based on differential gene expression analysis. Differential gene expression analysis has traditionally been used to determine genes that are statistically significantly differentially expressed between different experimental conditions based on different metrics, such as non-parametric generalized linear models, independent sample t-tests, and log2 fold changes [1]. Even though differential gene expression analysis is one of the most common methods for identifying disease pathways in various experimental conditions, it does not take into consideration the interactions of genes that work as a system to coordinate cellular functions. As a result, using only differential gene expression analysis would limit mechanistic interpretations of the data. mRNAs never act in isolation, but rather in concert with each other and other signaling molecules to define a particular biological pathway and function. Interactions of these signaling molecules can be viewed as networks of interconnected genes and their partners, that are up/down regulated under certain chemical or environmental conditions.
Many algorithms that utilize network theory have found applications in identifying and analyzing these molecular interactions [2,3,4]. Correlation networks are an example of such algorithms, and describe the co-expression of many genes in response to changing conditions, which can ultimately provide information about the underlying molecular mechanisms or biochemical pathways [5, 6]. In particular, the Weighted Gene Co-expression Network Analysis (WGCNA) method, which is provided as an R software package, has been widely used for performing different aspects of weighted correlation network analysis [7]. The co-expression networks used in WGCNA are constructed based on correlations between the quantitative measurements of each gene, and can be described by an n x m matrix X = [xij]. Here the row indices (i = 1,…,n) correspond to different genes, and the column indices (j = 1,…,m) correspond to different sample measurements. While co-expression networks integrate systems-level information to provide a mechanistic interpretation of the dataset, detecting modules (clusters) of closely related mRNAs within the co-expression networks has been a challenging problem. Significant pathways that are identified by different clustering methods often yield tens or hundreds of genes, making biological interpretation and validation challenging. Further, many clustering techniques such as Dynamic Tree Cut utilized in WGCNA rely on user-settable parameters, including minimum module size, and are sensitive to cluster splitting [8, 9]. While many of these module detection methods perform optimally on some datasets, they may fail to effectively detect patterns in other datasets. A practical challenge in terms of discovering modules and determining the total number of modules is the identification of the optimal number of modules in the network, such that the individual modules are neither too large, preventing meaningful interpretation, nor too small, allowing little to no general inference. In general, characterizing and detecting community structures within networks has been a challenging problem in the study of networks [10,11,12]. One of the most commonly used metrics to investigate community structure is a quality index for clustering known as Modularity [13,14,15]. In spite of its popularity, Modularity does have drawbacks. The resolution limit (RL) problem is one of the most significant drawbacks, referring to the problem of maximizing Modularity while hindering one’s ability to detect communities that contain fewer links [16]. To address this problem, several approaches have been introduced [17,18,19,20]. Of these approaches, Modularity Maximization, which utilizes modularity density measures, has been shown to eliminate rather than merely reduce the RL problem in a wide range of networks [20].
In this study, we propose a pipeline using Modularity Maximization [20] to effectively detect and evaluate modules from co-expression networks obtained from the adjacency matrix, utilizing WGCNA [4, 7]. We employ the above technique to characterize the effects of 56Fe irradiation on mice livers, in order to study the potential consequences of deep space travel. In particular, astronauts will be exposed to high-charge, high-energy ions (HZE) during deep space travel. Even at low doses, exposure to HZE can lead to cancer [21, 22]. However, the effects of ions found in the deep space environment on cancer formation is not well understood since very few people have been exposed to space irradiation. As human exploration into deep space increases in the future, characterization of and intervention in irradiation-induced diseases will become more important. Previous studies have shown that irradiation of mice with low-dose HZE, specifically 56Fe ions, significantly increases the incidences of hepatocellular carcinoma (HCC) [23, 24]. HCC is the most common type of liver cancer, and its formation has mainly been studied in the context of terrestrial risk factors such as chronic hepatitis B/C virus infection, exposure to aflatoxin, obesity, smoking, and heavy alcohol consumption [25,26,27]. However, there is limited knowledge of the effects of low-dose 56Fe ion irradiation on the formation of HCC. To better understand the molecular mechanisms of low-dose 56Fe induced HCC, we used RNA-Seq to determine gene expression changes in the hepatic micro-environment of 56Fe ion irradiated compared to non-irradiated control mice at 5 different time points post-irradiation. We hypothesized that mitochondrial pathways could be significantly affected, since mitochondria represent a substantial cellular target volume (4–25% depending on the cell) [28]. In this manuscript, we will describe how WGCNA can be integrated with Modularity Maximization to construct co-expression correlation networks of differentially expressed genes and detect modules using data obtained from RNA-Seq.
