Abstract
*
Background In the search for therapeutic peptides for disease treatments, many efforts have been made to identify various functional peptides from large numbers of peptide sequence databases. In this paper, we propose an effective computational model that uses deep learning and word2vec to predict therapeutic peptides (PTPD).
*
Results Representation vectors of all k-mers were obtained through word2vec based on k-mer co-existence information. The original peptide sequences were then divided into k-mers using the windowing method. The peptide sequences were mapped to the input layer by the embedding vector obtained by word2vec. Three types of filters in the convolutional layers, as well as dropout and max-pooling operations, were applied to construct feature maps. These feature maps were concatenated into a fully connected dense layer, and rectified linear units (ReLU) and dropout operations were included to avoid over-fitting of PTPD. The classification probabilities were generated by a sigmoid function. PTPD was then validated using two datasets: an independent anticancer peptide dataset and a virulent protein dataset, on which it achieved accuracies of 96% and 94%, respectively.
*
Conclusions PTPD identified novel therapeutic peptides efficiently, and it is suitable for application as a useful tool in therapeutic peptide design.
Similar content being viewed by others
Background
Cancer continues to a burden worldwide and its frequency is expected to double in the coming decades [1]. Available treatment regimens include radiation therapy, targeted therapy, and chemotherapy, all of which are often accompanied by harmful side effects and result in high financial costs for both individuals and society [2, 3]. Anticancer peptides (ACPs) provide a new cost-efficient approach to cancer treatment, have minimal side effects, and have been shown to be promising in the treatment of various tumours by targeting mitochondria or membranolytic mechanisms [4]. Although progress has been made in preclinical applications of peptide-based methods against cancer cells, the mechanism behind the success of ACP treatments are still elusive. It is therefore highly important to be able to efficiently identify ACPs in both cancer research and drug development purposes. Due to the high costs and lengthy process of identifying ACP experimentally, various computational models have been developed to identify ACPs from peptide sequences. These advances include iACP development by g-gap dipeptide component (DPC) optimization [5, 6], and SAP peptide identification by 400-dimensional features with g-gap dipeptide pruned by the maximum relevance-maximum distance method [7]. In addition, various types of amino acid compositions (AACs) of peptide sequences have been introduced to develop prediction models such as Chou’s pseudo amino acid composition (PseAAC) [8], combinations of AACs, average chemical shifts (acACS) and reduced AAC (RAAC) [6], pseudo g-Gap DPC, amphiphilic PseAAC, and reduced amino acid alphabet (RAAAC) [9]. Other methods include computational tools developed based on the q-Wiener graph indices for ACP predication [10]. In addition, machine learning methods were adopted to promote model efficiency [6, 9, 11]. Several models have utilized support vector machine (SVM) and random forest (RF) machine learning methods [11, 12], combinations of the quantitative outcomes of individual classifiers (RF, K-nearest neighbor, SVM, generalized neural network and probabilistic neural network) [9], or a pool of SVM-based models trained by sequence-based features [13].
Novel computational models based on machine learning have also been applied to identify virulent proteins in infection pathophysiology. Virulent proteins consist of a diverse set of proteins and are important for host invasion and pathogenesis. Drug resistance to bacterial pathogens has created an urgent need to identify novel virulent proteins that may facilitate drug target and vaccine developments. Several computational models have been developed to identify virulent proteins. The first methods were developed based on similarity search methods such as the Basic Local Alignment Search Tool (BLAST) [14] and Position-specific Iterated BLAST (PSI-BLAST) [15]. Machine learning algorithms for predicting virulent proteins have also been reported that apply SVM-based models based on AAC and DPC [16], an ensemble of SVM-based models trained with features extracted directly from amino acid sequences [17], a bi-layer cascade SVM model [18], and a model based on an SVM and a variant of input decimated ensembles and their random subspace [19]. Studies have also focused on conducting feature extraction of sequences such as protein presentations, by using amino acid sequence features and evolutionary information of a given protein [19]. Moreover, a computational tool based on the q-Wiener graph indices was also proposed to effectively predict virulent proteins [10]. Despite substantial progress, identifying specific peptides from massive protein databases remains challenging.
