Abstract
Background
Few studies have utilized whole blood samples to investigate the association between metal mixture exposure during early pregnancy and spontaneous preterm birth (SPB). We conduct this nested case–control study to investigate both the independent and joint effect of each metal, and identify critical metals in the metal mixture.
Results
A total of 120 pregnant women with SPB and 120 pregnant women with full-term delivery were selected from the prospective birth cohort. We measured 14 metal concentrations in maternal blood collected during 10–13 weeks gestation. Conditional logistic regression showed that high concentrations of vanadium (V), magnesium, and copper were positively associated with SPB (Adjusted OR = 5.76 (95% CI 2.46–13.53), 3.64 (95% CI 1.64–8.09), 2.88 (95% CI 1.29–6.41), respectively). Moderate manganese (Mn) concentration (50th–75th percentile) group had the lowest estimated OR (Adjusted OR = 0.32 (95% CI 0.13–0.76)). The high level of strontium (Sr) was negatively associated with SPB (Adjusted OR = 0.39 (95% CI 0.17–0.91)). The BKMR model showed a significant positive joint effect of metal mixture exposure on SPB, while V was the most important metal. The non-linear effects of V and lead (Pb) on SPB, and the interaction effects between V–Pb, Sr–Mn were also revealed.
Conclusions
Maternal blood metal mixtures in the first trimester were found to be positively associated with SPB, with V exhibiting the strongest independent association. Mn had a potential U-shaped association with SPB. Elimination of metal contamination in the environment has a positive impact on maternal and child health.
Similar content being viewed by others
Introduction
Preterm birth (PTB) is a significant public health concern that poses a threat to infant health and is the primary cause of perinatal mortality [1]. Despite significant advancements in medical and nursing care that have improved perinatal survival rates, premature infants still face increasing medical expenses and a heightened risk of social disability in adulthood, leading to significant medical and social burdens [2, 3]. Previous studies have pointed out that PTB negatively affects the development of the respiratory, nervous, and immune systems [4,5,6]. Compared to the term infants, the risk of cerebral palsy, mental retardation, blindness, deafness and other major disabilities is significantly increased for premature newborns [7, 8]. Clinically, PTBs are defined as live-born with a gestational age of less than 37 weeks, and divided into several subtypes, of which spontaneous preterm birth (SPB) accounts for the majority [9, 10]. The causes of SPB are complicated. Both environmental and biological factors can increase the risk of SPB, and most of these factors currently have no clear mechanism [10].
Metal exposure widely exists in populations and can affect fetuses via placental transfer [11]. Recent studies have suggested that maternal metal exposure may influence the incidence of PTB. However, most of them have focused on the association between PTB and single metal, especially toxic metals and metalloids including lead (Pb), mercury (Hg), cadmium (Cd) and arsenic (As), with relatively less research focused on metals such as vanadium (V) and calcium (Ca). In addition, to date, the associations between many metals and PTB were uncertain, with inconsistent findings across the research. A population-based cohort study conducted in Hubei Province indicated a significant association between urinary V levels and PTB [12], while another population-based cohort study conducted in Xiamen found no significant relationship between umbilical cord blood V levels and preterm birth [13]. Another recent study indicated that the essential trace element Zn may increase the risk of preterm birth, contrasting with conventional findings [14]. The relationship between the toxic metal Pb and PTB has also been reported with contradictory results across studies [15]. The inconsistency in conclusions across studies may be due to variations in the selection of biological samples for exposure detection and differences in the timing of sample collection, leading to a lack of comparability in results. Currently, the common biological samples used for exposure measurement in studies exploring the health effects of prenatal exposures include maternal blood, serum, urine, and umbilical cord blood. Although some studies have investigated the relationship between multiple metal exposure and SPB, many of these studies have used urine or serum samples in the late trimester [16, 17]. For certain metals such as Pb, blood is considered to be a better detection sample than urine and serum [18], yet studies regarding multi-metal exposure utilizing whole blood samples are relatively limited at present. Some studies have used cord blood or other samples collected before delivery as the research sample, but the conclusions may be subject to potential issues of reverse causality [19, 20], and samples collected during late pregnancy periods provide limited value in studying the prediction and preventive mechanisms of SPB. In addition, metal exposure tends to occur in the form of co-exposure rather than single metal exposure in the real world, which is also a critical issue in the field of metal exposure [21]. The interaction effects between these metals can potentially modify their effects on the human body. Low concentrations of metals that do not reach the threshold for significant effects may be amplified in their negative impact due to synergistic interactions with other metals [22, 23], hence, it is crucial to determine the potential interactions among metals. Currently, Bayesian kernel machine regression (BKMR) is one of the best approaches for estimating the joint effects and interactions of multiple exposures [24].
Based on the current state of research, we conducted this nested case–control study based on a large prospective birth cohort in Fujian, aimed to explore the effect of multi-metal exposure during early pregnancy on SPB, recognize the important metals in the metal mixture, and estimate the interactions between metals. The blood sample was prospectively collected during the first trimester of pregnancy and participants were followed until delivery. We estimated 14 maternal blood metal concentrations during the first trimester and employed various statistical methods to investigate the individual effects of each single metal as well as their independent and joint effects in metal mixture. This study aims to provide new evidence and insights into the effect of multi-metal exposure during early pregnancy on SPB.