Results
Differential gene expression analysis
Results of differential gene expression analysis are shown in Table 1, which includes the total number of differentially expressed genes at each time point, as well as whether genes are up/down-regulated.
Feature selection
A total of 2273 unique differentially expressed genes were identified in comparison between 56Fe irradiated and non-irradiated controls. Genes that were statistically significant with FDR ≤ 10− 5 were used for downstream network analysis; 487 unique genes met the filtration criteria. The significance cut off can be adjusted to a higher value if a researcher decides to investigate more genes, depending on the study goals, experimental conditions, and data variability.
WGCNA
We initially used the WGCNA Dynamic Tree Cut algorithm [7] to identify modules within the selected differentially expressed genes. Module identification with this algorithm requires two parameters to be determined prior to network construction: deepSplit, and minClusterSize. deepSplit can be either logical or an integer in the range 0 to 4. It controls the sensitivity to cluster splitting. Higher values result in smaller clusters. minClusterSize represents the minimum number of genes needed in a module to be considered a separate module. Table 2 shows the results of WGCNA module identification using different minClusterSize values, with a default deepSplit value of 2. As minClusterSize increases, the total number of modules decreases. These values produce different types of networks with differing numbers of unassigned genes. If a gene does not belong to a specific module, it is assigned to the Grey/Unassigned Module. The number of unassigned genes varied between 36 and 73 (shown in the last column of Table 2). At the same time, the total number of modules needs to be within a reasonable range, in order to be able to meaningfully investigate the relationship between genes; 70 different modules each containing a few genes may not provide meaningful information about these co-expression patterns. In our dataset, networks with a total of 11–18 modules provided interpretable co-expression patterns for further investigation, using pathway analysis tools as well as experimental validation. However, these clustering parameters resulted in 61–69 unassigned genes, representing ~ 12–14% of the 487 selected highly significant features.
WGCNA with Modularity Maximization
To optimize the number and size of identified modules as well as reduce the number of unassigned genes (~ 12–14%), we exploited the concept of Modularity Maximization, to assist in finding community structures, as an alternative to utilizing the Dynamic Tree Cut algorithm employed in the standard WGCNA pipeline. Dynamic Tree Cut relies on hierarchical clustering, which is based on the relative distance between genes and samples. Modules are detected by “cutting” these trees, which can lead to many different small modules or a few large modules, depending on the selection of the minClusterSize and deepSplit parameters. Using Modularity Maximization, we were able to identify modules without the need to set these parameters empirically. In particular, the adjacency matrix with a soft threshold beta of 16 (corresponding to R2 = 0.9) was first computed using WGCNA, then a clustering algorithm based on Modularity Maximization was used to automatically find community structures in our dataset. We chose the Modularity Maximization method, since the metric of Modularity has been widely used to detect and assess community structures in social and biological networks since its inception [20, 29,30,31,32].
Utilizing the modularity-based clustering algorithm to identify modules, 14 modules were discovered, and only 14 individual genes were unassigned. The final modularity score was Q = 0.696, which is indicative of a strong modular structure in the network. Figure 1 depicts the 14 modules in the network, and Table 3 shows the number of genes included in each module along with the enriched molecular pathways, as discussed below.
