Abstract
Toxicity of single pollutants or microplastics (MPs) on organisms have been widely reported. However, their combined toxicity with boron has not been investigated. This study examined effects of individual polypropylene microplastics (PP-MPs) or mixed PP-MPs and boron on biochemical biomarkers in red tilapia (Oreochromis niloticus). O. niloticus were exposed for 21 days to pristine PP-MPs concentrations (10 or 100 mg/L), concentrations of boron alone (30 or 70 mg/L), and identical concentrations of boron in the presence of PP-MPs in laboratory aquaria. Results showed that higher concentrations of individual PP-MPs lead to significantly decreased acetylcholinesterase (AChE) in the brain and malondialdehyde (MDA) in fish liver. In contrast, superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione (GSH) were significantly increased in fish liver exposed to higher concentrations of individual PP-MPs. Mixed concentrations of boron and PP-MPs significantly decreased AChE, GSH, and MDA activity in fish. In contrast, mixed concentrations of boron and PP-MPs significantly increased CAT, SOD, and GPx activity in fish. Findings highlight that PP-MPs may increase adverse effects of boron in O. niloticus. We present evidence that individual MPs in long-term exposure have a significant impact on biomarker responses in O. niloticus.
Similar content being viewed by others
Introduction
In recent decades, the demand for the use of plastics in a variety of industries has continuously grown [9, 28, 44]. Plastics are estimated to reach up to 54% (by mass) of anthropogenic waste materials discharged into the environment due to overuse and inappropriate management [22]. Microplastics (MPs; ranging from 1 μm to 5 mm) [16] are ubiquitous in all matrices of the environment [4], including seas [46], sediments [34], rivers [10, 31], soils [29, 61], and airs [1]. Ingestion of different MPs has been shown to be hazardous to a number of species in laboratory experiments, ranging from invertebrates to fish [14]. For example, Hanachi et al. [21] found that Zebrafish (Danio rerio) exposed to combined polyethylene terephthalate (PET) and abamectin for 96h exhibited alterations on glutathione (GSH) content, glutathione peroxidase (GPX), and superoxide dismutase (SOD) activity. Likewise, Ding et al. [12] found that red tilapia (Oreochromis niloticus) exposed to three sizes of PS-MPs (0.3, 5, and 70 − 90 μm) for 14 days had significant effects on oxidative stress in fish.
Biomarker responses have been applied to evaluate effects of environmental stressors on fish [50]. Some laboratory studies have reported MPs uptake in freshwater organisms including fish [13, 27], water flea (Daphnia magna) [24], and zebra mussel (Dreissena polymorpha) [35]. However, information on the mixed effects of MPs and chemical contaminants in freshwater fish is still limited. Zhang et al. [60] showed interactive effects of polystyrene (PS) MPs and roxithromycin on activities of cytochrome P450 (CYP) enzymes [7-ethoxyresorufin o deethylase (EROD) and 7-benzyloxy-4-trifluoromethyl-coumarin Odibenzyloxylase (BFCOD) O. niloticus.
Boron (including borates, boric acid, and boric oxide) can be found in rocks, soils, seawater and fresh water and is considered an essential micro-mineral [47]. Boron is also widely used in agricultural (e.g., fertilizer) and industrial applications (e.g., glass and antifreeze ingredients) [48]. Land-based anthropogenic activities can allow the release of boron into aquatic receiving environments from fertilizers, pesticides, and detergents [49]. Adverse effects on fish from boron contamination, including hematological, serum and DNA damage in Nile tilapia and Rainbow Trout (Oncorhynchus mykiss) have been reported by Acar et al. [2] and ÖZ et al. [41], respectively.
MPs may have a synergistic effect on organism health when combined with other pollutants, or they may serve as a transport vector for environmental pollutants. However, there is still a lack of understanding about the potential for pollutants to be incorporated into aquatic organisms by MP consumption [11]. To our knowledge, no study is available to investigate the combined effect of PP-MPs and boron on biomarker responses on organisms. The interaction of MPs and chemical contaminants has shown conflicting findings, with several studies reporting increased or decreased toxicity of mixed MPs with organic or inorganic chemicals [8, 18, 27]. For example, Karbalaei et al. [27] reported that PS-MPs increased toxicity of chlorpyrifos to O. mykiss. In another study, Guven et al. [18] found that MPs do not enhance the acute toxicity effects of pyrene on predatory performance of barramundi (Lates calcarifer).
Boron could adsorb on MPs, because B(OH)3 attaches to dissolved organic matter through surface complexations [53]. A recent study on the interaction between boron and MPs in aquatic environments showed boron adsorption capacity on aged PVC, aged PS, PVC, and PS. In addition, on aged PVC, 35.9% of the boron desorbed in the simulated gut of warm-blooded animals [53]. Another study also showed amino-modified polystyrene (PS-NH2) and excess boron inhibited the growth of Microcystis aeruginosa [59].
