Abstract
Background
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) represent a category of pervasive and enduring environmental pollutants that present a risk to human health. Although growing evidence suggests that probiotics can potentially alleviate the adverse effects of PFAS, large cross-sectional studies on the relationship between probiotic consumption and PFAS remain lacking.
Objective
The objective of this study is to assess the association between the exposure of probiotics and serum levels of PFAS.
Methods
This analysis included individuals aged 20 and above who took part in the National Health and Nutrition Examination Survey (NHANES) between 2003 and 2018. Probiotic consumption was considered when a participant reported consuming yogurt during the two 24-h dietary recall or using a probiotic supplement in dietary supplement questionnaires over the past 30 days.
Results
This study involved 9469 adults, out of which 1333 had been exposed to probiotics. We found negative associations between probiotic consumption and serum concentrations of perfluorooctanoic acid (PFOA) (β: − 0.19, 95% CI − 0.35 to − 0.02; P = 0.027), and perfluorooctane sulfonic acid (PFOS) (β: − 0.1.27, 95% CI − 2.23 to − 0.32; P = 0.010). The consumption of probiotic supplements alone was associated with reduced perfluorononanoic acid (PFNA) (β: − 0.19, 95% CI − 0.28 to − 0.10; P < 0.001). No statistically significant association was identified between probiotic consumption and perfluorohexane sulphonic acid (PFHxS).
Conclusion
In this cross-sectional, nationally representative study, probiotic ingestion was negatively associated with several serum PFAS compounds. These findings carry substantial implications for designing interventions that target the reduction of accumulated PFAS levels in the body and mitigating the resulting adverse health effects.
Similar content being viewed by others
Introduction
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) belong to a group of fluorinated aliphatic chemicals that offer great chemical and thermal stability, along with hydrophobic and oleophobic properties [1]. Thus, they have found wide-ranging applications in diverse industries and everyday consumer products, including industrial detergents, non-stick cookware, food packaging, and textiles [2, 3]. However, the remarkable stability of PFAS poses a challenge as they exhibit resistance to natural degradation. In addition, PFAS can accumulate in water, soil, plants, and organisms throughout the production and usage processes [4]. The persistence and ubiquity of PFAS result in their detection in populations worldwide [5, 6]. PFAS have raised high public health concerns due to their detrimental health effects, which encompass but are not limited to: liver and kidney injury, skeletal disorders, reproductive disorders, immunosuppression, hormonal imbalance, lipid dysregulation, and cancer [7,8,9].
Recently, the application standards for PFAS have become increasingly stringent in numerous countries [10, 11]. Perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) serum levels in the US population witnessed a decline of over 85% and 70% respectively between 1999 and 2018 based on the National Health and Nutrition Examination Survey (NHANES) [12]. Nevertheless, other PFAS may be released as PFOS and PFOA are phased out and replaced. The long-lasting nature of PFAS implies that the bioaccumulation in the human body may still endanger personal well-being. Therefore, the development of effective measures to alleviate individuals’ burden to PFAS is of utmost importance. Previous research has indicated that cholestyramine, a bile acid sequestrant, may reduce PFAS reabsorption in bile, leading to an elevated excretion of PFAS through feces [13, 14]. Lifestyle modifications aimed at lowering lipid levels could also potentially influence circulating levels of PFAS [15]. Additionally, numerous studies have suggested a negative association between PFAS and the ingestion of fiber-rich foods, including vegetables, fruits, legumes, and whole grains [16,17,18]. Interestingly, dietary fiber and folate, which are essential components of these foods, have been found to appear to decrease the concentration of PFAS in the bloodstream [19,20,21]. The intestinal microbiota has the ability to ferment dietary fiber, resulting in the production of short-chain fatty acids (SCFAs) that exert protective effects on the gut [22]. Consequently, it is worth considering whether the ingestion of probiotics could act as an important confounding factor in the relationship between dietary fiber intake and PFAS levels.
Probiotics, which are live microorganisms with a beneficial impact on human health [23], can be obtained from various sources, with yogurt being the most common dietary source [24]. The primary probiotic bacteria are lactic acid bacteria and bifidobacterial [25]. Previous studies have demonstrated a rise in the usage of probiotics among adults and children in the US from 1999 to 2018, which is opposite to the decreasing trend of certain PFAS levels [26]. Probiotics may potentially offer defense against the toxicity of PFAS [27, 28]. However, the precise mechanism by which probiotics mitigate the toxicity of PFAS remains unclear, and there is currently a lack of direct evidence supporting the effect of probiotics on serum PFAS levels. Furthermore, there is a lack of epidemiological studies to evaluate PFAS levels in populations consuming probiotics. This study seeks to examine the association between probiotic consumption and serum PFAS concentrations using NHANES data, providing new insights into the potential role of probiotics in mitigating PFAS toxicity.