Modularity Maximization Network. Modules identified by performing Modularity Maximization on the network obtained from WGCNA. The module numbers on the network correspond to the modules shown in Table 3. A total of 14 genes were unassigned
Module validation and properties
To explore the biological relevance of the modules, each module was investigated by Ingenuity Pathway Analysis (IPA). Specifically, module 1 was shown to be significant (−log10(p-value) ≥ 1.3) in mitochondrial pathways, such as the Sirtuin Signaling Pathway, Mitochondrial Dysfunction, and Oxidative Phosphorylation [31]. All the genes involved in mitochondrial dysfunction in our dataset were contained in module 1. In particular, Fig. 2 shows that 5 of these genes express different subunits of mitochondrial complex I and III. To validate these results, we performed an additional experimental technique to determine whether complex I activity is reduced in response to 56Fe irradiation. Complex I activity was observed to be decreased in response to exposure to 56Fe HZE ion across all time points as measured by mitochondrial complex I enzyme activity (Fig. 3). The downstream effects of irradiation on mitochondrial functions have been emphasized [33], as mitochondria have been shown to occupy a substantial fraction of the cell volume [28]. Therefore, they may be fairly easily targeted by irradiation as the 56Fe nuclei traverse the cell. The electron transport chain in the mitochondrion is composed of five protein complexes (I-V) that perform a series of oxidation-reduction reactions, in which O2 is the final electron acceptor and is reduced to a water molecule. One of the consequences of this process is the formation of reactive oxygen species (ROS), which is thought to arise from the leakage of electrons, specifically from complex I and III, and to a minor extent complex II [34,35,36]. Using oxygen as the final electron acceptor causes mitochondria to consume about 90% of the body’s oxygen but also become the richest source of ROS [36,37,38,39]. The upregulation of mitochondrial genes shown in Fig. 2, specifically in complex I and complex III, suggests that leakage of electrons from these two complexes results in increased Complex I and III enzyme activity. This leads to further the overexpression of these genes in response to 56Fe irradiation. Other modules could potentially be validated in future experimental designs, involving live animals and more fresh tissues. For example, module 2 can be tested for JAK/STAT signaling. STATs are ubiquitously expressed and mainly activated after stimulation of cytokine receptors. STATs function in the nucleus, but they are first activated in the cytoplasm and have then to be transported into the nuclear compartment [40]. This translocation can be assessed by indirect immunofluorescence. Additionally, STAT signaling can be experimentally validated by pharmacologically inhibiting STAT pathways with specific STAT inhibitors. Similarly, module 9 could be tested for Endoplasmic Reticulum (ER) stress pathways. Several molecular indicators of ER stress could be examined by Western Blots and/or proteomic analysis, which could demonstrate increased or decreased phosphorylation of ER stress proteins.
Mitochondrial Dysfunction Pathway Genes. Five of the genes from module 1 are involved in the mitochondrial dysfunction pathway. Specifically, 4 of them, MT-ND2, MT-ND4, MT-ND5, and MT-ND6 are different subunits of Complex I. MT-CYB, or cytochrome b is part of Complex III/bc which also regulates Complex I. Figure was made using Ingenuity Pathway Analysis (IPA), (QIAGEN Inc., Hilden, Germany)
Discussion
One of the current statistical challenges in identifying co-expression patterns in RNA-Seq data is a robust determination of the number and size of modules appropriate, across a variety of datasets. The choice of an appropriate clustering algorithm that yields the most biologically interpretable networks has been studied using different datasets and methods. For example, a study has investigated Recursive Indirect-Paths Modularity (RIP-M) for module detection in an RNA-Seq co-expression network. Using an influenza vaccine response study, the authors showed that RIP-M had higher cluster assignment accuracy as compared to Newman Modularity, and similar results to WGCNA [41]. We compared WGCNA, RIP-M, and the combined WGCNA-M method based on the Rand Index (RI) [29]. In calculating the RIs, we considered every unassigned gene, as a cluster by itself, since such genes are viewed as not being similar to each other. The RIs for WGCNA-M versus WGCNA, WGCNA-M versus RIP-M, and WGCNA versus RIP-M were 0.909, 0.892, 0.936, respectively. The numbers of genes unassigned to a cluster for WGCNA-M, WGCNA, and RIP-M were 14, 108, and 0, respectively. All of the 14 genes which were unassigned in WGCNA-M were also unassigned in WGCNA. Based on our observed RIs, RIP-M and WGCNA were the most similar when applied to our dataset. Like WGCNA, RIP-M also requires the parameter minModuleSize (minClusterSize) as well as an additional parameter, maxModuleSize, which specifies a target range of module sizes. All genes assigned to a module below minModuleSize are then grouped together and merged into a further module. Modules above maxModuleSize are split in subsequent iterations to arrive at the target range. RIP-M forces all genes to be assigned to a cluster; the 14 genes unassigned by WGCNA and the WGCNA-M approach for our dataset were placed into cluster 1 by RIP-M. Community detection method selection is an important issue in cluster analysis and may greatly influence the results of a study and their biological interpretability. Therefore, it is imperative to select the most suitable method for each specific experimental design, and for the nature of the data being investigated. A complete list of gene cluster-assignments for each method is provided in Supplemental Table 1.