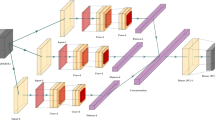
To date, deep learning applications have been successful in numerous fields other than medicine, including image classification and recognition [20–22], object detection [23, 24], scene recognition [25], character recognition [26], sentence classification [27], chromatin accessibility prediction [28] and so on. Inspired by these successful deep learning applications, we propose a novel computational model called PTPD, which is based on deep learning, to identify ACPs and virulent proteins from peptide sequences (Fig. 1). To verify the efficiency of our approach, we also performed ACP and virulent protein prediction on publicly available datasets [12, 18, 29]. Our results show that PTPD is able to identify ACPs and virulent proteins with high efficiency.
Methods
Datasets
The ACP datasets were extracted from publicly available resources [12, 29]. A total of 225 validated ACPs from the AMPs dataset and the database of Anuran defence peptides (DADP) [30] were used as positive samples, while 2,250 randomly selected proteins from the SwissProt protein database were used as negative samples. This dataset was used to build the model. An alternative dataset and two balanced datasets were employed to evaluate the model. To compare our methods with other existing methods, we also obtained an independent dataset (i.e. Hajisharifi-Chen (HC)) from a previous study [12]. The HC dataset, which contains 138 ACPs and 206 non-ACPs, was also employed to develop prediction models in [31, 32].
The virulent protein datasets were obtained from VirulentPred [18] and NTX-pred method [16]. We adopted the SPAAN adhesins dataset, which contains 469 adhesion and 703 non-adhesion proteins, to build the PTPD model for virulent protein prediction. The neurotoxin dataset was applied as an independent dataset to evaluate the model. It contains 50 neurotoxins (positive samples) and 50 non-virulent proteins (negative samples) obtained by the NTX-pred method [16].
Representation of k-mers by word2vec
Each peptide sequence was divided into k-mers by windowing method as previously described in [33, 34]. To represent the k-mers, we used the publicly available word2vec tool, which creates high-quality word embedding vectors according to a large number of k-mers.
The word2vec tool computes vector representations of words and has been widely applied in many natural language processing tasks as well as other research applications [35–38]. Two learning algorithms are available in word2vec: continuous bag-of-words and continuous skip-gram. These algorithms learn word representations to help to predict other words in the sentence. The skip-gram model in word2vec trains the word vectors of each word based on the given corpus. Given a word (W(t)) in a sentence, skip-gram can predict the probabilities P(W(t+i)|W(t)) of nearby words Wi(t−k≤i≤t+k) based on the probability of the current word W(t). Each word vector reflects the positions of the nearby words, as illustrated in Fig. 2. The goal of the skip-gram model is to maximize the following value:
where k denotes the size of the window, and W(t+i)(−k≤i≤k) denotes k words near the current word W(t), and n denotes the number of words.
Because word2vec can reflect the positional relationships of words in a sequence and preserve structural information, we treated the k-mers as the words. Using word2vec, the word embedding vector of each k-mer with 100 dimensions was obtained.
Input layer
After constructing the word representation of all the k-mers, we mapped the peptide sequence to numeric vectors. First, we used stride st to divide a peptide sequence S with length L0 into k-mers of length k. The number of k-mers and the subsequent number of vectors varied because the peptide sequences (S) had different original lengths (L0). The vectors for one peptide were set to be the same length L-the length of the longest vector for those peptide sequences. Vectors with lengths shorter than L zero-padded at the end as in the natural language process. Finally, the peptide sequence was converted to a vector \(\tilde {S}\) by the word vectors with dimensions L×100.
To prevent over-fitting and to improve model generalization, dropout was applied to a fraction of the inputs (i.e., a portion of the inputs was randomly set to zero).
Feature map
To extract features, a set of one-dimensional convolution filters was adopted to process the vectors of peptide sequences. The convolution kernel was a shape kernel with a size of (c×100). We used three types of convolution filters with sizes of three, four, and five. All the kernels performed convolutions on the entire representation vector. For example, using one convolution kernel with a size of (c×100), the feature map was constructed as follows:
where f(m) denotes the mth element of the feature map, ReLU denotes the rectified linear unit (ReLU) activation function, w(i, j) denotes the weight of the convolution kernel compiled by training, c denotes the size of filter, and \(\tilde {S}_{m}\) denotes the mth block of the representation vector of the peptide sequence. ReLU [39] was used to set the negative results of the convolution calculation to zero, and is defined as follows:
Multiple filters were used for each filter type. Let nc be the number of convolution filters, we applied
To reduce the spatial dimensions of the feature maps, max pooling was adopted following a convolution operation. A max pooling layer with a pooling window of size 2×1 and a stride of 2 was defined by the function
where
The results were finally merged concatenated as follows:
where c1=3, c2=4, and c3=5 denote the three filter sizes we used. Then FAm was processed by a fully connected hidden layer to produce FM=ReLU(FAmWft), where ReLU represents a rectified linear activation unit, and Wft is the weight matrix of the fully-connected layer.