Materials and methods
Study design and subject selection
This study was a nested case–control study based on the Birth-Cohort Project of Fujian Maternity and Child Health Hospital. During the first trimester prenatal check-up, pregnant women were recruited by birth cohort investigators. The inclusion criteria were as follows: (1) Gestational age of less than 13 weeks at first assessment; (2) Plan to have regular obstetric examinations and give birth in Fujian Maternity and Child Health Hospital; (3) Understand the study and complete informed consent, and are willing to cooperate with the investigation and research. After completing the informed consent, the participants will conduct the first trimester questionnaire and the measurement of physical conditions. Pregnant women who suffer from mental illnesses thus cannot cooperate with questionnaires or pregnant women with HIV and hepatitis B and other infectious diseases were excluded from research. Participants were followed until delivery, and their clinical information, including prenatal check-up data, gestational age of delivery, fetal health condition, and discharge diagnosis, were obtained from the electronic medical record system.
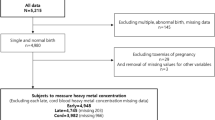
PTB was defined as live birth with the gestational age of delivery less than 37 weeks [9], and SPB was all PTB cases excluding iatrogenic PTBs. To exclude iatrogenic PTBs, participants with birth defects, stillbirth, abortion, and obstetric complications, including cervical insufficiency, fetal distress, placental abruption, pregnancy-induced hypertension and diabetes, placenta previa, and eclampsia [25] were excluded from the study. Multiple births and participants with baseline chronic hypertension and diabetes were also excluded. According to the above criteria, we included 120 participants with single live birth and birth gestational age < 37 weeks in the SPB group. The control group was selected from the rest of the available participants with a gestational age of delivery no less than 37 weeks, matched by age, race, body mass index (BMI), gravidity, and parity. 120 controls were included in this nested case–control study. The detailed flow diagram of subject selection is shown in Additional file 1: Fig. S1.
Covariates assessment
The baseline characteristics including age, height, and weight were assessed via the first trimester body assessments. BMI was calculated by dividing weight by squared height. The gestational age was determined through ultrasound scans during the first trimester check-up [26]. No current smoker was included in this study. Further information was collected through the questionnaire at the first trimester check-up, including the mother's education level, family income, exposure to passive smoking, and supplementation of folic acid during pregnancy. Family income was defined as the sum of the monthly income of both parents in Chinese Yuan, and passive smoking was defined as self-reported exposure to secondhand smoke at home and in the workplace in the three months before pregnancy. The sex of fetus and delivery mode were obtained from the medical and delivery records after delivery.
Measurement of the blood metal concentration
The blood sample was collected at the first trimester check-up for each participant after 8 h of fasting. The blood sample was stored at − 80 ℃ until analysis. Agilent Technologies 7800 inductively coupled plasma mass spectrometry (ICP-MS) and Agilent Technologies SPS4 Autosampler were used to quantify the blood concentrations of 14 metals that may be associated with the risk of SPB, including V, Ca, Cd, lithium (Li), magnesium (Mg), manganese (Mn), molybdenum (Mo), Pb, As, strontium (Sr), iron (Fe), copper (Cu), selenium (Se), and zinc (Zn).
Briefly, 2.5 mL of 75% nitric acid, 0.5 mL of Triton X-100 and were pipetted into a 500 mL volumetric flask, and filled with ultrapure water to obtain the dilution solution. 100 μL of calibration standards, quality control and test samples were diluted by 3.9 mL of dilution solution, shaken 20 times, vortex mixed for 5 min by a multi-tube vortex mixer, and injected for analysis. ICP-MS standard solutions (o2si smart solutions, USA) were used as standard reference materials. The recovery rate for 14 metals ranged from 88.24 to 106.47%, and both the inter-array coefficients of variations (CVs) and intra-array CVs for 14 metals were < 10%. When the concentration of a blood metal was below the limit of detection (LOD), it was imputed by LOD/√2.
Statistical analysis
Baseline analysis
The Spearman correlation was used to evaluate the relationship between the 14 metal concentrations. Chi-squared test, t-test and Fisher’s exact test were performed to analyze the crude difference in baseline characteristics between the SPB group and the control group. Subjects with missing covariates will be omitted from subsequent statistical analysis involving covariate adjustment.
Single metal analysis
Initially, the association between every single metal and SPB was analyzed using conditional logistic regression, including both a basic model and an adjusted model. Common factors that may have effects on SPB were included in the adjusted model, including sampling gestational age, sex of the fetus, education level of the mother, monthly family income, passive smoking and folate intake. In the adjusted model, in addition to the subjects missing covariates, their paired subjects were also omitted accordingly. The blood metal concentrations were divided into 4 groups according to the quartiles for analysis (Q1 < 25th quantile, Q2: 25th–50th quantile, Q3: 50th–75th quantile, Q4 > 75th quantile). The trend of each concentration quantile was tested by modelling the quantiles as continuous variables. The non-linear dose–response association between single metal concentration and SPB was further estimated using RCS regression, adjusted for sampling gestational age, sex of the fetus, education level of the mother, monthly family income, passive smoking and folate intake. The number of knots for each metal was selected based on the Akaike information criterion (AIC), and the knot was placed on the recommended quantiles [27]. The reference value used to calculate odds ratios (ORs) was the 50th quantile for each metal concentration. As the distribution of blood metal concentrations was right-skewed, a log10 transformation was applied to the concentration of each metal in the RCS regression. The significance of overall and nonlinear associations of each metal in the RCS model were evaluated using the Wald test.
