Abstract
The olfactory bulb receives cholinergic basal forebrain inputs as does the neocortex. With a focus on nicotinic acetylcholine receptors (nAChRs), this review article provides an overview and discussion of the following findings: (1) the nAChRs-mediated regulation of regional blood flow in the neocortex and olfactory bulb, (2) the nAChR subtypes that mediate their responses, and (3) their activity in old rats. The activation of the α4β2-like subtype of nAChRs produces vasodilation in the neocortex, and potentiates olfactory bulb vasodilation induced by olfactory stimulation. The nAChR activity producing neocortical vasodilation was similarly maintained in 2-year-old rats as in adult rats, but was clearly reduced in 3-year-old rats. In contrast, nAChR activity in the olfactory bulb was reduced already in 2-year-old rats. Thus, age-related impairment of α4β2-like nAChR function may occur earlier in the olfactory bulb than in the neocortex. Given the findings, the vasodilation induced by α4β2-like nAChR activation may be beneficial for neuroprotection in the neocortex and the olfactory bulb.
Similar content being viewed by others
Introduction
As background information for this review article, a brief history and scientific knowledge of basic and clinical research on the basal forebrain cholinergic system will be provided. In humans, the olfactory function starts declining after 65 years of age [1]. In addition to age-related impairment, olfactory decline is an early symptom of Alzheimer’s disease (AD) that appears before cognitive decline [2, 3]. The olfactory bulb, which is the first processing station of olfactory information in the brain, receives cholinergic basal forebrain input, as do the neocortex and hippocampus, which contribute to cognition and memory, respectively [4]. More specifically, magnocellular neurons in the basal forebrain provide widespread cholinergic innervation to the neocortex, hippocampus, and olfactory bulb. The bilateral horizontal and caudal portions of the cholinergic basal forebrain neurons, which are located in the substantia innominata (SI) and nucleus basalis of Meynert (NBM), project their axons to the neocortex. In turn, fibers of neurons located in the nucleus of the horizontal limb of the diagonal band of Broca (HDB) project to the olfactory bulb. The most rostral level of the cholinergic neurons of the basal forebrain, which is located in the medial septal nucleus (MS) and the nucleus of the vertical limb of the diagonal band of Broca (VDB), project mainly to the hippocampus [4, 5]. These cholinergic neurons of the basal forebrain undergo selective degeneration in patients with AD [6, 7]. Moreover, individual variations in cholinergic cell loss, from moderate to severe, are correlated with the degree of cognitive deterioration in these patients [7]. This indicates the importance of this cholinergic system for cognitive function. In vivo brain imaging studies have revealed a moderate structural decline in the gray matter volume of the basal forebrain cholinergic system during the adult life span, which worsens with advanced age [8]. A further decrease in the volume of the basal forebrain cholinergic system beyond the age effect alone were detected in early stages of AD [8, 9].
Presumed cholinergic terminations onto cerebral blood vessels from the magnocellular basal nucleus, in addition to interneuronal contacts, are evident in rodent studies that used the anterograde axonal-tracing technique [10]. The activation of cholinergic fibers originating in the NBM produces vasodilation, which leads to increases in regional blood flow in the neocortex in anesthetized rats [11,12,13]. Acetylcholine receptors consist of muscarinic and nicotinic receptors. The activation of not only muscarinic but also nicotinic receptors within the parenchyma of the neocortex is involved in the NBM cholinergic vasodilative system [11].
In the human neocortex, nicotinic acetylcholine receptors (nAChRs) exhibit a greater decline than do muscarinic acetylcholine receptors during the normal aging process as well as in patients with AD [14, 15]. Therefore, to understand the mechanisms of olfactory decline associated with cognitive decline in older adults and patients with AD, it is important to elucidate the regulation of regional blood flow mediated by the nicotinic cholinergic system in the neocortex and olfactory bulb, together with its aging process.
In this article, we focus on nAChRs, review results reported mainly by our research group using anesthetized rats and discuss on (1) nAChRs-mediated regulation of regional blood flow in the neocortex and the olfactory bulb, (2) nAChR subtype mediating their responses, and (3) their activity in old rats.
