Abstract
Objective
A systematic review of animal and human studies was conducted on genetically modified (GM) food consumption to assess its safety in terms of adverse effects/events to inform public concerns and future research.
Methods
Seven electronic databases were searched from January 1st 1983 till July 11th 2020 for in vivo, animal and human studies on the incidence of adverse effects/events of GM products consumption. Two authors independently identified eligible studies, assessed the study quality, and extracted data on the name of the periodical, author and affiliation, literature type, the theme of the study, publication year, funding, sample size, target population characteristics, type of the intervention/exposure, outcomes and outcome measures, and details of adverse effects/events. We used the Chi-square test to compare the adverse event reporting rates in articles funded by industry funding, government funding or unfunded articles.
Results
One crossover trial in humans and 203 animal studies from 179 articles met the inclusion criteria. The study quality was all assessed as being unclear or having a high risk of bias. Minor illnesses were reported in the human trial. Among the 204 studies, 59.46% of adverse events (22 of 37) were serious adverse events from 16 animal studies (7.84%). No significant differences were found in the adverse event reporting rates either between industry and government funding (χ2 = 2.286, P = 0.131), industry and non-industry funding (χ2 = 1.761, P = 0.185) or funded and non-funded articles (χ2 = 0.491, P = 0.483). We finally identified 21 GM food-related adverse events involving 7 GM events (NK603 × MON810 maize, GTS 40-3-2 soybean, NK603 maize, MON863 maize, MON810 maize, MON863 × MON810 × NK603 maize and GM Shanyou 63 rice), which had all been on regulatory approval in some countries/regions.
Conclusion
Serious adverse events of GM consumption include mortality, tumour or cancer, significant low fertility, decreased learning and reaction abilities, and some organ abnormalities. Further clinical trials and long-term cohort studies in human populations, especially on GM food-related adverse events and the corresponding GM events, are still warranted. It suggests the necessity of labelling GM food so that consumers can make their own choice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Genetic modification is defined as introducing transgene(s) with desired traits into the recipient organism’s genome by recombinant deoxyribonucleic acid (DNA) technology, and therefore it does not occur naturally [1,2,3]. Genetically modified (GM) crops are thought to address food security, sustainability and climate change solutions by improving crop yields, conserving biodiversity, providing a better environment in terms of the insect-resistant and herbicide-tolerant traits, reducing CO2 emissions and helping alleviate poverty through uplifting the economic situation [4]. Insect-resistant and herbicide-tolerant traits were first introduced into four types of crop, canola, cotton, maize and soybeans, at the beginning of GM production [5]. At present, the mainstream characteristics of new crops still pursue higher-yielding, more nutritious, pest- and disease-resistant and climate-smart to meet future demand for a yield increase of major crops such as wheat, rice and corn, due to the growing population [6].
Since 1996, the first year of commercialization of GM crops, 70 countries had adopted GM crops until 2018, including 26 countries that cumulatively planted 2.5 billion hectares of GM crops and an additional 44 countries that imported GM crops. During the 27 years (1992 to 2018), 4349 approvals for 387 GM events from 27 GM crops were granted by 70 countries involving 2063 for food (when the direct consumers are mainly humans), 1461 for feed (the products only intended for animal consumption) use and 825 for environmental release or cultivation [4, 7]. The major agricultural product exporting countries like the U.S.A., Brazil and Argentina show over 90% adoption of biotech crops [4]. For GM animal products, biotech salmon, considered to be the first genetically engineered animal for human consumption, was approved by the United States Department of Agriculture and Food & Drug Administration in 2015 [8]. In addition, it is illegal to grow major GM food crops in China while there are substantial investments in biotechnology research and GM maize, soybeans, and canola are allowed to import and eat [9].
Genetically modified food, however, is an example of the controversial relation between the inherent uncertainty of the scientific approach and the need of consumers to use products resulting from scientific developments thought to be safe [10]. Significant health risks have not been reported in peer-reviewed studies on GM food safety/security, which may cause some publication bias [11] but with a few exceptions, like the most famous “Monarch Butterfly controversy” [12], "Pusztai case" [13] and the "Séralini case" [14]. Unexpected effects of GM crops were reported in these studies, occupying an important place in the pages of scientific journals. Nevertheless, the above controversies severely impacted the public image, leading to full or partial bans in 38 countries including the European Union [15].
The complexity of risk evaluation is shown in these conflicting results, and concerns about the citizen-consumers have been raised against GM food [10]. Of most concern, aroused from the controversial events and some research results, is the potential of carcinogenesis, teratogenesis [16], lethal effects and adverse influences on fertility. GM agriculture is now widely discussed in both positive and negative frames and currently serves as a hotbed of debate in the public and policymakers. Although there are some reports and evidence from human and animal studies on the potential health effects of GM food/feed, the evidence is not conclusive and public concerns have not been resolved.
We aimed to conduct a systematic review of animal and human studies on GM food consumption to assess its safety in terms of adverse effects/events to inform public concerns and future research.
Methods
This study was a systematic review of previously published studies, conducted and reported in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [17] guideline.
Search strategy
China National Knowledge Infrastructure (CNKI), Wanfang, VIP Database, Chinese Biomedical Database (SinoMed), PubMed, the Cochrane Library and Embase databases were searched from January, 1st, 1983 till July, 11th, 2020, using a predefined search strategy (Additional file 1: Appendix S1). Reference lists of retrieved articles were also searched.
Eligibility criteria
Based on the evidence pyramid proposed by the Medical Center of State University of New York in 2001, we determined the type of research we included in the study. For a comprehensive evaluation of the literature, all in vivo animal studies and human studies (cross-sectional studies, case reports, case series, case–control studies, case–crossover studies, cohort studies, controlled clinical trials, including randomized trials, quasi-randomized trials and non-randomized trials) in multiple languages were included. Animal studies in all fields were included, that is, they could be clinical, agricultural and animal husbandry, veterinary medicine, life sciences, etc. Field studies were excluded.
The study population in animal studies was applied with inclusion criteria based on the categorization approach that highlights the actual use of them: laboratory animals and economical animals (livestock and aquatilia) were included, with no prespecified limitations on age, population, species/races, health status or others. Interventions/exposures of the genetically modified animal/plant/microorganism products included for animal/human ingestion referred to GM food, GM food ingredients and GM feed, regardless of their dosage or duration. The GM strain (line) and GM event were not limited. There was no restriction on whether controls were or were not included. The studies were excluded if they focused on the effects of GM food/feed on secondary or multilevel consumers in the food chain where GM food/feed was only consumed by primary consumers in the predator relationships. For instance, if non-GM fishes were fed with diet containing GM ingredients and then the fish was fed to the experimental cats, the study was excluded.
Outcomes focused on the incidence of adverse effects or adverse events in GM food/feed consumption, including primary outcomes on carcinogenesis, teratogenesis, lethal effect (all-cause mortality) and reproduction and secondary outcomes on other biomarkers were included. Toxicity studies of general toxicity studies (acute, sub-acute, sub-chronic, chronic and carcinogenicity toxicity studies) and specific toxicity studies (genotoxicity, reproductive and developmental toxicity, immunotoxicity and other toxicology studies) were included. Mortality in pups before weaning was considered as an outcome of reproductive toxicity but not as a lethal effect. Outcomes of adverse events in laboratory testing would not be included only when they could indicate tissue or organ toxicity. Outcomes of adverse events in breeding performance in animal husbandry studies, which focused on the economic benefits of the animal products, were included and these indicators were regarded as reproduction biomarkers in this research.
Outcomes of adverse events on growth performance, carcass traits, meat and fur production performance and meat quality for economic benefit evaluation of live stocks were excluded, of which the indicators included final body weight, weight gain, feed to gain ratio, half-eviscerated weight, eviscerated weight, percentage of eviscerated yield and muscle lean meat, sebum rate in some parts of the body, etc. Studies on the insecticidal effect of insect-resistant GM feed and outcomes of adverse events in gene fragments residual in the digestive tract were excluded. Besides, duplicate publications, studies with duplicate statistics, or references devoid of necessary information of participants, sample size, interventions/exposures or results were excluded.
Study selection and data extraction
Titles and abstracts of the retrieved articles were reviewed by 6 researchers in pair (C Shen, XC Yin, BY Jiao, J Peng, YZ Li, XH Cheng). 6 authors (C Shen, XC Yin, BY Jiao, JX Ren, J Li and XW Zhang) independently reviewed the full texts to identify the studies meeting eligibility criteria and then 8 researchers in pair (C Shen, XC Yin, BY Jiao, J Li, P Jia, XW Zhang, XH Cheng and JX Ren) independently extracted data from the included studies according to a predesignated extraction table. The discrepancies were resolved through consensus and if necessary, arbitrated by another author (JP Liu).
We extracted the name of the periodical, author and affiliation, literature type, the theme of the study, publication year, funding, sample size, target population characteristics, type of the intervention/exposure, outcomes and outcome measures. For those studies in which adverse effects/events occurred, details of interventions/exposures and control conditions (if any), dosage, duration, number of the generation, and the results were extracted.
