Abstract
Disinfectants and preservatives used as biocides may contain or release active substances (a.s.) that can form by-products with the surrounding matrices during their application which may be released into the environment. Over the past 40 years, several hundred of these so-called disinfection by-products (DBPs) have been detected after applications of biocides used for disinfection. Due to intensive research and further development of analytical capabilities, many new DBP classes, such as iodinated DBPs (I-DBPs), halonitromethanes (HNMs), haloacetamides (HaAms), or halomethanesulfonic acids were detected worldwide in various matrices and applications. Due to the possible hazards and risks for humans and the environment, frequently occurring DBP classes, such as trihalomethanes (THM), haloacetic acids (HAA) and nitrosamines (NDMA), have already been included in many legislations and given limit values. In the European Union, biocides are assessed under the Biocidal Products Regulation 528/2012 (BPR) regarding their efficacy, potential hazards, and risks to human health and the environment. However, the available guidance for the environmental risk assessment (ERA) of DBPs remains vague. To identify knowledge gaps and to further develop the assessment scheme for the ERA of DBPs, a literature search on the multiple uses of biocides and their formation potential of DBPs was performed and the existing process for ERA was evaluated. The results show knowledge gaps on the formation of DBP in non-aqueous systems and DBP formation by non-halogen-based biocidal active substances. Based on the literature research on biocides, a possible proposal of grouping a.s. to consider their DBP formation potential is presented to simplify future ERAs. However, this also requires further research. Until then, a pragmatic approach considering the DBPs formation potential of the active substances and the identified knowledge gaps need to be established for the environmental risk assessment of DBPs in the EU.
Graphical Abstract

Similar content being viewed by others
Background
The use of biocides against harmful organisms is an effective way to reduce and eliminate these organisms. Their use can be essential, especially in the control of infection and disease control, such as disinfection of water. In the European Union, Regulation (EU) No 528/2012 of the European Parliament and the Council of 22 May 2012 concerning the making available on the market and use of biocidal products (Biocidal Products Regulation, BPR) defines a biocide as a substance or preparation containing one or more active substances intended to destroy, deter, render harmless, prevent the action of or otherwise exert a controlling effect on harmful organisms [1]. It classifies biocidal products into 22 different product types (PT) grouped into four main groups. These main groups are disinfectants, preservatives, pest control and other biocidal products. Each PT has a specific description and application; for example, the "human hygiene" PT 1 includes biocidal products used for human hygiene purposes, applied on or in contact with human skin or scalps for the primary purpose of disinfecting the skin or scalp.
The BPR is based on the precautionary principle. The aim of the BPR is the identification, evaluation and prevention or at least decrease of adverse effects and risks on humans and the environment caused by biocidal active substances and products. An environmental risk assessment (ERA) is conducted for the respective active substance and the corresponding biocidal products to assess potential impacts on the environment. This assessment requires information on substance release patterns, fate, and behavior in the environment. An authorization will be granted only if the biocidal product, as well as its residues, has no unacceptable effects on the environment (BPR Article 19 (1), (iv)). In this case, the term residue refers to a substis present “in or on products of plant or animal origin, water resources, drinking water, food, feed or elsewhere in the environment and resulting from the use of a biocidal product, including such a substance’s metabolites, breakdown or reaction products.” (Article 3 (1) (h)) [2]. Some of the biocidal a.s. used as a disinfectant in the product types (PT) 1–5 as well as preservatives in PT 11 and 12 tend to react quickly with the organic matter present during the application and with each other, forming so-called disinfection by-products (DBPs). Therefore, based on the definition of residues, DBPs also need to be considered for an adequate risk assessment. This represents a major challenge because the type and the number of formed DBPs depend strongly on the application conditions. Hence, predicting the type and environmental concentration of the DBPs, as an important part of the risk assessment, is difficult, if not impossible. For the evaluation of DBPs under the BPR, a specific “Guidance on Disinfection By-Products” [2] was developed by the European Member States and ECHA and published in 2017. However, the ERA section of it is only focussing on the use of halogenated substances, as well as on PTs 2 (disinfectants and algaecides, not intended for direct application to humans or animals), 11 (preservatives for liquid-cooling and processing systems) and 12 (slimicides). In addition, detailed technical guidance is still missing for the implementation of an ERA of DBPs for those application types covered by it. Therefore, information and data submitted by the applicants during a. s. approval or product authorization can be interpreted differently by individual EU Member States making a harmonized ERA of DBPs currently questionable. One reason is that there is no detailed step-by-step process or “test scenario” in the current version for different PTs to demonstrate DBP formation potential; only a general three-step process for determining DBP formation risk is explained. Hence, the risk for environment may be evaluated differently by member states. Thus, the precautionary principle and the level of protection in the EU Member States are defined differently, which may also hamper the mutual recognition of product authorizations between member states.
To identify possibilities for further development of the guidance, the following study discusses the DBP formation potential by the biocidal a.s. in PTs 1-5, 11 and 12. Based on these findings, it summarizes the occurrence of DBPs in the literature and proposes a possible grouping of biocidal a.s. based on their DBP formation potential. The three-step approach on DBP-assessment contained in the current Guidance on Disinfection By-Products was reviewed in detail, and its advantages and disadvantages were compiled and discussed considering the literature results on DBPs and a.s. regarding the formation and ERA of DBPs. Supplemental approaches based on the results were suggested to improve the guidance.
Methods
Evaluation of literature on DBPs [Step 1]
Since there is no uniform definition for the term "disinfection by-product" given neither in BPR nor the current Guidance on Disinfection By-Products regarding when a by-product is considered a disinfection by-product, a working definition had to be established for the literature review. In the literature, disinfection by-products are often referred to as by-products from the reaction of biocides/disinfectants with organic/inorganic matrix. This definition is mainly based on aqueous applications [3]. The place and time of formation (during the application phase, after application, or at a later point in time) have not yet been further considered and defined in the literature. According to that, DBPs were defined as by-products that are formed directly during the application of the biocide. Further transformation or degradation of the biocide is beyond the scope of the BPR guidance on disinfection by-products. This study also considers only the application or use phase of the respective biocidal active substance/product. The production, formulation and disposal phases are not considered further either. The main part of the literature search on DBPs was conducted from 2013 to April 2019, including selected publications from before 2013 through references in newer literature. To support the findings from this main literature search, also review articles from 2019 to 2023 were taken into account. Both searches were done in the database Web of Science using the search terms „disinfection by-product* “ or „disinfection by product* “.
For the time period of 2013 to 2019, of almost 3000 search results, 154 were selected as relevant by screening titles and abstracts (Fig. 1). The 154 publications were selected manually to cover a wide range of formulation types (gaseous, liquid, solid), biocides (oxidizing/non-oxidizing biocides), matrices (aqueous/non-aqueous), as well as types of DBPs (non-halogenated/halogenated). If there was no specific reference to a biocidal active substance used in the experiment, the literature results were also discarded, e.g., novel AOP processes or multi-stage disinfection, which do not specifically appear as biocidal active substances in the ECHA database. This was necessary due to the regulatory context of our study. Studies where the experimental conditions, such as pH, temperature, biocide concentration, organic matrix, etc., were not evident were also not included. Studying the selected literature, 272 unique DBPs were identified, 186 of them being halogenated and 86 being non-halogenated [1.1]. The detailed literature review results were compiled (see Additional file 1; Sheet: Halogenated_DBPs; Nonhalogenated DBPs), including primary DBP data such as name, molecular structure, molecular formula and CAS No., formation conditions, name and CAS No. of applied biocide and treated matrix. The number of publications analyzing a specific DBP is recorded in Additional file 2: Table S1. To verify the consistency of identified knowledge gaps, recent review articles from 2019 to July 2023 were analyzed by searching the database Web of Science. This search gave 330 results for review articles for the search terms "disinfection by-product” and “disinfection by product".
Allocation of test matrices from literature and biocidal a.s. to PTs [Step 2]
The matrices investigated in literature (see Additional file 1) were manually assigned to the biocidal PTs (Additional file 2: Table S2). Since not all literature studies represented natural environmental conditions, but laboratory studies were also included, these were evaluated more closely and only assigned to a PT if realistic experimental conditions were provided. The laboratory studies included matrices such as artificial solutions like humic acid (HA), artificial seawater and algal organic material. If the experimental conditions were assumed unrealistic, the matrices were assigned to the "Other" category.
Using the ECHA database of biocidal active substances (access in November 2022, see Additional file 3), a list of all biocidal active substances belonging to PTs 1–5, 11 and 12 was created [4]. All active substances that were approved or under assessment were further considered. Active substances that were no longer supported, had cancelled applications or were not approved were not considered as products containing the respective a.s. will disappear from the European market sooner or later [4]. Following this step, the number of active substances for each PT was summed up, and a total of 382 active substance/PT-combinations were considered for the evaluation (Table 1).
Categorization of biocidal a.s. based on reactive molecules [Step 3]
As literature data often did not specify exactly which regulatory-defined biocidal a.s. was used to generate the DBP analyzed in the experiments, it was not possible within the study to directly link biocidal a.s, specified in the ECHA database to the DBPs from the literature. Literature data mainly focused on the reactive molecules driving the reactions (e.g., chlorination). To specify which DBP might result from using a biocidal a.s. from the ECHA database, a categorization system was developed that categorized the biocidal a.s. in a system of reactive molecules that suited the results from the literature. This categorization system assigned the results of the literature search and the ECHA database to categories of reactive molecules (Table 2). The number of DBPs also includes multiple findings of the corresponding unique 272 DBPs found. As an example, chloroform was observed several times in different studies and was therefore counted several times.
Criteria for the likeliness of DBP formation and categorization of active substances [Step 4]
An approach for assessing the DBP forming potential was developed for the respective biocidal a.s., and they were analyzed regarding their potential to form DBPs based on these criteria. The assessments of whether an active substance would generate DBPs during use were defined based on our literature research (publication, ECHA dossiers), their chemical structure and expert judgment. The criteria to classify the a.s. based on their chemical structure or “expert judgment” were only used if the literature or the ECHA dossier did not reveal any concrete information on DBP formation.
The chemical structure of a biocidal active substance (a.s.) can be used to categorize it into two main groups: halogenated (class 1) and non-halogenated substances (class 2):
-
Halogenated a.s.: Have a high potential of DBP formation due to their high reactivity (see literature).
-
Non-halogenated a.s.: More diverse in terms of their chemical structures. Non-halogenated a.s. include highly reactive oxidizing substances like ozone and peroxides.
For the categorization according to chemical structure, characteristic groups in the structural formula of the biocide were used, e.g., the hydroxyl group for the alcohols. Often, the assignment according to these characteristic molecular groups also corresponded to the type of disinfection mentioned in literature results, such as chlorine in the structure of the a.s. and chlorination as disinfection type.