Nicotinic cholinergic regulation of regional blood flow in the neocortex
Nicotine injection
Nicotine, a nAChR agonist, has been demonstrated to increase regional cerebral blood flow when injected intravenously, especially in the neocortex, independent of mean arterial pressure [16,17,18]. In our investigation, we measured the blood flow in the frontal cortex by laser Doppler flowmetry in urethane-anesthetized artificially ventilated rats, before and after intravenous bolus injection of nicotine [18]. Nicotine at doses of 3–30 µg/kg increased neocortical blood flow in a dose-dependent manner, without significant changes in mean arterial pressure. At 300 µg/kg, nicotine increased neocortical blood flow in parallel with a marked increase in arterial pressure. The rapid increase in neocortical blood flow after the injection of 300 µg/kg of nicotine appears to be due to a passive increase in neocortical blood flow in response to the increased arterial pressure. Accordingly, nicotine doses of 30 μg/kg or less increased the neocortical blood flow without affecting systemic blood pressure is due to active dilation of neocortical vessels. Nicotine at the same doses had a similar effect to the parietal cortex in our animals.
The finding mentioned above is in line with evidence that the activation of nAChRs is involved in vasodilation in the neocortex by excitation of intracranial cholinergic fibers originating in the NBM of the basal forebrain projecting to the neocortex [11,12,13, 19].
nAChR subtype
The increase in neocortical blood flow induced by intravenous injection of nicotine (30 μg/kg) is due to an activation of nAChRs in the brain; in fact, the response was not influenced by a nAChR antagonist (hexamethonium) which cannot transverse the blood–brain barrier, but was abolished by a nAChR antagonist (mechamylamine) that can cross it [18]. Activations of nAChRs in both the NBM and the neocortex are possibly involved in the nicotine-induced neocortical vasodilation [17, 18]. Furthermore, nitric oxide is necessary for this nicotine-induced increase in neocortical blood flow [20].
Of the various subtypes of nAChRs, the α4β2 and α7 subtypes are the most abundant and widespread in the mammalian brain, including the neocortex and NBM [21,22,23]. The increase in neocortical blood flow induced by nicotine was not influenced by methyllycaconitine, an α7-selective nAChR antagonist but was completely abolished by dihydro-β-erythroidine, an α4β2-preferring nAChR antagonist [24]. These results suggest that activation of α4β2-like nAChRs but not of α7 nAChRs in the NBM and the neocortex is responsible for the nicotine-induced neocortical vasodilation. However, although dihydro-β-erythroidine is often used as an α4β2-preferring nAChR antagonist [25, 26], we cannot exclude the possible contribution of other heterometric nAChRs, since dihydro-β-erythroidine can bind to heterometric neuronal nAChRs containing not only the α4β2 subtype but also the α4β4 [27], α3β2 [28], and α2β2 [29] subtypes.
Aging
The above-mentioned experiments showing an increase in neocortical blood flow induced by intravenous injection of nicotine (30 µg/kg) were performed in adult rats aged 3–10 months. Then we moved on to older animals [18, 30, 31]. In rats 23–26 months old (approximately 2 years old), a bolus injection of 30 µg/kg of nicotine increased neocortical blood flow to a similar extent as in the younger rats. A lower nicotine dose (3 µg/kg), however, was ineffective. In other words, the intensity of the response to nicotine remained unchanged in old rats, but the threshold became higher. In contrast, in 32–36 months old rats (approximately 3 years old), nicotine, at 3 or 30 µg/kg, had no significant effect on the neocortical blood flow (Fig. 1A). The decrease in the neocortical blood flow response in the very old animals is probably due to a decline in the number of nAChRs in the neocortex, as previously observed in both rodents and humans [14, 32].
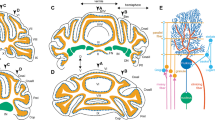
Schematic diagram showing the aging effects of α4β2-like nAChR-mediated neocortical vasodilation (A) and potentiation of vasodilation in the olfactory bulb (B). A Neocortical vasodilation induced by intravenous bolus injection of nicotine at a dose of 30 μg/kg was schematically illustrated [18]. B Vasodilation of the olfactory bulb induced by olfactory nerve stimulation before (black line) and after (red line) intravenous injection of nicotine at a dose of 30 μg/kg was schematically illustrated [54]. nAChR nicotinic acetylcholine receptor
Nicotinic cholinergic regulation of regional blood flow in the olfactory bulb
Nicotine injection
The effect of an intravenous bolus injection of nicotine on the blood flow in the olfactory bulb was investigated [33]. Nicotine at a dose of 30 µg/kg, increased neocortical blood flow [18], but did not increase blood flow in the olfactory bulb [33]. This result agrees with the fact that activation of the HDB in the basal forebrain, which is the main source of cholinergic input to the olfactory bulb [5, 34, 35], increased extracellular acetylcholine release in the olfactory bulb but failed to affect the blood flow in the olfactory bulb [36]. Our results suggest a functional difference between the olfactory bulb and neocortex regarding cerebral blood flow regulation through cholinergic activation.