Multi-metal analysis
Considering the correlation and interaction effects between metals, we utilized BKMR models to investigate the importance of each metal in metal mixtures and their joint effects on SPB. The BKMR model was a non-parametric method used to evaluate the importance of each metal in the metal mixture, which has advantages in evaluating nonlinear associations and the interaction effects of multiple exposures [28]. Since the BKMR model is sensitive to extreme values, the concentration of 14 metals was log10 transformed and scaled, and 5 participants with extreme concentrations of Mn (> 35.88 μg/L, n = 1), Pb (> 60.00 μg/L, n = 2), Sr (> 47.42 μg/L, n = 2) were excluded from the BKMR analysis. Covariates including sampling gestational age, sex of the fetus, education level of the mother, monthly family income, passive smoking, and folate intake were also included in the BKMR model. 50,000 iterations were run by a Markov Chain Monte Carlo (MCMC) algorithm in the BKMR model. Posterior inclusion probabilities (PIP) were used to evaluate the importance of each metal, and PIP = 0.5 was considered as the threshold of importance. Considering the effect of potential multicollinearity, we further performed a hierarchical variable selection for metals with PIP > 0.5 in the first model, and metals were grouped based on the correlation to further identify the critical metals. Sensitivity analyses were conducted by performing the BKMR model without excluding extreme values and with different prior distributions to verify the stability of the statistical results.
All statistical analysis was conducted by R version 4.2.1. Conditional logistic regression was performed using package “survival” (version 3.5–5), RCS regression was performed using package “rms” (version 6.7–1) and BKMR regression was conducted using package “BKMR” (version 0.2.2). Two-tailed P-value < 0.05 and 95% confidence interval (CI) not including null value were considered statistically significant in this study. This study was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital (Approval Number: 2019–200).
Results
Baseline characteristics of participants
The baseline characteristics of the included participants are summarized in Table 1. The average age of the participants included in this study was 29.9, with a range from 21.6 to 42.1. The characteristics of delivery mode, mother education level, family income, passive smoking, and folic acid supplementation were similar between the two groups. A total of 4 subjects with missing covariates were excluded from subsequent analysis with adjustment for covariates.
Associations between single critical metals and SPB
The distributions of blood metal concentrations are shown in Table 2 For Li, the rate of blood concentration higher than the LOD was 85.8%, while for other 13 metals was 100%. The LOD of each metal is shown in Additional file 1: Table S1. A significantly higher concentration of V, Mg and Cu was found in the SPB group (PV < 0.001, PMg = 0.013, PCu = 0.012).
The results of conditional logistic regression estimating the association between single metal and SPB are shown in Table 3. After adjusting for covariates, a positive association was found between V concentration and SPB (Q4 vs Q1: adjusted OR = 5.76 (95% CI 2.46–13.53), P < 0.001; Ptrend < 0.001). High blood levels of Mg (Q4 vs Q1: adjusted OR = 3.64 (95% CI 1.64–8.09), P = 0.002; Ptrend = 0.003) and Cu (Q4 vs Q1: adjusted OR = 2.88 (95% CI 1.29–6.41), P = 0.010; Ptrend = 0.010) were also positively associated with SPB. The moderate blood Mn level group (Q3) had the lowest estimated OR compared to other groups (Q3 vs Q1: adjusted OR = 0.32 (95% CI 0.13–0.76), P = 0.010; Ptrend = 0.095). High blood levels of Sr were negatively associated with SPB (Q4 vs Q1: adjusted OR = 0.39 (95% CI 0.17–0.91), P = 0.029; Ptrend = 0.078). The association between blood Mn level in Q2 group and SPB became significant after adjusted for covariates (Q2 vs Q1: adjusted OR = 0.42 (95% CI 0.18–0.96, P = 0.041; Ptrend = 0.095), while other estimates did not change significantly. Significant associations were not observed between other single metals and SPB.
The results of the dose–response association between single metals and SPB are presented in Fig. 1. V and Sr showed non-linear associations with SPB (Pnon-linear = 0.038 and 0.046, respectively). Blood Mg (Poverall = 0.027, Pnon-linear = 0.114) and Cu (Poverall = 0.011, Pnon-linear = 0.901) were positively associated with SPB, while Mn was negatively associated (Poverall = 0.010, Pnon-linear = 0.059), and the non-linear effect for the above three metals was not significant. Significant associations were not observed between other selected metals and SPB.
The dose–response association between log10 transformed maternal blood concentration of each metal and the estimated odds ratio (OR). The red shaded area indicated the 95% confidence interval of the estimated odds ratio, and the rug plot on the x-axis indicated the distribution of concentration. P-overall and P-nonlinear were estimated by the Wald test
Multi-metal models
Correlation between multiple metals
The Spearman correlations between blood metal concentrations are shown in Additional file 1: Fig. S2. Strong significant positive associations were found in the following metal pairs: V–Ca (Spearman ρ = 0.46), Mn–Li (Spearman ρ = 0.64), Mg–Fe (Spearman ρ = 0.49), Se–As (Spearman ρ = 0.51) and Fe–Zn (Spearman ρ = 0.42). Significant negative correlations were found in Ca–Cd (Spearman ρ = − 0.20), Ca–Fe (Spearman ρ = − 0.34), Ca–Zn (Spearman ρ = − 0.30), Fe–Cu (Spearman ρ = − 0.16) and Fe–Sr (Spearman ρ = − 0.13).