Utilizing Modularity Maximization to detect community structures provides an additional way to construct a network and explore various RNA-Seq datasets; however, WGCNA-M is not limited to this application domain, and can be applied to detect co-expression patterns amongst other omics studies. Protein or lipids can be linked together in networks via a defined functional relationship in a similar fashion. Methodologically, MS-based proteomics and lipidomics tend to have consistency, and coverage issues [42,43,44,45,46,47] as compared to RNA-based high throughput methods. As a result, some network analysis methods as applied to proteomic data may not capture the complexity and nuances underlying biological processes, and alternative approaches may be needed to complement the existing analytical tools. Similar to any other analytical method, the network-based WGCNA-M analysis method must be applied appropriately based on the inherent quality and nature of each dataset. This will allow us to gain robust biological insight and decipher the unique patterns in our data from which we can further understand the complexity and coordinated function of the system being investigated. In the current study, utilization of WGCNA with Modularity Maximization resulted in the identification of biologically interpretable and relevant modules, without the need for parameter optimization.
Conclusions
In this study, we proposed a new pipeline that combines the adjacency matrix notion of WGCNA with Modularity Maximization to find modules that are involved in specific biological pathways. To show the validity of the identified modules, we conducted gene enrichment analysis and experimental validation. Our results showed that mitochondrial pathways that were changed in response to irradiation were contained in the same module. Further, our data indicates that even after performing stringent feature selection focusing on significant genes (FDR ≤ 10− 5), WGCNA-M was still able to identify biologically relevant modules. The use of the WGCNA Dynamic Tree Cut clustering algorithm in our dataset resulted in a high number of unassigned genes (61–69). On the other hand, WGCNA-M reduced the number of unassigned genes to 14 while maintaining an optimal number of modules/specific pathways. The proposed pipeline enables the identification of network and community structures without requiring optimization of the minClusterSize and deepSplit parameters. The increasing number of high throughput genomic datasets, together with the use of appropriate network pipelines, will enable researchers to efficiently investigate molecular mechanisms and pathways involved in different disease processes.
Methods
In this section, we describe the WGCNA combined with Modularity Maximization for community detection pipeline used in the RNA-Seq dataset. Our evaluation strategy was targeted to analyze data from an RNA-Seq experiment of 56Fe irradiated and non-irradiated control mice liver lobes, designed to characterize the microenvironmental changes induced by HZE irradiation (similar to HZE ions encountered in deep space travel,) and that lead to induction of HCC. Our aim is to detect modules (clusters of genes) that are related by correlation across samples, and differ between experimental conditions. The resulting co-expression networks were analyzed using functional enrichment analysis and experimentally validated.
Animal experiments and sample preparation
C57BL/6NCrl mice purchased from Charles River (Wilmington, MA) were used in this experiment. Tumor induction studies and studies of molecular changes in the irradiated tissues can only be conducted in whole animals. Further, based on an extensive literature search and examination of studies previously approved by the institutional animal care and use committees (IACUCs), computer models or cell culture studies are not possible. The numbers of animals used were based on the expected numbers of irradiation-related tumors that would develop if animals were allowed to live out their lifespans. Power calculations for numbers in this study are based on the chi-square test for comparing two proportions, with a two-sided significance level set at 0.05 at 80% power.
The serial sacrifice study included 15 male mice with 3 mice per time point at five time-points (30, 60, 120, 270, and 360 days) post-exposure to HZE, specifically 56Fe irradiation. Additionally, 15 mice were used as controls, at the same time points, resulting in a total of 30 mice for this study. The two groups were: 600 Me V/n 56Fe (0.2 Gy) and non-irradiated/sham-irradiated control. The mice were shipped from the vendor to Brookhaven National Laboratories (BNL) and housed at the BNL animal facility until the time of irradiation at the NASA Space Radiation Laboratory (NSRL). Following irradiation, the animals were shipped to the UTMB Animal Resources Center (ARC), quarantined for 1 month, and maintained in the ARC for the duration of the experiment. Animals were housed in sterile cages with free access to food and water. Facilities at both BNL and UTMB are fully AAALAC accredited, ensuring adequacy of animal care at both animal facilities.