Multiple in vivo studies in rodents have described that natural olfactory stimulation increases regional blood flow in the olfactory bulb, in association with neuronal activities [37,38,39,40]. The vasodilation in the olfactory bulb induced by olfactory stimulation is due to neurovascular coupling mechanisms [41, 42].
Next, we investigated the effect of nAChR activation by nicotine injection on the blood flow response in the olfactory bulb induced by olfactory stimulation in urethane-anesthetized artificially ventilated rats [43]. Odor stimulation (5% amyl acetate, 30 s) produced an increase in olfactory bulb blood flow without changes in frontal cortical blood flow or mean arterial pressure. An intravenous injection of nicotine at a dose of 30 µg/kg potentiated the odor-induced increased olfactory bulb blood flow, without changing the basal blood flow level.
The olfactory nerve transmits smell information from the olfactory epithelium to the olfactory bulb. Rodent studies indicated that the olfactory nerve increases its firing frequency depending on the odor concentration [44,45,46]. By varying the stimulus frequencies of olfactory nerve electrical stimulation, we could quantify the strength of odor stimulation. Our experiments demonstrated that unilateral, electrical stimulation of olfactory nerve produced current (≥ 100 μA) and frequency-dependent (≥ 5 Hz) increases in blood flow in the olfactory bulb ipsilateral to the stimulus without changes in frontal cortical blood flow or mean arterial pressure [47]. Furthermore, we observed that an intravenous injection of nicotine (30 μg/kg) augmented the olfactory bulb blood flow response to nerve stimulation. Nicotine-induced potentiation of olfactory bulb blood flow responses occurred with olfactory nerve stimulation at 2 and 20 Hz but not at 100 Hz [47]. This finding provides additional evidence that nAChR activation potentiates olfactory bulb blood flow responses to olfactory input, this potentiation occurs for intermediate or weak, but not strong, input. In a Ca2+ imaging study in the mouse olfactory bulb, Bendahmane et al. [48] indicated that electrical stimulation of the HDB leads to the activity-dependent modulation of glomerular odor responses, whereby weak-to-moderate responses are enhanced and strong responses are reduced. Thus, our results [47], almost agree with those of Bendahmane et al. [48].
nAChR subtype
The above-mentioned nicotine-induced potentiation of olfactory bulb blood flow response to odor was negated by dihydro-β-erythroidine, an α4β2-preferring nAChR antagonist [43]. Thus, our results suggest that the activation of α4β2-like neuronal nAChRs in the brain potentiates olfactory sensory processing in the olfactory bulb. However, a contribution of not only α4β2-like nAChRs [49] but also α2- [50] and β4-containing nAChRs [51] in the olfactory bulb should be considered, since dihydro-β-erythroidine can bind to heterometric neuronal nAChRs other than the α4β2 subtype.
As described in “nAChR subtype” section, activation of α4β2-like nAChRs in the NBM and the neocortex is suggested to be responsible for the nicotine-induced neocortical vasodilation [18, 24]. The olfactory bulb receives cholinergic neural inputs originating in the HDB in the basal forebrain [5, 35]. Both in olfactory bulb and HDB cholinergic neurons, mRNA expression of both the α4 and β2 nAChR subunits has been identified in rats [21, 52, 53]. Thus, the nicotine-induced potentiation of olfactory sensory processing in the olfactory bulb could be due to activation of α4β2-like neuronal nAChRs in the olfactory bulb and/or in HDB cholinergic neurons.
Aging
The investigation of nicotine-induced potentiation of olfactory bulb blood flow responses induced by olfactory nerve stimulation in adult rats (4–8 months old) described above was then extended to animals of older age [54]. In old rats of 24–27 months (approximately 2 years old), olfactory nerve stimulation produces vasodilation in the olfactory bulb. However, the nicotine-induced potentiation of olfactory bulb vasodilation due to α4β2-like nAChR activation decreased considerably in old rats (Fig. 1B). In contrast, the olfactory bulb vasodilatory response to hypercapnic stimulation, indicating the vasodilatory ability of the olfactory bulb, was considerably greater than its response to olfactory nerve stimulation. Thus, we consider that with age, the olfactory bulb blood vessels maintain their vasodilatory ability but with lower reactivity to nicotine. This suggests a decline in α4β2-like nAChR function involving the nicotine-induced potentiation of olfactory bulb vasodilation in old rats.