The expert judgment was necessary because, e.g., substances like bromoacetic acid can act both as an acid, but also as an alkylating reagent. Since no literature on the use of bromoacetic acid as a disinfectant and DBP formation could be found and the ECHA dossier proposed the hydrolysis of bromoacetic acid in water to the bromide ion, it was included in the acid category. The reasoning is that the acid group here was considered to have a greater role as a biocidal effect than the releasing bromide ion.
Based on the assessments, an assignment was made into one of three groups: Y = DBP-formation likely; N = DBP-formation not likely, and U = DBP-formation possible. If DBP formation was described in the literature or ECHA dossier and could be additionally proven by chemical structure, or expert judgment, a DBP formation potential was expected (Y); if no evidence for DBP formation could be found in the literature or ECHA dossier, the a.s. was classified as non-DBP forming (N). If possible DBP formation of a.s. categories cannot be excluded due to their chemical structure, but no data was available, the last category was chosen (U).
Results and discussion
Literature review on DBPs
DBPs found in the literature search were discussed according to the number of studies found (see Additional file 1). A characterization of non-halogenated and halogenated DBP classes was carried out. According to the number of findings (n = 860), among the halogenated DBPs, the group of trihalomethanes (26%) followed by haloacetic acids (18%) > haloacetonitriles (15%) were most frequently mentioned in the literature. The classes oxohalides (2%) and chloramines (1%) were also included in the consideration because they are partially regulated and constituted of only a few representatives. The DBP classes of haloacetamides, haloacetaldehydes and halonitromethanes together also accounted for 13% of the findings but were not discussed further because most of the literature focused only on drinking water, swimming pool water and the findings consisted of many different representative substances compared to the chloramines or oxohalides. Among the non-halogenated DBP classes, nitrosamines were the most frequent, followed by the aldehydes. Besides the mentioned DBP classes, further DBPs could be found in literature that could not be assigned to any class. In the following, these DBP classes and their occurrence in the environment are explained in more detail based on the literature research.
Halogenated DBPs
Trihalomethanes (THM)
Trihalomethanes, which belong to the first DBP class discovered [5], form the group with the most findings. Trihalomethanes have the molecular formula CHX3, where the X represents any halogen (Br, Cl, I). It should be noted that only the four frequently regulated THM—chloroform, dibromochloromethane, bromodichloromethane and bromoform—have the most detections and not all trihalomethanes. The group of these 4 substances is called THM(4) and is often produced in greater quantities than other THM [6]. In addition to many drinking water and swimming pool water regulations [7, 8], THM(4) is also listed in the EU “Guidance on Disinfection By-Products” [2] as well as the GESAMP list for ballast water treatment [9]. Representatives of THM have already been detected in various matrices, including drinking water [10,11,12,13,14,15,16,17,18], swimming pool water (indoor [19,20,21,22,23,24,25,26,27,28], outdoor [29,30,31,32], air [33,34,35]), surface water [36,37,38,39,40,41,42,43], groundwater [39, 44,45,46], wastewater [47,48,49,50,51], ballast water [52,53,54,55,56,57,58,59] and food [60,61,62,63,64,65,66,67,68].
Haloacetic acids (HAA)
Haloacetic acids consist of the molecular formula CX3COOH, where X is replaced by a halogen (Br, Cl, I) or hydrogen. HAA belong to the second largest DBP group, which are also regulated as sum parameters either as HAA(5) or HAA(9) in many drinking water and swimming pool water regulations [7, 8]. HAA(5) includes monochloroacetic acid (MCAA), monobromoacetic acid (MBAA), dichloroacetic acid (DCAA), dibromoacetic acid (DBAA) and trichloroacetic acid (TCAA). Bromochloroacetic acid (BCAA), dibromochloroacetic acid (DBCAA), dichlorobromoacetic acid (DCBAA) and tribromoacetic acid (TBAA) are added to be grouped as HAA(9). HAA have already been detected in drinking water [10, 13,14,15, 36, 77], swimming pool water [27, 29,30,31, 75, 78, 79], surface water [40, 56, 80] and food [60,61,62]. In contrast to THM, HAA are polar, non-volatile DBPs and are formed preferentially under acidic conditions. HAA can often degrade at high temperatures and serve as precursor substances for THM [81]. Within the EU, this group of DBPs is listed with individual representatives in the EU “Guidance on Disinfection By-Products” and the GESAMP list for ballast water treatment [9], but not in the drinking water and swimming pool water regulations. Bromoacetic acid is also an approved biocidal a.s. in PT 4 (disinfectants for food and feed areas).
Haloacetonitriles (HAN)
Haloacetonitriles are the third largest group of DBP classes, and also here, a varying frequency of findings for the individual representatives can be seen. Haloacetonitriles consist of the basic structure of a nitrile (R–C≡N), where the R is a carbon that may be substituted with up to three halogens. HAN are formed by chlorinating free amino acids [69], proteinaceous materials, and combined amino acids bound to humic structures [69, 70] and were detected in various matrices of drinking water [10,11,12, 36, 71,72,73], swimming pool water [27, 30, 31, 74,75,76] and food process water [66]. Dichloroacetonitrile and dibromoacetonitrile account for over half of the findings and are regulated in many drinking and swimming pool water regulations [7, 8]. Bromochloroacetonitrile and trichloroacetonitrile have a similar frequency and are also considered in the WHO and EPA guidelines for drinking and swimming pool water [7, 8], but no limit values have been set yet. Bromochloroacetonitrile has been included in the EU “Guidance on Disinfection By-Products” [2] and the GESAMP list for ballast water treatment [9], but no limit values have been set.
Oxohalides
Other regulated halogenated DBPs are inorganic anions such as bromate or the oxohalides chlorite and chlorate. Due to its high carcinogenicity, bromate is regulated in drinking water, swimming pool water and wastewater treatment [7, 82, 83]. Oxohalides have already been found in swimming pool water [84,85,86], drinking water [87], wastewater [87], seawater [88] and food [89]. They are DBPs that result from disinfection with chlorine-releasing biocides or chlorine dioxide. They are also regulated in drinking and swimming pool water due to their toxic effect on human blood cells [7, 8]. They are also listed in the EU “Guidance on Disinfection By-Products” and the GESAMP list for ballast water treatment [2, 9]. Chlorate could also be formed as a by-product using chlorine, chlorine dioxide or hypochlorite for the disinfection of many fruits and vegetables [89].
Chloramines
Chloramines also form a subgroup of the halogenated DBP class and consist of the three representatives: mono-, di-, and trichloramine and are formed during the chlorination of nitrogenous compounds. The group of chloramines belongs to very volatile DBPs and, similar to trihalomethanes, can spread not only through water, but also mainly through the air as an exposure pathway [90]. Chloramines can be found in various matrices, including swimming pool water [91], wastewater [92] and drinking water [93]. They are also regulated as individual representatives or sum parameters in swimming pool water and are listed in the EU “Guidance on Disinfection By-Products” [2], and the GESAMP list for ballast water treatment [9].
Non-halogenated DBPs
Note: Non-halogenated DBPs are not considered so far in the EU “Guidance on Disinfection By-Products” [2].
Nitrosamines
Nitrosamines form the largest group of the non-halogenated DBP classes and account for 80 findings out of 299, with NDMA alone accounting for 19. NDMA is considered the most toxic of the nitrosamines and is strictly regulated under drinking water and wastewater directives [7, 8, 83]. Nitrosamines have also been detected in swimming pool water [94, 95], drinking water [96], wastewater [97] and wash water used in food production [66]. Nitrosamines are not yet included in the EU “Guidance on Disinfection By-Products” or the GESAMP list for ballast water treatment [2, 9]. In toxicological studies, NDMA has been shown to cause liver, lung, and renal cancer due to its potential binding to DNA [98].
Aldehydes
Aldehydes form the second largest group with 64 findings of the non-halogenated DBP classes. Aldehydes are known for their high toxicity and carcinogenicity and have been reported in many matrices such as swimming pool water [23], drinking water [10, 14, 99], lake water [100], wastewater [101], ballast water [102], and wash water in food production [103].
Categorization of biocidal a.s.
Categorization
For the categorization, the biocidal a.s. that are under approval or already approved in the EU were first divided into two main groups: halogenated and non-halogenated a.s.. Halogenated a.s. were further assigned into five groups represented by the corresponding reactive molecule: hypochlorite, hypobromite, iodine, monochloramine and chlorine dioxide. Where appropriate, a sub-categorization into organic, inorganic and in situ representatives of a reactive molecule was performed. The chlorine dioxide group shows that the categorization by chemical structure requires compromises. Although chlorine acts as a halogen-releasing biocide, chlorine dioxide reacts mainly as an oxidant [104, 105].
The leading group of non-halogenated a.s. is much more diverse concerning the included chemical structures responsible for the biocidal activity. The highly reactive oxidizing substances like ozone and peroxides and seven other groups with specific chemical structures accounting for the biocidal reactivity are included. In the last group, called “others”, all the a.s. not fitting into any other of the groups are included. The DBP formation potential of the non-halogenated a.s. differs depending on the reactive molecule. It is estimated to be fairly high for ozone and peroxides and moderate to low for the other groups of non-halogenated a.s. If necessary, some a.s. were assigned to more than one group (e.g., BCDMH). The categorization is summarized in Table 3.
Halogen-based biocides
Halogen-releasing biocides
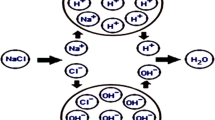
All halogen-releasing biocides have the same mode of action: they serve as a source of chlorine, bromine, or both. After the release of the halogen, the hypohalogenic acid of the respective halogen (HOCl, HOBr or HOI) is formed in the presence of water (Fig. 2). Hypohalogenic acids (HOCl, HOBr, HOI) and their hypohalogenites (OCl−, OBr−, OI−) have a high oxidation potential and, thus, the possibility to inactivate microorganisms. Chlorine is the most widely used disinfectant for controlling harmful microorganisms due to its high efficiency, low cost and residual disinfection effect. Chlorine can also form halogenated DBPs such as aldehydes [106] or nitrosamines [96]. Chlorination of water is associated with an increased health risk of bladder cancer [107] and the US EPA Stage 2 guideline assumes a lifetime cancer risk from chlorinated water of one per thousand persons [108]. Bromine-releasing biocides are often generated by bromine-containing substances such as BCDMH or by using a strong oxidizer, which causes bromide to be oxidized to hypobromite [86]. Bromine-based disinfections can only be used without light; otherwise, the remaining bromine is degraded. An advantage of bromine-based biocides in terms of usability is that they degrade more slowly than hypochlorous acid at high temperatures due to their lower vapor pressure, which is why they are specifically used in higher temperatures such as spas [6]. Chlorine cyanurates can release chlorine slower by releasing chlorine only after the existing chlorine has been consumed or diluted. A major advantage concerning their scope of application is the low degradation rates of chlorinated cyanurates compared to hypohalogenated acids [86]. All classes of DBP were detected in studies on the DBP formation of HOCl and HOBr [109]. For the iodine-based biocides such as polyvinylpyrrolidone iodine (25254-50-6) and iodine (7553-56-2), no DBPs are known to occur during application. Nevertheless, in the presence of oxidizing agents, iodide can be oxidized to hypoiodous acid and form I-DBPs [110].