The Canadian federal government has called for more research to improve our understanding of the ecotoxicological impacts of MPs [15]. Thus, these important knowledge gaps must be address to help inform government strategies to reduce adverse effects of environmental MP pollution [52]. Very limited studies examined the effects of MPs and boron on aquatic environments and organisms [53, 59]. This study is the second research to investigate the combined effects of MPs and boron on aquatic organisms. To improve our understanding on impacts of individual and mixed PP-MPs and boron exposure, this study used multiple biomarkers in O. niloticus including a nervous system enzyme acetylcholinesterase (AChE) in the brain to assess potential neurotoxicity; cytochrome P450 (CYP) enzymes (EROD, and BFCOD) in the liver of O. niloticus to assess metabolic disturbances; and an antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), GPX, GSH, and malondialdehyde (MDA) in the liver of O. niloticus to assess potential oxidative damage. The main objectives of this study were to: (1) examine the effect of pristine PP-MPs exposure on enzymatic activities in liver of O. niloticus; and, (2) investigate whether PP-MPs change impacts of boron on enzymatic activities in liver of O. niloticus.
Materials and methods
Sources of MPs
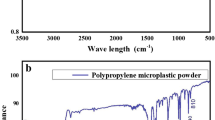
MPs used in this study were pristine polypropylene pellets purchased from commercial MP pellets Takht-e-Jamshid company. Original purity of PP was frozen in liquid nitrogen and crushed with a 0.5 mm sieve in an Ultra Centrifugal Mill ZM 200 (Germany). PP-MPs mixed with ethanol were pipetted onto aluminum stubs and gold sputtered, and considered in a scanning electron microscope (SEM; VEGA3 TESCAN; Czech Republic) for morphological observations (Fig. 1). Image J software was used to analyze the SEM image to determine the MPs particle size distributions. Sizes ranged from 5.12 to 398 μm, with 82% of particles < 100 μm, 13% between 100 and 250 μm, 3% between 250 and 300 μm, and 2% > 300 μm. MP composition was confirmed using Fourier transform infra-red spectroscopy (FTIR, Bruker tensor 27, Germany).
Experimental design
Fish samples
Tilapia is one of the most widely used farmed fish worldwide for aquaculture, marketability and stable market prices, and about ten species including Nile, blue, Mozambique tilapia, and red tilapia are the most commercially important species [51]. O. niloticus were bred from brood stock fish in a sterilised farm that used UV-treated water in tanks and the environment was cleaned with detergent and sterilised with 70% ethanol. Larvae were initially fed ad libitum with newly hatched Artemia nauplii, 4 times/day for 2 weeks. Fish were then fed 5–10% of body weight two times/day with fish pellets (crude protein: 38–40%). Early juveniles of tilapia were transported to the fish biology laboratory and acclimatized for 2 weeks at 28.5 ℃ in UV-treated water in a 2000 L fiberglass tank in photoperiod 12:12 light:dark. During the acclimatization period, no mortality was observed.
Fish exposure experiment
A total of 60 early juveniles O. niloticus (mean weight ± SD: 24.15 ± 9.21 g, mean total length ± SD: 8.91 ± 1.56 cm) were randomly distributed among 100 L glass aquaria (Seven fish per aquarium, one aquarium per treatment) 1 week prior to the exposure. A farmwork of this study is shown in Fig. 2. According to OECD guideline for testing chemicals, a minimum of seven fish must be used at each treatment and in the controls and no test tank replication is required [38]. Protocols for exposure, sampling and methods were approved by Laboratory Animal Center. Boron (purity: 99%, size: 44 µm), was selected as the pollutant model, purchased from Nanomaterial Powders, Turkey. Boron concentrations were below reported median lethal concentration (LC50) values for O. niloticus (141.42 mg/L) [2]. Boron concentrations were prepared from a boron standard stock diluted in boric acid (H3BO3, Merck This solution was prepared by dissolving 0.571 g of boric acid, then dried beforehand at 50 ℃ until constant weight, in 500 mL double distilled water and made up to 1 L. Suspension of PP-MPs was prepared by adding MPs particles (0.1 g) in Milli-Q water (1 L), then the bottle was placed for 30 min in an ultrasonic bath (Elma Schmidbauer GmbH, Singen, Germany) to achieve a homogeneous suspension. Chronic toxicity was conducted to identify adverse effects of individual PP-MPs, boron, and mixed boron and PP-MPs concentrations within 21 days exposure according to OECD guidelines [38] and modified by Karami et al. [26]. Treatments included: negative control (NC; no added PP particles), nominal PP-MP concentrations (10 mg/L PP-MPs, 100 mg/L PP-MPs), nominal boron concentrations (30 mg/L boron, 70 mg/L boron), and mixed PP-MP and boron concentrations (30 mg/L boron + 10 mg/L PP-MPs, 70 mg/L boron + 10 mg/L PP-MPs, 30 mg/L boron + 100 mg/L PP-MPs, 70 mg/L boron + 100 mg/L PP-MPs). Concentrations of PP-MPs tested in this study (i.e., 10 and 100 mg/L) were within reported ranges found in previous studies [3, 19]. For combination of boron and PP-MPs, target concentrations of boron (30 mg/L boron, 70 mg/L boron) were loaded to MPs (10 mg/L PP-MPs, 100 mg/L PP-MPs) in cleaned glass tube, then tubes were incubated in shaker for 28 h [27]. Water quality of experiment was as follows: temperature 28.5 ± 0.1 ℃, pH 7.2 ± 0.3, and dissolved oxygen 7.16 ± 0.3 mg/L. To reduce MP aggregation, glass aquaria were aerated gently with two air stones attached to the up and down parts of the aquaria. Fish were fed once a day at 2% of body weight during the 21-day exposure time. Water in aquaria was changed every 24 h with UV-treated water spiked with appropriate boron/PP-MP concentrations. No mortality was observed during exposure. The fish were fasted for 24 h prior to sampling to prevent vomiting during sampling. After a 21-day exposure, five fish per treatment were euthanized with clove oil, weighed and measured. Livers and brains were quickly sampled, washed in 0.15 M KCl, weighed, and then frozen in liquid nitrogen, and stored at – 80 °C until analysis of enzymatic activity.