In old rats of 24–27 months, electrical stimulation of unilateral olfactory nerve increased blood flow in the olfactory bulb ipsilateral to the stimulus without changes in mean arterial pressure [54]. The spatiotemporal blood flow response characteristics and the current and frequency dependence of prompt vasodilation of the olfactory bulb were identical to those observed in adult rats [47]. This is consistent with Kass et al. [55], who described that the odor-evoked synaptic output from the olfactory sensory neurons to the olfactory bulb glomeruli is relatively stable in anesthetized mice of 6–24 months old.
Comparison of aging effects on the blood flow responses in the neocortex and the olfactory bulb
As described above, the α4β2-like nAChR-mediated vasodilation in the neocortex induced by nicotine injection is relatively well maintained in old rats (23–26 months old) but markedly declines in very old rats (32–36 months old) [18, 24]. On the other hand, the α4β2-like nAChR-mediated potentiation of olfactory bulb vasodilation induced by nicotine injection is reduced in old rats (24–27 months old) [54]. Accordingly, we assume that the age-related impairment of α4β2-like nAChR function may affect the olfactory bulb earlier than the neocortex.
In rat brains, α4 and β2 mRNA levels decrease from 7 to 29 months of age, further decreasing at 32 months. This tendency is relatively constant across in different areas of the brain including neocortex, although olfactory bub is not analyzed [56]. Similarly, in human neocortex, decreases in α4 and β2 mRNA levels as well as α4β2 nAChR availability have been reported with normal aging [57, 58] and AD [59]. The diminished olfactory bulb blood flow potentiation effects of nicotine in old rats as well as diminished nicotine-induced neocortical vasodilation in very old rats may be due to the decline in α4β2 nAChRs in the brain. Further studies comparing the aging effects on neocortex and olfactory bulb, regarding α4β2 nAChR availability, are needed.
Age-related impairment of the regulation of blood flow in the olfactory bulb and neocortex mediated by α4β2-like nAChRs may not be comparable to the baseline regional cerebral blood flow, at least in rodents. This is because the baseline regional blood flow in the neocortex and olfactory bulb is not significantly different in 12-, 24-, and 34-month-old conscious rats when measured using the [14C]-iodoantipyrine method [60]. Similarly, using microsphere methods, the baseline regional blood flow in the olfactory bulb has been shown to remain unchanged in 6- and 24-month-old conscious rats [61]. In contrast, in the human brain, resting (baseline) gray matter cerebral blood flow, including that in the frontal regions, is decreased between the ages of 40 and 100 years, as measured using the 133X inhalation method [62]. Similar age-related reductions in gray matter cerebral blood flow have been reported by studies that used positron emission tomography [63] and pseudocontinuous arterial spin labeling (pCASL) MRI [64].
Clinical significance
This review emphasized the crucial role of α4β2-like nAChRs in the brain in neocortical vasodilation and potentiation of olfactory bulb vasodilation in response to olfactory stimulation. A considerable decrease of these α4β2-like nAChR functions occur in older animals at around 2 years old in the olfactory bulb, and later at around 3 years old in the neocortex. The age-related impairment of nicotinic cholinergic regulation of cerebral blood flow in the neocortex and olfactory bulb may explain the deterioration of olfactory and cognitive function in older people [65, 66]. The earlier decline of nAChR function in the olfactory bulb than in the neocortex may explain why the olfactory dysfunction is the earliest symptoms of AD [2, 3]. Human studies have described the relationship between olfaction, cognitive function, regional cerebral blood flow, and the nicotinic cholinergic system. Pilot studies among community-dwelling older adults have shown that older individuals with a higher olfactory identification threshold for rose odor exhibited a greater decline in cognitive function, particularly in attention and discrimination abilities [67, 68]. Attention and discrimination abilities are related to the basal forebrain cholinergic system [69, 70] and undergo early impairment related to AD [71, 72]. Cortical nAChRs, as assessed in vivo using 11C-nicotine binding in patients with mild AD, are robustly associated with attention cognitive function [73]. Moreover, the olfactory identification score was negatively correlated with regional cerebral blood flow in several brain areas including the bilateral frontal pole, in patients with mild cognitive impairment (MCI) and AD [74].