Classification
The last layer of PTPD adopted a fully-connected layer to obtain a single output. A sigmoid activation function was set to generate the output probability between zero and one, which was defined as
Loss function and optimizer
A binary cross entropy loss function was used to train the model. The model was trained with the RMSprop optimizer. The binary cross entropy loss function between the predictions and targets was defined as
The total cost of the two classes was
Model evaluation
The performance of PTPD was evaluated by various metrics, including the sensitivity (Sn), specificity (Sp), prediction accuracy (Acc), Matthew’s correlation coefficient (MCC), and the area under the curve (AUC) of the receiver-operating characteristic (ROC) curve. These metrics were defined as follows:
where TP denotes true positives, TN denotes true negatives, FP denotes false positives, FN denotes false negatives.
Results
Model performance
To verify the proposed method, we executed the proposed model on ACPs and virulent protein datasets. Each dataset was randomly divided into three groups. The first group, which consisted of 75% of the complete dataset, was used to train the model. The second group of data, 15% of the entire dataset, was used to minimize over-fitting. The third group, 10% of the entire dataset, was used to evaluate the performance of the trained PTPD model. For ACP identification, the performance of PTPD was first measured using the test data from the main dataset, and then further tested on an alternative dataset. Furthermore, we also evaluated the performance of PTPD on two types of balanced datasets (Table 1).
PTPD achieved high performance scores of Sn = 94.2%, Sp = 86.2%, Acc = 90.2%, Mcc = 0.8, and AUC = 0.97, respectively. Moreover, to evaluate the generalizability or robustness of the prediction model, we executed PTPD on the independent HC dataset, as shown in Table 1. The AUCs of the five data sets were all higher than 0.97. Thus, PTPD offers stable performance even on unbalanced data sets (Table 1).
To evaluate the performance of PTPD, we conducted an evaluation on the test data of the SPAAN adhesins dataset. We also tested the performance of PTPD on an independent Neurotoxins dataset (Table 2).
The five performance metrics (Sn, Sp, Acc, MCC, and AUC) achieved by PTPD on the virulent protein dataset are higher than 95.6%, 73.3%, 88.2%, 0.7, and 0.93, respectively, which confirms the good performance of PTPD. Sp on the SPAAN adhesins dataset had a relatively lower value (Table 2).
Comparison with the state-of-the-art methods
For verification purposes, we compared the proposed method with other state-of-the-art methods on the identification of ACPs and virulent proteins on two independent datasets.
Comparison performed on independent aCP dataset
To further evaluate the performance of PTPD to predict ACPs, we compared its performance with those of some state-of-the-art methods (i.e., AntiCP [29], MLACP [12], and mACPpred [40]) on an independent HC dataset (Table 3 and Fig. 3). PTPD performed equally as well as MLACP (RF) on the HC dataset. The proposed PTPD has the highest sensitivity, relatively higher AUC, ACC, and MCC, and intermediate specificity. Thus, PTPD offers relatively better generalizability on independent datasets than do the other tested state-of-the-art methods for identifying ACPs.
Comparison of different methods on the HC dataset. a Sn, Sp and Acc of different methods. b MCC and AUC of different methods. Sn: the sensitivity; Sp: the specificity; Acc: the prediction accuracy; MCC: Matthew’s correlation coefficient; AUC: the area under the curve of the receiver-operating characteristic curve
Comparison performed on an independent virulent protein dataset
We also compared the performance of PTPD with that of q-FP [10], AS and 2Gram [41], VirulentPred [18], and NTX-pred [16] on a bacterial neurotoxins dataset (Table 4 and Fig. 4).
Comparison of different methods on the neurotoxin virulent proteins dataset. a Sn, Sp and Acc of different methods. b MCC and AUC of different methods. Sn: the sensitivity; Sp: the specificity; Acc: the prediction accuracy; MCC: Matthew’s correlation coefficient; AUC: the area under the curve of the receiver-operating characteristic curve
Again, the overall performance of PTPD was relatively better than those of other methods. Thus, we can conclude that PTPD is able to predict potential virulent proteins with high accuracy.
Parameter settings
Because model convergence is related to the learning rate, we set the learning rate variously to 0.5, 0.1, 0.05, 0.01, 0.005, 0.001, 0.0005, 0.0001, 0.00005, and 0.00001 for ACP training. The accuracy and loss values under the different learning rates are shown in Fig. 5.