Methods
Study design and participants
The public data for this cross-sectional study was obtained from the NHANES 2003–2018 cycle. NHANES is a nationwide survey conducted every 2 years in the US to evaluate the nutritional and health conditions of the general population using questionnaires, physical exams, and biospecimen collection. The study included participants aged 20 and above who had completed probiotics consumption data, PFAS levels measuring, and data for all other covariates. Pregnant or breastfeeding women were excluded from the study because PFAS can be transferred to the fetus through cord blood during pregnancy, or to the baby through breast milk during lactation [29,30,31,32,33]. Participants undergoing dialysis were also excluded due to the potential excretion of PFAS through dialysis [34]. The study population flowchart is depicted in Additional file 1: Fig. S1. All individuals involved in the study provided written informed consent and all protocols were approved by the National Center for Health Statistics’s Research Ethics Review Board.
PFAS measurement
Briefly, the online-solid phase extraction combined with high-performance liquid chromatography-Turbo Ion Spray ionization-tandem mass spectrometry was employed to quantify PFAS. This study specifically examined four PFAS substances: PFOA, PFOS, perfluorononanoic acid (PFNA), and perfluorohexane sulfonic acid (PFHxS), which had detection frequencies above 98% in the serum samples. The concentrations below the limit of detection (LOD) were substituted with LOD/√2. The LODs for each chemical were as follows: PFOA LOD: 0.1 ng/mL (2003–2018). PFOS LOD: 0.4 ng/mL (2003–2004), 0.2 ng/mL (2005–2012), and 0.1 ng/mL (2013–2018). PFNA LOD: 0.82 ng/mL (2007–2010), 0.8 ng/mL (2011–2012), and 0.1 ng/mL (2003–2006 and 2013–2018). PFHxS LOD: 0.3 ng/mL (2003–2004) and 0.1 ng/mL (2005–2018). In the 2013–2018 data cycles, separate measurements were conducted for both the linear and branched isomers of PFOA and PFOS. To maintain consistency with previous cycles, the total concentration of PFOA and PFOS was calculated by summing the measured values of their respective linear and branched isomers.
Identifying probiotics
This study assessed probiotic consumption based on participants’ consumption of yogurt or probiotic-containing dietary supplements [35, 36]. Yogurt ingestion was evaluated using 24-h dietary recall on the first and second days, with USDA Food Code “114” indicating yogurt. Dietary supplements containing probiotics were assessed using the Dietary Supplement Use 30-day questionnaire, which contained data on dietary supplement and nonprescription antacid. Text mining methodologies were applied to identify probiotics by analyzing data from the dietary supplement products, ingredients, and blends information. The search terms used for identifying probiotics refer to previous research [26], as detailed in Additional file 1: Table S1.
Covariates
Covariates selected a priori for analysis consisted of age, gender, race, body mass index (BMI), highest educational level, marital, poverty income ratio (PIR), smoking status, alcohol drinking, NHANES wave, and the intake of fish, meat, egg, energy, dietary folate equivalent, and dietary fiber. Demographic variables were collected through questionnaires, encompassing age (continuous, in years), gender (categorized as male or female), race (classified as Mexican American, non-Hispanic White, non-Hispanic Black, or other), BMI (continuous, in kg/m2), highest educational level (categorized as ≤ high school or > high school), marital (categorized as married or other), and PIR (categorized as < 1.30, 1.30 to < 3.50, or ≥ 3.50). Respondents were grouped into current, former, or never smokers in accordance with whether they had smoked 100 cigarettes in their lives and whether they now smoked.
To take into potential confounding factors account, food items that have been identified as having high levels of PFAS or being associated with higher PFAS levels, such as fish, meat, and egg, were included in the analysis [37,38,39,40]. Food and nutrient calculations were based on data from two 24-h recalls, using the USDA Food Patterns Equivalents Database to determine food consumption. The fish variable (continuous, in ounce/d) encompassed fish, shellfish, and other seafood. The meat variable (continuous, in ounce/d) encompassed beef, pork, veal, lamb, and game, organ meats, frankfurters, sausages, luncheon meat, and poultry (chicken, turkey, and other). The egg variable (continuous, in ounce/d) encompassed eggs and egg substitutes. Nutrient intake covariates included dietary fiber (continuous, in g/d), energy (continuous, in kcal/d), dietary folate equivalent (continuous, μg/d), and alcohol consumption. Dietary fiber and folate have been found to be negatively associated with PFAS levels in adult serum [19, 20]. Adjusting for total energy intake allows for a relative rather than an absolute representation of dietary intake, considering the variation in energy intake among individuals engaged in different physical activities or with varying body weights [41]. Subjects were classified as heavy alcohol consumption if their daily ethanol consumption exceeded 10 g for females or 20 g for males; otherwise, they were classified as no heavy alcohol consumption.