Analysis of enzymatic activity
Liver and brain of each sample were homogenized by 0.15 M KCl, 0.1 M Tris–HCl at pH 7.4, and centrifuged (10,000 ×g; 25 min) at 4 °C. The remaining supernatant was collected for determination of enzymatic activity using a microplate reader (Biotek, USA). Analysis of AChE (in brain), CAT, SOD, GPx, GST, MDA activities and protein content were performed according to Diagnostic Reagent Kits (Comin Biotechnology Co., Ltd., Suzhou, China) according to manufacture instruction. All treatments were repeated three times. CAT activity of fish samples was assayed according to the method of ammonium vanadate–molybdate. A unit of CAT enzyme activity defined by catalytic degradation of 1 nmol H2O2 per minute. The xanthine oxidase method (hydroxylamine method) was used to measure SOD activity, and the absorbance was read at 550 nm. The dithio-binitrobenzoic acid method was used to measure the GPx activity, and the absorbance was read at 412 nm. GST activity was determined by measuring the substrates of 1-chloro2,4-dinitrobenzene (CDNB) and glutathione (GSH), and the absorbance was read at 340 nm. The MDA level was measured by the thiobarbituric acid method in the absorbance at 532 nm and 600 nm. EROD and BFCOD activities of the livers were quantified based on the method described by Mayeaux and Winston [37] with a slight modification [13]. The excitation and emission filters for EROD and BFCOD activities were set at 530 and 585 nm, respectively. Briefly, liver homogenate (10 mL), buffer (30 mL), and 40 mmol/L alkyl-substituted resorufin in Tris buffer (12.5 mL) were mixed and the reactions were initiated by adding 10 mmol/L NADPH (10mL). Reactions were stopped by adding ice-cold methanol (150 mL). For quantification, the fluorescence values were compared to authentic resorufin standards.
Data analysis
All data were checked for normality (Shapiro–Wilks test) and homoscedasticity (Levene's test) prior to analysis. Data of enzyme activities were analyzed using one-way ANOVAs and If ANOVA indicate a significant difference (p ˂ 0.05), treatments were compared by Tukey multiple comparison tests. Two-way ANOVA with interaction was used to compare effects of boron in absence and presence of PP-MPs (main factors: boron concentrations and presence of PP-MPs). Data were analyzed with IBM SPSS Statistics (V. 23).
Results
Biomarkers in O. niloticus exposed to individual PP-MPs
As shown in Fig. 3, obvious reduction was observed in the AChE activity in fish brains exposed to high concentration of PP-MPs (p < 0.05). In addition, AChE activity has no significant difference in the lower concentration of PP-MPs and control group (p > 0.05). PP-MPs do not change activity of CAT, SOD, and GPx (p > 0.05) except in higher concentration of MPs that SOD and GPx activity was increased in fish liver (p < 0.05; Fig. 4a–c). Both individual MPs significantly increased GSH activity in fish liver (p < 0.05; Fig. 4d). MDA contents was significantly decreased in higher concentration of individual PP-MPs (p < 0.05; Fig. 5). No significant difference was observed in EROD and BFCOD activity of fish liver in all treatments of PP-MPs (p > 0.05; Fig. 6a, and b).