Since the α4β2-like nAChRs in the brain decline with age as well as in AD, activation of the nAChRs involved in cortical vasodilatation or potentiation of olfactory bulb vasodilation could be beneficial for older people and AD patients. To this end, administering nicotinic receptor agonists or physical therapies such as somatosensory stimulation and walking, known to activate basal forebrain cholinergic system in both adult and old rats [75,76,77,78,79,80], may have therapeutic values. Furthermore, the increased cerebral blood flow in the neocortex and olfactory bulb induced by α4β2-like nAChR activation could improve oxygen and glucose delivering to those brain areas, and those sufficient nourishments appear to be beneficial for neuronal protection and maintaining cognitive function and olfaction.
Availability of data and materials
Not applicable.
Abbreviations
- AD:
-
Alzheimer’s disease
- HDB:
-
Horizontal limb of the diagonal band of Broca
- MCI:
-
Mild cognitive impairment
- MS:
-
Medial septal nucleus
- NBM:
-
Nucleus basalis of Meynert
- SI:
-
Substantia innominate
- VDB:
-
Ventral limb of the diagonal band of Broca
- nAChR:
-
Nicotinic acetylcholine receptor
References
Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L (1984) Smell identification ability: changes with age. Science 226:1441–1443
Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH (2008) Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry 64:871–879
Murphy C (2019) Olfactory and other sensory impairments in Alzheimer disease. Nat Rev Neurol 15:11–24
Mesulam MM, Mufson EJ, Wainer BH, Levey AI (1983) Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6). Neuroscience 10:1185–1201
Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB (1984) Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience 13:627–643
Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, DeLong MR (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215:1237–1239
Lehéricy S, Hirsch EC, Cervera-Piérot P, Hersh LB, Bakchine S, Piette F, Duyckaerts C, Hauw JJ, Javoy-Agid F, Agid Y (1993) Heterogeneity and selectivity of the degeneration of cholinergic neurons in the basal forebrain of patients with Alzheimer’s disease. J Comp Neurol 330:15–31
Grothe M, Heinsen H, Teipel SJ (2012) Atrophy of the cholinergic basal forebrain over the adult age range and in early stages of Alzheimer’s disease. Biol Psychiatry 71:805–813
Grothe M, Zaborszky L, Atienza M, Gil-Neciga E, Rodriguez-Romero R, Teipel SJ, Amunts K, Suarez-Gonzalez A, Cantero JL (2010) Reduction of basal forebrain cholinergic system parallels cognitive impairment in patients at high risk of developing Alzheimer’s disease. Cereb Cortex 20:1685–1695
Luiten PGM, Gaykema RPA, Traber J, Spencer DG Jr (1987) Cortical projection patterns of magnocellular basal nucleus subdivisions as revealed by anterogradely transported Phaseolus vulgaris leucoagglutinin. Brain Res 413:229–250
Biesold D, Inanami O, Sato A, Sato Y (1989) Stimulation of the nucleus basalis of Meynert increases cerebral cortical blood flow in rats. Neurosci Lett 98:39–44
Hotta H (2016) Neurogenic control of parenchymal arterioles in the cerebral cortex. Prog Brain Res 225:3–39
Sato A, Sato Y (1992) Regulation of regional cerebral blood flow by cholinergic fibers originating in the basal forebrain. Neurosci Res 14:242–274
Nordberg A, Alafuzoff I, Winblad B (1992) Nicotinic and muscarinic subtypes in the human brain: changes with aging and dementia. J Neurosci Res 31:103–111
Geula C, Dunlop SR, Ayala I, Kawles AS, Flanagan ME, Gefen T, Mesulam MM (2021) Basal forebrain cholinergic system in the dementias: vulnerability, resilience, and resistance. J Neurochem 158:1394–1411
Crystal GJ, Downey HF, Adkins TP, Bashour FA (1983) Regional blood flow in canine brain during nicotine infusion: effect of autonomic blocking drugs. Stroke 14:941–947