Chlorine dioxide
Chlorine dioxide is a yellowish gas often produced in situ at the point of use. It is not considered a chlorine disinfectant, as it acts differently without producing residual chlorine through conversion to chlorite and chlorate ions that remain in the solution [111]. In water, it remains a dissolved gas and does not hydrolyze or form hypochlorous acid. With the reaction of electron-rich molecules, it can form chlorite via a radical mechanism, which can decompose to chloride or chlorates. Chlorine dioxide is effective over a wide pH range, unlike chloramines or hypochlorite/hypobromite. The amount of chlorite and chlorate formed increases with higher concentrations and times of action. A direct THM formation by chlorine dioxide could not yet be determined. Nevertheless, in studies in the presence of a matrix, halogenated DBPs could be detected [112]. Apart from the formation of chlorates and chlorites, further studies are needed on forming direct DBPs when used in the food industry [113].
Inorganic chloramines
Inorganic chloramines consist mainly of three representatives: monochloramine (NH2Cl), dichloramine (NHCl2) and trichloramine (NCl3) (Fig. 3). Chloramines are often produced by hypochlorous acid (HOCl) and a nitrogenous source such as ammonium. The ratio of chlorine to nitrogen and the pH-value is decisive for the amount and the species of chloramines formed. At neutral to alkaline pH, monochloramine is the dominant species. Chloramines have a lower oxidation potential than hypohalogenic acids and are relevant due to their high longevity [114]. Dichloramines (NHCl2) and trichloramine (NCl3) are unstable compounds, so only monochloramine is produced and used in practice. Disinfection of aqueous matrices by chloramines can lead to the formation of THMs, HAAs, N-DBPs, chloral hydrates, cyanogen compounds, nitrates, nitrites, organic chloramines and haloketones [115, 116]. The formation of nitrosamines during chloramination is one of the main disadvantages of this disinfection method [117, 118].
Non-halogen-based biocides
Ozone
Ozone has a very high oxidation potential and thus acts as a strong oxidizing agent [119]. Ozone can be produced by oxygen using UV light and electrical discharge (Fig. 4). Nowadays, ozone is only generated through discharge. Ozone can react as both a nucleophile and an electrophile. Ozone has no residual disinfection effect like chlorine and has the disadvantage of oxidizing bromide to bromate [120]. Ozone is often used in low concentrations for trace contaminant elimination and as a disinfectant in drinking water treatment. Even though ozone does not produce halogenated DBPs, downstream ozonation increases THM and HAA [121]. Carboxylic acids could be detected in comparison in six times higher concentration than other DBP classes after ozonation of drinking water [122].
Peroxide-group-based biocides
Among the peroxide-based biocides, hydrogen peroxide is the most relied upon and has the most applications [123]. In general, they contain a peroxy group (–O–O–) in their structure. Hydrogen peroxide and other hydroperoxide-based biocides develop their oxidizing effect using hydroxyl radicals (OH) and have a greater oxidizing potential than chlorine or chlorine dioxide. Despite this, high amounts of peroxides are required for disinfection compared to halogenated biocides. As with chlorine dioxide, although no direct formation of halogenated DBPs was observed, halogenated DBPs were indeed detected in the presence of the matrix [68, 124]. In a study on washing lettuce with peracetic acid, halogenated by-products were also found [68]. An advantage of peroxide-based biocides is their decomposition into the harmless degradation products of water and oxygen, which are not dangerous. An advantage of organic peroxides is that they produce hydrogen peroxide only in contact with water and therefore do not produce residues or gasses. Peroxides are often used with other disinfection techniques such as silver, ozone, QACs or UV.
Aldehydes and formaldehyde releasers
The class of aldehydes contains the characteristic terminal carbonyl group (CHO). They are also formed as endogenous intermediates in biological metabolism and occur in the environment. So far, no studies on the formation of DBPs by aldehydes are known, and the formation potential is estimated to be low due to their chemical reactivity. They are highly effective broad-spectrum disinfectants and can be used in gaseous and volatile forms [125]. In addition, they are effective at high organic loads and are non-corrosive. The most prominent two representatives, formaldehyde and glutaraldehyde, are also volatile and toxic to humans. Aldehydes also belong to the DBPs, which are formed when oxidizing biocides such as ozone are used. Aldehydes can denature proteins and thus damage them [126]. Their mode of action has not been fully elucidated but is thought to consist of cross-linking proteins, DNA or RNA. Formaldehyde-releasing biocides can also have carcinogenic effects and have been declared as such since 2015 on the recommendation of the ECHA at a total concentration higher than 0.1% of possible formable formaldehyde [127].
Quaternary ammonium compounds (QACs)
Quaternary ammonium compounds all have the following structure: NR4+X− (R: organic residue; X: anion, e.g., chloride, hydroxide, bromide). Their mode of action is due to a cytoplasmic effect leading to cell death. It has been shown that they are preferentially found in wastewater and sediments, where they are generally largely biodegraded [128]. Despite degradability, they can also be found in surface water [129], soils and sediment [130, 131]. Besides the direct toxicity of QACs, they can form nitrosamines [132], primarily by reaction with chloramines. In a study on the disinfection of polycarbonate surfaces with diluted disinfectant solutions of QACs, chemical damage to the surface due to a change in the molecular structure was confirmed [133]. A direct formation of DBPs during using QACs is not known in the literature.
Isothiazolinones biocides
Isothiazolinones consist of a five-membered ring based on the structure of cyclopentane. The group of isothiazolinones has been banned by the EU in many applications due to the potential toxicity to cells and possible carcinogenicity hazard to humans and is expected to be replaced in the future [134]. Stability studies showed that the degradation of isothiazolinones has greater environmental relevance than the direct formation of DBPs. In the presence of an aqueous environment and nucleophiles, ring opening occurs, which can lead to a variety of transformation products (CaCl2, loss of Cl and S, N-methylmalonamic acid, malonamic, malonic, acetic, and formic acids, 5-chloro-2-methyl-4-isothiazolin-1-oxide, N-methylglyoxylamide, ethylene glycol, and urea) but also to mineralization (CO2) [135]. It is believed that for some isothiazolinones, the degradation products have higher toxicity than the biocide. No direct DBP formation by isothiazolinones has been studied or found in the literature.
Alcohol- and acid-based biocides
Alcohols all have in common that they contain a hydroxy group (-OH) in their structure; alcohol-based disinfectants have a wide range of applications, such as hand disinfection, surface disinfection, food disinfection and many more [136, 137]. Carboxylic acids have the characteristic carboxyl group (-COOH) and can exist in protonated or deprotonated forms. Due to their high rapid antimicrobial activity, ease of application and low cost, both alcohols and acids are very popular [138]. The formation of DBPs is also not known in the literature to occur using alcohols and acids as disinfectants.
Silver-, inorganic-based and other biocides
Many biocidal active substances, such as silver-containing biocides, are only represented with a few a.s./PT-combinations in the ECHA database. Most are not persistent, degraded to non-toxic products or partially mineralized [8]. For example, magnesium-based biocides (calcium magnesium oxide) are converted into their respective ionic components, which are part of the existing chemical cycles [139, 140]. In terms of silver-based biocides, the active species is often a silver cation (Ag+) which is released and is not suspected of forming DBPs [141]. Even though the mechanisms behind disinfection by silver ions are not fully understood, it is assumed that they do not produce toxic DBPs [142]. Biocides that have a high chemical reactivity and are categorized in “others” as well are, e.g., sulfur dioxide, ethylene oxide and free radicals (generated in situ from ambient air or water). Those a.s. categorized in the category “others” show various reactivities, and their DBP formation potential would have to be investigated individually. In the ECHA dossiers on biocides in the category “others”, studies on behavior in different compartments such as air, water or soil are included, but the focus here is not on DBP formation and in some cases, data are very old or incomplete. No literature studies on these a.s. were found during our search.
Comparison of findings from literature to ECHA database
DBPs found in the literature are summarized within the categories of reactive molecules in Fig. 5 and can also be seen in Table 2. For halogenated DBPs, n = 860 entries, and for non-halogenated DBPs, n = 294 entries were considered. In literature, the largest proportion of DBPs can be attributed to hypochlorite-based biocides, with more than 55% [n = 1154] making them responsible for the largest fraction of DBPs detected. It can be seen that a large part of the other reactive molecules still needs to be investigated in more detail concerning their potential to form DBPs. Therefore, the share of reactive molecules does not necessarily relate to the potency of DBP formation of the respective reactive molecule, but also relates to study design preferences in research.
Looking at the shares of a.s. in the ECHA database, hypochlorite alone as a reactive molecule account for 16% of the active substance/PT-combinations [n = 382], being the reactive molecule with the most a.s. in all investigated PTs. Summing the proportions of the potentially non-DBP-forming biocide categories (QACs, acids, alcohols, isothiazolinones, aldehydes, MITC, iodine-, silver-, inorganic-, other-biocides), the result is 46% of all a.s. considered in the relevant PTs. However, it has to be kept in mind that the number of a.s. that are approved or under approval does not necessarily reflect the highest amounts of a.s. used on the market.
Overall, it can be shown that for most of the biocidal a.s. in the ECHA database, information on their DBP formation potential is not yet available in literature. For some major groups (hypochlorite-based biocides, monochloramines, chlorine dioxide), many studies on DBP formation are already available. But even if these groups of biocidal a.s. are represented in the literature, it does not mean that all PTs are also represented regarding their DBP formation.