Statistical analysis
Baseline characteristics were presented using median and quartiles for continuous variables, while percentages were utilized for categorical variables. To investigate the relationship between probiotic consumption and various PFAS compounds (PFOA, PFNA, PFOS, and PFHxS), a generalized linear regression model was applied. Model 1 was unadjusted, Model 2 adjusted for age, gender, and BMI, and Model 3 adjusted for all covariates. To investigate whether the associations differed depending on the source of probiotics (probiotics supplement or yogurt), we conducted further analysis. Subgroup analyses were performed for different factors, including age (< 54 y, ≥ 54 y), gender, race, BMI (< 20, 20–< 25, 25–< 30, 30+), educational level, marital status, PIR (< 1.30, 1.30–< 3.50, 3.50+), smoking status, alcohol drinking, NHANES wave (2003–2005, 2007–2009, 2011–2013, 2015–2017), and the intake of fish (Yes or No), meat (< 2.19, 2.19–< 3.71, 3.71–< 5.74, 5.74+), egg (< 0.035, 0.035–< 0.230, 0.230–< 0.885, 0.885+), energy (< 1545, 1545–< 1972.5, 1972.5–< 2559, 2559+), dietary folate equivalent (< 333, 333–< 466, 466–< 657, 657+), and dietary fiber (< 10.75, 10.75–< 15.25, 15.25–< 21.35, 21.35+). Likelihood ratio tests were conducted to assess the presence of interaction effects by comparing models with and without interaction terms. The statistical analysis was conducted utilizing R version 4.3.1 and the “survey” package. Statistical significance was determined based on a significance level of P < 0.05.
Results
The baseline population characteristics regarding probiotic consumption are outlined in Table 1. This study included 9469 adults aged 20 years or above, of whom 1333 had been exposed to probiotics. The median age of the probiotic exposure group, 50 years, was higher than that of the non-exposure group, which had a median age of 46 years. In the probiotic exposure group, there was a higher proportion of female participants (62.28%) compared to the non-exposure group (49.28%). The individuals in the non-exposure group had a median BMI of 28.10 kg/m2, whereas those in the probiotic consumption group had a median BMI of 27.49 kg/m2. The probiotic exposure group had higher proportions of non-Hispanic White individuals (79.71%) and married participants (61.41%). Probiotic consumption also had higher socioeconomic status, as indicated by both PIR and education. For instance, 76.52% of individuals with an education level beyond high school reported probiotic consumption, compared to 58.66% of those who did not. In the non-probiotic exposure group, the daily intake of meat, dietary fiber, and dietary folate equivalents was 3.77 oz, 14.70 g and 462.00 mcg, respectively, while in the probiotic exposure group, it was 3.30 oz, 17.95 g and 480.00 mcg, respectively. In the group exposed to probiotics, participants demonstrated a higher preference for dietary fiber and dietary folate equivalents rather than meat. The ingestion of energy, egg, and the proportion of heavy alcohol consumption were similar between the two groups. Individuals exposed to probiotics had higher percentages of never-smoker statuses (62.68%) and lower proportions of current smokers (9.70%) compared to those not exposed to probiotics. Among the PFAS compounds studied, the serum levels of PFOA, PFNA, and PFOS showed significant differences between the two groups, with lower median concentrations by 0.30 ng/mL, 0.09 ng/mL, and 2.30 ng/mL respectively, compared to the non-exposure group. However, the concentrations of PFHxS did not differ significantly between the two groups.
The study utilized baseline and multivariate design-based survey linear regression models to analyze the association between probiotic consumption and individual PFAS, as shown in Table 2. In the unadjusted analyses (Model 1), it was observed that probiotic consumption exhibited a statistically significant association with the reductions in PFOA levels, PFNA levels, and PFOS levels. After controlling for age, gender, and BMI (Model 2), the statistical significance of the association between probiotic consumption and decreased levels of PFOA (β: − 0.43, 95% CI − 0.63 to − 0.23; P < 0.001), PFNA (β: − 0.13, 95% CI − 0.21 to − 0.05; P = 0.002), and PFOS (β: − 3.21, 95% CI − 4.23 to − 2.19; P < 0.001) was maintained. After full adjustment (Model 3), although the association between probiotic consumption and PFNA disappeared, probiotic consumption remained negatively associated with PFOA and PFOS. Notably, no significant association was found between probiotic consumption and PFHxS across all models.
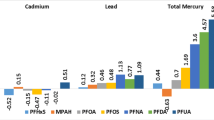
In Model 3, further analysis was undertaken to assess the association between probiotic supplements, yogurt consumption, and serum PFAS concentrations (Fig. 1). Among the participants who were exposed to probiotics, a total of 1194 individuals were exposed to yogurt, 139 individuals were exposed to probiotic supplements, and 50 participants were exposed to both simultaneously. Any type of probiotics and probiotic supplements were associated with reduced PFOA concentration. Serum PFNA concentration was not associated with exposure to any type of probiotics or yogurt but showed a significant association with exposure to probiotic supplements alone (β: − 0.19, 95% CI − 0.28 to − 0.10; P < 0.001). Irrespective of the type of probiotics, a negative association was observed with serum PFOS levels.