At each of the five time-points, 3 animals from each group were randomly selected and euthanized using CO2 asphyxiation, as per current AVMA guidelines for euthanasia. Prior to euthanasia, animals were weighed and weights recorded. Post euthanasia, tissues of the left lobe of livers were collected, snap-frozen on either dry ice or liquid nitrogen, and stored at − 80 °C until tissues could be extracted for RNA analysis. Livers were sampled by taking two 40-μm thick slices using a cryotome at − 20 °C.
Acquisition of RNA-Seq data
Total RNA was isolated from the liver slices using RNAqueous™ Total RNA Isolation Kit (ThermoFisher Scientific, Waltham, MA), and rRNA was removed using the Ribo-Zero™ rRNA Removal Kit (Illumina, San Diego, CA), prior to library preparation with the Illumina TruSeq RNA Library kit. Samples were sequenced in a paired-end 50 base format on an Illumina HiSeq 1500. FastQC was utilized for the quality evaluation of FASTQ files [48]. All FastQC reports were examined prior to the analysis of RNA-Seq samples. The total number of reads used in analysis varied between 23 and 35 million. A complete list of samples, and related reads information is available in Table 4.
Reads were aligned to the mouse GRCm38 reference genome using the STAR alignment program, version 2.5.3a, with the recommended ENCODE options [49]. The-quantMode GeneCounts option was used to obtain read counts per gene based on the Gencode release M14 annotation file [50].
Differential gene expression analysis
Raw RNA-Seq data of 51,826 genes from 15 non-irradiated control and 15 56Fe irradiated C57 mice liver tissue samples were subjected to differential gene expression analysis. All calculations and statistics were performed using statistical software R (R Foundation for Statistical Computing, Vienna, Austria) (version 3.5.1) [51]. Differential gene expression analysis was conducted using R software package edgeR [52, 53]. First, normalization factors were calculated to scale the raw library sizes. In addition, dispersion parameters based on generalized linear models (GLM) were estimated; in particular, the common dispersion for negative binomial GLMs, trended dispersion for negative binomial GLMs using the power method, and empirical Bayes tagwise dispersions for negative binomial GLMs [53, 54]. Statistical tests were then conducted for every time point, to compare between 56Fe irradiated and non-irradiated control samples, using a quasi-likelihood negative binomial generalized log-linear model for count data [55,56,57]. The Benjamini-Hochberg correction was applied, and genes with FDR ≤ 0.05 & fold change ≥1.5 (|(log2(Fold Change)| ≥ 0.59—up/down regulated) were extracted.
Feature selection (FS)
Final differential gene expression analyses for all time points were combined. For genes differentially expressed at multiple time points, the lowest FDR was kept. The list was further filtered to keep only genes with FDR ≤ 10− 5. For the final selected gene list, raw variance stabilized normalized count data were retrieved from every RNA-Seq sample (n = 30) using the R package DESeq2 [58] This variance stabilized normalization method was specifically selected because it has proven useful for network construction using WGCNA methodology (The WGCNA FAQ).
WGCNA
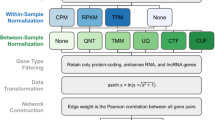
The gene expression profiles were comprised of 51,826 genes from 30 samples. Constructing a co-expression network on this original list without filtering could not meet a power threshold that corresponded to (R2 = 0.9) as recommended by WGCNA, and did not yield any biologically interpretable network. As a result, we first performed the feature selection based on differential gene expression analysis and FDR rank list (step 1–2 in Fig. 4, and described above) and then constructed the WGCNA network on genes given by this feature selection (step 3 in Fig. 4). WGCNA was performed on differentially expressed genes with FDR ≤ 10− 5 & fold change ≥1.5 (up/down-regulated). WGCNA analysis was performed per the methodology publication (step 4–7 in Fig. 4) [7].
WGCNA with Modularity Maximization
To evaluate the effect of feature selection on the median cluster size, we performed Modularity Maximization analysis on co-expression data derived by WGCNA applied to gene lists filtered over a range of FDR values. As shown in Fig. 5, the FDR value of 10− 5 led to the largest median cluster size, in this particular dataset. The features’ significance threshold can be optimized by plotting median cluster sizes at different FDR values. To derive clusters, the following steps were used. An adjacency matrix based on the Pearson correlation with the soft threshold was calculated by WGCNA [7]. The power threshold parameter was set to 16, corresponding to an R2 value of 0.9, which reflects a scale-free topology in which adjacency between all differential genes was calculated by a power function (step 3 in Fig. 4). The adjacency matrix was then filtered to only keep pairs of genes with a Pearson correlation of ≥0.7 (step 4 in Fig. 4). Then, module identification was performed using the Modularity Maximization clustering method (step 5 in Fig. 4) [20, 59]. Final modules were visualized using the ExplodeLayout algorithm (step 6 in Fig. 4) [60].