Application matrices: aqueous-based vs. non-aqueous-based applications
The amount of water present during disinfection use is another relevant factor for DBP formation, and common disinfection uses can be divided into two cases. In the first case, the disinfection occurs in an aqueous solution. All substances reacting with each other or influencing the reaction process are dissolved in water over the entire disinfection use. This is, for example, the case in drinking water (PT5), preservatives for liquid-cooling and processing systems (PT11) and swimming pool water (PT2). In the second case, the amount of water is much more limited in the disinfection of surfaces (PT2) or the food industry (PT4). These disinfection uses will usually not occur without water, as residual water will be present on the treated surface and the a.s. formulation will mainly include water. However, the reacting substances responsible for DBP formation will not necessarily be in solution and thus might be unavailable for reaction over the entire disinfection use. The DBP formation may be influenced by the solubility of the different components of the matrix, both the organic components being the source for DBPs and inorganic components (e.g., bromide or iodide) influencing the reaction process [143]. In addition to its function as a solvent, water also affects the reactivity of numerous functional groups by protonating or deprotonating them. These effects may differ in a mixture with limited water amount. Consequently, compared to the same reaction mixture in an aqueous solution, alternative reaction may occur in a non-aqueous setting, leading to divergent results concerning the resulting DBPs. No studies investigating DBP formation under conditions of surface disinfection could be identified in the literature [3]. In a recently published review of the use of ozone for disinfection during the Covid crisis, it was found that masks could become damaged by repeated disinfection with ozone and that the polymer bonds could be degraded. Also, ozone is suspected to promote oxidative destruction of natural rubber or plastic surfaces and unsaturated organic compounds [144, 145]. The authors did not perform further investigations on DBPs. In addition, all literature studies were assessed regarding the matrices used in the experiments to match literature data with the possible uses. If possible, the investigated matrices were assigned to application types (Additional file 3: Table S2). These application types were assigned to the product types PT to compare registrations in the ECHA database and the generally studied PTs in our literature search. Laboratory studies, which used artificial matrices as a basis, were not considered in this compilation since they cannot be related to realistic use scenarios of PTs.
It can be seen that only a very specific part of the possible uses is covered in the literature (Fig. 6, bottom). Drinking water (PT 5) is one of the most well-studied matrices due to the diversity of different studies concerning DBP formation, which is why many field studies can already be referred to here and legal limit values have already been established [146]. In addition, PT 2 plays a significant role and is also well-studied in terms of DBPs, with a proportion of almost 46% of all findings. However, these studies focus on swimming pool water and some other types of water disinfection. Important to mention is that PT 2 also includes different application types, such as surface disinfection, textile disinfection, aquariums, air conditioning systems, walls, floors and air, which still need to be investigated for DBPs in the literature (Fig. 6, bottom) Another product type accounting for 18% of all a.s./PT-combinations in the ECHA database and, to a minimal extent, also present in the literature search is PT 4, which covers disinfection in the food and feed sector. Studies such as that of Cardador et al. [60, 64, 147] and Bao Loan et al. [62] have investigated washing solutions and the food treated with chlorine that is used in the disinfection processes (cheese [60, 61, 147], vegetables [148]) juices and soft drinks [149], meat, fish).
It should be noted, however, that only aqueous matrices were investigated for DBP formation and not solids or surfaces. Disinfectants or disinfecting solutions are often used in the food industry for sanitizing, washing, blanching, cooling and transporting the final product. It would be important to conduct further studies in all PTs with little or no water-containing matrices, as the conditions (contact time, temperature, concentration, pH, DOC/COD load, etc.) defined as key parameters in the BPR guidance can be quite different. An example is the disinfection of post-harvest foodstuffs in washing plants where contact times of 1–5 min are achieved. A review article on the application of silver-based nanomaterials showed that in contrast to classical disinfection, such as chlorine, additional parameters, such as the morphology of the nanosilver and the scaffold material, must be taken into account [142]. In the disinfection of drinking water/swimming pool water, chlorine remains in the water for several hours [150]. Several studies have shown that the use of disinfectants can have an impact on essential nutrient components such as vitamins and secondary plant compounds [151]. The authors did not do any research on the possible formation of DBPs. A recent review article on the use of ozone also reported negative changes in color, odor, firmness, weight, and texture in fruits and vegetables [152]. Despite this, there is a high need for research in the study of processing fresh-cut vegetables using biocides. The insufficient availability of data regarding DBP residues in fresh-cut products poses a significant challenge when it comes to a comprehensive assessment of the associated food safety risk [153].
PT 1 (Human hygiene) and 3 (Veterinary hygiene) have yet to be investigated concerning DBP formation and thus represent a major unexplored field of research. An example is using disinfectants in the veterinary area, e.g., potable water supplies, medical equipment disinfection, rubber mannequin, syringes, needles, blood- or body-fluid spill, environmental surfaces, laundry, dental therapy and medical waste [154]. In addition, a.s. are used in human hygiene, e.g., hand or scalp disinfection, and body and oral hygiene. Many of these applications have mainly organic matrices, such as amino acids in the blood plasma. DBP formation thus does not seem unlikely. Among the results from the literature search, several studies on essential amino acids have shown that some can react with hypochlorite. In past studies, a wide variety of DBPs have already been demonstrated in the chlorination of peptides in the water of the seven essential amino acids such as leucine [106, 155, 156], isoleucine [106, 156], histidine [157], lysine [158], methionine [159], threonine [160, 161], phenylalanine [106, 156], tryptophan, and valine [106, 156, 158].
The analysis of review articles from 2019 to 2023 showed that they mainly were related to aqueous applications such as (such as (drinking water [162,163,164], wastewater [165, 166], washing water, fruit vegetables or health effects [163, 167], advanced oxidation processes [168,169,170,171]). The results of the search in the review articles did not reveal any further findings on DBP formation in other application types (surface, steel disinfection, etc.) or by non-oxidizing biocides tested concerning DBPs.
Consideration of use volumes of the active substances on DBP formation volumes
To assess potential risks by DBPs, information on their amounts potentially released to the environment would be needed. The preliminary information necessary to evaluate would be the total use amount of the respective DBP-forming biocidal a.s. This data needs to include the volumes of biocidal a.s. used in each particular PT or even better, a specific use, as both the number of DBPs formed and the amount entering the environment will, besides the applied volume, depend on the PT or the particular use. However, this data is not available. COWI performed the best comprehensive data compilation for the European market on behalf of the European Commission [5]. The industry submitted the information within the notification procedure following the entry into force of the Biocidal Product Directive 98/8/EC. The evaluated data originate from the time period 1998–2001; therefore, conclusions for the present situation must be regarded with care. Nevertheless, some results are consistent with the current situation or are at least expected to be reasonably similar.
The information misses important details, as only the overall tonnage per PT is given, without detailed volumes of biocidal a.s. used within particular PTs, due to confidentiality reasons. The total annual volume of sales of biocidal a.s. (production and imports) in the EU was estimated to be about 400,000 tones. The majority of nearly 90% of those amounts were produced or imported in PTs 1–5, 11 and 12. Most relevant was the production/import in PT2, accounting for 50.4% of the volume, followed by PT 5 and 11, accounting for > 12% each. The actual amount is expected to be higher due to the entry of eastern European countries into the EU in 2004 and 2007.
Although generally no volumes for single substances were given, the sum of used sodium hypochlorite, chlorine and hydrogen peroxide, the three substances with the highest production volumes, was stated to be approximately 54% across all PTs. As the a.s. are used in the relevant PTs 1–5 and 11–12, it can be assumed that about half of the amounts of biocidal a.s. produced or imported are proven to generate DBPs potentially. For well-founded decisions on the relevance of a risk assessment for DBPs, a much more detailed data source on the use of biocidal active substances would be necessary.
Current approaches and challenges to ERA for DBPs in the regulation
The current BPR “Guidance on Disinfection By-Products” [2] provides a general approach for the environmental risk assessment of DBPs. The risk assessment includes three steps, which are required, to underpin the absence of unacceptable effects:
-
An initial worst-case risk assessment for a set of known marker DBPs, using aPEC (predicted environmental concentration)/PNEC (predicted no effect concentration) approach assuming 100% conversion of the biocidal active substance;
-
Chemical assessments in which (changes in) group parameters (e.g., AOX (adsorbable organic halogens)) are determined;
-
A refined risk assessment for known marker DBPs, appended with a whole effluent testing (WET)-approach to cover unknown DBPs.
The current BPR guidance correctly describes the potential complexity when dealing with DBPs, but the proposed approach is challenging and although it needs much effort from authorities and industry parties, it is questionable whether DBPs are considered adequately. Furthermore, due to the generic character of the existing guidance and the lack of detailed instructions and requirements, a consistent interpretation by all competent authorities is questionable (Table 4). Consequently, a harmonized scientific-based ERA of DBPs is currently not possible, which may also hamper the mutual recognition of product authorization between member states.
To offer a practical approach to simplifying the current three-step process, based on our findings, a grouping of the a.s. and an allocation of DBPs to these groups can be discussed. This allocation of DBPs is based on the reactive molecule related to an a.s. (see Table 3) and independent of the PT, which facilitates the DBP allocation by disregarding the complexity of application types within the PTs. This approach could help to simplify the assessment of disinfectants/preservatives and their DBP formation potential. Moreover, the approach can be combined with the current guidance, i.e., as a supplement to the initial worst-case approach in the first step.
As an example, for the category of hydrogen peroxide, some a.s. of the 42 a.s. associated with this category could be initially tested for their DBP formation potential. In the next step, if DBPs are formed, these DBPs would be considered as relevant DBPs/DBP classes for all future a.s. approvals in this a.s. category. At the moment, without this grouping approach, all hydrogen peroxides-based a.s. would need to be tested individually regarding formed DBPs, i.e., for each a.s. the potentially formed DBPs have to be identified, and marker DBPs defined. An exemplary schematic diagram of the proposed grouping approach in comparison to the current approach is shown in Fig. 7.
However, it has to be kept in mind that it was not possible until now to define a worst-case scenario for DBP formation as this is a complex reaction system influenced by various parameters. The definition of worst-case applications would thus not be possible at the moment. Similarly, the effort for biocides grouped in the category “Others” remains the same. As DBP formation and the quantity depend on PT/application type, consideration of DBP risks via a grouping of a.s. may not represent the environmental worst-case. Also, the initial selection of a.s. for DBP formation determination would have to be done as representative as possible.
Conclusion
The literature, as summarized here, shows that there is still a lack of knowledge on DBPs and their impact on the environment. A strict differentiation between the definition of DBPs and transformation products is necessary, this should be included in the EU “Guidance on Disinfection By-Products”. Based on the results of the present study DBPs should be considered as by-products that are formed during the use phase.
Based on the types of applications, DBP classes and biocidal a.s. used, it can also be seen that the focus of the literature to date has been on the disinfection of aqueous matrices and applications such as drinking water, wastewater, swimming pool water, or process water in the food industry. For this reason, environmental conditions defined as key parameters in the current Guidance on Disinfection By-Products are based primarily on aqueous application types. In the future, other major application types, such as surface disinfections used to disinfect and sanitize surfaces such as floors, furniture, washrooms, tiles, walls, instruments, and clothes, should also be investigated regarding the DBP formation potential and environmental risk. These non-aqueous application types could have unknown DBPs or a different distribution of DBP classes formed during disinfection, resulting in the need to modify or to extend the prioritization of Guidance on Disinfection By-Products.