Biomarkers in O. niloticus exposed to individual boron
AChE activity decreased in higher concentration of boron compared to control and lower concentration of boron in fish brain (p < 0.05; Fig. 2). CAT activity was significantly increased in both concentrations of boron in comparison with control group of fish liver (p < 0.05; Fig. 3a). In SOD activity, no significant differences were observed in in both concentrations of boron and control group of fish liver (p > 0.05; Fig. 3b). There was no significant difference in GPx activity among boron concentrations and control group (p > 0.05; Fig. 3c). Higher concentration of born significantly increased GSH activity (p < 0.05; Fig. 3d) and decreased MDA contents in fish liver (p < 0.05; Fig. 4). No significant difference was observed in EROD and BFCOD activity of fish liver in all treatments of boron (p > 0.05; Fig. 5a and b).
Biomarkers in O. niloticus exposed to mixed concentrations of boron and PP-MPs
Similar to the higher concentration of individual PP-MPs, three mixed concentrations of boron and PP-MPs (30 mg/L boron + 100 mg/L PP-MPs, 70 mg/L boron + 10 mg/L PP-MPs, 70 mg/L boron + 100 mg/L PP-MPs) significantly decreased AChE activity in fish brains (p < 0.05; Fig. 2). Higher concentration of PP-MPs and boron was significantly increased CAT activity in fish liver (p < 0.05; Fig. 3a), while no changed observed in other individual and mixed concentrations. Similar to higher concentration of individual PP-MPs, both concentration of boron mixed with higher concentration of PP-MPs were significantly increased SOD activity in fish liver compared to control groups (p < 0.05; Fig. 3b). GPx activity was increased in 30 mg/L boron + 100 mg/L PP-MPs, 70 mg/L boron + 10 mg/L PP-MPs, and 70 mg/L boron + 100 mg/L PP-MPs (p < 0.05; Fig. 3c). In contrast, GSH activity was decreased in 30 mg/L boron + 100 mg/L PP-MPs, 70 mg/L boron + 10 mg/L PP-MPs, and 70 mg/L boron + 100 mg/L PP-MPs (p < 0.05; Fig. 3d). All mixed concentrations of PP-MPs and boron were significantly decreased MDA contents in fish (p < 0.05; Fig. 4). EROD was significantly increased in mixed concentrations of PP-MPs with higher concentration of boron (p < 0.05; Fig. 5a). However, BFCOD activity was significantly increased in all mixed treatments of PP-MPs and boron (p < 0.05; Fig. 5b).
Discussion
Cholinesterases (ChE) belong to a family of enzymes that hydrolyze acetylcholine into choline and acetic acid, which can block acetylcholine metabolism causing acetylcholine to accumulate in the synaptic cleft, causing nerve impulse transmission to be disrupted [36]. Neurotoxic effects of individual MPs and combined MPs with other contaminants have been widely reported [5, 6, 40, 58]. The activity of AChE in the brain was measured to indicate impacts of individual PP-MPs, boron, and mixed boron and PP-MPs on neural activity. In this study, significant reductions found in AChE activity in the brain of O. niloticus in mixed contaminants concentrations, suggesting that mixed PP-MPs and boron can suppress catalytic capacity of this enzyme and lead to neurotoxicity in fish. In fact, AChE activity inhibition due to individual MPs, contaminants, and MPs load other contaminants has been found in various marine organisms, such as brain juvenile seabass (Dicentrarchus labrax) [6], larvae zebrafish (Danio rerio) [6], liver benthic crustacean (Eriocheir sinensis) [58], and liver mice [58]. Oliveira et al. [39] showed individual polyethylene microplastics (1–5 μm) and combined MPs with pyrene were able to inhibit AChE activity in common goby (Pomatoschistus microps).
SOD, CAT, and GPx endogenous antioxidant defense systems, are important for fish health by scavenging, neutralizing, and/or detoxifying ROS. Both CAT and SOD act as first antioxidant defense enzymes that eliminate ROS induced by xenobiotics. SOD can also catalyze partitioning of superoxide into hydrogen peroxide (H2O2) and molecular oxygen (O2). Any subsequent H2O2 can be removed by CAT to nonharmful products [43]. Changes of CAT activity in individual concentration of boron and mixed concentration of boron and PP-MPs showed MPs and boron led to CAT activation, probably due to the antioxidative response to increased H2O2 production. Some studies showed individual MPs and combined with other contaminants increased CAT and SOD activity in fish [20, 54, 55]. For example, CAT and SOD activity were significantly increased in Channa argus exposed to 80 nm and 0.5 μm PS-MPs (200 μg/L), and Cd (50 μg/L)[54].
GPx aids in the conversion of peroxides into less-toxic hydroxyl compounds and prevents the accumulation of ROS [57]. The higher GPX activity in individual PP-MPs and mixed PP-MPs/boron might have been the result of de novo synthesis, which was potentially induced by increased oxidative stress of liver in response to the contaminants [17] and required further investigation. Similarly, increased GPx activity in the marine copepod (Paracyclopina nana) with 20 μg/mL PS-MPs (0.05 μm) [25] and juvenile guppy (Poecilia reticulata) with low and high concentration of PS-MPs (32 − 40 μm)[23] were observed. In a study conducted by Magara et al. [33], GPx activity increased in combined exposures of polyethylene MPs and fluoranthene in fish gills.