Linville DG, Williams S, Raszkiewicz JL, Arneric SP (1993) Nicotinic agonists modulate basal forebrain control of cortical cerebral blood flow in anesthetized rats. J Pharmacol Exp Ther 267:440–448
Uchida S, Kagitani F, Nakayama H, Sato A (1997) Effect of stimulation of nicotinic cholinergic receptors on cortical cerebral blood flow and changes in the effect during aging in anesthetized rats. Neurosci Lett 228:203–206
Hotta H, Uchida S, Kagitani F, Maruyama N (2011) Control of cerebral cortical blood flow by stimulation of basal forebrain cholinergic areas in mice. J Physiol Sci 61:201–209
Uchida S, Kawashima K, Lee TJ (2002) Nicotine-induced NO-mediated increase in cortical cerebral blood flow is blocked by beta2-adrenoceptor antagonists in the anesthetized rats. Auton Neurosci 96:126–130
Azam L, Winzer-Serhan U, Leslie FM (2003) Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience 119:965–977
Gaimarri A, Moretti M, Riganti L, Zanardi A, Clementi F, Gotti C (2007) Regulation of neuronal nicotinic receptor traffic and expression. Brain Res Rev 55:134–143
Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL (2001) Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol Ther 92:89–108
Uchida S, Hotta H, Kawashima K (2009) Long-term nicotine treatment reduces cerebral cortical vasodilation mediated by alpha4beta2-like nicotinic acetylcholine receptors in rats. Eur J Pharmacol 609:100–104
Nott A, Levin ED (2006) Dorsal hippocampal alpha7 and alpha4beta2 nicotinic receptors and memory. Brain Res 1081:72–78
Wang Y, Sherwood JL, Miles CP, Whiffin G, Lodge D (2006) TC-2559 excites dopaminergic neurones in the ventral tegmental area by stimulating alpha4beta2-like nicotinic acetylcholine receptors in anaesthetised rats. Br J Pharmacol 147:379–390
Harvey SC, Maddox FN, Luetje CW (1996) Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. J Neurochem 67:1953–1959
Dwoskin LP, Crooks PA (2001) Competitive neuronal nicotinic receptor antagonists: a new direction for drug discovery. J Pharmacol Exp Ther 298:395–402
Xiao Y, Kellar KJ (2004) The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J Pharmacol Exp Ther 310:98–107
Sato A, Sato Y, Uchida S (2002) Regulation of cerebral cortical blood flow by the basal forebrain cholinergic fibers and aging. Auton Neurosci 96:13–19
Uchida S, Hotta H (2009) Cerebral cortical vasodilatation mediated by nicotinic cholinergic receptors: effects of old age and of chronic nicotine exposure. Biol Pharm Bull 32:341–344
Araujo DM, Lapchak PA, Meaney MJ, Collier B, Quirion R (1990) Effects of aging on nicotinic and muscarinic autoreceptor function in the rat brain: relationship to presynaptic cholinergic markers and binding sites. J Neurosci 10:3069–3078
Shiba K, Machida T, Uchida S, Hotta H (2006) Effects of nicotine on regional blood flow in the olfactory bulb in rats. Eur J Pharmacol 546:148–151
Hamamoto M, Kiyokage E, Sohn J, Hioki H, Harada T, Toida K (2017) Structural basis for cholinergic regulation of neural circuits in the mouse olfactory bulb. J Comp Neurol 525:574–591
Záborszky L, Carlsen J, Brashear HR, Heimer L (1986) Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band. J Comp Neurol 243:488–509
Uchida S, Kagitani F (2018) Effect of basal forebrain stimulation on extracellular acetylcholine release and blood flow in the olfactory bulb. J Physiol Sci 68:415–423
Boido D, Rungta RL, Osmanski BF, Roche M, Tsurugizawa T, Le Bihan D, Ciobanu L, Charpak S (2019) Mesoscopic and microscopic imaging of sensory responses in the same animal. Nat Commun 10:1110. https://doi.org/10.1038/s41467-019-09082-4
Chaigneau E, Tiret P, Lecoq J, Ducros M, Knöpfel T, Charpak S (2007) The relationship between blood flow and neuronal activity in the rodent olfactory bulb. J Neurosci 27:6452–6460
Iadecola C (2017) The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96:17–42
Poplawsky AJ, Fukuda M, Murphy M, Kim SG (2015) Layer-specific fMRI responses to excitatory and inhibitory neuronal activities in the olfactory bulb. J Neurosci 35:15263–15275