Plot to visualize different FDR thresholds using the Modularity Maximization Algorithm. The plot shows the change in the median number of clusters detected using Modularity Maximization, as the FDR cutoff is varied. The numbers next to each point designate the number of genes and the number of modules in the corresponding network. The module with the largest median size was chosen for further analysis, since small clusters are difficult to interpret
Module statistical analysis
To determine whether the modules were observed by chance, the significance of the results was evaluated by comparing them to the average modularity of 1000 permutations of the weighted and thresholded co-expression network adjacency matrix. Each permutation of the network would preserve the number and weight of all the links but randomly shuffle them; thus it should still meet the scale-free network distribution criteria. Based on the 1000 permutations, we obtained a z-score of 86.8 for our modularity, indicating a strongly significant modular structure in the co-expression network as compared to random.
Mitochondrial complex I enzyme activity assay
The mitochondria isolation kit for tissue (Abcam, ab110168) was used to isolate mitochondria from mice liver lobes. Complex 1 enzyme activity was monitored with a colorimetric microplate assay (Abcam, ab110168) using the isolated mitochondria from the liver.
Functional Enrichment Analysis
To determine whether the co-expression modules were biologically meaningful, functional enrichment analysis was performed separately on every module. Significant functional pathways (−log10(p-value) ≥ 1.3) for each module were evaluated using Ingenuity Pathway Analysis (IPA) (QIAGEN Inc., Hilden, Germany) [31].
Availability of data and materials
The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (Nia et al., 2020) and are accessible through GEO Series accession number GSE136165 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE136165). The R and C scripts written for this publication are accessible through https://github.com/annamnia/Efficient-Identification-of-Multiple-Pathways-RNA-Seq-Analysis-of-Livers-from-56Fe-Ion-Irradiated-M
Abbreviations
- WGCNA:
-
Weighted Gene Co-Expression Network Analysis
- WGCNA-M:
-
Weighted Gene Co-Expression Network Analysis with Modularity Maximization
- HZE:
-
High Charge High Energy Ions
- IPA:
-
Ingenuity Pathway Analysis
References
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47.
Barabasi AL, Oltvai ZN. Network biology: understanding the cell's functional organization. Nat Rev Genet. 2004;5(2):101–13.
Hartwell LH, Hopfield JJ, Leibler S, Murray AW. From molecular to modular cell biology. Nature. 1999;402(6761 Suppl):C47–52.
Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4:Article17.
Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95(25):14863–8.
Stuart JM, Segal E, Koller D, Kim SK. A gene-coexpression network for global discovery of conserved genetic modules. Science (New York, NY). 2003;302(5643):249–55.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9(1):559.
Langfelder P, Zhang B, Horvath S. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics (Oxford, England). 2008;24(5):719–20.
Jiang B, Gribskov M. Assessment of subnetwork detection methods for breast cancer. Cancer Informat. 2014;13(Suppl 6):15–23.
Newman MEJ. Detecting community structure in networks. Eur Physical J B Condensed Matter. 2004;38(2):321–30.
Danon L, Díaz-Guilera A, Duch J, Arenas A. Comparing community structure identification. J Stat Mechanics Theory and Experiment. 2005;2005(09):P09008.
Fortunato S. Community detection in graphs. Phys Rep. 2010;486(3–5):75–174.
Girvan M, Newman MEJ. Community structure in social and biological networks. Proc Natl Acad Sci. 2002;99(12):7821–6.
Newman MEJ. The structure and function of complex networks. SIAM Rev. 2003;45(2):167–256.
Newman ME, Girvan M. Finding and evaluating community structure in networks. Phys Rev E Stat Nonlinear Soft Matter Phys. 2004;69(2 Pt 2):026113.
Fortunato S, Barthelemy M. Resolution limit in community detection. Proc Natl Acad Sci. 2007;104(1):36–41.