Also, there is a strong bias in literature towards biocidal a.s. acting through chlorination compared to other reactive molecules. The proposed categorization of biocidal a.s. concerning their DBP formation potential could lead to considerable relief in terms of future ERA as many biocidal a.s. might not require a DBP assessment due to their missing formation potential. From the grouping of a.s., whether an a.s. is likely or unlikely to be classified as a DBP former, it follows that more stringent or extended criteria (e.g., by exact determination of the mode of action, oxidation potential, and halogenation potential) may be necessary for classification as a DBP former.
Furthermore, more ecotoxicity studies are required to identify emerging DBP classes of concern for the environment and sort out irrelevant DBPs. To determine the ecotoxicity as well as the fate and distribution of DBPs and a.s., experiences from computational simulation studies could also be used as a first approach. In addition, updated data on the production quantities and consumption of a.s., capable of forming DBP, would be necessary.
The idea of a grouping of a.s. with regard to DBP formation potential is challenging, and more research was identified that needs to be addressed before the guidance can be improved. Several findings from this research could contribute to this discussion and improvement. Until then, a pragmatic approach taking into account the DBPs formation potential of the active substances and the identified knowledge gaps need to be established for the environmental risk assessment of DBPs in the EU.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files. The authors declare that data supporting the findings of this study are available within the references given in the article and its supplementary information files.
References
ECHA (2012) Biocidal Products Regulation. https://echa.europa.eu/de/regulations/biocidal-products-regulation/legislation. Accessed 10 Aug 2023
ECHA (2017) Guidance on the BPR: Guidance on Disinfection By-Products. https://doi.org/10.2823/72847
Richardson SD, Plewa MJ, Wagner ED et al (2007) Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res 636:178–242. https://doi.org/10.1016/j.mrrev.2007.09.001
ECHA (2023) Information on biocides—ECHA. https://echa.europa.eu/de/information-on-chemicals/biocidal-active-substances. Accessed 23 Feb 2023
European Commission Environment Directorate-GeneralI (2009) Assessment of different options to address risks from the use phase of biocides Final report. https://ec.europa.eu/environment/archives/ppps/pdf/final_report0309.pdf. Accessed 18 Mar 2023
Yang L, Chen X, She Q et al (2018) Regulation, formation, exposure, and treatment of disinfection by-products (DBPs) in swimming pool waters: a critical review. Environ Int. https://doi.org/10.1016/j.envint.2018.10.024
EPA (2010) Disinfectants and disinfection byproducts rules (Stage 1 and Stage 2). https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=P1009Q0H.txt. Accessed 14 Jan 2022
WHO (2006) Guidelines for safe guidelines for safe recreational water environments creational water environments. https://apps.who.int/iris/handle/10665/42274. Accessed 15 Jul 2022
GESAMP Working Group (2019) methodology for the evaluation of ballast water management systems using active substances. http://www.gesamp.org/site/assets/files/2041/rs101e.pdf. Accessed 15 Jul 2022
Serrano M, Montesinos I, Cardador MJ et al (2015) Seasonal evaluation of the presence of 46 disinfection by-products throughout a drinking water treatment plant. Sci Total Environ 517:246–258. https://doi.org/10.1016/j.scitotenv.2015.02.070
Bond T, Templeton MR, Mokhtar Kamal NH et al (2015) Nitrogenous disinfection byproducts in English drinking water supply systems: occurrence, bromine substitution and correlation analysis. Water Res 85:85–94. https://doi.org/10.1016/j.watres.2015.08.015
Lyon BA, Milsk RY, Deangelo AB et al (2014) Integrated chemical and toxicological investigation of UV-chlorine/chloramine drinking water treatment. Environ Sci Technol 48:6743–6753. https://doi.org/10.1021/es501412n
Zhang X, Talley JW, Boggess B et al (2008) Fast selective detection of polar brominated disinfection byproducts in drinking water using precursor ion scans. Environ Sci Technol 42:6598–6603. https://doi.org/10.1021/es800855b
Selvam R, Muniraj S, Duraisamy T, Muthunarayanan V (2018) Identification of disinfection by-products (DBPs) halo phenols in drinking water. Appl Water Sci 8:1–8. https://doi.org/10.1007/s13201-018-0771-1
Hammami B, Ben Hessin S, Bahri M, Driss MR (2014) Assessment of haloacetic acids in drinking water in Bizerte, Tunisia. Clean (Weinh) 42:1052–1059. https://doi.org/10.1002/clen.201300094
Al-Otoum F, Al-Ghouti MA, Ahmed TA et al (2016) Disinfection by-products of chlorine dioxide (chlorite, chlorate, and trihalomethanes): occurrence in drinking water in Qatar. Chemosphere 164:649–656. https://doi.org/10.1016/j.chemosphere.2016.09.008
Domínguez-Tello A, Arias-Borrego A, García-Barrera T, Gómez-Ariza JL (2015) Seasonal and spatial evolution of trihalomethanes in a drinking water distribution system according to the treatment process. Environ Monit Assess. https://doi.org/10.1007/s10661-015-4885-8
Jeong CH, Postigo C, Richardson SD et al (2015) Occurrence and comparative toxicity of Haloacetaldehyde disinfection Byproducts in drinking water. Environ Sci Technol 49:13749–13759. https://doi.org/10.1021/es506358x
Kanan A, Selbes M, Karanfil T (2015) Occurrence and formation of disinfection by-products in indoor U.S. swimming pools. ACS Symp Ser 1190:405–430. https://doi.org/10.1021/bk-2015-1190.ch021
Weaver WA, Li J, Wen Y et al (2009) Volatile disinfection by-product analysis from chlorinated indoor swimming pools. Water Res 43:3308–3318. https://doi.org/10.1016/j.watres.2009.04.035
Simard S, Tardif R, Rodriguez MJ (2013) Variability of chlorination by-product occurrence in water of indoor and outdoor swimming pools. Water Res 47:1763–1772. https://doi.org/10.1016/j.watres.2012.12.024
Erdinger L, Kühn KP, Kirsch F et al (2004) Pathways of trihalomethane uptake in swimming pools. Int J Hyg Environ Health 207:571–575. https://doi.org/10.1078/1438-4639-00329
Richardson SD, DeMarini DM, Kogevinas M et al (2010) What’s in the pool? A comprehensive identification of disinfection by-products and assessment of mutagenicity of chlorinated and brominated swimming pool water. Environ Health Perspect 118:1523–1530. https://doi.org/10.1289/ehp.1001965
Parinet J, Tabaries S, Coulomb B et al (2012) Exposure levels to brominated compounds in seawater swimming pools treated with chlorine. Water Res 46:828–836. https://doi.org/10.1016/j.watres.2011.11.060
Villanueva CM, Cantor KP, Grimalt JO et al (2006) Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am J Epidemiol 165:148–156. https://doi.org/10.1093/aje/kwj364
Aggazzotti G, Fantuzzi G, Righi E, Predieri G (1995) Environmental and biological monitoring of chloroform in indoor swimming pools. J Chromatogr A 710:181–190. https://doi.org/10.1016/0021-9673(95)00432-M
Lee J, Jun MJ, Lee MH et al (2010) Production of various disinfection byproducts in indoor swimming pool waters treated with different disinfection methods. Int J Hyg Environ Health 213:465–474. https://doi.org/10.1016/j.ijheh.2010.09.005
Usman M, Hüben M, Kato T et al (2022) Occurrence of brominated disinfection by-products in thermal spas. Sci Total Environ 845:157338. https://doi.org/10.1016/j.scitotenv.2022.157338
Zwiener C, Richardson SD, De Marini DM et al (2007) Drowning in disinfection byproducts? Assessing swimming pool water. Environ Sci Technol 41:363–372. https://doi.org/10.1021/es062367v
Manasfi T, De Méo M, Coulomb B et al (2016) Identification of disinfection by-products in freshwater and seawater swimming pools and evaluation of genotoxicity. Environ Int 88:94–102. https://doi.org/10.1016/j.envint.2015.12.028
Yeh RYL, Farré MJ, Stalter D et al (2014) Bioanalytical and chemical evaluation of disinfection by-products in swimming pool water. Water Res 59:172–184. https://doi.org/10.1016/j.watres.2014.04.002
Yang L, Schmalz C, Zhou J et al (2016) An insight of disinfection by-product (DBP) formation by alternative disinfectants for swimming pool disinfection under tropical conditions. Water Res 101:535–546. https://doi.org/10.1016/j.watres.2016.05.088
Schmalz C, Frimmel FH, Zwiener C (2011) Trichloramine in swimming pools—formation and mass transfer. Water Res 45:2681–2690. https://doi.org/10.1016/j.watres.2011.02.024
Lourencetti C, Grimalt JO, Marco E et al (2012) Trihalomethanes in chlorine and bromine disinfected swimming pools: air-water distributions and human exposure. Environ Int 45:59–67. https://doi.org/10.1016/j.envint.2012.03.009
Boudenne JL, Parinet J, Demelas C et al (2017) Monitoring and factors affecting levels of airborne and water bromoform in chlorinated seawater swimming pools. J Environ Sci (China) 58:262–270. https://doi.org/10.1016/j.jes.2017.05.022
Guilherme S, Rodriguez MJ (2014) Occurrence of regulated and non-regulated disinfection by-products in small drinking water systems. Chemosphere 117:425–432. https://doi.org/10.1016/j.chemosphere.2014.08.002
Zhang TY, Xu B, Hu CY et al (2015) A comparison of iodinated trihalomethane formation from chlorine, chlorine dioxide and potassium permanganate oxidation processes. Water Res 68:394–403. https://doi.org/10.1016/j.watres.2014.09.040
Werner D, Valdivia-Garcia M, Weir P, Haffey M (2016) Trihalomethanes formation in point of use surface water disinfection with chlorine or chlorine dioxide tablets. Water Environ J 30:271–277. https://doi.org/10.1111/wej.12209
Guo W, Shan Y, Yang X (2014) Factors affecting the formation of iodo-trihalomethanes during oxidation with chlorine dioxide. J Hazard Mater 264:91–97. https://doi.org/10.1016/J.JHAZMAT.2013.10.064
Goslan EH, Krasner SW, Villanueva CM et al (2014) Disinfection by-product occurrence in selected European waters. J Water Supply Res Technol AQUA 63:379–390. https://doi.org/10.2166/aqua.2013.017