Hosford PS, Gourine AV (2019) What is the key mediator of the neurovascular coupling response? Neurosci Biobehav Rev 96:174–181
Petzold GC, Albeanu DF, Sato TF, Murthy VN (2008) Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron 58:897–910
Uchida S, Ito Y, Kagitani F (2019) Effects of nicotine on odor-induced increases in regional blood flow in the olfactory bulb in rats. J Physiol Sci 69:425–431
Connelly T, Savigner A, Ma M (2013) Spontaneous and sensory-evoked activity in mouse olfactory sensory neurons with defined odorant receptors. J Neurophysiol 110:55–62
Duchamp-Viret P, Chaput MA, Duchamp A (1999) Odor response properties of rat olfactory receptor neurons. Science 284(5423):2171–2174
Reisert J, Matthews HR (2001) Response properties of isolated mouse olfactory receptor cells. J Physiol 530(Pt 1):113–122
Uchida S, Kagitani F (2020) Effects of nicotine on regional blood flow in the olfactory bulb in response to olfactory nerve stimulation. J Physiol Sci 70:30. https://doi.org/10.1186/s12576-020-00758-x
Bendahmane M, Ogg MC, Ennis M, Fletcher ML (2016) Increased olfactory bulb acetylcholine bi-directionally modulates glomerular odor sensitivity. Sci Rep 6:25808. https://doi.org/10.1038/srep25808
D’Souza RD, Vijayaraghavan S (2014) Paying attention to smell: cholinergic signaling in the olfactory bulb. Front Synaptic Neurosci 6:21. https://doi.org/10.3389/fnsyn.2014.00021
Burton SD, LaRocca G, Liu A, Cheetham CE, Urban NN (2017) Olfactory bulb deep short-axon cells mediate widespread inhibition of tufted cell apical dendrites. J Neurosci 37:1117–1138
Spindle MS, Parsa PV, Bowles SG, D’Souza RD, Vijayaraghavan S (2018) A dominant role for the beta 4 nicotinic receptor subunit in nicotinic modulation of glomerular microcircuits in the mouse olfactory bulb. J Neurophysiol 120:2036–2048
Keiger CJ, Walker JC (2000) Individual variation in the expression profiles of nicotinic receptors in the olfactory bulb and trigeminal ganglion and identification of alpha2, alpha6, alpha9, and beta3 transcripts. Biochem Pharmacol 59:233–240
Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW (1989) Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol 284:314–335
Uchida S, Moriya J, Morihara D, Kagitani F (2023) Nicotinic cholinergic regulation of olfactory bulb blood flow response in aged rats. J Physiol Sci 73:1. https://doi.org/10.1186/s12576-022-00859-9
Kass MD, Czarnecki LA, McGann JP (2018) Stable olfactory sensory neuron in vivo physiology during normal aging. Neurobiol Aging 69:33–37
Ferrari R, Pedrazzi P, Algeri S, Agnati LF, Zoli M (1999) Subunit and region-specific decreases in nicotinic acetylcholine receptor mRNA in the aged rat brain. Neurobiol Aging 20:37–46
Mitsis EM, Cosgrove KP, Staley JK, Bois F, Frohlich EB, Tamagnan GD, Estok KM, Seibyl JP, van Dyck CH (2009) Age-related decline in nicotinic receptor availability with [123I]5-IA-85380 SPECT. Neurobiol Aging 30:1490–1497
Tohgi H, Utsugisawa K, Yoshimura M, Nagane Y, Mihara M (1998) Age-related changes in nicotinic acetylcholine receptor subunits alpha4 and beta2 messenger RNA expression in postmortem human frontal cortex and hippocampus. Neurosci Lett 245:139–142
Sabri O, Meyer PM, Gräf S, Hesse S, Wilke S, Becker GA, Rullmann M, Patt M, Luthardt J, Wagenknecht G, Hoepping A, Smits R, Franke A, Sattler B, Tiepolt S, Fischer S, Deuther-Conrad W, Hegerl U, Barthel H, Schönknecht P, Brust P (2018) Cognitive correlates of α4β2 nicotinic acetylcholine receptors in mild Alzheimer’s dementia. Brain 141:1840–1854
Ohata M, Sundaram U, Fredericks WR, London ED, Rapoport SI (1981) Regional cerebral blood flow during development and ageing of the rat brain. Brain 104:319–332
Salter JM, Cassone VM, Wilkerson MK, Delp MD (1998) Ocular and regional cerebral blood flow in aging Fischer-344 rats. J Appl Physiol 85:1024–1029
Shaw TG, Mortel KF, Meyer JS, Rogers RL, Hardenberg J, Cutaia MM (1984) Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology 34:855–862