The model achieved its highest accuracy (98.5%) and the lowest loss (0.03) when the learning rate was set to 0.0001, which was subsequently selected for model training. The detailed parameter settings are shown in Table 5.
Discussion
The model performance presented in this study suggests that PTPD possesses good generalizability and robustness. The comparison between PTPD and other methods showed that PTPD outperformed the other tested state-of-the-art methods for independent data analysis.
The performance of PTPD benefits from several major factors: (1) word2vec was applied to extract representation vectors of k-mers to consider the co-existence information of k-mers in peptide sequences. (2) For the feature map, a convolution neural network (CNN) architecture was used to automatically extract features without domain experts. (3) Dropout and max-pooling operations were adopted to avoid over-fitting.
Conclusions
Identifying new ACPs and virulent proteins is an extremely labour-intensive and time-consuming process. In this paper, we proposed a computational model based on deep learning that predicts therapeutic peptides with in a highly efficient manner. We then present a new deep learning-based prediction model that achieves better recognition performances compared to those of other state-of-the-art methods. We first trained a model to extract feature vectors of all k-mers using word2vec. Next, the peptide sequences were converted into k-mers, and each peptide sequence was represented by the vectors compiled by word2vec. The CNN then automatically extracted features without expert assistance, which decreases the reliance on domain experts for feature construction. The CNN was configured with three types of filters, and dropout and max-pooling operations were applied to avoid over-fitting. After fusing the features, ReLU activation was used to replace any negative values in the output of the CNN layer with zeros. Finally, the sigmoid function was used to classify the peptide.
The performance and generalizability of PTPD were verified on two independent datasets. The trained model achieved AUCs of 0.99 and 0.93, respectively, which confirmed that the proposed model can effectively identify ACPs and virulent proteins.
In summary, the PTPD model presented in this paper outperformed other tested methods. Nevertheless, the approach still suffers because the inability to assign values regarding which features are most important for identifying favourable bioactivity. In future studies on potential structures and feature selection methods, we may consider other available network architectures such as generative adversarial networks. Some new methods that have been successfully applied to natural language processes might also facilitate further research. Our study confirmed that PTPD is an effective means for identifying and designing novel therapeutic peptides. Our approach might be extensible to other peptide sequence-based predictions, including antihypertensive [42, 43], cell-penetrating [44], and proinflammatory [45].
Abbreviations
- AAC:
-
Amino acid composition
- Acc:
-
Accuracy
- ACP:
-
Anticancer peptide
- AUC:
-
The area under ROC curve
- CNN:
-
Convolution neural networks
- DADP:
-
Defence peptide
- FN:
-
False negative
- FP:
-
False positive
- MCC:
-
Matthew’s correlation coefficient
- Pse-g-Gap DPC:
-
Pseudo g-Gap dipeptide composition
- PseAAC:
-
Pseudo amino acid composition
- PSI-BLAST:
-
Position specific iterated BLAST
- PSSM:
-
Position Specific Scoring Matrices
- PTPD:
-
Prediction of therapeutic peptide by deep learning and word2Vec
- RAAAC:
-
Reduced amino acid alphabet
- RAAC:
-
Reduced amino acid composition
- ReLU:
-
Rectified linear unit
- RF:
-
Random Forest
- SAP:
-
Sequence-based model
- Sn:
-
Sensitivity
- Sp:
-
Specificity
- SVM:
-
Support vector machine
- TN:
-
True negative
- TP:
-
True positive
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. Ca-Cancer J Clin. 2015; 65(2):87—108.
Al-Benna S, Shai Y, Jacobsen F, Steinstraesser L. Oncolytic Activities of Host Defense Peptides. Int J Mol Sci. 2011; 12(11):8027.
Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamraju S. Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem. 2002; 234(1):119–24.
Huang Y, Feng Q, Yan Q, Hao X, Chen Y. Alpha-helical cationic anticancer peptides: a promising candidate for novel anticancer drugs. Mini-Rev Med Chem. 2015; 15(1):73–81.
Chen W, Ding H, Feng P, Lin H, Chou KC. iACP: a sequence-based tool for identifying anticancer peptides. Oncotarget. 2016; 7(13):16895–909.
Li FM, Wang XQ. Identifying anticancer peptides by using improved hybrid compositions. Sci Rep. 2016; 6:33910.
Xu L, Liang G, Wang L, Liao C. A Novel Hybrid Sequence-Based Model for Identifying Anticancer Peptides. Genes. 2018; 9(3):158.