BKMR model
In the component-wise variable selection model, 14 metals were initially included, and V, Ca, Mg, Mn, Pb, Sr, Fe, Cu, and Zn were selected with PIP > 0.5, with V, Ca, Mn, and Sr having the highest PIP (PIPV = 1.00, PIPCa = 0.99, PIPMn = 0.98, PIPSr = 0.95). Figure 2A reveals the significant joint effect of the metal mixture when the scaled log10 concentration (log10C) of each metal was higher than their 55th percentile. Figure 2B shows the independent effect of each metal when the concentrations of other metals were fixed at the 25th, 50th and 75th percentile. The positive effect of V on SPB was significant regardless of other metal concentrations. The positive effect of Cu was significant when fixing other metal concentrations at the 50th and 75th percentiles, while the effect of Sr was negative. The negative effects of Ca and Mn were significant when fixing other concentrations at the 25th and 50th quantiles. When the concentration of other metals increases, the negative effect of Mn increased while the negative effect of Sr decreased, these observations suggested the potential existence of interactions among the metal mixtures Fig. 2.
The association between overall metal mixture exposure and spontaneous preterm birth (A) and the independent effect of each metal (B). A The effect was estimated by fixing all scaled log10-transformed metal concentration(log10C) at the same quantile. B The effects were estimated by comparing the effect for each metal at the 75th percentile versus the 25th percentile of scaled log10C while holding the scaled log10C for other metals fixed at the 25th, 50th, and 75th percentiles
We further performed a secondary BKMR model with hierarchical variable selection for metals with PIP > 0.5 in the first model. According to the correlation, V and Ca were assigned to group 1, Mg, Fe and Zn were assigned to group 2, and other metals were assigned to each single group individually from group 3 to group 6. The summary of the PIPs is shown in Table 4. Group posterior inclusion probability (groupPIP) indicated group importance for each metal group, and conditional posterior inclusion probability (condPIP) indicated the importance of each metal given the group it belongs was important. The effect of Ca vanished after being assigned to the same group as V. The univariate exposure–response curve from the BKMR model is shown in Fig. 3. The figure revealed a strong positive relationship between SPB and V at moderate concentrations, although the confidence interval widened at high and low concentrations due to reduced sample size. Mn and Sr were negatively associated with SPB. Cu, Zn, Fe, and Mg were positively associated with SPB, but the associations were not significant. Additional file 1: Fig. S3 shows the bivariate exposure–response curve and the potential interaction effect between metals by fixing the other metal concentrations at different percentiles. The slope of the effect curve of Sr changed when the concentration of Mn was fixed at different percentiles, and the slope of the curve of Pb also changed with the change of V concentration, indicating the potential existence of V-Pb and Sr-Mn interactions. Approximately paralleled lines were observed in other metal combinations, suggesting that these co-exposure effects on SPB were mostly additive without interaction effect Fig. 3.
Sensitivity analysis
Sensitivity analyses were conducted by performing the BKMR model without excluding extreme values and with different prior distributions, and the results are presented in Additional file 1: Fig. S4–6. Excluding extreme values did not significantly affect the results, and the effects of each metal were generally consistent across the BKMR models with different prior distributions, indicating that the results from the BKMR model were valid.
Discussion
In general, we selected SPB cases and their paired controls based on the large prospective birth cohort and further measured 14 blood metal concentrations for each participant during their first trimester. We used multiple statistical methods to identify critical metals and estimated the independent and joint effects of metals on SPB. V was identified as the most important agent, with the highest PIP in the BKMR model, and both conditional logistic regression and RCS regression revealed a significant positive association between of V and SPB. Cu and Mg were also found to be positively associated with SPB. Mn was negatively associated with SPB at moderate concentrations, but the estimated OR tended to increase at higher concentrations. On the other hand, Sr was a potential protective agent for SPB, with a significant negative association. The results of the BKMR model showed a positive association between overall metal mixture exposure levels and SPB, and the bivariate exposure–response curve suggested the potential existence of V-Pb and Sr-Mn interactions.
In our study, V exhibited the strongest positive association with SPB, which was consistent with most previous studies. A study based on a birth cohort from Guangdong, China, used cord blood V concentration as the indicator and obtained similar results [19]. Another study that utilized urine for the exposure measurement also indicated that V was the most important element in metal mixtures [16]. A birth cohort study in Wuhan, China, observed a positive association between urinary V concentration and premature rupture of membranes, which is one of the main factors leading to PTB [29]. The above evidence supported the conclusion that V exposure during pregnancy increases the risk of PTB, and we obtained similar results using blood samples. It is worth noting that, for V, while blood samples used in our study and urine samples are preferred over hair samples for measurement, the measurement can only serve as biomarkers for internal exposure and cannot accurately quantify external exposure levels [30]. V is a transition metal that occurs in the human body as a super-trace element and is widely found in nature [31]. Many studies have demonstrated that one of the toxic mechanisms for V is stimulating the production of excessive reactive oxygen species (ROS) and further inducing oxidative stress, which is closely related to the incidence of SPB [32,33,34]. Oxidative stress intensifies during pregnancy as the developing fetus requires more nutrition and oxygen, generating more ROS and exacerbating oxidative stress [35]. In addition, V also stimulates immune activity, causing increased production of cytokines and further inducing inflammatory responses, which is a recognized risk factor for SPB [1, 36, 37]. The inflammatory response and oxidative stress mentioned above may be part of the biological mechanisms by which V increases the risk of SPB and requires further exploration. China is one of the world's largest producers of V, which brings a more serious burden of vanadium pollution [38, 39]. Because of the mobility through the soil into plants, V can accumulate in the food chain and eventually enter the human body [40]. Atmospheric and marine pollution is also a pathway for human exposure [41]. The treatment of V pollution may have positive effects on the prevention of PTBs.