Quality assessment
The methodological quality for animal studies was assessed, using criteria from the SYRCLE’s risk of bias tool for animal studies. The quality of animal studies was categorized into low risk of bias, unclear risk of bias, or high risk of bias according to the risk for each important outcome within included studies, including the adequacy of generation of the sequence generation, baseline characteristics, allocation concealment, random housing, blinding (performance bias), random outcome assessment, blinding (detection bias), incomplete outcome data, selective outcome reporting, or other sources of bias. The judgment of other risk of bias was based on whether there were contamination (pooling drugs), inappropriate influence of funders, unit of analysis errors, design-specific risks of bias or new animals added to the control and experimental groups to replace drop-outs from the original population.
Statistical synthesis and analyses
Statistical analyses were carried out using Microsoft Excel 2016 and SPSS 20.0. The findings were reported mainly in two parts, characteristics of the included studies and detailed information on the studies in which adverse effects/events occurred. Initially, descriptive statistics, frequencies, and percentages were calculated to summarize the data. Subsequently, studies that evaluated similar populations, interventions, controls (if any) and outcomes were pooled using a random-effects meta-analysis, and data from other studies were presented in tables and described in a narrative summary. The incidence of adverse events reported in articles funded by industry funding, government funding or unfunded articles were, respectively, counted and the Chi-square test was used for the comparisons.
Besides, we figured the incidence of serious adverse events (SAEs) by percentage. With reference to the Food and Drug Administration’s definition [18], our study defined SAEs as death, life-threatening, hospitalization (initial or prolonged), disability or permanent change, disruption, impairment or damage in a body function or structure (including cancer or tumour), in physical activities or quality of life, congenital anomaly or birth defect in the newborn child or pups, infertility or significant low in the number of deliveries or live birth rate than the non-GM commercial, conventional or blank controls, and an event resulting in intervention/treatment to prevent permanent impairment, damage or to prevent one of the other outcomes.
Meanwhile, the adverse events which cannot be ruled out that it has nothing to do with GM food (hereinafter abbreviated as GM food-related adverse events) were identified and the percentages under each outcome were calculated.
Results
Description of studies
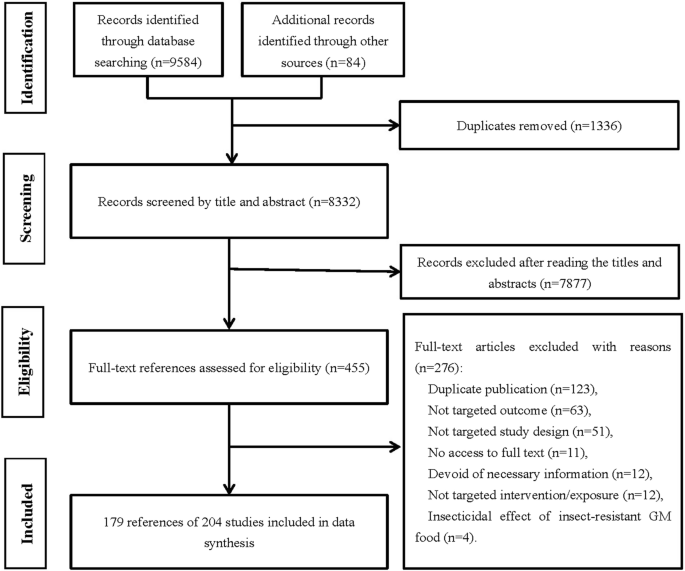
The flow diagram of the literature selection is shown in Fig. 1. A total of 9668 records were identified, including 9584 from the initial search through seven databases and 84 from other sources. After removal of duplicates and exclusion of references by reading titles and abstracts, 455 full-text articles were screened and 276 references were excluded with reasons (seen in the flow chart). Finally, 204 studies from 179 articles [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197] (153 journal articles, 22 dissertations, 3 conference proceedings and 1 unpublished report) were included in data synthesis, since there were more than one study conducted in each of the 2 included dissertations [107, 127], 11 journal articles [19, 33, 35, 63, 67, 88, 102, 118, 132, 172, 184] and 1 unpublished report [32]. The included studies were of 203 in vivo animal studies and 1 crossover trial [97] in humans.
Study characteristics
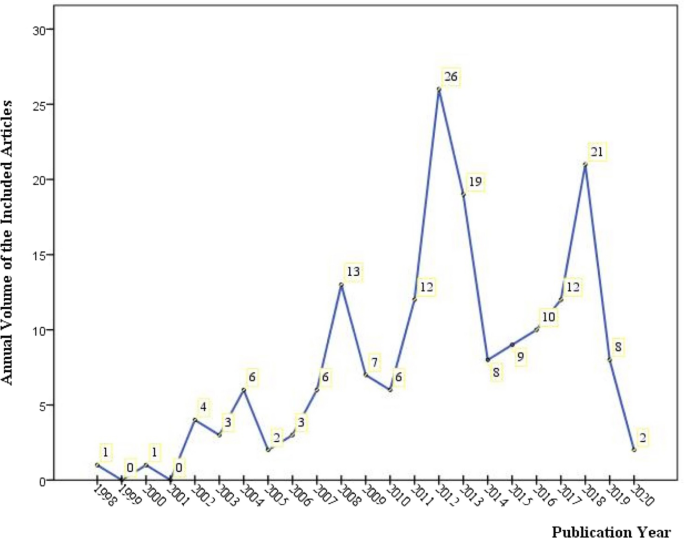
Of the 179 included articles, 94 were in English [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112], 83 were published in Chinese [113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195], and 2 in Japanese [196, 197]. The earliest included reference dated back to 1998 [153] (shown in Fig. 2), after which the remaining articles were distributed from 2000 to 2020 (45 articles in the 2000s, while 131 in the 2010s and 2 in the 2020s). The year 2012 witnessed the largest volume of publication (n = 26 articles, 14.53%). For funding sources or sponsors (Additional file 1: Appendix S2), in addition to 57 articles not mentioning the funding/sponsor (hereinafter as non-funded articles), there were 116 articles (64.8% of the 179 articles) supported by 56 kinds of government funding from 12 countries/government organizations and, still, 9 articles (5.03%) by 10 kinds of industry/institute funding sources/sponsors from 4 countries (America, Australia, French and German). Among them, 3 articles [29, 62, 74] claimed to have been funded or sponsored by both government and industry. China had undertaken the most government/school-level funding projects (39 of 56 projects, 69.64%).
The periodicals that have published more than 5 included articles were Food and Chemical Toxicology (published 25 included articles), EFSA Journal (13), Regulatory Toxicology and Pharmacology (9), Journal of Hygiene Research (9) and Chinese Journal of Food Hygiene (8). 11 of 13 authors, who have published ten or more included studies, were from European Food Safety Authority and published 12 included articles as co-authors. They were Christina Tlustos (published 12 included articles), Claudia Bolognesi (12), Konrad Grob (12), Vittorio Silano (12), Andre Penninks (11), Gilles Riviere (11), Holger Zorn (11), Karl-Heinz Engel (11), Yi Liu (11), Natalia Kovalkovicova (10), Sirpa Karenlampi (10). In addition to the above 12 articles, the top 3 of the 11 authors who published five or more included studies was Yang Xiao-Guang (from Chinese Center for Disease Control and Prevention, published 11 included articles), Wang Jing (from Tianjin Centre for Disease Control and Prevention, published 10 included articles) and Zhuo Qin (from Chinese Center for Disease Control and Prevention, published 7 included articles). The top 5 affiliations which published included articles were Chinese Center for Disease Control and Prevention (published 16 included articles), Tianjin Centre for Disease Control and Prevention (12), European Food Safety Authority (12), National Chung Hsing University (10), International Rice Research Institute (9).
Of the 204 included studies, one was a double-blind crossover trial (n = 36) in humans and the others were all animal studies. Individual sample sizes of the total 54,392 study population ranged from 4 (cats) [153] to 21,000 (Atlantic salmon) [23]. The studies involved 14 different kinds of animals (see Table 1). Apart from the most commonly used rats/mice (in 160 studies, 78.82%), pigs and chicks were two of the most extensively studied animals (in 23 studies, 11.33%). For themes of the 178 included animal studies, 158 were on clinical and 20 were on agricultural and animal husbandry. For the ones on clinical, 117 were on general toxicity (8 on acute, 6 sub-acute, 84 sub-chronic, 16 chronic toxicity, and still 3 on both acute, sub-acute and sub-chronic toxicity), 35 on specific toxicity (15 on reproductive and developmental toxicity, 16 on immunotoxicity, 3 on teratogenic effect and 1 on mutagenicity), 3 on allergenicity, 1 on learning and memory ability, 1 on athletic ability and 1 on both sub-chronic toxicity and allergenicity.
For interventions/exposures, 31 kinds of GM food were identified, including 18 kinds of GM plant food, 7 kinds of GM animal food and 6 kinds of GM microorganism food. Each included study covered one intervention/exposure, except for one study, Chen [29], that involved two kinds of GM products (sweet pepper and tomato) modified with the same gene (coat protein gene of cucumber mosaic virus), respectively, in two experimental groups. Maize, rice and soybean were the three most popular kinds of GM plant food (taken 79.38%) in research while milk/milk powder and animal-derived protein occupied the top two in GM animal food (56.25%). As for GM microorganism products, 5 kinds of food/feed enzyme derived from 5 different kinds of GM fungi or bacteria as well as 1 kind of microorganism-derived protein were among included studies.