Association between different sources of probiotics and serum PFAS concentration. Adjusted for age, gender, race, body mass index, highest educational level, marital status, poverty income ratio, smoking status, alcohol drinking, NHANES wave, and the intake of fish, meat, egg, energy, dietary folate equivalent, and dietary fiber
The stratified analysis based on population characteristics (Table 3 and Additional file 1: Table S2) revealed significant interactions between probiotic consumption and serum PFOA levels by race (P-interaction = 0.009), education (P-interaction = 0.012), PIR (P-interaction = 0.021), and meat intake (P-interaction = 0.046). Additionally, significant interactions were observed between probiotic consumption and PFNA by race (P-interaction = 0.038), as well as between probiotic consumption and PFOS by PIR (P-interaction = 0.022) and heavy alcohol consumption (P-interaction = 0.036). Other associations did not yield statistically significant differences. Notably, the negative association between probiotic consumption and PFOA levels was more apparent among non-Hispanic White individuals, those with an education level beyond high school, high household income, and the highest quartile of meat intake. A statistically significant association between probiotic consumption and decreased PFNA concentration was also observed among non-Hispanic White individuals. In terms of PFOS, participants with moderate family incomes or heavy alcohol consumption were more susceptible to the association.
Discussion
This representative large-scale cross-sectional study suggested a strong negative association between the use of probiotics and serum concentrations of PFOA and PFOS. While there were no associations found between exposure to any type of probiotics and serum PFNA concentration, exposure to probiotic supplements alone was significantly associated with reduced PFNA levels. The negative associations between probiotic consumption and PFOA (among non-Hispanic White individuals, those with an education level beyond high school, high household income, and the highest quartile of meat intake), PFNA (among non-Hispanic White individuals), and PFOS (among moderate family incomes and heavy alcohol consumption) were more pronounced.
As far as we know, this study is the first large cross-sectional analysis investigating the association between probiotic consumption (through supplements or yogurt) and serum PFAS levels. Previous research on the association between probiotics and PFAS has been limited to animal and cell-based studies, which have found that probiotics can mitigate the toxicity of PFAS. Specifically, exposure to PFOA toxicity in mouse models has been shown to induce liver damage, reduces the abundance of beneficial probiotics, and lowers levels of SCFAs in feces, but these effects can be alleviated through the administration of probiotics or synbiotics [42,43,44]. Probiotics may alleviate PFOA toxicity by absorbing the compound, exerting antioxidant effects, and regulating gut microbiota [43]. Additionally, an in vitro study assessed the binding capacity of 25 Lactobacillus strains to PFOA and found that Lactobacillus plantarum CCFM738 exhibited the highest binding capability. This strain also demonstrated excellent cellular antioxidative properties, tolerance to acidic and bile salt conditions, and adhesion to intestinal epithelial cells, which are important factors in assessing the functional properties of probiotics [45]. In mice, PFOS has been demonstrated to cause liver damage, alter lipid and glucose metabolism, and disturb intestinal homeostasis [46, 47]. Lactic acid bacteria can help mitigate PFOS toxicity by binding to PFOS, exerting antioxidant effects, and restoring the intestinal environment through up-regulating the content of SCFAs in the cecum and enhancing the expression of tight junction proteins in the intestines [48]. Our study reveals a negative association between probiotic consumption and serum PFAS concentrations, suggesting that probiotics may have the potential to alleviate the burden of PFAS in the human body and mitigate their detrimental effects.
According to the analysis of probiotic sources, the consumption of probiotic supplements, as opposed to yogurt, was observed to be associated with a decrease in serum levels of PFOA and PFNA. Both types of probiotics were significantly associated with a reduction in PFOS concentration. In this study, the consumption of a dietary supplement containing probiotics within the past 30 days was considered probiotic supplement consumption, which reflected a relatively long-term pattern of probiotic consumption. In contrast, yogurt consumption referred to consuming yogurt 1 day between 2 days, which had a certain degree of chance and did not accurately reflect a long-term habit of consuming yogurt. The quantity of probiotics ingested by individuals through yogurt consumption may vary depending on the brand, type, and serving size. Considering the persistence of PFAS compounds, this finding suggested that long-term probiotic consumption may have a stronger association with the reduction of serum PFAS concentrations.