GSH antioxidant enzymes can also play a major role in the maintenance of redox status. The GSH level increased in O. niloticus exposed to individual PP-MPs and higher concentration of boron, suggesting a protective response of fish to MPs concentrations. Such an increase in GSH may show the activation of the glutathione-dependent system of antioxidant defense caused by MPs [55]. However, the GSH content was reduced by mixed PP-MPs and boron, probably due to an antagonistic interaction between PP-MPs and boron. In addition, Wen et al. [55] reported that the mixture of PS-MPs and cadmium resulted in a decreased GSH content in discus fish (Symphysodon aequifasciatus).
Changes in MDA levels, indicate ROS production and intense oxidation, accompanied by severe damage to cell structure and function [56]. Kim et al. [30] found that SOD activity was negatively correlated with MDA levels, owing to the ability of SOD to metabolize and neutralize ROS. This study showed an increase in SOD activity in O. niloticus liver following exposure of individual and mixed PP-MPs and boron treatments. Similarly, MDA content was inhibited in O. niloticus liver in this study. Zhang et al. (2021) reported single and combined effects of phenanthrene and PS-MPs also showed negative correlation of SOD activity and MDA levels in the clam (Mactra veneriformis). Although this study demonstrated that exposure to individual and mixed MPs and boron treatments increases levels of intracellular ROS and, induced oxidative stress, more studies are required to improve our understanding of the biological effects of individual MPs or combined effects with other environmental stressors on aquatic organisms.
Both EROD and BFCOD activities in fish liver showed an increase in mixed concentrations of PP-MPs and boron, indicating disturbance of fish metabolism, but the disturbance mechanism is still unknown. Increase in CYP enzyme activity could be attributed to a period of contaminant adaptation [13]. No significant effects of individual PP-MPs on CYP enzyme activity observed in this study corroborates similar studies that showed CYP enzymes, such as EROD activity, were not sensitive to MPs exposure (e.g., [7, 32, 42]). In contrast to our study, Pannetier et al. [45] reported ingestion of individual MPs increased EROD activity in Japanese medaka. Conflicting results of EROD activity and CYP enzyme metabolism in organisms exposed to MPs pollution required further studies.
In General, MPs may exhibit different effects on O. niloticus, particularly when combined with other pollutants. CAT, SOD, GPx, EROD, and BFCOD activity showed similar results, with a significant increase observed in combined MPs and boron treatments. In contrast, AChE, GSH activity and MDA content decreased in combined MPs and boron treatments. Induced perturbations in antioxidant enzymes observed in this study, may suggest that aquatic organisms activate their antioxidant defense systems to cope with oxidative stress induced by mixed MPs and boron exposure. Recent study showed the charges on MPs affected boron adsorption on MPs and the aggregation of MPs with algal (Microcystis aeruginosa) cells, showing that the charge on MPs is a dominant factor influencing the combined effects of MPs and excess boron on M. aeruginosa [59]. Previous studies have also shown individual or mixed MPs combined with contaminants may induce complex, or even contradictory responses in fish due to the complex suite of contaminants or polymers used in exposure studies. Therefore, further studies are required to verify long-term or short-term biochemical responses in fish exposed to mixed MPs and other contaminants.
Conclusion
This study shows that individual PP-MPs, boron, and mixed PP-MPs and boron exposure induces complex biochemical response in O. niloticus. However, biochemical effects were observed more in mixed PP-MPs and boron treatments. Inhibition of AChE activity suggests potential neurotoxicity of mixed MPs and contaminants in fish. Alterations in biochemical biomarkers highlight that under oxidative stress from individual MPs and in combination with contaminant exposure, antioxidative enzymatic systems could be activated and hamper oxidative damage from occurring in fish. Further studies are required to expand our knowledge on the biological effects of individual MPs or combined effects with other environmental stressors on aquatic organisms.
Availability of data and materials
All data are publicly available, with sources described in the manuscript and supplementary material.