Methodological quality of the animal studies
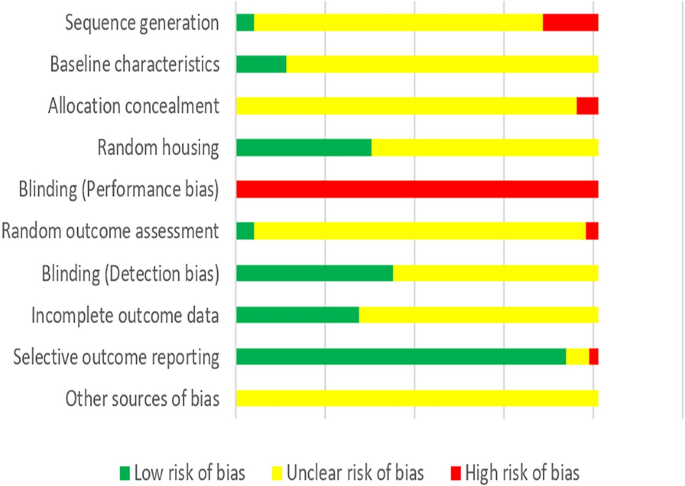
According to our predefined quality assessment criteria, all of the studies were identified as being unclear or having a high risk of bias (Fig. 3). None of the studies were reported to blind researchers from knowing which intervention each animal received. None of the studies reported prior sample-size calculation, 31 studies (15.27%) described wrong randomization procedures or did not mention the method of “randomization”, and 12 studies (5.91%) did not report adequate allocation concealment. 28 studies (13.79%) described that the groups were similar at baseline and 76 studies (37.44%) claimed that the housing conditions of animals from the various experimental groups were identical. 10 studies (4.93%) described randomly pick an animal during outcome assessment while 7 studies (3.45%) failed to select animals at random for outcome assessment. 88 studies (43.35%) completely used objective outcome indicators for outcome measurement. 185 studies (91.13%) reported consistent outcomes in the method and result sections while 5 studies did not, but none of the study protocols were available.
Incidence of adverse events/effects
No meta-analysis was conducted due to the significant heterogeneity of the primary studies. Among the 204 studies, a total of 29 studies (14.22%) from 23 articles reported 37 adverse events, involving 13 on mortality, 6 on reproductive toxicity, 3 on carcinogenesis and 15 on other biomarkers (including one human trial). It is worth noting that when, in one study, there were multiple aspects of adverse events on “other biomarkers”, we recorded it as 1 adverse event. Then, 22 serious adverse events (59.46% of adverse events) were identified in 16 studies (7.84% of the included studies and 55.17% of the studies reporting adverse events, marked in the tables with double asterisks). The SAEs mainly rested on mortality (13 studies), tumour or cancer (3), significant low in the number of pup deliveries (2), decreased learning and reaction abilities (1), severe stomach inflammation (1), intestinal adenoma lesions (1), and other pathology abnormalities (1) as hypertrophies and hyperplasia in mammary glands and pituitary, liver congestions and necrosis as well as severe chronic progressive nephropathies.
The incidence of adverse events reporting in government funding, industry funding and non-funded articles were 10.34% (12 of 116), 33.33% (3 of 9) and 15.79% (9 of 57), respectively. When comparing the adverse event reporting rates using the Chi-square test, we found that there were no significant differences either between industry funding and government funding (χ2 = 2.286, P = 0.131), industry funding and non-industry funding (χ2 = 1.761, P = 0.185) or funded and non-funded articles (χ2 = 0.491, P = 0.483).
Incidence of adverse events/effects in human trial
As for the human trial [97], shown in Table 2, a randomized double-blind crossover design was conducted for acute consumption of two single breakfasts, with a 14-day washout period, containing either seed oil generated from transgenic Camelina sativa plants or commercially blended fish oil. 36 healthy people were randomly allocated into two groups and venous blood samples were collected after the postprandial session, 8 h after each meal. No follow-up was reported. No major adverse symptoms or health effects were reported but some unrelated minor illnesses for the 72 postprandial sessions from 36 participants, such as minor upper respiratory tract infections (2.78%), minor nose bleed (1.39%), pyelonephritis (1.39%) and headaches (8.33%).
Incidence of adverse events/effects in animal studies
For the 203 animal studies, 28 studies (13.79%) from 22 articles reported 36 adverse events, including 13 on mortality (Table 3, 36.11%), 6 on reproductive toxicity (Table 4, 16.67%), 3 on carcinogenesis (Table 5, 8.33%) and 14 on other biomarkers (Additional file 1: Appendix S3, 38.89%).
All causes of death were included in this analysis and 11 of the 13 studies claimed that the mortality was not significantly different between the groups or had nothing to do with GM food. One study (Ermakova [37]) reported higher pup mortality in the Roundup-Ready soya (40.3.2 line) group compared with the controls. In Séralini [74], the general cause of death was large mammary tumours in females and other organ problems in males. Besides, rats in the Roundup-tolerant GM NK603 maize groups were 2–3 times more likely to die than controls, and more rapidly.
With respect to effects on reproduction, 5 animal feeding studies were reported to trigger reproductive toxicity but one study (Cisterna [31]) claimed to have no substantial impact on fertility. The reproductive toxicity manifested in the significant low in the number of deliveries, survival rate (from birth to weaning), litter weight, litter size and weight of some organs in the pups. For example, in Ermakova I 2005, the rats fed with Roundup-Ready soya had a 55.6% pup mortality rate during lactation periods compared to 9% in the control of traditional soya and 6.8% in the reference group. The pups kept dying during the lactation period while pups from the control group only died during the first week. Cyran N 2008 a and Cyran N 2008 c [32] were two rat feeding studies reported in one article, both given NK603 × MON810 maize. A multi-generation study was conducted as Cyran N 2008 a while Cyran N 2008 c did a continuous breeding study. Both of them indicated that fewer sum of pups was born and weaned in the GM groups. Pup losses, in Cyran N 2008 a, overall generations were about twice as many pups lost as compared to the control group (14.59% vs 7.4%) but was not significantly different and significantly lower litter weight was also reported in Cyran N 2008 c.
Three mouse/rat feeding studies reported triggering cancers/tumours when Tang [156] attributed the incidence of the tumour to the elder age of rats. Séralini 2014 (on Roundup-tolerant GM maize) found that females in the treatment groups almost always developed large mammary tumours more often than and controls. As for males, 4 times larger palpable tumours than controls were presented which emerged up to 600 days earlier. Cyran 2008 b [32] revealed a life term study where mice in the three groups were fed with transgenic maize NK603xMON810 (from 33.0% in the diet), control isoline diet and GM-free Austrian corn reference diet, respectively. The survival rate was not significantly different while cancer (leucosis) was the common cause of death.
GM food-related adverse events
Among the 37 adverse events reported, 16 of them claimed to have nothing to do with GM food, while the rest 21 (from 17 studies) did not, still leaving the question open. The GM food-related adverse events existed in mortality (2 studies), reproductive toxicity (5), carcinogenesis (2), and other biomarkers (12).
By gathering evidence, we identified 3 kinds of GM food associated with adverse events, GM soybean, GM maize as well as GM rice. For the 17 studies involved in the GM food-related adverse events, 4 studies were absent of information on the GM event of their test substance and the remainder concentrated on 7 GM events (3 studies on NK603 × MON810 maize, 2 on GTS 40-3-2 soybean, 2 on NK603 maize, 2 on MON863 maize, 2 on MON810 maize, 1 on maize mixed with MON863 × MON810 × NK603, NK603 × MON810 and NK603 and 1 on GM Shanyou 63 rice). When searching in the GM Approval Database on the ISAAA website, we found that all of the first 6 GM events listed, all developed by Monsanto Company, had been on regulatory approval for food, feed and cultivation in multiple countries/regions, including the European Union. GM -39 Shanyou 63 was developed in China and given approval for food, feed, and cultivation only by China in 2009.
Discussion
Summary of findings
We included 203 in vivo animal studies and 1 human trial, and all of the studies were identified as being unclear or having a high risk of bias. Overall, we reported two main findings. First, we identified 37 adverse events for GM food consumption while 22 of them (59.46%) were serious adverse events extracted from 16 animal studies (7.84%). SAEs were mortality, tumour or cancer, significantly low in the number of pup deliveries, decreased learning and reaction abilities, severe stomach inflammation, intestinal adenoma lesions, and other pathological abnormalities in the mammary glands, pituitary, liver and kidney.
Second, there were 21 GM food-related adverse events indicating that GM food may have effects on increased mortality (2 studies), reproductive toxicity (5 studies), which referred to significantly low fertility in parental generation and low survival rate, litter weight, litter size and weight of some organs in the pups, carcinogenesis (2 studies) and other biomarkers (12 studies). The effect-related GM food included 7 GM events (NK603 × MON810 maize, GTS 40-3-2 soybean, NK603 maize, MON863 maize, MON810 maize, MON863 × MON810 × NK603 maize and GM Shanyou 63 rice), which had all been on regulatory approval for food, feed and cultivation in some countries/regions.
