Abstract
Information on transformation and persistence of chemical substances in the environment is important for hazard and risk assessment within a regulatory context or as a decision criterion in a safe and sustainable by design framework. Half-lives for human and veterinary medicinal products available from marketing authorization applications were compared between soil (OECD 307) and aquatic water/sediment systems (OECD 308). The comparison shows, that there is no obvious correlation between the total system half-lives in the two different compartments and that surpassing persistence criteria is compartment-specific in 45% of the cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Information on transformation and persistence of chemical substances in the environment is important for risk and hazard assessment within a regulatory context or as a decision criterion in a safe and sustainable by design framework [1]. Data requirements and adherence to the requirements is different for regulatory frameworks. While for plant protection products different environmental compartments are covered, for other frameworks like pharmaceuticals or REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) chemicals only one compartment is tested initially, and only in some cases additional information on other compartments is required, depending on the outcome of the test, substance properties and regulatory framework [2, 3]. Therefore, it is interesting to examine if testing one compartment is sufficient or if differing results might be observed in aquatic and terrestrial compartments.
Methods
This question was examined using end point data from applications for marketing authorization for pharmaceuticals (21 human and one veterinary medicinal products) which are intended to be publicly available [4]. The terrestrial compartment was assessed by tests according to OECD (Organisation for Economic Cooperation and Development) TG (test guideline) 307 (transformation in soil, [5]). The aquatic compartment was examined in studies according to OECD TG 308 with water/sediment systems [6]. Studies conform to Good Laboratory Practice (GLP). All active ingredients (ai) from human pharmaceuticals and veterinary medicinal products were considered for which both data for soil and water/sediment systems were available (22 ai in total). Half-lives were calculated based on SFO (single first order) kinetics (DFOP (dual first order in parallel) for ceritinib in OECD 308) as geometric mean of different soils (n = 1–4) and different sediments (n = 2) for each ai. Only aerobic studies were considered, however, it has to be taken into account that the sediment might contain anaerobic regions [7]. All studies were conducted at 20 °C. Half-lives were temperature corrected to 12 °C as described in [3]. The resulting total system (ts) half-lives are reported as DT50wsts for OECD 308 studies and DT50soil for OECD 307 studies. If half-lives could not be derived, because a plateau was observed or very high values were reported extrapolating far above the study duration, the values are given as > 1000 days and are marked in Fig. 1 by an arrow. Non-extractable residues were considered as sink (i.e., as equal to mineralized) in the present study. Additionally, Koc (organic carbon normalized adsorption coefficient) values reported as part of the applications were collected. It should be noted that the available data are biased towards high Koc values as generally only for compounds with a Koc > 10,000 L/kg a terrestrial assessment is required. Table 1 gives an overview of the examined ai. As preference was given to use SFO kinetics to derive half-lives for better comparability between different substances and mean values are used, the results do not strictly correspond to a regulatory persistence classification which requires best fit kinetics (that may deviate from SFO kinetics more often) and worst case values from parallel test systems (instead of the geometric mean) to be used [8]. Pearson’s coefficient of correlation was calculated using MSExcel 2019™.
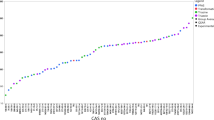
Comparison of half-lives (@12 °C in d) for 22 ai (see Table 1) in soil and water/sediment systems; ai abbreviations (see Table 1) are given adjacent to the data point (the respective total system half-life for soil (DT50soil) and water/sediment (DT50wsts) is given in parentheses in days (d)). The 1:1 line is shown as dotted line. Values given as > 1000 d are marked with an arrow (arbitrary length). Persistence in water/sediment (total system) is indicated by blue shading of the region exceeding 120 d while persistence in soil is indicted by brown shading of the region exceeding 120 d. Individual ai names are color coded according to logKoc (3–4 light green; 4–5 dark green; 5–6 black; ≥ 6 violet; no data, light grey)
Results and discussion
The comparison of terrestrial and aquatic half-lives is shown in Fig. 1 (see also Table S1 in the supplemantary information).