Roccaro P, Vagliasindi FGA, Korshin GV (2014) Relationships between trihalomethanes, haloacetic acids, and haloacetonitriles formed by the chlorination of raw, treated, and fractionated surface waters. J Water Supply Res Technol AQUA 63:21–30. https://doi.org/10.2166/aqua.2013.043
de la Rubia Á, Rodríguez M, León VM, Prats D (2008) Removal of natural organic matter and THM formation potential by ultra- and nanofiltration of surface water. Water Res 42:714–722. https://doi.org/10.1016/j.watres.2007.07.049
Golea DM, Upton A, Jarvis P et al (2017) THM and HAA formation from NOM in raw and treated surface waters. Water Res 112:226–235. https://doi.org/10.1016/j.watres.2017.01.051
Carter JM, Moran MJ, Zogorski JS, Price CV (2012) Factors associated with sources, transport, and fate of chloroform and three other trihalomethanes in untreated groundwater used for drinking water. Environ Sci Technol 46:8189–8197. https://doi.org/10.1021/es301839p
O’Driscoll C, McGillicuddy E, Croot P et al (2020) Tracing sources of natural organic matter, trihalomethanes and metals in groundwater from a karst region. Environ Sci Pollut Res 27:12587–12600. https://doi.org/10.1007/S11356-020-07855-9
Salameh E, Alawi M, Batarseh M et al (2002) Determination of trihalomethanes and the ionic composition of groundwater at Amman City, Jordan. Springer 10:332–339. https://doi.org/10.1007/s10040-001-0187-z
Doederer K, Gernjak W, Weinberg HS, Farré MJ (2014) Factors affecting the formation of disinfection by-products during chlorination and chloramination of secondary effluent for the production of high quality recycled water. Water Res 48:218–228. https://doi.org/10.1016/j.watres.2013.09.034
Hanigan D, Truong L, Simonich M et al (2017) Zebrafish embryo toxicity of 15 chlorinated, brominated, and iodinated disinfection by-products. J Environ Sci 58:302–310. https://doi.org/10.1016/j.jes.2017.05.008
Yang F, Yang Z, Li H et al (2018) Occurrence and factors affecting the formation of trihalomethanes, haloacetonitriles and halonitromethanes in outdoor swimming pools treated with trichloroisocyanuric acid. Environ Sci (Camb) 4:218–225. https://doi.org/10.1039/c7ew00245a
Tan Y, Lin T, Jiang F et al (2017) The shadow of dichloroacetonitrile (DCAN), a typical nitrogenous disinfection by-product (N-DBP), in the waterworks and its backwash water reuse. Chemosphere 181:569–578. https://doi.org/10.1016/j.chemosphere.2017.04.118
Tak S, Kumar A (2017) Chlorination disinfection by-products and comparative cost analysis of chlorination and UV disinfection in sewage treatment plants: Indian scenario. Environ Sci Pollut Res 24:26269–26278. https://doi.org/10.1007/s11356-017-0568-z
Lee J, Sim W, Im Y et al (2018) Optimization of disinfection by-product analysis methods for IMO G9 approval. Mar Pollut Bull 126:402–412. https://doi.org/10.1016/j.marpolbul.2017.10.082
Hernandez MR, Ismail N, Drouillard KG, MacIsaac HJ (2017) Ships’ Ballast water treatment by chlorination can generate toxic trihalomethanes. Bull Environ Contam Toxicol 99:194–199. https://doi.org/10.1007/s00128-017-2125-3
Zhang N, Ma B, Li J, Zhang Z (2013) Factors affecting formation of chemical by-products during ballast water treatment based on an advanced oxidation process. Chem Eng J 231:427–433. https://doi.org/10.1016/j.cej.2013.07.055
Zhu Y, Ling Y, Peng Z, Zhang N (2020) Formation of emerging iodinated disinfection by-products during ballast water treatment based on ozonation processes. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.140805
Shah AD, Liu ZQ, Salhi E et al (2015) Formation of disinfection by-products during ballast water treatment with ozone, chlorine, and peracetic acid: influence of water quality parameters. Environ Sci (Camb) 1:465–480. https://doi.org/10.1039/c5ew00061k
Dock A, Linders J, David M et al (2020) Are workers on board vessels involved with chemicals from treated ballast water sufficiently protected?—A decadal perspective and risk assessment. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.125824
Zhu Y, Ling Y, Peng Z, Zhang N (2020) Formation of emerging iodinated disinfection by-products during ballast water treatment based on ozonation processes. Sci Total Environ 743:140805. https://doi.org/10.1016/j.scitotenv.2020.140805
Zhang H, Xue J, Wang Q et al (2022) Formation of halogenated disinfection by-products during ballast water chlorination. Environ Sci (Camb) 8:648–656. https://doi.org/10.1039/D1EW00674F
Cardador M, Gallego M, Chemistry LC-F (2016) Detection of regulated disinfection by-products in cheeses. Food Chem 204:306–313. https://doi.org/10.1016/j.foodchem.2016.02.146
Cardador MJ, Prados F, Fernandez-Salguero J (2017) Article in food additives and contaminants-part A chemistry, analysis, control, exposure and risk assessment. Taylor Francis 34:928–938. https://doi.org/10.1080/19440049.2017.1311421
Bao Loan HN, Jacxsens L, Kurshed AAM, De Meulenaer B (2016) 3-Chlorotyrosine formation in ready-to-eat vegetables due to hypochlorite treatment and its dietary exposure and risk assessment. Food Res Int 90:186–193. https://doi.org/10.1016/j.foodres.2016.10.048
Cardador MJ, Gallego M (2018) Determination of several common disinfection by-products in frozen foods. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 35:56–65. https://doi.org/10.1080/19440049.2017.1382731
Cardador MJ, Gallego M, Cabezas L, Fernández-Salguero J (2016) Detection of regulated disinfection by-products in cheeses. Food Chem 204:306–313. https://doi.org/10.1016/j.foodchem.2016.02.146
Dos Santos MS, Martendal E, Carasek E (2011) Determination of THMs in soft drink by solid-phase microextraction and gas chromatography. Food Chem 127:290–295. https://doi.org/10.1016/j.foodchem.2010.12.115
Lee WN, Huang CH, Zhu G (2018) Analysis of 40 conventional and emerging disinfection by-products in fresh-cut produce wash water by modified EPA methods. Food Chem 256:319–326. https://doi.org/10.1016/j.foodchem.2018.02.134
Lee WN, Huang CH, Zhu G (2019) Analytical methods for conventional and emerging disinfection by-products in fresh-cut produce. Food Chem 291:30–37. https://doi.org/10.1016/j.foodchem.2019.03.150
Lee W-N, Huang C-H (2019) Formation of disinfection byproducts in wash water and lettuce by washing with sodium hypochlorite and peracetic acid sanitizers. Food Chem. https://doi.org/10.1016/j.fochx.2018.100003
Reckhow DA, Singer PC, Malcolm RL (1990) Chlorination of Humic materials: byproduct formation and chemical interpretations. Environ Sci Technol 24:1655–1664. https://doi.org/10.1021/es00081a005
Young MS, Uden PC (1994) Byproducts of the aqueous chlorination of purines and pyrimidines. Environ Sci Technol 28:1755–1758. https://doi.org/10.1021/es00058a029
Huang H, Wu QY, Tang X et al (2016) Formation of haloacetonitriles and haloacetamides and their precursors during chlorination of secondary effluents. Chemosphere 144:297–303. https://doi.org/10.1016/j.chemosphere.2015.08.082
Huang H, Wu QY, Tang X et al (2013) Formation of haloacetonitriles and haloacetamides during chlorination of pure culture bacteria. Chemosphere 92:375–381. https://doi.org/10.1016/j.chemosphere.2013.01.031
Oliver BG (1983) Dihaloacetonitriles in drinking water: algae and Fulvic acid as precursors. Environ Sci Technol 17:80–83. https://doi.org/10.1021/es00108a003
Smith EM, Plewa MJ, Lindell CL et al (2010) Comparison of byproduct formation in waters treated with chlorine and iodine: Relevance to point-of-use treatment. Environ Sci Technol 44:8446–8452. https://doi.org/10.1021/es102746u
Tardif R, Catto C, Haddad S et al (2016) Assessment of air and water contamination by disinfection by-products at 41 indoor swimming pools. Environ Res 148:411–420. https://doi.org/10.1016/j.envres.2016.04.011
Weng SC, Blatchley ER (2011) Disinfection by-product dynamics in a chlorinated, indoor swimming pool under conditions of heavy use: national swimming competition. Water Res 45:5241–5248. https://doi.org/10.1016/j.watres.2011.07.027
Wei X, Chen X, Wang X et al (2013) Occurrence of regulated and emerging iodinated DBPs in the shanghai drinking water. PLoS ONE 8:e59677. https://doi.org/10.1371/journal.pone.0059677
Xiao F, Zhang X, Zhai H et al (2012) New halogenated disinfection byproducts in swimming pool water and their permeability across skin. Environ Sci Technol 46:7112–7119. https://doi.org/10.1021/es3010656
Wang X, MI GL, Zhang X et al (2014) Haloacetic acids in swimming pool and spa water in the United States and China. Front Environ Sci Eng 8:820–824. https://doi.org/10.1007/s11783-014-0712-7
Guilherme S, Rodriguez MJ (2017) Models for estimation of the presence of non-regulated disinfection by-products in small drinking water systems. Environ Monit Assess. https://doi.org/10.1007/s10661-017-6296-5
Alexandrou L, Meehan BJ, Jones OAH (2018) Regulated and emerging disinfection by-products in recycled waters. Sci Total Environ 637–638:1607–1616. https://doi.org/10.1016/j.scitotenv.2018.04.391
WHO (2005) Chlorite and chlorate in drinking water, background document for development of WHO guidelines for drinking-water quality. http://www.who.int/water_sanitation_health/dwq/chemicals/chlorateandchlorite0505.pdf. Accessed 15 Jan 2022
EU Directive (2003) Council Directive 98/83/EC on the quality of water intended for human consumption. In: Official Journal of the European Union. http://data.europa.eu/eli/dir/1998/83/oj. Accessed 15 Jan 2022
Righi E, Fantuzzi G, Predieri G, Aggazzotti G (2014) Bromate, chlorite, chlorate, haloacetic acids, and trihalomethanes occurrence in indoor swimming pool waters in Italy. Microchem J 113:23–29. https://doi.org/10.1016/j.microc.2013.11.007
Lin T, Wu S, Chen W (2014) Formation potentials of bromate and brominated disinfection by-products in bromide-containing water by ozonation. Environ Sci Pollut Res 21:13987–14003. https://doi.org/10.1007/s11356-014-3329-2
Carter RAA, Joll CA (2017) Occurrence and formation of disinfection by-products in the swimming pool environment: a critical review. J Environ Sci 58:19–50. https://doi.org/10.1016/j.jes.2017.06.013