Agreements and disagreements with other reviews
To our knowledge, there have been 3 previous systematic reviews (SRs) [198,199,200] and 6 conventional reviews [16, 201,202,203,204,205] addressing similar research questions on the unexpected effects of GM food consumption. Keshani et al. [198], searching in 4 English databases, included experimental studies on GM crops’ potential effects on sperm parameters. The study finally included 7 rat feeding studies, which were all identified in our study, and indicated no harm to GM plants consumers. Edge et al. [199] addressed 30 review questions for including human studies, published in recent 20 years (1994–2014), on health effects of genetically engineered (GE) food crops, but found no human study on 25 questions. The remaining 5 questions, related to allergenicity and nutrient adequacy, were answered based on 21 human studies. The human studies were all excluded in our research because of no direct ingestion of GE food in the allergenicity assessment studies or no targeted outcomes in the nutrient assessment trial. To illustrate, the above-mentioned nutrient assessment clinical trial evaluated the effect of carrots containing twofold higher calcium content on calcium absorption and we thought it was not on outcome related to adverse events/effects. The conclusion of the research also supported that there were no clear adverse health effects associated with the consumption of GE food. Moreover, Dunn et al. [200] included both human and animal studies for examining the allergenicity of GM organisms and finally found 34 human studies and 49 animal studies eligible. In addition to 32 human studies which involved human serum for IgE binding or inhibition studies and not direct consumption of GM product, the rest 2 [206, 207]studies were on actual ingestion of a GM food. However, they were not included in our research because of not targeted study type and unrelated outcomes. The conclusion agreed with the first two SRs that GM foods did not appear to be more allergenic than their conventional counterparts.
As for conventional reviews, Domingo showed special attention to the safety of GM food and published four literature reviews in 2000 [203], 2007 [204], 2011 [205] and 2016 [16]. Domingo searched two databases, PubMed and Scopus, to assess adverse/toxic effects of GM plants. In the latest updated review, he addressed the conclusion that GM soybeans, rice, corn/maize and wheat would be as safe as the parental species of these plants. However, our results may not be consistent with Domingo’s conclusion: we focus on a summarization of adverse events for GM food consumption through a systematic search in 7 databases; we identified 37 adverse events, 22 serious adverse events and 21 GM food-related adverse events; GM maize, soybean and rice with some specific GM events were all related to GM food-related adverse events. In addition, Domingo found a notable advance of studies published in scientific journals by biotechnology companies. Coincidentally, we did a Chi-square test to compare the adverse event reporting rates and found no significant differences between industry funding, government funding and non-funded articles. Besides, our systematic review validated Domingo’s findings that some GM plants were studied scarcely in recent years including GM potatoes discussed in the controversy of Pusztai case.
Strengths and limitations
In this review, a systematic search of major databases was conducted to identify all available studies in all languages on the adverse effects/events of GM food consumption. To make the inclusion and data synthesis comprehensive, both in vivo human and animal studies in all fields were included, with no limitations on the type of participant, type of intervention/exposure or whether control was included. The terms used for searching, containing all kinds of names of GM food, were based on a basic search on the internet by the researchers and the list was perfected as much as possible. With respect to additional searching, we went through multifarious news which reported controversy of GM food and thus we identified several hot studies by following the clue. In order to trace the potential conflicts of interest, we performed a Chi-square test for comparing adverse events report rates in articles funded by industry funding, government funding or unfunded articles, but found no statistical significance. Nevertheless, it was hard to conduct a quantitative data synthesis for the effects of GM food consumption on the adverse events because of the significant heterogeneity of the primary studies.
There are several limitations in this review. The methodological quality of the included studies is generally poor, which indicates a high or unclear risk of bias resulting from insufficient reporting of methodological components in the studies. Methodological quality may not be fully reflected based solely on the reporting of the manuscript. There were unclear descriptions of randomization procedures and a lack of blinding in all of the studies, which may have created potential performance biases and detection biases, as researchers might have been aware of the effects of interventions. The ability to perform meta-analysis was limited because of the heterogeneity of the participants, interventions (GM food in various GM events), comparisons, feeding doses, administration time, other exposure factors, and the variance of composite outcome measures used in the 204 included studies. When we did the manual search, we found that related publications were retracted sometimes, under the name of inadequate experimental designs or statistical analysis. For example, Séralini 2012 was retracted by Food and Chemical Toxicology, but subsequently republished in another journal [14, 74]. This indicates that it was hard for us to find the original full-text papers of the retracted publications and articles provided by databases still have some unavoidable publication bias. The retraction on controversial researches may also cause the controversy for the public to doubt the reality of the studies published and to concern the safety of GM food. In addition, the lack of human studies is another key limitation of this research. As for the searching strategy, we did not include publication types as newspaper articles and comments. This was thought to be a limitation of this research because these sources may give us clues of related researches and can help us to do a manual search comprehensively. It is also an implication for future systematic reviews.
Implications for research
Future research should be conducted in humans, especially observational cohort studies. High-quality animal studies according to the ARRIVE reporting standard focusing on reproductive toxicity and carcinogenesis are still needed. Trials or studies should be registered prospectively, and be accessible. Furthermore, to address public concerns, future studies should focus on SAEs and GM food-related adverse events reported in this research such as NK603 maize, MON863 maize and MON810 maize. Meanwhile, some implications of findings still could be explored such as how GM food affects people’s eating habits, labelling of GM food and public choice. Some of the included studies conducted an intergenerational or multigenerational evaluation of the safety of GM food, but only two studies (Cyran N 2008 a and Cyran N 2008 c) in one article reported adverse events related to fertility. The differences in the results may be due to different interventions/exposures (GM food in certain GM events), laboratory animals, intervention/exposure time, experiment environment, etc. Therefore, it is necessary for subsequent studies to start with intergenerational or multigenerational research to verify the safety of GM food in terms of study design.
Conclusion
Serious adverse events accounted for 59.46% of the total 37 identified adverse events of GM consumption, which include: mortality, tumour or cancer, significantly lower number of pup deliveries, decreased learning and reaction abilities, and organ abnormalities in the stomach, intestinal adenoma, mammary glands, pituitary, liver and kidney. The interventions/exposures in the adverse event related studies emphasized on GM soybean, maize and rice in specific GM events. Animal studies occupy the lowest hierarchy of evidence, and there are flaws in study design and is not convincing enough. The evidence on the effect of GM consumption on humans is still insufficient. Further clinical trials and long-term cohort studies in human populations, especially on GM food-related adverse events and the corresponding GM events, are still warranted. It is better to prove the safety before they are approved for food consumption and it also suggests the necessity of labelling on GM food so that consumers can make their own choice.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- GM:
-
Genetically modified
- DNA:
-
Deoxyribonucleic acid
- CNKI:
-
China National Knowledge Infrastructure
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SAE:
-
Serious adverse event
- CSO:
-
Camelina sativa Seed oil
- BFO:
-
Blended fish oil
- bw:
-
Body weight
- SR:
-
Systematic reviews
- GE:
-
Genetically engineered
References
Hundleby PA, Harwood WA (2019) Impacts of the EU GMO regulatory framework for plant genome editing. Food Energy Secur. https://doi.org/10.1002/fes3.161
Sprink T, Eriksson D, Schiemann J et al (2016) Regulatory hurdles for genome editing: process- vs. product-based approaches in different regulatory contexts. Plant Cell Rep 35:1493–1506. https://doi.org/10.1007/s00299-016-1990-2
Georges F, Ray H (2017) Genome editing of crops: a renewed opportunity for food security. GM Crops Food 8:1–12. https://doi.org/10.1080/21645698.2016.1270489
ISAAA (2018) Global status of commercialized biotech/GM Crops in 2018: biotech crops continue to help meet the challenges of increased population and climate change. ISAAA Brief No. 54 [Online]. http://www.isaaa.org/resources/publications/briefs/54/default.asp. Accessed 17 July 2020
Andrew J, Ismail NW, Djama M (2018) An overview of genetically modified crop governance, issues and challenges in Malaysia. J Sci Food Agric 98:12–17. https://doi.org/10.1002/jsfa.8666
Hickey LT, Hafeez AN, Robinson H et al (2019) Breeding crops to feed 10 billion. Nat Biotechnol 37(7):744–754. https://doi.org/10.1038/s41587-019-0152-9
Giraldo PA, Shinozuka H, Spangenberg GC et al (2019) Safety assessment of genetically modified feed: is there any difference from food? Front Plant Sci 10:1592. https://doi.org/10.3389/fpls.2019.01592
ISAAA (2016) Global status of commercialized biotech/GM crops: 2016. ISAAA Brief No. 52 [Online]. http://www.isaaa.org/resources/publications/briefs/52/default.asp. Accessed 17 July 2020
Pray C, Huang J, Hu R, Deng H et al (2018) Prospects for cultivation of genetically engineered food crops in China. Global Food Secur Agric Policy Econ Environ 16:133–137. https://doi.org/10.1016/j.gfs.2018.01.003
Martinelli L, Karbarz M, Siipi H (2013) Science, safety, and trust: the case of transgenic food. Croat Med J 54:91–96. https://doi.org/10.3325/cmj.2013.54.91
Klümper W, Qaim M (2014) A meta-analysis of the impacts of genetically modified crops. PLoS ONE. https://doi.org/10.1371/journal.pone.0111629
Losey JE, Rayor LS, Carter ME (1999) Transgenic pollen harms monarch larvae. Nature 399:214. https://doi.org/10.1038/20338
Ewen SW, Pusztai A (1999) Effect of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat small intestine. Lancet 354:1353–1354. https://doi.org/10.1016/S0140-6736(98)05860-7
Séralini GE, Clair E, Mesnage R et al (2012) Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize [retracted in: Food Chem Toxicol. 2014 Jan;63:244]. Food Chem Toxicol 50(11):4221–4231. https://doi.org/10.1016/j.fct.2012.08.005
Genetic Literacy Project (2017) Where are GMOs grown and banned? [Online]. https://gmo.geneticliteracyproject.org/FAQ/where-are-gmos-grownand-banned/. Accessed 5 Sept 2020
Domingo JL (2016) Safety assessment of GM plants: an updated review of the scientific literature. Food Chem Toxicol 95:12–18. https://doi.org/10.1016/j.fct.2016.06.013 (Epub 2016 Jun 16)
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta analyses: the PRISMA statement. BMJ. https://doi.org/10.1136/bmj.b2535
FDA (2016) What is a serious adverse event? [Online]. https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event. Accessed 28 Sept 2020
Al-Harbi A, Lary S, Edwards MG et al (2019) A proteomic-based approach to study underlying molecular responses of the small intestine of Wistar rats to genetically modified corn (MON810). Transgenic Res 28(5–6):479–498. https://doi.org/10.1007/s11248-019-00157-y
Appenzeller LM, Munley SM, Hoban D et al (2009) Subchronic feeding study of grain from herbicide-tolerant maize DP-Ø9814Ø-6 in Sprague-Dawley rats. Food Chem Toxicol 47:2269–2280. https://doi.org/10.1016/j.fct.2009.06.014
Bai H, Wang Z, Hu R et al (2015) A 90-day toxicology study of meat from genetically modified sheep overexpressing TLR4 in Sprague-Dawley rats. PLoS ONE. https://doi.org/10.1371/journal.pone.0121636
Bakke-Mckellep AM, Koppang EO, Gunnes G et al (2007) Histological, digestive, metabolic, hormonal and some immune factor responses in Atlantic salmon, Salmo salar L, fed genetically modified soybeans. J Fish Dis 30:65–79. https://doi.org/10.1111/j.1365-2761.2007.00782.x
Bakke-McKellep AM, Sanden M, Danieli A et al (2008) Atlantic salmon (Salmo salar L.) parr fed genetically modified soybeans and maize: histological, digestive, metabolic, and immunological investigations. Res Vet Sci 84:395–408. https://doi.org/10.1016/j.rvsc.2007.06.008
Buzoianu SG, Walsh MC, Rea MC et al (2012) Effect of feeding genetically modified Bt MON810 maize to ∼ 40-day-old pigs for 110 days on growth and health indicators. Animal 6:1609–1619. https://doi.org/10.1017/S1751731112000249
Buzoianu SG, Walsh MC, Rea MC et al (2012) Effects of feeding Bt maize to sows during gestation and lactation on maternal and offspring immunity and fate of transgenic material. PLoS ONE. https://doi.org/10.1371/journal.pone.0047851
Carman JA, Vlieger HR, Ver Steeg LJ et al (2013) A long-term toxicology study on pigs fed a combined genetically modified (GM) soy and GM maize diet. J Organic Systems 1:1–12
Chen X, Gao MQ, Liang D et al (2017) Safety assessment of genetically modified milk containing human beta-defensin-3 on rats by a 90-day feeding study. Food Chem Toxicol 100:34–41. https://doi.org/10.1016/j.fct.2016.12.012
Chen YN, Hwang WZ, Fang TJ et al (2011) The impact of transgenic papaya (TPY10-4) fruit supplementation on immune responses in ovalbumin-sensitised mice. J Sci Food Agric 91:539–546. https://doi.org/10.1002/jsfa.4218
Chen ZL, Gu H, Li Y et al (2003) Safety assessment for genetically modified sweet pepper and tomato. Toxicology 188:297–307. https://doi.org/10.1016/s0300-483x(03)00111-2
Chukwudebe A, Privalle L, Reed A et al (2012) Health and nutritional status of Wistar rats following subchronic exposure to CV127 soybeans. Food Chem Toxicol 50:956–971. https://doi.org/10.1016/j.fct.2011.11.034
Cisterna B, Flach F, Vecchio L et al (2008) Can a genetically-modified organism-containing diet influence embryo development? A preliminary study on pre-implantation mouse embryos. Eur J Histochem 52:263–267. https://doi.org/10.4081/1226
Cyran N, Gülly C, Handl S et al (2008) Biological effects of transgenic maize NK603xMON810 fed in long term reproduction studies in mice. FiBL, Wien
de Vendômois JS, Roullier F, Cellier D et al (2009) A comparison of the effects of three GM corn varieties on mammalian health. Int J Biol Sci 5:706–726. https://doi.org/10.7150/ijbs.5.706
Delaney B, Appenzeller LM, Munley SM et al (2008) Subchronic feeding study of high oleic acid soybeans (Event DP-3Ø5423-1) in Sprague-Dawley rats. Food Chem Toxicol 46:3808–3817. https://doi.org/10.1016/j.fct.2008.10.003
EFSA Panel on Genetically Modified Organisms (GMO), Naegeli H, Birch AN et al (2018) Assessment of genetically modified soybean MON 87751 for food and feed uses under Regulation (EC) No 1829/2003 (application EFSA-GMO-NL-2014-121). EFSA J 16(8):e05346. https://doi.org/10.2903/j.efsa.2018.5346
El-Shamei ZS, Gab-Alla AA, Shatta AA (2012) Histopathological changes in some organs of male rats fed on genetically modified corn (Ajeeb YG). Am J Sci 10(8):684–696
Ermakova I (2005) Influence of genetically modified soya on the birth-weight and survival of rat pups. In: Epigenetics, transgenic plants& risk assessment.
Gao MQ, Zhang R, Yang Y et al (2018) A subchronic feeding safety evaluation of transgenic milk containing human β-defensin 3 on reproductive system of C57BL/6J mouse. Food Chem Toxicol 115:198–204. https://doi.org/10.1016/j.fct.2018.03.007
Gu J, Krogdahl Å, Sissener NH et al (2013) Effects of oral Bt-maize (MON810) exposure on growth and health parameters in normal and sensitised Atlantic salmon, Salmo salar L. Br J Nutr 109:1408–1423. https://doi.org/10.1017/S000711451200325X
Guo QY, He LX, Zhu H et al (2015) Effects of 90-day feeding of transgenic maize BT799 on the reproductive system in male Wistar rats. Int J Environ Res Public Health 12:15309–15320. https://doi.org/10.3390/ijerph121214986