References
Abbasi S, Keshavarzi B, Moore F et al (2019) Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ Pollut 244:153–164. https://doi.org/10.1016/J.ENVPOL.2018.10.039
Acar Ü, İnanan BE, Zemheri F et al (2018) Acute exposure to boron in Nile tilapia (Oreochromis niloticus): median-lethal concentration (LC50), blood parameters, DNA fragmentation of blood and sperm cells. Chemosphere 213:345–350. https://doi.org/10.1016/J.CHEMOSPHERE.2018.09.063
Ahmadifar E, Kalhor N, Dawood MAO et al (2020) Effects of polystyrene microparticles on inflammation, antioxidant enzyme activities, and related gene expression in Nile tilapia (Oreochromis niloticus). Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-11731-x
Allen S, Allen D, Karbalaei S et al (2022) Micro(nano)plastics sources, fate, and effects: what we know after ten years of research. J Hazard Mater Adv 6:100057. https://doi.org/10.1016/J.HAZADV.2022.100057
Avio CG, Gorbi S, Milan M et al (2015) Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ Pollut 198:211–222. https://doi.org/10.1016/J.ENVPOL.2014.12.021
Barboza LGA, Vieira LR, Branco V et al (2018) Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat Toxicol 195:49–57. https://doi.org/10.1016/J.AQUATOX.2017.12.008
Batel A, Linti F, Scherer M et al (2016) Transfer of benzo[a]pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ Toxicol Chem 35:1656–1666. https://doi.org/10.1002/ETC.3361
Bellas J, Gil I (2020) Polyethylene microplastics increase the toxicity of chlorpyrifos to the marine copepod Acartia tonsa. Environ Pollut 260:114059. https://doi.org/10.1016/J.ENVPOL.2020.114059
Cao Y, Zhao M, Ma X et al (2021) A critical review on the interactions of microplastics with heavy metals: mechanism and their combined effect on organisms and humans. Sci Total Environ 788:147620. https://doi.org/10.1016/J.SCITOTENV.2021.147620
Capparelli MV, Molinero J, Moulatlet GM et al (2021) Microplastics in rivers and coastal waters of the province of Esmeraldas, Ecuador. Mar Pollut Bull 173:113067. https://doi.org/10.1016/J.MARPOLBUL.2021.113067
de Sá LC, Oliveira M, Ribeiro F et al (2018) Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci Total Environ 645:1029–1039. https://doi.org/10.1016/J.SCITOTENV.2018.07.207
Ding J, Huang Y, Liu S et al (2020) Toxicological effects of nano- and micro-polystyrene plastics on red tilapia: are larger plastic particles more harmless? J Hazard Mater 396:122693. https://doi.org/10.1016/J.JHAZMAT.2020.122693
Ding J, Zhang S, Razanajatovo RM et al (2018) Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ Pollut 238:1–9. https://doi.org/10.1016/J.ENVPOL.2018.03.001
Duis K, Coors A (2016) Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ Sci Eur 281(28):1–25. https://doi.org/10.1186/S12302-015-0069-Y
ECCC, Health Canada (2020) Draft Science Assessment of Plastic Pollution. Health Canada. Environment and Climate Change Canada, Gatineau, Quebec, Canada. https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/draft-sc. Accessed 20 Apr 2020
Frias JPGL, Nash R (2019) Microplastics: finding a consensus on the definition. Mar Pollut Bull 138:145–147. https://doi.org/10.1016/J.MARPOLBUL.2018.11.022
Gehringer MM, Shephard EG, Downing TG et al (2004) An investigation into the detoxification of microcystin-LR by the glutathione pathway in Balb/c mice. Int J Biochem Cell Biol 36:931–941. https://doi.org/10.1016/J.BIOCEL.2003.10.012
Guven O, Bach L, Munk P et al (2018) Microplastic does not magnify the acute effect of PAH pyrene on predatory performance of a tropical fish (Lates calcarifer). Aquat Toxicol 198:287–293. https://doi.org/10.1016/J.AQUATOX.2018.03.011
Hamed M, Soliman HAM, Osman AGM, Sayed AEDH (2019) Assessment the effect of exposure to microplastics in Nile Tilapia (Oreochromis niloticus) early juvenile: I. blood biomarkers. Chemosphere 228:345–350. https://doi.org/10.1016/j.chemosphere.2019.04.153
Hamed M, Soliman HAM, Osman AGM, Sayed AEDH (2020) Antioxidants and molecular damage in Nile Tilapia (Oreochromis niloticus) after exposure to microplastics. Environ Sci Pollut Res 27:14581–14588. https://doi.org/10.1007/S11356-020-07898-Y/FIGURES/3
Hanachi P, Kazemi S, Zivary S et al (2021) The effect of polyethylene terephthalate and abamectin on oxidative damages and expression of vtg and cyp1a genes in juvenile zebrafish. Environ Nanotechnol Monit Manag 16:100565. https://doi.org/10.1016/J.ENMM.2021.100565
Hodson ME, Duffus-Hodson CA, Clark A et al (2017) Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ Sci Technol 51:4714–4721. https://doi.org/10.1021/acs.est.7b00635