A significant association between blood Mn and SPB was observed in RCS regression, and the result from logistic regression suggested that individuals with moderate blood Mn levels had the lowest estimated OR. According to the dose–response curve, visually, the curve is approximately U shaped. Although the confidence interval at high concentrations has widened and lost significance, the estimate was increasing. Mn is an essential element and also a toxic metal that is involved in the activation and synthesis of multiple enzymes, including Mn superoxide dismutase (MnSOD), which is a crucial antioxidant component [42]. MnSOD clears ROS in mitochondria and is highly dependent on Mn, thus Mn deficiency can increase oxidative stress, thereby raising the risk of SPB [43]. However, a positive association between Mn and PTB was reported in a previous study, which reflected the toxicity of Mn [14]. Excessive Mn production can also generate free radicals, reactive oxygen species, and toxic metabolites, causing oxidative balance disruption in cells [44]. As a result, both Mn deficiency and excess can disturb the oxidative balance and induce oxidative stress, leading to a U-shaped association between Mn concentration and oxidative stress levels, which is consistent with our findings. This U-shaped association had also been reported in a previous study conducted in Wuhan [45]. Notably, the whole blood Mn concentrations in our study were lower than those in the North Puerto Rican study [14], thus our finding mainly reflected the protective effect of Mn, the negatively correlated part of the U-shaped curve, while the North Puerto Rican study mainly reflects the toxicity of Mn at high blood concentrations.
Cu was found to be positively associated with SPB, which was consistent with most of the previous research [19, 46, 47]. A potential mechanism could be the catalytic activity of free Cu in generating highly reactive hydroxyl radicals and inducing oxidative stress [48]. Notably, we found a protective association between higher concentrations of Sr on SPB. This nonlinear protective association has also been mentioned in previous studies but has not been extensively studied [19]. A prospective cohort study in Bangladesh also showed that exposure to Sr in early pregnancy reduced the SPB risk [49]. Another prior study also revealed a significant association between higher levels of Sr in serum metal mixtures and reduced risk of SPB [17]. These studies, along with our results, suggested the potential for Sr to be a protective factor against SPB, and a possible mechanism of this effect may also related to oxidative stress, as Sr can eliminate lipid peroxidation and thereby reduce the level of oxidative stress [50].
Unexpectedly, blood Mg concentration was found to be positively associated with SPB in our study, as hypomagnesemia is an important precursor to PTB, and magnesium sulfate is also used as a tocolytic and neuroprotection drug for PTB infants [51, 52]. However, many studies point out that Mg supplements cannot prevent the incidence of PTB or increase the gestational age of birth, but only have neuroprotective effects [53, 54]. A U-shaped exposure–response relationship between cord blood Mg and risk of PTB was identified in the study based on the Guangdong Birth Cohort, which suggested that not only Mg deficiency, but high exposure to Mg can also increase the risk of PTB [19]. Combined with the results of our study, it is suggested that high exposure to Mg may increase the risk of SPB, which was rarely been mentioned in other studies. Higher blood Mg concentration is associated with placental inflammation and oxidative stress levels, and further research is needed to elucidate the underlying mechanisms [55]. Pb showed a non-monotonic nonlinear relationship with SPB in BKMR model, which was inconsistent with the recognition of Pb as a toxic metal. However, previous studies have also reported non-significant associations between Pb and SPB, particularly when Pb concentrations were within normal ranges [56, 57]. Besides, Pb was even found to be negatively correlated with PTB in a cohort study conducted in Guangzhou, China, which the distribution of blood Pb concentrations was similar to ours [58]. These findings may suggest that the effect of Pb on SPB was limited when it had not reached the threshold concentration. Besides, we did not observe significant correlations between other single metals and SPB, although significant correlations between some of these metals and PTB have been reported in earlier studies, especially toxic metals such as As [15]. The absence of significant exposure effects of As may be attributed to the unsuitability of blood as a sample for detecting arsenic exposure [59].
The results of the BKMR model demonstrated a positive joint effect of overall metal mixture exposure on SPB. Furthermore, our findings revealed potential antagonistic effects between V and Pb, and synergistic protective effects between Mn and Sr, which is rarely mentioned in other studies. The underlying mechanisms remain unclear and require replication experiments to validate and further explore comprehensively.
There are still some limitations in this study. First, it should be noted that most recruited participants in this study were of Han ethnicity and were recruited from the same hospital in the provincial capital. Therefore, need to be cautious when pushing the results to populations of other ethnicities and areas. Second, while blood samples have advantages in measuring the exposure of certain metals such as Pb, urine samples are more recommended for some other metals, such as As. Third, we have identified associations between several metals and SPB, however, the underlying biological mechanisms remain unclear. Further investigations including in vitro experiments are necessary to explore the underlying mechanisms. Besides, blood samples were only collected once during the first trimester, which may not fully capture the dynamic changes in metal concentrations throughout pregnancy, thus we could not explore the critical window of metal exposure.
This study had several strengths. First, this nested case–control study was based on a prospective cohort, where the information collection and specimen acquisition followed standardized procedures and quality control processes, ensuring the credibility of the data. The SPB group and the control group were selected from the same population, the baseline characteristics of participants between the two groups were relatively similar. Moreover, we performed matching on baseline characteristics including age, BMI, gravidity and parity between the SPB and control groups to control for potential confounding biases. This matching design effectively reduced the influence of common confounders, thereby enabling a better exploration of the relationship between metals and SPB. Additionally, using blood metal concentrations in the first trimester as an indicator had better predictive value, the findings may facilitate the early identification of high-risk individuals for intervention and reducing the risk of SPB. Furthermore, we used multiple statistical methods, including conditional logistic regression, RCS regression, and BKMR models. By combining the results of these statistical methods, we gained a more comprehensive understanding of the correlation between metals and SPB risk, including linear and nonlinear associations, the independent effects of metals, and the interactions between metals.