Hammond B, Dudek R, Lemen J et al (2004) Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem Toxicol 42:1003–1014. https://doi.org/10.1016/j.fct.2004.02.013
Hammond B, Lemen J, Dudek R et al (2006) Results of a 90-day safety assurance study with rats fed grain from corn rootworm-protected corn. Food Chem Toxicol 44:147–160. https://doi.org/10.1016/j.fct.2005.06.008
He XY, Tang MZ, Luo YB et al (2009) A 90-day toxicology study of transgenic lysine-rich maize grain (Y642) in Sprague-Dawley rats. Food Chem Toxicol 47:425–432. https://doi.org/10.1016/j.fct.2008.11.032
Healy C, Hammond B, Kirkpatrick J (2008) Results of a 13-week safety assurance study with rats fed grain from corn rootworm-protected, glyphosate-tolerant MON 88017 corn. Food Chem Toxicol 46:2517–2524. https://doi.org/10.1016/j.fct.2008.04.005
Ibrahim MA, Okasha EF (2016) Effect of genetically modified corn on the jejunal mucosa of adult male albino rat. Exp Toxicol Pathol 68:579–588. https://doi.org/10.1016/j.etp.2016.10.001
Kiliç A, Akay MT (2008) A three generation study with genetically modified Bt corn in rats: Biochemical and histopathological investigation. Food Chem Toxicol 46:1164–1170. https://doi.org/10.1016/j.fct.2007.11.016
Kiliçgün H, Gürsul C, Sunar M et al (2013) The comparative effects of genetically modified maize and conventional maize on rats. J Clin Anal Med 4:136–139
Lee NJ, Yang BC, Hwang JS et al (2010) Effects of cloned-cattle meat diet on reproductive parameters in pregnant rabbits. Food Chem Toxicol 48:871–876. https://doi.org/10.1016/j.fct.2009.12.025
Lee NJ, Yang BC, Im GS et al (2013) No long-term feeding toxicities on the health status in rats fed with cloned Korean native beef cattle (Hanwoo) meat. Toxicol Pathol 41:872–879. https://doi.org/10.1177/0192623312470762
Lin HT, Lee WC, Tsai YT et al (2016) Subchronic immunotoxicity assessment of genetically modified virus-resistant papaya in rats. J Agric Food Chem 64:5935–5940. https://doi.org/10.1021/acs.jafc.6b02242
Liu P, He X, Chen D et al (2012) A 90-day subchronic feeding study of genetically modified maize expressing Cry1Ac-M protein in Sprague-Dawley rats. Food Chem Toxicol 50:3215–3221. https://doi.org/10.1016/j.fct.2012.06.009
Liu Q, Yang W, Li M et al (2017) Effects of 60-week feeding diet containing Bt rice expressing the Cry1Ab protein on the offspring of inbred Wuzhishan Pigs fed the same diet. J Agric Food Chem 65:10300–10309. https://doi.org/10.1021/acs.jafc.7b04067
Liu S, Li CX, Feng XL et al (2013) Safety assessment of meat from transgenic cattle by 90-day feeding study in rats. Food Chem Toxicol 57:314–321. https://doi.org/10.1016/j.fct.2013.04.00
Liu S, Liu HB, Wang HL et al (2019) Evaluation of behavioral profiles in mice fed with milk supplemented diets derived from human lactoferrin gene-modified cows. Regul Toxicol Pharmacol 104:133–140. https://doi.org/10.1016/j.yrtph.2019.03.008
Liu Y, Zhang S, Zhou Q et al (2020) Subchronic feeding toxicity studies of drought-tolerant transgenic wheat MGX11-10 in Wistar Han RCC rats. Food Chem Toxicol 137:111129. https://doi.org/10.1016/j.fct.2020.111129
MacKenzie SA, Lamb I, Schmidt J et al (2007) Thirteen week feeding study with transgenic maize grain containing event DAS-Ø15Ø7-1 in Sprague-Dawley rats. Food Chem Toxicol 45:551–562. https://doi.org/10.1016/j.fct.2006.09.016
Malatesta M, Boraldi F, Annovi G et al (2008) A long-term study on female mice fed on a genetically modified soybean: effects on liver ageing. Histochem Cell Biol 130(5):967–977. https://doi.org/10.1007/s00418-008-0476-x
Malatesta M, Caporaloni C, Gavaudan S et al (2002) Ultrastructural morphometrical and immunocytochemical analyses of hepatocyte nuclei from mice fed on genetically modified soybean [published correction appears in Cell Struct Funct. 2002 Oct;27(5):399]. Cell Struct Funct 27(4):173–180. https://doi.org/10.1247/csf.27.173
Malatesta M, Caporaloni C, Rossi L et al (2002) Ultrastructural analysis of pancreatic acinar cells from mice fed on genetically modified soybean. J Anat 201(5):409–415. https://doi.org/10.1046/j.0021-8782.2002.00103.x
Mao J, Sun X, Cheng JH et al (2016) A 52-week safety study in cynomolgus macaques for genetically modified rice expressing Cry1Ab/1Ac protein. Food Chem Toxicol 95:1–11. https://doi.org/10.1016/j.fct.2016.06.015
Nouri-Ellouz O, Zeghal N, Makni S et al (2015) New food from a potato somatic hybrid: nutritional equivalence and safety assessment by a feeding study on rats. J Sci Food Agric 95(9):1911–1917. https://doi.org/10.1002/jsfa.6898
Oliva N, Florida Cueto-Reaño M, Trijatmiko KR et al (2020) Molecular characterization and safety assessment of biofortified provitamin A rice. Sci Rep 10(1):1376. https://doi.org/10.1038/s41598-020-57669-5
Papineni S, Golden RM, Thomas J (2017) The aryloxyalkanoate dioxygenase-12 (AAD-12) protein is not acutely toxic in mice[J]. Food Chem Toxicol 110:200–203. https://doi.org/10.1016/j.fct.2017.10.036
Papineni S, Murray JA, Ricardo E et al (2017) Evaluation of the safety of a genetically modified DAS-444Ø6-6 soybean meal and hulls in a 90-day dietary toxicity study in rats. Food Chem Toxicol 109(Pt 1):245–252. https://doi.org/10.1016/j.fct.2017.08.048
Papineni S, Passage JK, Ekmay RD et al (2018) Evaluation of 30% DAS-444Ø6-6 soybean meal in a subchronic rat toxicity study. Regul Toxicol Pharmacol 94:57–69. https://doi.org/10.1016/j.yrtph.2018.01.005
Poulsen M, Kroghsbo S, Schrøder M et al (2007) A 90-day safety study in Wistar rats fed genetically modified rice expressing snowdrop lectin Galanthus nivalis (GNA). Food Chem Toxicol 45(3):350–363. https://doi.org/10.1016/j.fct.2006.09.002
Qian ZY, Zhang SJ, Zhang L et al (2018) Subchronic toxicity study in rats evaluating genetically modified DAS-81419-2 soybean. Regul Toxicol Pharmacol 96:48–56. https://doi.org/10.1016/j.yrtph.2018.04.019
Qian ZY, Bultman J, Papineni S et al (2018) Safety evaluation of DAS-44406-6 soybeans in Wistar rats. Regul Toxicol Pharmacol 92:152–164. https://doi.org/10.1016/j.yrtph.2017.11.016
Tudisco R, Calabrò S, Cutrignelli MI et al (2015) Genetically modified soybean in a goat diet: Influence on kid performance. Small Rumin Res 126:67–74. https://doi.org/10.1016/j.smallrumres.2015.01.023
Richards HA, Han CT, Hopkins RG et al (2003) Safety assessment of recombinant green fluorescent protein orally administered to weaned rats. J Nutr 133(6):1909–1912. https://doi.org/10.1093/jn/133.6.1909
Sanden M, Ornsrud R, Sissener NH et al (2013) Cross-generational feeding of Bt (Bacillus thuringiensis)-maize to zebrafish (Danio rerio) showed no adverse effects on the parental or offspring generations. Br J Nutr 110(12):2222–2233. https://doi.org/10.1017/S0007114513001748
Schrøder M, Poulsen M, Wilcks A et al (2007) A 90-day safety study of genetically modified rice expressing Cry1Ab protein (Bacillus thuringiensis toxin) in Wistar rats. Food Chem Toxicol 45(3):339–349. https://doi.org/10.1016/j.fct.2006.09.001
Séralini GE, Cellier D, de Vendomois JS (2007) New analysis of a rat feeding study with a genetically modified maize reveals signs of hepatorenal toxicity. Arch Environ Contam Toxicol 52(4):596–602. https://doi.org/10.1007/s00244-006-0149-5
Séralini GE, Clair E, Mesnage R et al (2014) Republished study: long-term toxicity of a roundup herbicide and a roundup-tolerant genetically modified maize. Environ Sci Eur 26(1):14. https://doi.org/10.1186/s12302-014-0014-5
Sheng Y, Qi X, Liu Y et al (2014) Subchronic toxicity study in vivo and allergenicity study in vitro for genetically modified rice that expresses pharmaceutical protein (human serum albumin). Food Chem Toxicol 72:242–246. https://doi.org/10.1016/j.fct.2014.07.030
EFSA Panel on Food Contact Materials, Enzymes, Processing Aids (EFSA CEP Panel) et al (2019) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Bacillus licheniformis (strain NZYM-CE). EFSA J 17(4):e05685. https://doi.org/10.2903/j.efsa.2019.5685 (Published 2019 Apr 30)
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Bacillus subtilis (strain LMG S-24584). EFSA J 16(10):e05447. https://doi.org/10.2903/j.efsa.2018.5447 (Published 2018 September 27)
EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP), Silano V et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Aspergillus oryzae (strain NZYM-FA). EFSA J 16(11):e05480. https://doi.org/10.2903/j.efsa.2018.5480 (Published 2018 Nov 16)
EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP), Silano V et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Trichoderma reesei (strain DP-Nzd22). EFSA J 16(11):e05479. https://doi.org/10.2903/j.efsa.2018.5479 (Published 2018 Nov 30)