Hajisharifi Z, Piryaiee M, Mohammad Beigi M, Behbahani M, Mohabatkar H. Predicting anticancer peptides with Chou’s pseudo amino acid composition and investigating their mutagenicity via Ames test. J Theor Biol. 2014; 341:34–40.
Akbar S, Hayat M, Iqbal M, Jan MA. iACP-GAEnsC: Evolutionary genetic algorithm based ensemble classification of anticancer peptides by utilizing hybrid feature space. Artif Intell Med. 2017; 79:62–70.
Xu C, Ge L, Zhang Y, Dehmer M, Gutman I. Computational prediction of therapeutic peptides based on graph index. J Biomed Inf. 2017; 75:63–9.
Manavalan B, Basith S, Shin TH, Choi S, Kim MO, Lee G. MLACP: machine-learning-based prediction of anticancer peptides. Oncotarget. 2017; 8(44):77121–36.
Manavalan B, Basith S, Shin TH, Choi S, Kim MO, Lee G. MLACP: machine-learning-based prediction of anticancer peptides. Oncotarget. 2017; 8(44):77121.
Wei L, Zhou C, Chen H, Song J, Su R. ACPred-FL: a sequence-based predictor using effective feature representation to improve the prediction of anti-cancer peptides. Bioinformatics. 2018; 34(23):4007–16.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990; 215(3):403–10.
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al.Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997; 25(17):3389–402.
Saha S, Raghava GPS. Prediction of neurotoxins based on their function and source. In Silico Biol. 2007; 7(4-5):369–87.
Nanni L, Lumini A. An ensemble of support vector machines for predicting virulent proteins. Expert Syst Appl. 2009; 36(4):7458–62.
Garg A, Gupta D. VirulentPred: a SVM based prediction method for virulent proteins in bacterial pathogens. BMC Bioinf. 2008; 9(1):62.
Nanni L, Lumini A, Gupta D, Garg A. Identifying Bacterial Virulent Proteins by Fusing a Set of Classifiers Based on Variants of Chou’s Pseudo Amino Acid Composition and on Evolutionary Information. IEEE/ACM Trans Comput Biol Bioinf. 2012; 9(2):467–75.
Krizhevsky A, Sutskever I, Hinton GE. ImageNet Classification with Deep Convolutional Neural Networks In: Pereira F, Burges CJC, Bottou L, Weinberger KQ, editors. Advances in Neural Information Processing Systems 25. Red Hook: Curran Associates, Inc.: 2012. p. 1097–105.
Szegedy C, Liu W, Jia Y, Sermanet P, Reed S, Anguelov D, et al.Going deeper with convolutions. In: 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). Boston: IEEE: 2015. p. 1–9.
He K, Zhang X, Ren S, Sun J. Deep Residual Learning for Image Recognition. In: The IEEE Conference on Computer Vision and Pattern Recognition (CVPR). Las Vegas: IEEE: 2016. p. 770–8.
Girshick R, Donahue J, Darrell T, Malik J. Region-based convolutional networks for accurate object detection and segmentation. IEEE T Pattern Anal. 2016; 38(1):142–58.
Ren S, He K, Girshick R, Sun J. Faster R-CNN: towards real-time object detection with region proposal networks. IEEE T Pattern Anal. 2017; 6:1137–49.
Tang P, Wang H, Kwong S. G-MS2F: GoogLeNet based multi-stage feature fusion of deep CNN for scene recognition. Neurocomputing. 2017; 225:188–97.
Zhong Z, Jin L, Xie Z. High performance offline handwritten chinese character recognition using googlenet and directional feature maps. In: Document Analysis and Recognition (ICDAR), 2015 13th International Conference on. Tunis: IEEE: 2015. p. 846–50.
Zhang Y, Roller S, Wallace BC. MGNC-CNN: A Simple Approach to Exploiting Multiple Word Embeddings for Sentence Classification. In: Proceedings of NAACL-HLT. San Diego: Association for Computational Linguistics: 2016. p. 1522–7.
Min X, Zeng W, Chen N, Chen T, Jiang R. Chromatin accessibility prediction via convolutional long short-term memory networks with k-mer embedding. Bioinformatics. 2017; 33(14):i92–i101.
Tyagi A, Kapoor P, Kumar R, Chaudhary K, Gautam A, Raghava G. In silico models for designing and discovering novel anticancer peptides. Sci Rep. 3; 2013:2984.