Conclusion
In summary, this study explored the association between multiple blood metal concentrations during the first three months of pregnancy and SPB based on a large birth cohort, identified critical metals, and further investigated the effects of joint exposure. V, Cu, and Mg were positively associated with SPB, with V being the most important toxic agent. Pregnant women with moderate blood Mn concentration had the lowest SPB risk, and the underlying relationship between Mn and SPB could be approximately U shaped. Sr was a potential protective agent of SPB. Significant non-linear associations were observed in the association between V, Sr and SPB, and overall metal exposure was positively associated with SPB. The study also revealed potential antagonistic effects between V and Pb, and synergistic protective effects between Mn and Sr. These findings contribute to future studies investigating the mechanisms underlying the impact of metal exposure on SPB, while also indicating the potential positive public health effects that may result from reducing environmental metal contamination.
Availability of data and materials
The data and materials used in this study are not publicly available due to the restrictions of availability under the license for this study. Data are, however, available from the authors upon reasonable request and with permission of Fujian Maternity and Child Health Hospital.
Abbreviations
- AIC:
-
Akaike information criterion
- As:
-
Arsenic
- BMI:
-
Body mass index
- BKMR:
-
Bayesian kernel machine regression
- Ca:
-
Calcium
- Cd:
-
Cadmium
- CI:
-
Confidence interval
- condPIP:
-
Conditional posterior inclusion probability
- Cu:
-
Copper
- CV:
-
Coefficients of variation
- Fe:
-
Iron
- groupPIP:
-
Group posterior inclusion probability
- Hg:
-
Mercury
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- Li:
-
Lithium
- LOD:
-
The limit of detection
- MCMC:
-
Markov Chain Monte Carlo
- Mg:
-
Magnesium
- Mn:
-
Manganese
- MnSOD:
-
Manganese superoxide dismutase
- Mo:
-
Molybdenum
- OR:
-
Odds ratio
- Pb:
-
Lead
- PIP:
-
Posterior inclusion probability
- PTB:
-
Preterm birth
- RCS:
-
Restricted cubic splines
- ROS:
-
Reactive oxygen species
- SD:
-
Standard deviation
- Se:
-
Selenium
- SPB:
-
Spontaneous preterm birth
- Sr:
-
Strontium
- V:
-
Vanadium
- Zn:
-
Zinc
- λmin :
-
Lambda with the minimum misclassification error
References
Goldenberg RL, Culhane JF, Iams JD, Romero R (2008) Epidemiology and causes of preterm birth. The Lancet 371(9606):75–84
Moster D, Lie RT, Markestad T (2008) Long-term medical and social consequences of preterm birth. N Engl J Med 359(3):262–273
Rawnsley KL, Cheong JLY, Spittle AJ (2021) Impact of very preterm birth on maternal employment and socio-economic status. Acta Paediatr 110(4):1383–1384. https://doi.org/10.1111/apa.15722
Melville JM, Moss TJ (2013) The immune consequences of preterm birth. Front Neurosci 7:79. https://doi.org/10.3389/fnins.2013.00079
Moss TJ (2006) Respiratory consequences of preterm birth. Clin Exp Pharmacol Physiol 33(3):280–284. https://doi.org/10.1111/j.1440-1681.2006.04359.x
Ream MA, Lehwald L (2018) Neurologic consequences of preterm birth. Curr Neurol Neurosci Rep 18(8):48. https://doi.org/10.1007/s11910-018-0862-2
Goldenberg RL, Culhane JF, Iams JD, Romero R (2008) Epidemiology and causes of preterm birth. Lancet 371(9606):75–84. https://doi.org/10.1016/s0140-6736(08)60074-4
Saigal S, Doyle LW (2008) An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371(9608):261–269. https://doi.org/10.1016/s0140-6736(08)60136-1
Moutquin JM (2003) Classification and heterogeneity of preterm birth. Int J Obstet Gynaecol 110:30–33
Vogel JP, Chawanpaiboon S, Moller A-B, Watananirun K, Bonet M, Lumbiganon P (2018) The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol 52:3–12. https://doi.org/10.1016/j.bpobgyn.2018.04.003
Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G et al (2014) Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J Eposure Sci Environ Epidemiol 24(5):537–544. https://doi.org/10.1038/jes.2014.26
Hu J, Xia W, Pan X, Zheng T, Zhang B, Zhou A et al (2017) Association of adverse birth outcomes with prenatal exposure to vanadium: a population-based cohort study. The Lancet Planet Health 1(6):e230–e241. https://doi.org/10.1016/S2542-5196(17)30094-3
Zheng G, Zhong H, Guo Z, Wu Z, Zhang H, Wang C et al (2014) Levels of heavy metals and trace elements in umbilical cord blood and the risk of adverse pregnancy outcomes: a population-based study. Biol Trace Elem Res 160(3):437–444. https://doi.org/10.1007/s12011-014-0057-x
Ashrap P, Watkins DJ, Mukherjee B, Boss J, Richards MJ, Rosario Z et al (2020) Maternal blood metal and metalloid concentrations in association with birth outcomes in Northern Puerto Rico. Environ Int 138:105606. https://doi.org/10.1016/j.envint.2020.105606
Khanam R, Kumar I, Oladapo-Shittu O, Twose C, Islam AA, Biswal SS et al (2021) Prenatal environmental metal exposure and preterm birth: a scoping review. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph18020573