EFSA Panel on Food Contact Materials, Enzymes, Processing Aids (CEP) et al (2019) Safety evaluation of the food enzyme pullulanase from a genetically modified Bacillus licheniformis (strain DP-Dzp39). EFSA J 17(1):e05554. https://doi.org/10.2903/j.efsa.2019.5554 (Published 2019 Jan 10)
EFSA Panel on Food Contact Materials, Enzymes, Processing Aids (CEP) et al (2018) Safety evaluation of the food enzyme α-amylase from a genetically modified Aspergillus niger (strain NZYM-MC). EFSA J 16(10):e05451. https://doi.org/10.2903/j.efsa.2018.5451 (Published 2018 Oct 31)
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme alpha-amylase from a genetically modified Bacillus licheniformis (strain NZYM-AN). EFSA J 16(7):e05317. https://doi.org/10.2903/j.efsa.2018.5317 (Published 2018 Jul 6)
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme glucose oxidase from a genetically modified Aspergillus oryzae (strain NZYM-KP). EFSA J 16(7):e05319. https://doi.org/10.2903/j.efsa.2018.5319 (Published 2018 Jul 6)
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Aspergillus niger (strain XEA). EFSA J 16(4):e05228. https://doi.org/10.2903/j.efsa.2018.5228 (Published 2018 Apr 27)
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme aqualysin 1 from a genetically modified Bacillus subtilis (strain LMGS 25520). EFSA J 16(5):e05170. https://doi.org/10.2903/j.efsa.2018.5170 (Published 2018 May 2)
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme xylanase from a genetically modified Bacillus subtilis strain TD160(229). EFSA J 16(1):e05008. https://doi.org/10.2903/j.efsa.2018.5008 (Published 2018 Jan 22)
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of food enzyme xylanase from a genetically modified Bacillus subtilis (strain LMG S-27588). EFSA J 16(5):e05169. https://doi.org/10.2903/j.efsa.2018.5169 (Published 2018 May 2)
Talyn B, Lemon R, Badoella M et al (2019) Roundup ®, but not roundup-ready ® corn, increases mortality of drosophila melanogaster. Toxics. https://doi.org/10.3390/toxics7030038
Tang M, Xie T, Cheng W et al (2012) A 90-day safety study of genetically modified rice expressing rhIGF-1 protein in C57BL/6J rats [published correction appears in Transgenic Res. 2012 Aug;21(4):927]. Transgenic Res 21(3):499–510. https://doi.org/10.1007/s11248-011-9550-6
Tang X, Han F, Zhao K et al (2012) A 90-day dietary toxicity study of genetically modified rice T1C–1 expressing Cry1C protein in Sprague Dawley rats. PLoS ONE 7(12):e52507. https://doi.org/10.1371/journal.pone.0052507
Teshima R, Watanabe T, Okunuki H et al (2002) Effect of subchronic feeding of genetically modified corn (CBH351) on immune system in BN rats and B10A mice. Shokuhin Eiseigaku Zasshi 43(5):273–279. https://doi.org/10.3358/shokueishi.43.273
Trabalza Marinucci M, Brandi G, Rondini C (2008) A three-year longitudinal study on the effects of a diet containing genetically modified Bt176 maize on the health status and performance of sheep. Livest Sci 2008(113):178–190
Walsh MC, Buzoianu SG, Gardiner GE et al (2011) Fate of transgenic DNA from orally administered Bt MON810 maize and effects on immune response and growth in pigs. PLoS ONE 6(11):e27177. https://doi.org/10.1371/journal.pone.0027177
Walsh MC, Buzoianu SG, Gardiner GE et al (2012) Effects of short-term feeding of Bt MON810 maize on growth performance, organ morphology and function in pigs. Br J Nutr 107(3):364–371. https://doi.org/10.1017/S0007114511003011
Walsh MC, Buzoianu SG, Rea MC et al (2012) Effects of feeding Bt MON810 maize to pigs for 110 days on peripheral immune response and digestive fate of the cry1Ab gene and truncated Bt toxin. PLoS ONE 7(5):e36141. https://doi.org/10.1371/journal.pone.0036141
Wang X, He X, Zou S et al (2016) A subchronic feeding study of dicamba-tolerant soybean with the dmo gene in Sprague-Dawley rats. Regul Toxicol Pharmacol 77:134–142. https://doi.org/10.1016/j.yrtph.2016.02.001
West AL, Miles EA, Lillycrop KA et al (2019) Postprandial incorporation of EPA and DHA from transgenic Camelina sativa oil into blood lipids is equivalent to that from fish oil in healthy humans. Br J Nutr 121(11):1235–1246. https://doi.org/10.1017/S0007114519000825
Wu Y, Xu Y, Du Y et al (2017) Dietary safety assessment of genetically modified rice EH rich in β-carotene. Regul Toxicol Pharmacol 88:66–71. https://doi.org/10.1016/j.yrtph.2017.05.019
Xiao GJ, Jiang SW, Qian LL et al (2016) A 90-day feeding study in rats to assess the safety of genetically engineered pork. PLoS ONE 11(11):e0165843. https://doi.org/10.1371/journal.pone.0165843
Xie Z, Zou S, Xu W et al (2018) No subchronic toxicity of multiple herbicide-resistant soybean FG72 in Sprague-Dawley rats by 90-days feeding study. Regul Toxicol Pharmacol 94:299–305. https://doi.org/10.1016/j.yrtph.2018.02.004
Yang L, Sun Y, Wang Y et al (2014) Effects of dietary transgenic poplar leaf pellets on performance and tissues in rabbits. J Sci Food Agric 94(6):1163–1167. https://doi.org/10.1002/jsfa.6388
Yen GC, Lin HT, Cheng YH et al (2011) Food safety evaluation of papaya fruits resistant to papaya ring spot virus. J Food Drug Anal 19(2):269–377
Yong L, Liu YM, Jia XD et al (2012) Subchronic toxicity study of GH transgenic carp. Food Chem Toxicol 50(11):3920–3926. https://doi.org/10.1016/j.fct.2012.07.064
Zeljenková D, Aláčová R, Ondrejková J et al (2016) One-year oral toxicity study on a genetically modified maize MON810 variety in Wistar Han RCC rats (EU 7th Framework Programme project GRACE). Arch Toxicol 90(10):2531–2562. https://doi.org/10.1007/s00204-016-1798-4
Zeljenková D, Ambrušová K, Bartušová M et al (2014) Ninety-day oral toxicity studies on two genetically modified maize MON810 varieties in Wistar Han RCC rats (EU 7th Framework Programme project GRACE). Arch Toxicol 88(12):2289–2314. https://doi.org/10.1007/s00204-014-1374-8
Zhou C, Wang JW, Huang KL et al (2011) A 90-day safety study in Sprague-Dawley rats fed milk powder containing recombinant human lactoferrin (rhLF) derived from transgenic cloned cattle. Drug Chem Toxicol 34(4):359–368. https://doi.org/10.3109/01480545.2010.542465
Zhou XH, Dong Y, Xiao X et al (2011) A 90-day toxicology study of high-amylose transgenic rice grain in Sprague-Dawley rats. Food Chem Toxicol 49:3112–3118. https://doi.org/10.1016/j.fct.2011.09.024
Zhu HJ, Chen Y, Li YH et al (2015) A 90 day safety assessment of genetically modified rice expressing Cry1Ab/1Ac protein using an aquatic animal model [published correction appears in J Agric Food Chem. 2015 Aug 26;63(33):7462]. J Agric Food Chem 63(14):3627–3633. https://doi.org/10.1021/jf5055547
Zhu Y, He X, Luo Y et al (2013) A 90-day feeding study of glyphosate-tolerant maize with the G2-aroA gene in Sprague-Dawley rats. Food Chem Toxicol 51:280–287. https://doi.org/10.1016/j.fct.2012.09.008
Zou S, Tang M, He X et al (2015) A 90-day subchronic study of rats fed lean pork from genetically modified pigs with muscle-specific expression of recombinant follistatin. Regul Toxicol Pharmacol 73(2):620–628. https://doi.org/10.1016/j.yrtph.2015.09.009
Dong SS, Zhang DN, Zhang ZH et al (2019) Ecotoxicological effects of transgenic mCry1Ac maize (BT799) on zebrafish. Ying Yong Sheng Tai Xue Bao 30(8):2845–2853. https://doi.org/10.13287/j.1001-9332.201908.031
Hu Y, Piao J, Yang X et al (2012) Nutritional components and sub-chronic toxicity of genetically modified rice expressing human lactoferrin. Wei Sheng Yan Jiu 41(1):6–12. https://doi.org/10.2166/wst.2012.090
Bai H (2015) Bio-Safety assessment of transgenic sheep overexpressing oTLR4. PhD Thesis, China Agricultural University, Beijing, China
Bao ZK (2016) Safety assessment of GH-Transgenic dairy goats. MS Thesis, Nanjing Agricultural University, Nanjing, China
Cao ZH (2014) Safety assessment of transgenic Bt rice in growing pig diet. MS Thesis, Henan University of Science and Technology, Henan, China
Chen XP, Zhuo Q, Piao JH et al (2004) Immunotoxicologic assessment of transgenetic rice. J Hyg Res 33(1):77–80
Chu HH, Si QQ, Xu Y et al (2016) Effect of genetically modified soybean meal on the immune function, intestinal digestive enzyme activities and serum biochemical indexes of growing pigs. J Qingdao Agric Univ Nat Sci 33(02):119–123. https://doi.org/10.3969/J.ISSN.1674-148X.2016.02.008
Dai YN, Yang XZ, Liu Y et al (2018) Acute oral toxicity and 90 days feeding test of recombinant human lactoferrin. J Hyg Res 47(02):286–311
Du HF (2006) Safety assessment of transgenic rice used in broiler diet. PhD Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Feng XL, Wang HL, Li CX et al (2017) Safety assessment of meat from transgenic cattle by 90-day feeding study in rats. J Hyg Res 29(1):19–25. https://doi.org/10.13590/j.cjfh.2017.01.005