Race, education, income, meat intake, and alcohol abuse may influence the relationship between probiotic consumption and specific PFAS concentrations. Compared to other races, a stronger negative association was observed between the consumption of probiotics and serum levels of PFOA and PFNA in non-Hispanic White individuals. This could be due to genetic, environment, and dietary differences among different races, as well as variations in their gut microbiota composition [49,50,51]. It is worth noting that the sample size of non-Hispanic White individuals was approximately 2–3 times larger than that of other races. In a study utilizing NHANES data from 1999 to 2018 to investigate the trends in probiotic usage among adults and children in the US, it was observed that individuals with higher levels of education and income exhibited greater prevalence of probiotic use [26]. The effect heterogeneity by education and income in our study possibly because individuals with higher levels of education and income tend to have a greater interest in health-related knowledge and more disposable income. As a result, they are more likely to develop habits of consuming probiotic-rich foods or foods that promote the growth of beneficial gut bacteria. However, these possible confounding factors cannot be comprehensively addressed in our study. Previous studies have found a link between meat consumption and higher levels of PFAS [39, 40]. The observation of a stronger negative association between probiotic consumption and serum PFAS levels in populations with higher meat intake requires further investigation and careful evaluation. Heavy alcohol consumption has been found to potentially interact with PFAS in relation to liver function biomarkers [52]. Our finding that associations were more apparent among individuals with heavy alcohol consumption should be interpreted with caution, given the small sample size of the population with heavy alcohol consumption. One possible explanation is that the antagonistic effect of probiotics on PFAS levels could be influenced by impaired liver function due to alcohol abuse. Alternatively, unmeasured confounding variables may contribute to difference by groups.
When interpreting the results, it is important to take into account several limitations of the present study. Firstly, the cross-sectional study design hinders the establishment of temporal relationships between probiotic exposure and PFAS concentrations in serum, and therefore, causality cannot be determined. It is not possible to infer whether probiotic consumption leads to a decrease in PFAS concentration. Although we made efforts to control for potential confounding factors, there may still be unaccounted or residual confounding. It is possible that other dietary sources of probiotics not considered in this study could also influence the association between yogurt consumption and serum PFAS levels. Future studies could examine the effects of other fermented foods to expand on our findings. Additionally, the study population was categorized based on whether they consumed probiotics (either through supplements or yogurt), but information regarding the specific types, quantity and quality of probiotics consumed was unavailable. Therefore, we were unable to investigate the potential relationship between the dosage and types of probiotics ingestion and PFAS levels. Finally, the available information regarding the exposure of other PFAS compounds released into the environment as PFOS and PFOA are phased out and replaced remains unclear. It is important to recognize the need for further monitoring to understand the potential implications of exposure to these emerging PFAS compounds.
Conclusion
In summary, this large nationally representative study observed negative associations between probiotic consumption and several serum PFAS levels. Considering the widespread exposure, persistent nature, and harmfulness of PFAS, these findings present a promising approach to mitigating the body burden of PFAS. Further prospective and experimental studies are needed to confirm or refute the association between probiotic consumption and PFAS concentration, and to explore the underlying mechanisms involved.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available in the NHANES repository, https://www.cdc.gov/nchs/nhanes/ [53]. Further information is available from the corresponding author upon request.
Abbreviations
- BMI:
-
Body mass index
- LOD:
-
Limit of detection
- NHANES:
-
National Health and Nutrition Examination Survey
- PFHxS:
-
Perfluorohexane sulfonic acid
- PFNA:
-
Perfluorononanoic acid
- PFOA:
-
Perfluorooctanoic acid
- PFOS:
-
Perfluorooctane sulfonic acid
- PIR:
-
Poverty income ratio