Four of the 22 compounds consistently show half-lives below the persistence threshold of 120 d [3] for both soil and water/sediment systems. Another eight compounds also consistently produce half-lives above the persistence threshold, independent of simulation study type. Six compounds are only classified as persistent when relying on data from OECD 308 studies and four compounds only exceed the 120 d persistence criterion when data from OECD 307 studies are used. For 55% of the compounds, persistence assessment shows the same outcome, regardless whether the terrestrial or the aquatic compartment is tested. Pearson’s coefficient of correlation between soil and water/sediment is < 0.1 (without values ≥ 1000 d), i.e., there is no correlation observable. The different logKoc values of the compounds are color coded in Fig. 1. There is also no obvious correlation visible between half-lives and logKoc. Calculated Pearson’s coefficients of correlation are 0.3 for soil half-lives and Koc, and − 0.1 for water/sediment half-lives and Koc. A tendency might be observable that extremely high logKoc values (≥ 6) correspond to longer half-lives in water/sediment tests compared to soil, as all logKoc ≥ 6 compounds are classified as persistent in OECD 308 studies but not necessarily in OECD 307 studies (see violet compounds in Fig. 1, n = 4). It should be noted that some types of ai seem to be overrepresented among the study compounds. This is true for tyrosine kinase inhibitors, which are a class of antineoplastic agents (name ends in -ib). Additionally, antiviral compounds (ending in -vir) are also overrepresented. This should not be generalized however, as the sample of 22 ai is just a small subset of pharmaceutical ai on the market. It should be considered, that the sample of ai is biased for recently authorized compounds, as there are no or less data available for older ai, and for high Koc compounds, as otherwise only the aquatic compartment is examined and data for soil are not available [2].
Conclusion and outlook
The results show that persistence assessment might yield different outcomes depending on the environmental compartment that is examined. In the studied examples, this concerns 45% of the test substances (10 out of 22 ai). It is therefore important to consider all compartments in persistence assessment to which emissions or subsequent transfer might occur. This has also been noted for REACH chemicals [9]. Consequently, skipping simulation type testing (i.e., OECD TG 308) for human pharmaceutical ai for the aquatic compartment in Phase II Tier A, as proposed by the revised draft guideline for environmental assessment for human medicinal products [10] will result in data gaps for persistence assessment of pharmaceutical compounds.
Availability of data and materials
Data are included in the manuscript or are available at https://www.ema.europa.eu/en, materials: not applicable.
References
EC (2023) Safe and Sustainable by Design chemicals and materials Review of safety and sustainability dimensions, aspects, methods, indicators, and tools. https://publications.jrc.ec.europa.eu/repository/handle/JRC127109, accessed 13.02.23
EMA (2006) Guideline on the environmental risk assessment of medicinal products for human use. EMEA/CHMP/SWP/4447/00 corr2
ECHA (2017) Guidance on Information Requirements and Chemical Safety Assessment Chapter R.11: PBT/vPvB Assessment. https://doi.org/10.2823/128621
Oelkers K, Floeter C (2019) The accessibility of data on environmental risk assessment of pharmaceuticals: is the marketing authorisation procedure in conflict with the international right of access to environmental information? Environ Sci Eur 31(1)
OECD (2002) Test No 307: Aerobic and Anaerobic Transformation in Soil. OECD Guidelines for the Testing of Chemicals, Section 3, OECD Publishing, Paris. https://doi.org/10.1787/9789264070509-en
OECD (2002) Test No 308: Aerobic and Anaerobic Transformation in Aquatic Sediment Systems. OECD Guidelines for the Testing of Chemicals, Section 3, OECD Publishing, Paris. https://doi.org/10.1787/9789264070523-en
EMA (2016) Questions and answers on ‘Guideline on the environmental risk assessment of medicinal products for human use’. EMA/CHMP/SWP/44609/2010 Rev. 1
FOCUS (2006) “Guidance Document on Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration” Report of the FOCUS Work Group on Degradation Kinetics, EC Document Reference Sanco/10058/2005 version 2.0
Holzmann H, Claßen D, Ackermann J, Schäffer A (2022) Fate of 14C-labelled ionic organic chemicals in a water-sediment system and surface water. Chemosphere 303:134885. https://doi.org/10.1016/j.chemosphere.2022.134885
EMA (2018) Draft guideline on the environmental risk assessment of medicinal products for human use—Revision 1. EMEA/CHMP/SWP/4447/00 Rev.1
Acknowledgements
We would like to thank Kim Teppe for her advice.
Funding
Open Access funding enabled and organized by Projekt DEAL. Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: SB; data analysis: UB, SB; writing original manuscript: SB; review and editing: UB, SB.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
Identities, pharmacological groups, identifiers, physico-chemical parameters and half-lives for the studied pharmaceutical ai.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berkner, S., Brandt, U. Comparing pharmaceutical persistence across terrestrial and aquatic environments: do studies according to OECD 307 and OECD 308 lead to similar outcomes?. Environ Sci Eur 35, 72 (2023). https://doi.org/10.1186/s12302-023-00783-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-023-00783-8