Ronhovde P, Nussinov Z: Local resolution-limit-free Potts model for community detection. Phys Rev E. 2010;81(4):046114.
Arenas A, Fernández A, Gómez S. Analysis of the structure of complex networks at different resolution levels. New J Phys. 2008;10(5):053039.
Aldecoa R, Marín I: Deciphering Network Community Structure by Surprise. PloS one. 2011;6(9):e24195.
Chen T, Singh P, Bassler KE. Network community detection using modularity density measures. J Stat Mechanics Theory and Experiment. 2018;2018(5):053406.
Cucinotta FA, Durante M. Cancer risk from exposure to galactic cosmic rays: implications for space exploration by human beings. Lancet Oncol. 2006;7(5):431–5.
Durante M, Cucinotta FA. Heavy ion carcinogenesis and human space exploration. Nat Rev Cancer. 2008;8(6):465–72.
Weil MM, Bedford JS, Bielefeldt-Ohmann H, Ray FA, Genik PC, Ehrhart EJ, Fallgren CM, Hailu F, Battaglia CL, Charles B, et al. Incidence of acute myeloid leukemia and hepatocellular carcinoma in mice irradiated with 1 GeV/nucleon (56)Fe ions. Radiat Res. 2009;172(2):213–9.
Weil MM, Ray FA, Genik PC, Yu Y, McCarthy M, Fallgren CM, Ullrich RL. Effects of 28Si ions, 56Fe ions, and protons on the induction of murine acute myeloid leukemia and hepatocellular carcinoma. PLoS One. 2014;9(7):e104819.
Yu MW, Lien JP, Chiu YH, Santella RM, Liaw YF, Chen CJ. Effect of aflatoxin metabolism and DNA adduct formation on hepatocellular carcinoma among chronic hepatitis B carriers in Taiwan. J Hepatol. 1997;27(2):320–30.
de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–15.
El-Serag HB. Hepatocellular Carcinoma. N Engl J Med. 2011;365(12):1118–27.
Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001;61(10):3894–901.
Rand WM. Objective criteria for the evaluation of clustering methods. J Am Stat Assoc. 1971;66(336):846–50.
Hubert L, Arabie P. Comparing partitions. J Classif. 1985;2(1):193–218.
Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics (Oxford, England). 2013;30(4):523–30.
Chauhan R, Ravi J, Datta P, Chen T, Schnappinger D, Bassler KE, Balázsi G, Gennaro ML. Reconstruction and topological characterization of the sigma factor regulatory network of mycobacterium tuberculosis. Nat Commun. 2016;7:11062.
Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327(1–2):48–60.
Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237(2):408–14.
Nohl H. A novel superoxide radical generator in heart mitochondria. FEBS Lett. 1987;214(2):269–73.
Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochemical J. 1973;134(3):707.
Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29(3–4):222–30.
Babior BM. NADPH Oxidase: An Update. Blood. 1999;93(5):1464.
Los M, Schenk H, Hexel K, Baeuerle PA, Dröge W, Schulze-Osthoff K. IL-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J. 1995;14(15):3731–40.
Behrmann I, Smyczek T, Heinrich PC, Schmitz-Van de Leur H, Komyod W, Giese B, Müller-Newen G, Haan S, Haan C. Janus Kinase (Jak) Subcellular Localization revisited THE exclusive membrane localization of endogenous janus kinase 1 by cytokine receptor interaction uncovers the Jak·receptor complex to be equivalent to a receptor tyrosine kinase. J Biol Chem. 2004;279(34):35486–93.
Rahmani B, Zimmermann MT, Grill DE, Kennedy RB, Oberg AL, White BC, Poland GA, McKinney BA. Recursive indirect-paths modularity (RIP-M) for detecting community structure in RNA-Seq co-expression networks. Front Genet. 2016;7:80.
Bell AW, Deutsch EW, Au CE, Kearney RE, Beavis R, Sechi S, Nilsson T, Bergeron JJM. A HUPO test sample study reveals common problems in mass spectrometry–based proteomics. Nat Methods. 2009;6(6):423–30.
Liu H, Sadygov RG, Yates JR. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76(14):4193–201.
White MY, Brown DA, Sheng S, Cole RN, O'Rourke B, Van Eyk JE: Parallel Proteomics to Improve Coverage and Confidence in the Partially Annotated Oryctolagus cuniculus Mitochondrial Proteome 2011, 10(2):M110.004291-M110M004110.