Weinberg HS, Delcomyn CA, Unnam V (2003) Bromate in chlorinated drinking waters: occurrence and implications for future regulation. Environ Sci Technol 37:3104–3110. https://doi.org/10.1021/es026400z
Gonçalves AA, Gagnon GA (2018) Seawater ozonation: effects of seawater parameters on oxidant loading rates, residual toxicity, and total residual oxidants/by-products reduction during storage time. Ozone Sci Eng 40:399–414. https://doi.org/10.1080/01919512.2018.1448705
Benford D, Ceccatelli S, Cottrill B et al (2015) Risks for public health related to the presence of chlorate in food. EFSA J 13:4135. https://doi.org/10.2903/j.efsa.2015.4135
Lourencetti C, Ballester C, Fernández P et al (2010) New method for determination of trihalomethanes in exhaled breath: applications to swimming pool and bath environments. Anal Chim Acta 662:23–30. https://doi.org/10.1016/j.aca.2009.12.040
Villanueva CM, Font-Ribera L (2012) Health impact of disinfection by-products in swimming pools. Ann Ist Super Sanita 48:387–396. https://doi.org/10.4415/ANN_12_04_06
Mitch WA, Oelker GL, Hawley EL et al (2005) Minimization of NDMA formation during chlorine disinfection of municipal wastewater by application of pre-formed chloramines. Environ Eng Sci 22:882–890. https://doi.org/10.1089/EES.2005.22.882
Poleneni SR (2020) Recent research trends in controlling various types of disinfection by-products in drinking water: detection and treatment. Disinfection By-products in drinking water: detection and treatment. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-08-102977-0.00015-9
Mustapha S, Tijani JO, Ndamitso MM et al (2021) The occurrence of N -nitrosodimethylamine (NDMA) in swimming pools: an overview. Environ Health Insights 15:117863022110365. https://doi.org/10.1177/11786302211036520
Walse SS, Mitch WA (2008) Nitrosamine carcinogens also swim in chlorinated pools. Environ Sci Technol 42:1032–1037. https://doi.org/10.1021/es702301p
Krasner SW, Mitch WA, McCurry DL et al (2013) Formation, precursors, control, and occurrence of nitrosamines in drinking water: a review. Water Res 47:4433–4450. https://doi.org/10.1016/j.watres.2013.04.050
Garcia-Rodríguez A, Fontàs C, Matamoros V (2014) Formation potential of N-nitrosamines during the disinfection of treated wastewaters with sodium hypochlorite. Desalination Water Treat 52:3019–3026. https://doi.org/10.1080/19443994.2013.797663
Kadmi Y, Favier L, Wolbert D (2015) N-nitrosamines, emerging disinfection by-products of health concern: an overview of occurrence, mechanisms of formation, control and analysis in water. Water Sci Technol Water Supply 15:11–25. https://doi.org/10.2166/WS.2014.095
Papageorgiou A, Voutsa D, Papadakis N (2014) Occurrence and fate of ozonation by-products at a full-scale drinking water treatment plant. Sci Total Environ 481:392–400. https://doi.org/10.1016/j.scitotenv.2014.02.069
Monarca S, Zani C, Richardson SD et al (2004) A new approach to evaluating the toxicity and genotoxicity of disinfected drinking water. Water Res 38:3809–3819. https://doi.org/10.1016/j.watres.2004.07.003
Park KY, Choi SY, Lee SH et al (2016) Comparison of formation of disinfection by-products by chlorination and ozonation of wastewater effluents and their toxicity to Daphnia magna. Environ Pollut 215:314–321. https://doi.org/10.1016/j.envpol.2016.04.001
Dock A, Linders J, David M et al (2020) Are workers on board vessels involved with chemicals from treated ballast water sufficiently protected?—A decadal perspective and risk assessment. Chemosphere 247:125824. https://doi.org/10.1016/j.chemosphere.2020.125824
Lee WN, Huang CH (2019) Formation of disinfection byproducts in wash water and lettuce by washing with sodium hypochlorite and peracetic acid sanitizers. Food Chem X1:100003. https://doi.org/10.1016/j.fochx.2018.100003
Andrés CMC, de la Lastra JMP, Andrés Juan C et al (2022) Chlorine dioxide: friend or foe for cell biomolecules? A chemical approach. Int J Mol Sci. https://doi.org/10.3390/IJMS232415660
Gan W, Huang H, Yang X et al (2016) Emerging investigators series: disinfection by-products in mixed chlorine dioxide and chlorine water treatment. Environ Sci (Camb) 2:838–847. https://doi.org/10.1039/C6EW00061D
Froese KL, Wolanski A, Hrudey SE (1999) Factors governing odorous aldehyde formation as disinfection by-products in drinking water. Water Res 33:1355–1364. https://doi.org/10.1016/S0043-1354(98)00357-1
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2004) Some drinking-water disinfectants and contaminants, including arsenic. In: IARC Monogr Eval Carcinog Risks Hum. http://www.ncbi.nlm.nih.gov/pubmed/15645577. Accessed 10 May 2021
Bull RJ, Reckhow DA (2008) Use of chemical models and structure-activity relationships to identify novel disinfection by products of potential toxicological concern. ACS Symp Ser 995:51–64. https://doi.org/10.1021/BK-2008-0995.CH004
Richardson SD (2021) Tackling unknown disinfection by-products: lessons learned. J Hazardous Mater Lett 2:100041. https://doi.org/10.1016/j.hazl.2021.100041
Bichsel Y, Von Gunten U (1999) Oxidation of iodide and hypoiodous acid in the disinfection of natural waters. Environ Sci Technol 33:4040–4045. https://doi.org/10.1021/es990336c
Aprea M-C, Banchi B, Lunghini L et al (2010) Disinfection of swimming pools with chlorine and derivatives: formation of organochlorinated and organobrominated compounds and exposure of pool personnel and swimmers. Nat Sci (Irvine) 02:68–78. https://doi.org/10.4236/ns.2010.22011
Li JW, Yu Z, Cai X et al (1996) Trihalomethanes formation in water treated with chlorine dioxide. Water Res 30:2371–2376. https://doi.org/10.1016/0043-1354(96)00146-7
EFSA Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Treatment of poultry carcasses with chlorine dioxide, acidified sodium chlorite, trisodium phosphate and peroxyacids. EFSA Journal. 10.2903/j. efsa.2006.297
Askenaizer D (2003) Drinking water quality and treatment. Encyclopedia Phys Sci Technol. https://doi.org/10.1016/B0-12-227410-5/00186-1
Kirmeyer GJ, Friedman M, Martel KD et al (2001) Practical guidelines for maintaining distribution system water qualitY. J Am Water Works Assoc 93:62–73. https://doi.org/10.1002/j.1551-8833,2001.tb09244.x
Vasil D, Selleck RE (1992) Reactions and products of chloramination. Environ Sci Technol 26:808–814. https://doi.org/10.1021/es00028a022
Choi J, Valentine RL (2002) Formation of N-nitrosodimethylamine (NDMA) from reaction of monochloramine: a new disinfection by-product. Water Res 36:817–824. https://doi.org/10.1016/S0043-1354(01)00303-7
Choi J, Duirk SE, Valentine RL (2002) Mechanistic studies of N-nitrosodimethylamine (NDMA) formation in chlorinated drinking water. J Environ Monit 4:249–252. https://doi.org/10.1039/B200622G
Morrison CM, Hogard S, Pearce R et al (2022) Ozone disinfection of waterborne pathogens and their surrogates: a critical review. Water Res 214:118206. https://doi.org/10.1016/J.WATRES.2022.118206
Bonacquisti TP (2006) A drinking water utility’s perspective on bromide, bromate, and ozonation. Toxicology 221:145–148. https://doi.org/10.1016/j.tox.2006.02.010
Hansen KMS, Willach S, Mosbæk H, et al (2011) Effect of selection of pH in swimming pools on formation of chlorination by-products. In: Fourth international conference swimming pool & spa, Porto, Portugal. http://www.isep.ipp.pt/swimmingconference/
Jurado-Sánchez B, Ballesteros E, Gallego M (2014) Occurrence of carboxylic acids in different steps of two drinking-water treatment plants using different disinfectants. Water Res 51:186–197. https://doi.org/10.1016/j.watres.2013.10.059
McDonnell G (2014) The use of hydrogen peroxide for disinfection and sterilization applications. PATAI’S Chem Function Groups. https://doi.org/10.1002/9780470682531.PAT0885
Farinelli G, Coha M, Vione D et al (2022) Formation of halogenated byproducts upon water treatment with peracetic acid. Environ Sci Technol 56:5123–5131. https://doi.org/10.1021/acs.est.1c06118
Rutala WA, Weber DJ (2016) Disinfection and sterilization in health care facilities. Infect Dis Clin North Am 30:609–637. https://doi.org/10.1016/j.idc.2016.04.002
Kim BR, Anderson JE, Mueller SA et al (2002) Literature review—efficacy of various disinfectants against Legionella in water systems. Water Res 36:4433–4444. https://doi.org/10.1016/S0043-1354(02)00188-4
ECHA (2017) Investigation report formaldehyde and formaldehyde releasers substance name: formaldehyde IUPAC name: Methanal EC number: 200-001-8 CAS NUMBER: 50-00-0. https://echa.europa.eu/documents/10162/13641/annex_xv_report_formaldehyde_en.pdf/58be2f0a-7ca7-264d-a594-da5051a1c74bwww.echa.europa. Accessed 11 Apr 2023
Zhang C, Cui F, Zeng G et al (2015) Quaternary ammonium compounds (QACs): a review on occurrence, fate and toxicity in the environment. Sci Total Environ 518–519:352–362. https://doi.org/10.1016/j.scitotenv.2015.03.007
Martínez-Carballo E, Sitka A, González-Barreiro C et al (2007) Determination of selected quaternary ammonium compounds by liquid chromatography with mass spectrometry. Part I. Application to surface, waste and indirect discharge water samples in Austria. Environ Pollut 145:489–496. https://doi.org/10.1016/j.envpol.2006.04.033
Li X, Brownawell BJ (2010) Quaternary ammonium compounds in urban estuarine sediment environments—a class of contaminants in need of increased attention? Environ Sci Technol 44:7561–7568. https://doi.org/10.1021/es1011669
Ruan T, Song S, Wang T et al (2014) Identification and composition of emerging quaternary ammonium compounds in municipal sewage sludge in china. Environ Sci Technol 48:4289–4297. https://doi.org/10.1021/es4050314
Hora PI, Pati SG, McNamara PJ, Arnold WA (2020) Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: consideration of environmental implications. Environ Sci Technol Lett 7:622–631. https://doi.org/10.1021/acs.estlett.0c00437
Dewey HM, Jones JM, Keating MR, Budhathoki-Uprety J (2022) Increased use of disinfectants during the COVID-19 pandemic and its potential impacts on health and safety. ACS Chem Health Safety 29:27–38. https://doi.org/10.1021/acs.chas.1c00026