Huang JN, Wen B, Meng LJ et al (2020) Integrated response of growth, antioxidant defense and isotopic composition to microplastics in juvenile guppy (Poecilia reticulata). J Hazard Mater 399:123044. https://doi.org/10.1016/J.JHAZMAT.2020.123044
Jemec A, Horvat P, Kunej U et al (2016) Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ Pollut 219:201–209. https://doi.org/10.1016/J.ENVPOL.2016.10.037
Jeong CB, Kang HM, Lee MC et al (2017) (2017) Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci Reports 71(7):1–11. https://doi.org/10.1038/srep41323
Karami A, Karbalaei S, Zad Bagher F et al (2016) Alterations in juvenile diploid and triploid African catfish skin gelatin yield and amino acid composition: effects of chlorpyrifos and butachlor exposures. Environ Pollut 215:170–177. https://doi.org/10.1016/j.envpol.2016.05.014
Karbalaei S, Hanachi P, Rafiee G et al (2021) Toxicity of polystyrene microplastics on juvenile Oncorhynchus mykiss (rainbow trout) after individual and combined exposure with chlorpyrifos. J Hazard Mater 403:123980. https://doi.org/10.1016/J.JHAZMAT.2020.123980
Karbalaei S, Hanachi P, Walker TR, Cole M (2018) Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ Sci Pollut Res 25:36046–36063. https://doi.org/10.1007/s11356-018-3508-7
Koutnik VS, Leonard J, Alkidim S et al (2021) Distribution of microplastics in soil and freshwater environments: global analysis and framework for transport modeling. Environ Pollut 274:116552. https://doi.org/10.1016/J.ENVPOL.2021.116552
Kim W-K, Lee S-K, Park J-W et al (2014) Integration of multi-level biomarker responses to cadmium and benzo[k]fluoranthene in the pale chub (Zacco platypus). Ecotoxicol Environ Saf 110C:121–128. https://doi.org/10.1016/j.ecoenv.2014.08.025
Li J, Ouyang Z, Liu P et al (2021) Distribution and characteristics of microplastics in the basin of Chishui River in Renhuai, China. Sci Total Environ 773:145591. https://doi.org/10.1016/J.SCITOTENV.2021.145591
Luís LG, Ferreira P, Fonte E et al (2015) Does the presence of microplastics influence the acute toxicity of chromium(VI) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations. Aquat Toxicol 164:163–174. https://doi.org/10.1016/J.AQUATOX.2015.04.018
Magara G, Khan FR, Pinti M et al (2019) Effects of combined exposures of fluoranthene and polyethylene or polyhydroxybutyrate microplastics on oxidative stress biomarkers in the blue mussel (Mytilus edulis). J Toxicol Environ Health 82:616–625. https://doi.org/10.1080/15287394.2019.1633451
Maghsodian Z, Sanati AM, Ramavandi B et al (2021) Microplastics accumulation in sediments and Periophthalmus waltoni fish, mangrove forests in southern Iran. Chemosphere 264:128543. https://doi.org/10.1016/J.CHEMOSPHERE.2020.128543
Magni S, Gagné F, André C et al (2018) Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci Total Environ 631–632:778–788. https://doi.org/10.1016/J.SCITOTENV.2018.03.075
Massoulié J, Pezzementi L, Bon S et al (1993) Molecular and cellular biology of cholinesterases. Prog Neurobiol 41:31–91. https://doi.org/10.1016/0301-0082(93)90040-Y
Mayeaux MH, Winston GW (1998) Antibiotic effects on cytochromes P450 content and mixed-function oxygenase (MFO) activities in the American alligator, Alligator mississippiensis. J Vet Pharmacol Ther 21:274–281. https://doi.org/10.1046/J.1365-2885.1998.00134.X
OECD (2018) Test No. 452: chronic toxicity studies. OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris
Oliveira M, Ribeiro A, Hylland K, Guilhermino L (2013) Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol Indic 34:641–647. https://doi.org/10.1016/J.ECOLIND.2013.06.019
Oliveira P, Barboza LGA, Branco V et al (2018) Effects of microplastics and mercury in the freshwater bivalve Corbicula fluminea (Müller, 1774): filtration rate, biochemical biomarkers and mercury bioconcentration. Ecotoxicol Environ Saf 164:155–163. https://doi.org/10.1016/J.ECOENV.2018.07.062
Öz M, Karașahin T, Aksoy NH et al (2020) Harmful effects of dietary supplementation of boron on blood parameters of Rainbow Trout (Oncorhynchus mykiss). J Hell Vet Med Soc 71:2227–2234. https://doi.org/10.12681/jhvms.24169
Pannetier P, Cachot J, Clerandeau C, et al (2016) Toxicity assessment of pollutants sorbed on microplastics using various bioassays on two fish cell lines. In: MICRO 2016: fate and Impact of Microplastics in Marine Ecosystems: from the Coastline to the Open Sea. https://books.google.nl/books?hl=en&lr=&id=geLxDAAAQBAJ&oi=fnd&pg=PA140&ots=d_nNdcCWsH&sig=_14dbQs4fHmJSVJagnTtjuTRRGM&redir_esc=y#v=onepage&q&f=false. Accessed 31 Mar 2022