Liu J, Ruan F, Cao S, Li Y, Xu S, Xia W (2022) Associations between prenatal multiple metal exposure and preterm birth: comparison of four statistical models. Chemosphere 289:133015
Deng Y, Kang H, Li N, Li L, Li Y, Li X et al (2024) Inverse association between maternal serum concentrations of trace elements and risk of spontaneous preterm birth: a nested case–control study in China. British J Nutr. https://doi.org/10.1017/S0007114523003070
Bergdahl IA, Skerfving S (2008) Biomonitoring of lead exposure—alternatives to blood. J Toxicol Environ Health A 71(18):1235–1243
Wang Z, Huang S, Zhang W, Zeng X, Chu C, Li Q et al (2022) Chemical element concentrations in cord whole blood and the risk of preterm birth for pregnant women in Guangdong. China Ecotoxicol Environ Saf 247:114228. https://doi.org/10.1016/j.ecoenv.2022.114228
Lee M-S, Eum K-D, Golam M, Quamruzzaman Q, Kile ML, Mazumdar M et al (2021) Umbilical cord blood metal mixtures and birth size in Bangladeshi children. Environ Health Perspect 129(5):057006
Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OI et al (2017) The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20–40 months of age: evidence from rural Bangladesh. Environ Health Perspect 125(6):067015
Altenburger, R. (2010). Understanding combined effects for metal co-exposure in ecotoxicology. In Metal Ions in Toxicology: Effects, Interactions, Interdependencies (pp. 1–26): Royal Society of Chemistry Great Britain.
Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L (2016) A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res 23:8244–8259
Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M et al (2015) Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16(3):493–508. https://doi.org/10.1093/biostatistics/kxu058
Bukowski R, Malone FD, Porter FT, Nyberg DA, Comstock CH, Hankins GD et al (2009) Preconceptional folate supplementation and the risk of spontaneous preterm birth: a cohort study. PLoS Med 6(5):e1000061
Butt K, Lim KI (2019) Guideline No. 388-determination of gestational age by ultrasound. J Obst Gynaecol Canada 41(10):1497–1507. https://doi.org/10.1016/j.jogc.2019.04.010
Harrell FE (2015). Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis: Springer International Publishing.
Bobb JF, Claus Henn B, Valeri L, Coull BA (2018) Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 17(1):67. https://doi.org/10.1186/s12940-018-0413-y
Jin S, Xia W, Jiang Y, Sun X, Huang S, Zhang B et al (2018) Urinary vanadium concentration in relation to premature rupture of membranes: a birth cohort study. Chemosphere 210:1035–1041. https://doi.org/10.1016/j.chemosphere.2018.07.110
Cseh L, Ingerman L, Keith S, Taylor J (2012) Toxicological profile for vanadium.
Tripathi D, Mani V, Pal RP (2018) Vanadium in biosphere and its role in biological processes. Biol Trace Elem Res 186(1):52–67. https://doi.org/10.1007/s12011-018-1289-y
Ścibior A, Kurus J (2019) Vanadium and oxidative stress markers–in vivo model: a review. Curr Med Chem 26(29):5456–5500. https://doi.org/10.2174/0929867326666190108112255
Rojas-Lemus M, Bizarro-Nevares P, López-Valdez N, González-Villalva A, Guerrero-Palomo G, Cervantes-Valencia ME et al (2020) Oxidative stress and vanadium. Genotoxic Mutagen Mechan Test Methods 3:1–19
Zhao Y, Ye L, Liu H, Xia Q, Zhang Y, Yang X et al (2010) Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. J Inorg Biochem 104(4):371–378. https://doi.org/10.1016/j.jinorgbio.2009.11.007
Toboła-Wróbel K, Pietryga M, Dydowicz P, Napierała M, Brązert J, Florek E (2020) Association of oxidative stress on pregnancy. Oxidat Med Cell Longev 2020:6398520
Vadillo-Ortega F, Osornio-Vargas A, Buxton MA, Sánchez BN, Rojas-Bracho L, Viveros-Alcaráz M et al (2014) Air pollution, inflammation and preterm birth: a potential mechanistic link. Med Hypotheses 82(2):219–224. https://doi.org/10.1016/j.mehy.2013.11.042
Zwolak I (2014) Vanadium carcinogenic, immunotoxic and neurotoxic effects: a review of in vitro studies. Toxicol Mech Methods 24(1):1–12. https://doi.org/10.3109/15376516.2013.843110
Bai X, Luo L, Tian H, Liu S, Hao Y, Zhao S et al (2021) Atmospheric vanadium emission inventory from both anthropogenic and natural sources in China. Environ Sci Technol 55(17):11568–11578
Yang J, Teng Y, Wu J, Chen H, Wang G, Song L et al (2017) Current status and associated human health risk of vanadium in soil in China. Chemosphere 171:635–643
Imtiaz M, Rizwan MS, Xiong S, Li H, Ashraf M, Shahzad SM et al (2015) Vanadium, recent advancements and research prospects: a review. Environ Int 80:79–88
Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D (2012) Biochemical and medical importance of vanadium compounds. Acta Biochim Pol 59(2):195–200
Miriyala S, Spasojevic I, Tovmasyan A, Salvemini D, Vujaskovic Z, St. Clair D et al (2012) Manganese superoxide dismutase, MnSOD and its mimics. Biochimica et Biophysica Acta Mol Basis Dis 1822(5):794–814. https://doi.org/10.1016/j.bbadis.2011.12.002
Bresciani G, da Cruz IBM, González-Gallego J (2015) Chapter four–manganese superoxide dismutase and oxidative stress modulation. In G. S. Makowski (Ed.), Advances in Clinical Chemistry (Vol. 68, pp. 87–130): Elsevier.