Novković M, Simunić J, Bojović V, Tossi A, Juretić D. DADP: the database of anuran defense peptides. Bioinformatics. 2012; 28(10):1406–7.
Hajisharifi Z, Piryaiee M, Beigi MM, Behbahani M, Mohabatkar H. Predicting anticancer peptides with Chouś pseudo amino acid composition and investigating their mutagenicity via Ames test. J Theor Biol. 2014; 341:34–40.
Chen W, Ding H, Feng P, Lin H, Chou KC. iACP: a sequence-based tool for identifying anticancer peptides. Oncotarget. 2016; 7(13):16895.
Compeau PEC, Pevzner PA, Tesler G. How to apply de Bruijn graphs to genome assembly. Nat Biotechnol. 2011; 29:987.
Aggarwala V, Voight BF. An expanded sequence context model broadly explains variability in polymorphism levels across the human genome. Nat Genet. 2016; 48(4):349–55.
Hinton GE. Learning distributed representations of concepts In: Morris RGM, editor. Parallel distributed processing: Implications for psychology and neurobiology. New York: Oxford University Press: 1989. p. 46–61.
Hu B, Tang B, Chen Q, Kang L. A novel word embedding learning model using the dissociation between nouns and verbs. Neurocomputing. 2016; 171:1108–17.
Mikolov T, Sutskever I, Chen K, Corrado G, Dean J. Distributed Representations of Words and Phrases and Their Compositionality. In: Proceedings of the 26th International Conference on Neural Information Processing Systems - Volume 2. NIPS’13. USA: Curran Associates Inc.: 2013. p. 3111–9.
Zhang D, Xu H, Su Z, Xu Y. Chinese comments sentiment classification based on word2vec and SVMperf. Expert Syst Appl. 2015; 42(4):1857–63.
Nair V, Hinton GE. Rectified Linear Units Improve Restricted Boltzmann Machines. In: Proceedings of the 27th International Conference on International Conference on Machine Learning. ICML’10. Omnipress: USA: 2010. p. 807–14.
Boopathi V, Subramaniyam S, Malik A, Lee G, Manavalan B, Yang DC. mACPpred: A Support Vector Machine-Based Meta-Predictor for Identification of Anticancer Peptides. International journal of molecular sciences. 2019; 20(8):1964.
Nanni L, Lumini A, Brahnam S. An Empirical Study of Different Approaches for Protein Classification. Sci World J. 2014; 2014:17.
Manavalan B, Basith S, Shin TH, Wei L, Lee G. mAHTPred: a sequence-based meta-predictor for improving the prediction of anti-hypertensive peptides using effective feature representation. Bioinformatics. 2018;12.
Win TS, Schaduangrat N, Prachayasittikul V, Nantasenamat C, Shoombuatong W. PAAP: a web server for predicting antihypertensive activity of peptides. Future Med Chem. 2018; 10(15):1749–67.
Su R, Hu J, Zou Q, Manavalan B, Wei L. Empirical comparison and analysis of web-based cell-penetrating peptide prediction tools. Brief Bioinform. 2019.
Manavalan B, Shin TH, Kim MO, Lee G. PIP-EL: A New Ensemble Learning Method for Improved Proinflammatory Peptide Predictions. Front Immunol. 2018; 9:1783.
Acknowledgments
The authors sincerely thank Dr. Zhi-Ping Liu (School of Control Science and Engineering at Shandong University) for his valuable suggestions.
Funding
This research was supported by the National Natural Science Foundation of China (NSFC) (Grant Nos. U1806202, 61533011, and 61877064). This study was also supported by the Swedish Research Council, Strategic Research Area Exodiab, Dnr 2009-1039, and the Swedish Foundation for Strategic Research Dnr IRC15-0067. Furthermore, the research was supported with a project grant from the Swedish Research Council to LG (2015-02558), a European Foundation for the Study of Diabetes (EFSD) grant, and a Hjelt Foundation grant to YDM. No funding body played a role in the design of the study, analysis and interpretation of data, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
CW developed the prediction method, designed and implemented the experiments, and wrote the paper. RG conceived and led the project, analysed the results and wrote the paper. YZ evaluated the methods, suggested improvements and analysed the results. YDM drafted the manuscript. All the authors edited the manuscript, read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wu, C., Gao, R., Zhang, Y. et al. PTPD: predicting therapeutic peptides by deep learning and word2vec. BMC Bioinformatics 20, 456 (2019). https://doi.org/10.1186/s12859-019-3006-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12859-019-3006-z