- SCFAs:
-
Short-chain fatty acids
References
Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG (2019) A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131–147. https://doi.org/10.1038/s41370-018-0094-1
Dhore R, Murthy GS (2021) Per/polyfluoroalkyl substances production, applications and environmental impacts. Bioresour Technol 341:125808. https://doi.org/10.1016/j.biortech.2021.125808
Glüge J, Scheringer M, Cousins IT, DeWitt JC, Goldenman G, Herzke D, Lohmann R, Ng CA, Trier X, Wang Z (2020) An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ Sci Process Impacts 22(12):2345–2373. https://doi.org/10.1039/d0em00291g
Evich MG, Davis MJB, McCord JP, Acrey B, Awkerman JA, Knappe DRU et al (2022) Per- and polyfluoroalkyl substances in the environment. Science 375(6580):eabg9065. https://doi.org/10.1126/science.abg9065
Calafat AM, Kato K, Hubbard K, Jia T, Botelho JC, Wong LY (2019) Legacy and alternative per- and polyfluoroalkyl substances in the US general population: paired serum-urine data from the 2013–2014 national health and nutrition examination survey. Environ Int 131:105048. https://doi.org/10.1016/j.envint.2019.105048
Fan X, Tang S, Wang Y, Fan W, Ben Y, Naidu R, Dong Z (2022) Global exposure to per- and polyfluoroalkyl substances and associated burden of low birthweight. Environ Sci Technol 56(7):4282–4294. https://doi.org/10.1021/acs.est.1c08669
Radke EG, Wright JM, Christensen K, Lin CJ, Goldstone AE, Lemeris C, Thayer KA (2022) Epidemiology evidence for health effects of 150 per- and polyfluoroalkyl substances: a systematic evidence map. Environ Health Perspect 130(9):96003. https://doi.org/10.1289/ehp11185
Panieri E, Baralic K, Djukic-Cosic D, Buha Djordjevic A, Saso L (2022) PFAS molecules: a major concern for the human health and the environment. Toxics 10(2):44. https://doi.org/10.3390/toxics10020044
Jane LEL, Yamada M, Ford J, Owens G, Prow T, Juhasz A (2022) Health-related toxicity of emerging per- and polyfluoroalkyl substances: comparison to legacy PFOS and PFOA. Environ Res 212(Pt C):113431. https://doi.org/10.1016/j.envres.2022.113431
Brennan NM, Evans AT, Fritz MK, Peak SA, von Holst HE (2021) Trends in the regulation of per- and polyfluoroalkyl substances (PFAS): a scoping review. Int J Environ Res Public Health 18(20):10900. https://doi.org/10.3390/ijerph182010900
Scheringer M (2023) Innovate beyond PFAS. Science 381(6655):251. https://doi.org/10.1126/science.adj7475
ATSDR. PFAS in the U.S. population. Per- and polyfluoroalkyl substances (PFAS) and your health (2023) https://www.atsdr.cdc.gov/pfas/health-effects/us-population.html. Accessed 9 Aug 2023
Genuis SJ, Curtis L, Birkholz D (2013) Gastrointestinal elimination of perfluorinated compounds using cholestyramine and Chlorella pyrenoidosa. ISRN Toxicol 2013:657849. https://doi.org/10.1155/2013/657849
Genuis SJ, Birkholz D, Ralitsch M, Thibault N (2010) Human detoxification of perfluorinated compounds. Public Health 124(7):367–375. https://doi.org/10.1016/j.puhe.2010.03.002
Morgan S, Mottaleb MA, Kraemer MP, Moser DK, Worley J, Morris AJ, Petriello MC (2023) Effect of lifestyle-based lipid lowering interventions on the relationship between circulating levels of per-and polyfluoroalkyl substances and serum cholesterol. Environ Toxicol Pharmacol 98:104062. https://doi.org/10.1016/j.etap.2023.104062
Sultan H, Buckley JP, Kalkwarf HJ, Cecil KM, Chen A, Lanphear BP, Yolton K, Braun JM (2023) Dietary per- and polyfluoroalkyl substance (PFAS) exposure in adolescents: the HOME study. Environ Res 231(Pt 1):115953. https://doi.org/10.1016/j.envres.2023.115953
Liu Y, Su J, van Dam RM, Prem K, Hoong JYS, Zou L, Lu Y, Ong CN (2017) Dietary predictors and plasma concentrations of perfluorinated alkyl acids in a Singapore population. Chemosphere 171:617–624. https://doi.org/10.1016/j.chemosphere.2016.12.107
Skuladottir M, Ramel A, Rytter D, Haug LS, Sabaredzovic A, Bech BH, Henriksen TB, Olsen SF, Halldorsson TI (2015) Examining confounding by diet in the association between perfluoroalkyl acids and serum cholesterol in pregnancy. Environ Res 143(Pt A):33–38. https://doi.org/10.1016/j.envres.2015.09.001
Dzierlenga MW, Keast DR, Longnecker MP (2021) The concentration of several perfluoroalkyl acids in serum appears to be reduced by dietary fiber. Environ Int 146:106292. https://doi.org/10.1016/j.envint.2020.106292
Zhang Y, Mustieles V, Wang YX, Sun Y, Agudelo J, Bibi Z, Torres N, Oulhote Y, Slitt A, Messerlian C (2023) Folate concentrations and serum perfluoroalkyl and polyfluoroalkyl substance concentrations in adolescents and adults in the USA (national health and nutrition examination study 2003–16): an observational study. Lancet Planet Health 7(6):e449–e458. https://doi.org/10.1016/s2542-5196(23)00088-8