Martinez-Finley EJ, Gavin CE, Aschner M, Gunter TE (2013) Manganese neurotoxicity and the role of reactive oxygen species. Free Radical Biol Med 62:65–75. https://doi.org/10.1016/j.freeradbiomed.2013.01.032
Zhang W, Chen H, Xia W, Ma J, Yang C, Yu L et al (2023) Associations of plasma manganese with adverse pregnancy outcomes: nested case-control studies in a Chinese birth cohort. Chemosphere 345:140550. https://doi.org/10.1016/j.chemosphere.2023.140550
Hao Y, Pang Y, Yan H, Zhang Y, Liu J, Jin L et al (2019) Association of maternal serum copper during early pregnancy with the risk of spontaneous preterm birth: a nested case-control study in China. Environ Int 122:237–243. https://doi.org/10.1016/j.envint.2018.11.009
Chiudzu G, Choko AT, Maluwa A, Huber S, Odland J (2020) Maternal serum concentrations of selenium, copper, and zinc during pregnancy are associated with risk of spontaneous preterm birth: a case-control study from Malawi. J Pregn 2020:9435972
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189(1–2):147–163. https://doi.org/10.1016/s0300-483x(03)00159-8
Huang H, Wei Y, Xia Y, Wei L, Chen X, Zhang R et al (2021) Child marriage, maternal serum metal exposure, and risk of preterm birth in rural Bangladesh: evidence from mediation analysis. J Eposure Sci Environ Epidemiol 31(3):571–580
Barneo-Caragol C, Martínez-Morillo E, Rodríguez-González S, Lequerica-Fernández P, Vega-Naredo I, Álvarez Menéndez FV (2018) Strontium and oxidative stress in normal pregnancy. J Trace Elem Med Biol 45:57–63. https://doi.org/10.1016/j.jtemb.2017.09.021
Kurzel RB (1991) Serum magnesium levels in pregnancy and preterm labor. Am J Perinatol 8(02):119–127
Chollat C, Sentilhes L, Marret S (2019) Protection of brain development by antenatal magnesium sulphate for infants born preterm. Dev Med Child Neurol 61(1):25–30
Crowther CA, Brown J, McKinlay CJ, Middleton P (2014) Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst Rev 2014(8):cd001060. https://doi.org/10.1002/14651858.CD001060.pub2
Haas DM, Benjamin T, Sawyer R, Quinney SK (2014) Short-term tocolytics for preterm delivery-current perspectives. Int J Womens Health 6:343–349. https://doi.org/10.2147/ijwh.S44048
Wang J-Q, Hu Y-B, Liang C-M, Xia X, Li Z-J, Gao H et al (2020) Aluminum and magnesium status during pregnancy and placenta oxidative stress and inflammatory mRNA expression: China Ma’anshan birth cohort study. Environ Geochem Health 42(11):3887–3898. https://doi.org/10.1007/s10653-020-00619-x
Yıldırım E, Derici MK, Demir E, Apaydın H, Koçak Ö, Kan Ö et al (2019) Is the concentration of cadmium, lead, mercury, and selenium related to preterm birth? Biol Trace Elem Res 191:306–312
Sowers M, Jannausch M, Scholl T, Li W, Kemp FW, Bogden JD (2002) Blood lead concentrations and pregnancy outcomes. Archiv Environ Health An Int J 57(5):489–495
Wang Z, Huang S, Zhang W, Zeng X, Chu C, Li Q et al (2022) Chemical element concentrations in cord whole blood and the risk of preterm birth for pregnant women in Guangdong. China Ecotoxicol Environ Safety 247:114228
Chou C-H, Harper C (2007) Toxicological profile for arsenic.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of Fujian Province of China for Youths (Grant No.2021J05081) (Yibing Zhu), the Key Project on Science and Technology Program of Fujian Health Commission (Grant No.2021ZD01002) (Hua Cao). The funders had no role in study design, data collection, data analysis, the decision to publication and preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
ZQW and YBZ: study design. YBZ, BS, and HC: revision of the manuscript. ZQW, HC and XRW: drafting of the manuscript. ZQW, CM and HBL: data analysis. HYG and WJL: data collection. BS, WJL and WL: participating in the data governance and manuscript drafting. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital (Approval Number: 2019–200), all participants were informed and signed an informed consent form before participating.
Consent for publication
Not applicable.
Competing interests
All authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Limit of detection (LOD) for each metal included in the study. Figure S1. Flow diagram of the selection of the SPB group and matched control group from the birth cohort. Figure S2. Spearman correlation between blood metal concentrations. Figure S3. The Bivariate exposure-response curve for each metal. Figure S4. The univariate exposure-response curve from an alternative BKMR model without the exclusion of extreme values. Figure S5. The univariate exposure-response curve from BKMR model with an alternative prior distribution (r.b=50). Figure S6. The univariate exposure-response curve from BKMR model with an alternative prior distribution (r.b=200).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Z., Cao, H., Wang, X. et al. Associations between maternal blood metal concentrations during the first trimester and spontaneous preterm birth: a nested case-control study. Environ Sci Eur 36, 82 (2024). https://doi.org/10.1186/s12302-024-00904-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-024-00904-x