Pantano P, Baron JC, Lebrun-Grandié P, Duquesnoy N, Bousser MG, Comar D (1984) Regional cerebral blood flow and oxygen consumption in human aging. Stroke 15:635–641
Leidhin CN, McMorrow J, Carey D, Newman L, Williamson W, Fagan AJ, Chappell MA, Kenny RA, Meaney JF, Knight SP (2021) Age-related normative changes in cerebral perfusion: data from the Irish longitudinal study on ageing (TILDA). Neuroimage 229:117741
Attems J, Walker L, Jellinger KA (2015) Olfaction and aging: a mini-review. Gerontology 61:485–490
Schubert CR, Fischer ME, Pinto AA, Klein BEK, Klein R, Cruickshanks KJ (2017) Odor detection thresholds in a population of older adults. Laryngoscope 127:1257–1262
Uchida S, Shimada C, Sakuma N, Kagitani F, Kan A, Awata S (2020) The relationship between olfaction and cognitive function in the elderly. J Physiol Sci 70:48. https://doi.org/10.1186/s12576-020-00777-8
Uchida S, Shimada C, Sakuma N, Kagitani F, Kan A, Awata S (2022) Olfactory function and discrimination ability in the elderly: a pilot study. J Physiol Sci 72:8. https://doi.org/10.1186/s12576-022-00832-6
Baxter MG, Chiba AA (1999) Cognitive functions of the basal forebrain. Curr Opin Neurobiol 9:178–183
Schilit Nitenson A, Manzano Nieves G, Poeta DL, Bahar R, Rachofsky C, Mandairon N, Bath KG (2019) Acetylcholine regulates olfactory perceptual learning through effects on adult neurogenesis. iScience 22:544–556
Perry RJ, Hodges JR (1999) Attention and executive deficits in Alzheimer’s disease. A critical review. Brain 122:383–404
Yang J, Ogasa T, Ohta Y, Abe K, Wu J (2010) Decline of human tactile angle discrimination in patients with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 22:225–234
Kadir A, Almkvist O, Wall A, Långström B, Nordberg A (2006) PET imaging of cortical 11C-nicotine binding correlates with the cognitive function of attention in Alzheimer’s disease. Psychopharmacology 188:509–520
Kashibayashi T, Takahashi R, Fujita J, Fujito R, Kamimura N, Okutani F, Kazui H (2021) Correlation between cerebral blood flow and olfactory function in mild cognitive impairment and Alzheimer’s disease. Int J Geriatr Psychiatry 36:1103–1109
Akaishi T, Kimura A, Sato A, Suzuki A (1990) Responses of neurons in the nucleus basalis of Meynert to various afferent stimuli in rats. NeuroReport 1:37–39
Kimura A, Okada K, Sato A, Suzuki H (1994) Regional cerebral blood flow in the frontal, parietal and occipital cortices increases independently of systemic arterial pressure during slow walking in conscious rats. Neurosci Res 20:309–315
Kurosawa M, Sato A, Sato Y (1992) Cutaneous mechanical sensory stimulation increases extracellular acetylcholine release in cerebral cortex in anesthetized rats. Neurochem Int 21:423–427
Kurosawa M, Okada K, Sato A, Uchida S (1993) Extracellular release of acetylcholine, noradrenaline and serotonin increases in the cerebral cortex during walking in conscious rats. Neurosci Lett 161:73–76
Uchida S, Kagitani F, Suzuki A, Aikawa Y (2000) Effect of acupuncture-like stimulation on cortical cerebral blood flow in anesthetized rats. Jpn J Physiol 50:495–507
Uchida S, Kagitani F (2015) Effect of acupuncture-like stimulation on cortical cerebral blood flow in aged rats. J Physiol Sci 65:67–75
Acknowledgements
Not applicable.
Funding
This work was supported by JSPS KAKENHI Grant Number JP21K11717 to SU.
Author information
Authors and Affiliations
Contributions
SU drafted the manuscript; both authors edited and revised manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
SU is an editorial board member of the Journal of Physiological Sciences. Another author has no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Uchida, S., Kagitani, F. Influence of age on nicotinic cholinergic regulation of blood flow in rat’s olfactory bulb and neocortex. J Physiol Sci 74, 18 (2024). https://doi.org/10.1186/s12576-024-00913-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12576-024-00913-8