Zhang Y, Mustieles V, Wang YX, Sun Q, Coull B, Sun Y, Slitt A, Messerlian C (2023) Red blood cell folate modifies the association between serum per- and polyfluoroalkyl substances and antibody concentrations in US adolescents. Environ Sci Technol 57(6):2445–2456. https://doi.org/10.1021/acs.est.2c07152
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341(6145):569–573. https://doi.org/10.1126/science.1241165
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B et al (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506–514. https://doi.org/10.1038/nrgastro.2014.66
Fernandez MA, Marette A (2017) Potential health benefits of combining yogurt and fruits based on their probiotic and prebiotic properties. Adv Nutr 8(1):155s-s164. https://doi.org/10.3945/an.115.011114
Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K et al (2020) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 17(11):687–701. https://doi.org/10.1038/s41575-020-0344-2
O’Connor LE, Gahche JJ, Herrick KA, Davis CD, Potischman N, Vargas AJ (2021) Nonfood prebiotic, probiotic, and synbiotic use has increased in US adults and children from 1999 to 2018. Gastroenterology 161(2):476–86.e3. https://doi.org/10.1053/j.gastro.2021.04.037
Baralić K, Živančević K, Bozic D, Đukić-Ćosić D (2023) Probiotic cultures as a potential protective strategy against the toxicity of environmentally relevant chemicals: state-of-the-art knowledge. Food Chem Toxicol 172:113582. https://doi.org/10.1016/j.fct.2022.113582
Mirza Alizadeh A, Hosseini H, MollakhaliliMeybodi N, Hashempour-Baltork F, Alizadeh-Sani M, Tajdar-Oranj B, Pirhadi M, Mousavi KA (2022) Mitigation of potentially toxic elements in food products by probiotic bacteria: a comprehensive review. Food Res Int 152:110324. https://doi.org/10.1016/j.foodres.2021.110324
Mamsen LS, Björvang RD, Mucs D, Vinnars MT, Papadogiannakis N, Lindh CH, Andersen CY, Damdimopoulou P (2019) Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ Int 124:482–492. https://doi.org/10.1016/j.envint.2019.01.010
Cai D, Li QQ, Chu C, Wang SZ, Tang YT, Appleton AA et al (2020) High trans-placental transfer of perfluoroalkyl substances alternatives in the matched maternal-cord blood serum: evidence from a birth cohort study. Sci Total Environ 705:135885. https://doi.org/10.1016/j.scitotenv.2019.135885
Liu Y, Liu K, Zheng P, Yin S, Jin H, Bai X et al (2021) Prenatal exposure and transplacental transfer of perfluoroalkyl substance isomers in participants from the upper and lower reaches of the Yangtze River. Environ Pollut 270:116202. https://doi.org/10.1016/j.envpol.2020.116202
Blomberg AJ, Norén E, Haug LS, Lindh C, Sabaredzovic A, Pineda D, Jakobsson K, Nielsen C (2023) Estimated transfer of perfluoroalkyl substances (PFAS) from maternal serum to breast milk in women highly exposed from contaminated drinking water: a study in the Ronneby mother–child cohort. Environ Health Perspect 131(1):17005. https://doi.org/10.1289/ehp11292
Blomberg AJ, Haug LS, Lindh C, Sabaredzovic A, Pineda D, Jakobsson K, Nielsen C (2023) Changes in perfluoroalkyl substances (PFAS) concentrations in human milk over the course of lactation: a study in Ronneby mother–child cohort. Environ Res 219:115096. https://doi.org/10.1016/j.envres.2022.115096
Huang JK, Chuang YS, Wu PH, Tai CJ, Lin JR, Kuo MC et al (2023) Decreased levels of perfluoroalkyl substances in patients receiving hemodialysis treatment. Sci Total Environ 896:165184. https://doi.org/10.1016/j.scitotenv.2023.165184
Liu X, Gao W, Yang J, Mao G, Lu H, Xing W (2022) Association between probiotic, prebiotic, and yogurt consumption and chronic kidney disease: the NHANES 2010–2020. Front Nutr 9:1058238. https://doi.org/10.3389/fnut.2022.1058238
Lau E, Neves JS, Ferreira-Magalhães M, Carvalho D, Freitas P (2019) Probiotic ingestion, obesity, and metabolic-related disorders: results from NHANES, 1999–2014. Nutrients 11(7):1482. https://doi.org/10.3390/nu11071482
Nyström J, Benskin JP, Plassmann M, Sandblom O, Glynn A, Lampa E, Gyllenhammar I, Lignell S, Moraeus L (2022) Healthy eating index and diet diversity score as determinants of serum perfluoroalkyl acid (PFAA) concentrations in a national survey of Swedish adolescents. Environ Res 212(Pt A):113170. https://doi.org/10.1016/j.envres.2022.113170
Soleman SR, Li M, Fujitani T, Harada KH (2023) Plasma eicosapentaenoic acid, a biomarker of fish consumption, is associated with perfluoroalkyl carboxylic acid exposure in residents of Kyoto, Japan: a cross-sectional study. Environ Health Prev Med 28:38. https://doi.org/10.1265/ehpm.22-00302
Huo X, Liang W, Tang W, Ao Y, Tian Y, Zhang Q, Zhang J (2023) Dietary and maternal sociodemographic determinants of perfluoroalkyl and polyfluoroalkyl substance levels in pregnant women. Chemosphere 332:138863. https://doi.org/10.1016/j.chemosphere.2023.138863