Goh WWB, Lee YH, Chung M, Wong L: How advancement in biological network analysis methods empowers proteomics. Proteomics. 2012;12(4–5):550–563.
Lay JO, Liyanage R, Borgmann S, Wilkins CL. Problems with the “omics”. TrAC Trends Anal Chem. 2006;25(11):1046–56.
Navas-Iglesias N, Carrasco-Pancorbo A, Cuadros-Rodríguez L. From lipids analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part II: Analytical lipidomics. 2009;28(4):393–403.
Andrews S: FastQC. In., 0.11.8 edn; 2018.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics (Oxford, England). 2013;29(1):15–21.
Frankish A, Diekhans M, Ferreira A-M, Johnson R, Jungreis I, Loveland J, Mudge JM, Sisu C, Wright J, Armstrong J, et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2018;47(D1):D766–73.
Team RC. R: a language and enviornment for statistical computing. Vienna: R Foundation for Statistical Computing. 2013.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics (Oxford, England). 2010;26(1):139–40.
McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40(10):4288–97.
Cox DR, Reid N. Parameter Orthogonality and approximate conditional inference. J R Stat Soc Ser B Methodol. 1987;49(1):1–39.
Lun ATL, Chen Y, Smyth GK. It’s DE-licious: A Recipe for Differential Expression Analyses of RNA-seq Experiments Using Quasi-Likelihood Methods in edgeR. In: Mathé E, Davis S, editors. Statistical Genomics: Methods and Protocols. New York: Springer New York; 2016. p. 391–416.
Lund Steven P, Nettleton D, McCarthy Davis J, Smyth Gordon K. Detecting Differential Expression in RNA-sequence Data Using Quasi-likelihood with Shrunken Dispersion Estimates. In: Statistical applications in genetics and molecular biology, vol. 11; 2012.
Phipson B, Lee S, Majewski IJ, Alexander WS, Smyth GK. Robust hyperparameter estimation protects against hypervariable genes and improves power to detect differential expression. Ann Appl Stat. 2016;10(2):946–63.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
Treviño S, Nyberg A, Del Genio CI, Bassler KE. Fast and accurate determination of modularity and its effect size. J Stat Mechanics Theory Exper. 2015;2015(2):P02003.
Bhavnani SK, Chen T, Ayyaswamy A, Visweswaran S, Bellala G, Rohit D, Kevin EB. Enabling comprehension of patient subgroups and characteristics in large bipartite networks: implications for precision medicine. AMIA Jt Summits Transl Sci Proc. 2017;2017:21–9.
Acknowledgments
The authors would like to thank the NASA Ground-Based Studies in Space Radiobiology (NNX15AD65G), NASA/Texas Space Grant Consortium Fellowship (BLB), Shirley Patricia Parker & Katherina Siebert Award for Excellence in Oncologic Scholarship (AMN and BLB) for funding of this research and UTMB for support of this research.
Funding
This work was supported by the Ground-Based Studies in Space Radiobiology through the National Aeronautics and Space Administration NASA (NNX15AD65G). The funders did not participate in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
AMN analyzed the data, developed the computational methodology, and wrote the manuscript. AMN, TC, and MRE conceived and designed the experiment and methodology. SKB participated in the design of the computational methodology. RLU participated in the design of the biological study. BLB conceieved the experimental methodology (tissue collection, RNA extraction/purification, complex 1 assay) and performed the experiments. KK helped with processing the RNA-Seq data. All authors participated in the analysis methodology and interpretation of results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The national or institutional guidelines were used for the care and use of animals, and approval for the experiments was obtained from the ethical committees of The University of Texas Medical Branch.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Supplementary Table 1.
Gene cluster-assignment comparison. Complete list of gene cluster-assignments using Recursive Indirect-Paths Modularity (RIP-M), Weighted Gene Co-Expression Network Analysis (WGCNA), and Weighted Gene Co-Expression Network Analysis with Modularity Maximization (WGCNA-M).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nia, A.M., Chen, T., Barnette, B.L. et al. Efficient identification of multiple pathways: RNA-Seq analysis of livers from 56Fe ion irradiated mice. BMC Bioinformatics 21, 118 (2020). https://doi.org/10.1186/s12859-020-3446-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12859-020-3446-5