Piddington DL, Fang FC, Laessig T et al (2001) Cu, Zn superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst. Infect Immun 69:4980–4987. https://doi.org/10.1128/IAI.69.8.4980-4987.2001/ASSET/B52D24FD-D7F7-433D-B847-7F5C16605299/ASSETS/GRAPHIC/II0811795005.JPEG
PlasticsEurope (2020) Plastics—the facts 2020 by PlasticsEurope. https://issuu.com/plasticseuropeebook/docs/plastics_the_facts-web-dec2020. Accessed 5 Dec 2021
Pannetier P, Morin B, Le Bihanic F et al (2020) Environmental samples of microplastics induce significant toxic effects in fish larvae. Environ Int 134:105047. https://doi.org/10.1016/j.envint.2019.105047
Russell M, Webster L (2021) Microplastics in sea surface waters around Scotland. Mar Pollut Bull 166:112210. https://doi.org/10.1016/J.MARPOLBUL.2021.112210
Samman S, Naghii MR, Lyons Wall PM, Verus AP (2013) The nutritional and metabolic effects of boron in humans and animals. Biol Trace Elem Res 661(66):227–235. https://doi.org/10.1007/BF02783140
Schoderboeck L, Mühlegger S, Losert A et al (2011) Effects assessment: boron compounds in the aquatic environment. Chemosphere 82:483–487. https://doi.org/10.1016/J.CHEMOSPHERE.2010.10.031
Türker OC, Vymazal J, Türe C (2014) Constructed wetlands for boron removal: a review. Ecol Eng 64:350–359. https://doi.org/10.1016/J.ECOLENG.2014.01.007
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149. https://doi.org/10.1016/S1382-6689(02)00126-6
Vazirzadeh A (2017) Tilapia farming in Iran: savior of aquaculture or destroyer of environment. Shil 5:104–110
Walker TR, Xanthos D (2018) A call for Canada to move toward zero plastic waste by reducing and recycling single-use plastics. Resour Conserv Recycl 133:99–100
Wang H, Huang W, Zhang Y et al (2021) Unique metalloid uptake on microplastics: the interaction between boron and microplastics in aquatic environment. Sci Total Environ 800:149668. https://doi.org/10.1016/J.SCITOTENV.2021.149668
Wang S, Xie S, Wang Z et al (2021) Single and combined effects of microplastics and cadmium on the cadmium accumulation and biochemical and immunity of Channa argus. Biol Trace Elem Res 2021:1–11. https://doi.org/10.1007/S12011-021-02917-6
Wen B, Jin SR, Chen ZZ et al (2018) Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ Pollut 243:462–471. https://doi.org/10.1016/J.ENVPOL.2018.09.029
Wirnitzer U, Töpfer R, Rosenbruch M (1998) Altered p53 expression in early stages of chemically induced rodent hepatocarcinogenesis. Toxicol Pathol 26:636–645. https://doi.org/10.1177/019262339802600507
Yonar ME, Yonar SM, Çoban MZ, Eroǧlu M (2014) Antioxidant effect of propolis against exposure to chromium in Cyprinus carpio. Environ Toxicol 29:155–164. https://doi.org/10.1002/TOX.20782
Yu P, Liu Z, Wu D et al (2018) Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat Toxicol 200:28–36. https://doi.org/10.1016/J.AQUATOX.2018.04.015
Zhang C, Lin X, Gao P et al (2023) Combined effects of microplastics and excess boron on Microcystis aeruginosa. Sci Total Environ. https://doi.org/10.1016/J.SCITOTENV.2023.164298
Zhang S, Ding J, Razanajatovo RM et al (2019) Interactive effects of polystyrene microplastics and roxithromycin on bioaccumulation and biochemical status in the freshwater fish red tilapia (Oreochromis niloticus). Sci Total Environ 648:1431–1439. https://doi.org/10.1016/j.scitotenv.2018.08.266
Zhou Y, Liu X, Wang J (2019) Characterization of microplastics and the association of heavy metals with microplastics in suburban soil of central China. Sci Total Environ 694:133798. https://doi.org/10.1016/J.SCITOTENV.2019.133798
Acknowledgements
The authors would like to acknowledge Dr. Abbas Abdollahi for his critical review and valuable suggestions.
Funding
This study was not supported by any internal or external funding agencies.
Author information
Authors and Affiliations
Contributions
JY: investigation, review and editing. SK: conceptualization, writing original draft, visualization, formal analysis, investigation. KS: investigation, SMH: formal analysis, review and editing, AFA: formal analysis, review and editing, TRW: review and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Protocols for exposure, sampling and methods were approved by Laboratory Animal Center.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, J., Karbalaei, S., Hussein, S.M. et al. Biochemical effects of polypropylene microplastics on red tilapia (Oreochromis niloticus) after individual and combined exposure with boron. Environ Sci Eur 35, 71 (2023). https://doi.org/10.1186/s12302-023-00771-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-023-00771-y