Menzel J, Abraham K, Dietrich S, Fromme H, Völkel W, Schwerdtle T, Weikert C (2021) Internal exposure to perfluoroalkyl substances (PFAS) in vegans and omnivores. Int J Hyg Environ Health 237:113808. https://doi.org/10.1016/j.ijheh.2021.113808
Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65(4 Suppl):1220S-1228S. https://doi.org/10.1093/ajcn/65.4.1220S. (discussion 9S–31S)
Wang G, Pan R, Liang X, Wu X, Wu Y, Zhang H, Zhao J, Chen W (2021) Perfluorooctanoic acid-induced liver injury is potentially associated with gut microbiota dysbiosis. Chemosphere 266:129004. https://doi.org/10.1016/j.chemosphere.2020.129004
Shi L, Pan R, Lin G, Liang X, Zhao J, Zhang H, Chen W, Wang G (2021) Lactic acid bacteria alleviate liver damage caused by perfluorooctanoic acid exposure via antioxidant capacity, biosorption capacity and gut microbiota regulation. Ecotoxicol Environ Saf 222:112515. https://doi.org/10.1016/j.ecoenv.2021.112515
Soltani M, Pourfarzam M, Sharifabad AH, Neisiani AK, Mousavi MK, Aliomrani M (2023) Effect of pretreatment with a synbiotic on perfluorooctanoic acid-induced liver damage after sub-acute oral exposure in C57BL/6J mice. Toxicol Appl Pharmacol 459:116360. https://doi.org/10.1016/j.taap.2022.116360
Xing J, Wang F, Xu Q, Yin B, Fang D, Zhao J, Zhang H, Chen YQ, Wang G, Chen W (2016) Screening of potential probiotic lactic acid bacteria based on gastrointestinal properties and perfluorooctanoate toxicity. Appl Microbiol Biotechnol 100(15):6755–6766. https://doi.org/10.1007/s00253-016-7535-3
Wang G, Sun S, Wu X, Yang S, Wu Y, Zhao J, Zhang H, Chen W (2020) Intestinal environmental disorders associate with the tissue damages induced by perfluorooctane sulfonate exposure. Ecotoxicol Environ Saf 197:110590. https://doi.org/10.1016/j.ecoenv.2020.110590
Jiang L, Hong Y, Xiao P, Wang X, Zhang J, Liu E, Li H, Cai Z (2022) The role of fecal microbiota in liver toxicity induced by perfluorooctane sulfonate in male and female mice. Environ Health Perspect 130(6):67009. https://doi.org/10.1289/ehp10281
Chen Q, Sun S, Mei C, Zhao J, Zhang H, Wang G, Chen W (2022) Capabilities of bio-binding, antioxidant and intestinal environmental repair jointly determine the ability of lactic acid bacteria to mitigate perfluorooctane sulfonate toxicity. Environ Int 166:107388. https://doi.org/10.1016/j.envint.2022.107388
Byrd DA, Carson TL, Williams F, Vogtmann E (2020) Elucidating the role of the gastrointestinal microbiota in racial and ethnic health disparities. Genome Biol 21(1):192. https://doi.org/10.1186/s13059-020-02117-w
Borrello K, Lim U, Park SY, Monroe KR, Maskarinec G, Boushey CJ et al (2022) Dietary intake mediates ethnic differences in gut microbial composition. Nutrients 14(3):660. https://doi.org/10.3390/nu14030660
Park SK, Peng Q, Ding N, Mukherjee B, Harlow SD (2019) Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: evidence of racial/ethnic and geographic differences in PFAS exposure. Environ Res 175:186–199. https://doi.org/10.1016/j.envres.2019.05.028
Ma X, Fisher JA, VoPham T, Vasiliou V, Jones RR (2023) Associations between per- and polyfluoroalkyl substances, liver function, and daily alcohol consumption in a sample of US adults. Environ Res 235:116651. https://doi.org/10.1016/j.envres.2023.116651
Prevention CfDCa. National health and nutrition examination survey (2023) https://www.cdc.gov/nchs/nhanes/
Acknowledgements
This study analyzed using the data provided by the National Health and Nutrition Examination Survey (2003–2018).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
YL designed research, analyzed data and drafted the manuscript; JC analyzed data and interpreted data; JL and JW revised the manuscript; LC and MG conceived research, revised the manuscript and had primary responsibility for final content. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All individuals provided written informed consent and all protocols were approved by the Research Ethics Review Board of the National Center for Health Statistics.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
A schematic flow chart of study design. Table S1. Probiotic search terms. Table S2. Association between probiotic consumption and PFAS serum concentrations according to different subgroups.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liao, Y., Chen, J., Li, J. et al. Association between probiotic consumption and serum perfluoroalkyl and polyfluoroalkyl substances (PFAS): results from NHANES, 2003–2018. Environ Sci Eur 35, 105 (2023). https://doi.org/10.1186/s12302-023-00808-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-023-00808-2