Silva V, Silva C, Soares P et al (2020) Isothiazolinone biocides: chemistry, biological, and toxicity profiles. Molecules. https://doi.org/10.3390/molecules25040991
Krzeminski SF, Brackett CK, Fisher JD, Spinnler JF (1975) Fate of microbicidal 3-isothiazolone compounds in the environment. Products of degradation. J Agric Food Chem 23:1068–1075. https://doi.org/10.1021/jf60202a054
Boyce JM (2018) Alcohols as surface disinfectants in healthcare settings. Infect Control Hosp Epidemiol 39:323–328. https://doi.org/10.1017/ICE.2017.301
Ribeiro MM, Neumann VA, Padoveze MC, Graziano KU (2015) Efficacy and effectiveness of alcohol in the disinfection of semi-critical materials: a systematic review. Rev Lat Am Enfermagem 23:741. https://doi.org/10.1590/0104-1169.0266.2611
Suchomel M, Gnant G, Weinlich M, Rotter M (2009) Surgical hand disinfection using alcohol: the effects of alcohol type, mode and duration of application. J Hosp Infect 71:228–233. https://doi.org/10.1016/j.jhin.2008.11.006
Seitz JM, Eifler R, Bach FW, Maier HJ (2014) Magnesium degradation products: effects on tissue and human metabolism. J Biomed Mater Res A 102:3744–3753. https://doi.org/10.1002/JBM.A.35023
Registration Dossier—Calcium magnesium oxide. https://echa.europa.eu/de/registration-dossier/-/registered-dossier/16185/5/5/1. Accessed 10 Mar 2023
Akter M, Sikder MdT, Rahman MdM et al (2018) A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J Adv Res 9:1–16. https://doi.org/10.1016/j.jare.2017.10.008
Bahcelioglu E, Unalan HE, Erguder TH (2021) Silver-based nanomaterials: a critical review on factors affecting water disinfection performance and silver release. Crit Rev Environ Sci Technol 51:2389–2423. https://doi.org/10.1080/10643389.2020.1784666
Hua G, Reckhow DA, Kim J (2006) Effect of bromide and iodide ions on the formation and speciation of disinfection byproducts during chlorination. Environ Sci Technol 40:3050–3056. https://doi.org/10.1021/es0519278
Cai Y, Zhao Y, Yadav AK et al (2023) Ozone based inactivation and disinfection in the pandemic time and beyond: taking forward what has been learned and best practice. Sci Total Environ 862:160711. https://doi.org/10.1016/j.scitotenv.2022.160711
Shen J, Gao Z (2018) Ozone removal on building material surface: a literature review. Build Environ 134:205–217. https://doi.org/10.1016/j.buildenv.2018.02.046
Sadiq R, Rodriguez M (2004) Disinfection by-products (DBPs) in drinking water and predictive models for their occurrence: a review. Sci Total Environ 321:21–46. https://doi.org/10.1016/j.scitotenv.2003.05.001
Cardador MJ, Fernández-Salguero J, Gallego M (2015) Simultaneous quantification of trihalomethanes and haloacetic acids in cheese by on-line static headspace gas chromatography–mass spectrometry. J Chromatogr A 1408:22–29. https://doi.org/10.1016/j.chroma.2015.07.007
Cardador MJ, Gallego M (2017) Control of disinfection by-products in canned vegetables caused by water used in their processing. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 34:10–23. https://doi.org/10.1080/19440049.2016.1241897
Cardador MJ, Gallego M (2015) Haloacetic acids content of fruit juices and soft drinks. Food Chem 173:685–693. https://doi.org/10.1016/j.foodchem.2014.10.105
Simpson AM-A, Mitch WA (2022) Chlorine and ozone disinfection and disinfection byproducts in postharvest food processing facilities: a review. Crit Rev Environ Sci Technol 52:1825–1867. https://doi.org/10.1080/10643389.2020.1862562
Vandekinderen I, Van Camp J, Devlieghere F et al (2008) Effect of decontamination agents on the microbial population, sensorial quality, and nutrient content of grated carrots (Daucus carota L.). J Agric Food Chem 56:5723–5731. https://doi.org/10.1021/jf800681a
Epelle EI, Macfarlane A, Cusack M et al (2023) Ozone application in different industries: a review of recent developments. Chem Eng J 454:140188. https://doi.org/10.1016/J.CEJ.2022.140188
Raffo A, Paoletti F (2022) Fresh-cut vegetables processing: environmental sustainability and food safety issues in a comprehensive perspective. Front Sustain Food Syst 5:681459. https://doi.org/10.3389/fsufs.2021.681459
Rutala WA, Weber DJ (1997) Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin Microbiol Rev 10:597. https://doi.org/10.1128/CMR.10.4.597
Cai L, Yu S, Li L (2022) Formation of odorous aldehydes, nitriles and N-chloroaldimines from free and combined leucine during chloramination. Water Res 210:117990. https://doi.org/10.1016/j.watres.2021.117990
Scully FE, Mazina KE, Ringhand HP et al (1990) Identification of organic N-chloramines in vitro in stomach fluid from the rat after chlorination. Chem Res Toxicol 3:301–306. https://doi.org/10.1021/tx00016a005
Choe JK, Hua LC, Komaki Y et al (2021) Evaluation of histidine reactivity and byproduct formation during peptide chlorination. Environ Sci Technol 55:1790–1799. https://doi.org/10.1021/acs.est.0c07408
How ZT, Linge KL, Busetti F, Joll CA (2017) Chlorination of amino acids: reaction pathways and reaction rates. Environ Sci Technol 51:4870–4876. https://doi.org/10.1021/acs.est.6b04440
Na C, Olson TM (2007) Relative reactivity of amino acids with chlorine in mixtures. Environ Sci Technol 41:3220–3225. https://doi.org/10.1021/es061999e
Wang T, Deng L, Dai W et al (2022) Formation of brominated halonitromethanes from threonine involving bromide ion during the UV/chlorine disinfection. J Clean Prod 373:133897. https://doi.org/10.1016/j.jclepro.2022.133897
Ding C, Zhang M, Zou B, Li N (2016) Mechanism of chlorination of threonine disinfection by-product trichloroacetone in drinking water. Huagong Xuebao/CIESC J 67:3010–3015. https://doi.org/10.11949/j.issn.0438-1157.20151906
Mazhar MA, Khan NA, Ahmed S et al (2020) Chlorination disinfection by-products in municipal drinking water—a review. J Clean Prod. https://doi.org/10.1016/J.JCLEPRO.2020.123159
Aslani H, Hosseini MS, Mohammadi S, Naghavi-Behzad M (2019) Drinking water disinfection by-products and their carcinogenicity; a review of an unseen crisis. Int J Cancer Manag. https://doi.org/10.5812/IJCM.88930
Kali S, Khan M, Ghaffar MS et al (2021) Occurrence, influencing factors, toxicity, regulations, and abatement approaches for disinfection by-products in chlorinated drinking water: a comprehensive review. Environ Pollut. https://doi.org/10.1016/J.ENVPOL.2021.116950
Collivignarelli MC, Abbà A, Miino MC et al (2021) Disinfection of wastewater by uv-based treatment for reuse in a circular economy perspective. Where are we at? Int J Environ Res Public Health 18:1–24. https://doi.org/10.3390/IJERPH18010077
Rizzo L, Malato S, Antakyali D et al (2019) Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci Total Environ 655:986–1008. https://doi.org/10.1016/J.SCITOTENV.2018.11.265
Pandian AMK, Rajamehala M, Singh MVP et al (2022) Potential risks and approaches to reduce the toxicity of disinfection by-product—a review. Sci Total Environ. https://doi.org/10.1016/J.SCITOTENV.2022.153323
Zhou J, Hung YC, Xie X (2023) Application of electric field treatment (EFT) for microbial control in water and liquid food. J Hazard Mater. https://doi.org/10.1016/J.JHAZMAT.2022.130561
Sinha R, Ghosal PS (2023) A comprehensive appraisal on status and management of remediation of DBPs by TiO2 based-photocatalysts: insights of technology, performance and energy efficiency. J Environ Manage. https://doi.org/10.1016/J.JENVMAN.2022.117011
Rayaroth MP, Aravindakumar CT, Shah NS, Boczkaj G (2022) Advanced oxidation processes (AOPs) based wastewater treatment—unexpected nitration side reactions—a serious environmental issue: a review. Chem Eng J. https://doi.org/10.1016/J.CEJ.2021.133002
Kumar R, Raizada P, Verma N et al (2021) Recent advances on water disinfection using bismuth based modified photocatalysts: strategies and challenges. J Clean Prod. https://doi.org/10.1016/J.JCLEPRO.2021.126617
ISO (2009) ISO 11348-3—2009-05—water quality—determination of the inhibitory effect of water samples on the light emission of Vibrio fischeri (Luminescent bacteria test). https://doi.org/10.31030/1495363
ISO (2012) ISO 8692—2012-06—water quality—fresh water algal growth inhibition test with unicellular green algae. https://doi.org/10.31030/1858340
Acknowledgements
The authors would like to thank our colleagues from the German Environment Agency (UBA) for proofreading. Furthermore, we would like to thank Dr. M. Diehle and J. Wolters from ISA RWTH Aachen for their help concerning the literature review. Figures were created with BioRender.com.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors thank the Umweltbundesamt for the research project Consideration of disinfection by-products in the context of environmental risk assessment of biocidal products—Inventory & development of recommendations for the assessment (FKZ 3718 65 403 0).
Author information
Authors and Affiliations
Contributions
MU: conceptualization, investigation, methodology, formal analysis, writing—original draft, writing—review and editing. MH: investigation, data curation, writing—original draft, writing—review and editing. SH: investigation, data curation, writing—original draft, writing—review and editing. SW: investigation, data curation, writing—original draft, writing—review and editing. AK-B: investigation, data curation, writing—original draft, writing—review and editing. VL: resources, supervision, conceptualization. TW: resources, supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Summary of the DBP literature search.
Additional file 2. Table S1.
Number of publications considering the respective DBPs. Table S2. Overview of the number of different DBPs by product types (PTs) and investigated matrix.
Additional file 3.
Entries from the ECHA database.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Usman, M., Hüben, M., Hahn, S. et al. Evaluation of the DBP formation potential of biocides and identification of knowledge gaps in environmental risk assessment. Environ Sci Eur 35, 77 (2023). https://doi.org/10.1186/s12302-023-00781-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-023-00781-w