Feng YQ, Hu J, Zhi Y et al (2013) The effect of exposure to transgenic Bt rice on the immune system of parental female rats. Chin J Food Hyg 25(4):298–302
Feng YQ, Wang EH, Zhi Y et al (2013) The effect of exposure to transgenic Bt rice on reproductive system of male offspring rats. Chin J Food Hyg 25(02):113–117
Guo MF (2018) A three generation study to evaluate reproductive and neurodevelopmental toxicity of genetically modified maize with Cry1Ab and epsps genes in SD rats. MS Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Hu J (2013) Establish of a rat model for development immunotoxicity and its application in safety evaluation of transgenic CryAb/Ac Rice. MS Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Huang Q, Xu HB, Gao F et al (2009) Anaphylactic reactions in WZS minipig orally induced by glycinin. J Hyg Res 38(05):531–534
Jia XD, Li N, Wang W et al (2005) Assessment of allergenicity of genetically modified rice S86 by BN rat model. Chin J Food Hyg 01:7–9. https://doi.org/10.13590/j.cjfh.2005.01.003
Li M (2012) Safety evaluation of recombinant herbicide-resistant protein AROa-CC-M and transgenic insect-resistant maize BT-799. MS Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Li M, Piao JH, Yang XG (2010) Subchronic toxicity test of genetically modified rice with double antisense starch-branching enzyme gene. J Hyg Res 39(04):436–443
Li R, Wang J, Jiang WL et al (2012) Effects of rice genetically modified with HJC-1 and G6-EPSPS genes on immunological parameters in Wuzhishan minipigs. J Environ Health 29(08):689–692. https://doi.org/10.16241/j.cnki.1001-5914.2012.08.017
Li YH, Piao JH, Chen XP et al (2004) Immunotoxicologic assessment on transgenic rice. China Public Health 20(04):20–22
Li YH, Piao JH, Zhuo Q et al (2004) Study on the teratogenicity effects of genetically modified rice with Xa21 on rats. J Hyg Res 33(06):710–712
Liang LQ, Wang J, Cao XC et al (2010) Toxicity analysis of common carp transferred salmon growth hormone gene. Food Sci 31(05):261–265
Liu HT, Wang CR, Liu L et al (2018) Acute toxicity of fresh leaves of insect resistant transgenic populus nigra to mice. J Anhui Agric Sci 46(01):94–136. https://doi.org/10.13989/j.cnki.0517-6611.2018.01.028
Liu HL, Wang J, Zeng Q et al (2012) Effects of rapeseed genetically modified with bar gene on immunological indicators in WZS minipigs. J Environ Health 29(11):977–980. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.020
Liu SS, Tan JZ, Sun Z et al (2011) Effects of glyphosate—resistant soybean meal on immune function of AA broilers. Chin J Anim Sci 47(13):41–46
Liu S, Wang XD, Feng XL et al (2013) Twenty-eight days feeding study on human lactoferrin expressed by cattle mammary bioreactor in mice. Chin J Public Health 29(02):230–232
Liu YH, Jiang SQ, Zhang J et al (2018) Subchronic toxicity of genetically modified Herbicide-resistant maize MON87427 with Cp4epsps gene in Wistar rats. J Public Health Prevent Med 29(06):17–20. https://doi.org/10.3969/j.issn.1006-2483.2018.06.004
Liu YF, Liu WH, He L et al (2008) Acute toxicity and mutagenic effect of transgenic rice on mice. J Hunan Univ Sci Technol Nat Sci Ed 23(04):111–117
Liu YF, Liu WH, He L et al (2008) Effects of resistant insects transgenic hybrid rice 21S/MSB on behavior and physiology of SD rats. Life Sci Res 12(03):257–261. https://doi.org/10.16605/j.cnki.1007-7847.2008.03.013
Lu MJ, Li F, Zhou GL et al (2008) Assessment of an anti-LeETR1 genetically modified tomato on reproductive-development toxicity and transferability of the transgene. J Toxicol 22(04):272–274. https://doi.org/10.16421/j.cnki.1002-3127.2008.04.006
Lu CB, Lin ZB, Zhang Y et al (2016) Effects of glyphosate-resistant transgenic soybean on physical enginery of male mice. Acta Agriculturae Zhejiangensis 28(07):1115–1120. https://doi.org/10.3969/j.issn.1004-1524.2016.07.04
Lu CB, Yang DY, Gao Z et al (2012) Safety assessment of reproductive system in male mice fed with genetically modified soybeans. J Yangzhou Univ Agric Life Sci Ed 33(01):23–27. https://doi.org/10.16872/j.cnki.1671-4652.2012.01.006
Lu CB, Zhang Y, Chen BH et al (2017) Effects of glyphosate-resistant transgenic soybean on in vitro fertilization of male mice with reproductive damage. Acta Agriculturae Zhejiangensis 29(06):910–916. https://doi.org/10.3969/j.issn.1004-1524.2017.06.08
Lu CB, Zhou W, Liu B et al (2013) Effects of transgenic soybean on reproductive system in male mice. Soybean Sci 32(01):119–123
Lv L, Guo J, Li SF et al (2013) Effects of transphytase gene maize on organ development and pathological changes of broilers. Chin J Anim Sci 49(05):31–34
Ma BT, Yuan XY, Wang XD et al (2017) Animal experiment of recombinant human lactoferrin based on the 28 days repeated oral toxicity. J Hyg Res 46(03):443–454
Ma YM, Wang J, Jiang WL et al (2012) Subchronic oral toxicity of genetically modified cottonseed with FBP7-iaaM gene in rats. J Environ Health 29(11):1001–1007. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.024
Qi XZ, Wang J, Zhou C et al (2010) Effect of transferred human lactoferrin milk powder on serum iron and ferritin in rats. Food Sci 31(23):340–343
Qin HF (2012) Safety assessment of rice genetically modified with Cry1Ac and sck feeding studies on broilers. PhD Thesis, Chinese Academy of Agricultural Sciences, Beijing, China.
Qiu ZL, Sun N, Wang J et al (2011) Sub-Chronic toxicity study of transgenic cottonseed in SD rats. Progr Mod Biomed 11(12):2215–2220. https://doi.org/10.13241/j.cnki.pmb.2011.12.010
Song LS, Gao GQ, Wei ZY et al (2017) Effects of Fat1 transgenic milk on the health and reproductive ability of mice. Lab Anim Sci 34(03):28–37
Song Y (2013) Establishment of immunotoxicity screening system in rodents and its application in immunotoxicity evaluation of pesticides and genetically modified foods. PhD Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Sun XW, Liang LQ, Yan XC et al (1998) Research on transgenic carp as food. High Technol Lett 03:3–5
Sun Z, Liu SS, Tan JZ et al (2011) Effects of glyphosate—resistant soybean meal on immune function of AA broilers. Chin J Anim Sci 23:836–841
Tan JZ (2011) The feed safety assessment of Glyphosate-Tolerant soybean meal in broilers. MS Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Tang XQ, Wang YF, Pei LJ et al (2019) Long-term toxicity study on transgenic rice T2A–1 with cry2A* gene. Chin J Food Hyg 31(6):510–516. https://doi.org/10.13590/j.jfh.2019.06.002
Tao R (2008) Detection of transgenic ingredients in feed and primary assessment on the safety of aquatic livestock fed transgenic soybean. MS Thesis, Chinese Academy of Sciences, Shandong, China
Wang EH (2014) A study to access effects of transgenic Cry1Ab/Ac Rice TT51 on reproductive and neural development in rats. PhD Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Wang EH, Yu Z, Fang HQ et al (2013) Effect of transgenic Bt rice TT51 on early physiological and neurological development of rats offspring. Chin J Food Hyg 25(06):485–488. https://doi.org/10.13590/j.cjfh.2013.06.008
Wang HM, Yin JY, Zhai WS, et al (2015) Toxic pathology of cynomolgus monkeys fed transgenic rice for 52 weeks. In: The 7th National Toxicology Conference of China Toxicology Society and the 8th Hubei Science and Technology Forum, Wuhan, China, pp 442–443
Wang J, Jiang WL, Wang XJ et al (2002) Toxicological safety evaluation of transgenic T5 line pepper. Chin J Urban Rural Ind Hyg 01:46
Wang J, Jiang WL, Wang Y et al (2012) Subacute oral toxicity of recombinant human lactoferrin from transgenic cows in rats on 28d. J Toxicol 26(05):393–397
Wang J, Li R, Liu HL (2012) Assessment of allergenicity of rice genetically modified with HJC-1 and G6-EPSPS genes by BN rats model. J Environ Health 29(11):967–970. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.014
Wang J, Liu HL, Zeng Q et al (2012) Subacute toxicity of rapeseed genetically modified with bar gene in WZS minipigs. J Environ Health 29(11):980–984. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.021
Wang J, Zhou C, Che HL et al (2010) Study on sub-chronic toxicity of powered milk containing transgenic lactoferrin on SD rats. Progr Mod Biomed 10(15):2809–2813. https://doi.org/10.13241/j.cnki.pmb.2010.15.002
Wang RY (2017) The effect of genetically modified feed on structure of mice testes. Livestock Poult Ind 28(06):6–7. https://doi.org/10.19567/j.cnki.1008-0414.2017.06.004
Wang R, Hu YC, Li M et al (2017) Study on the subchronic toxicity of transgenic DBN9978 herbicide resistant maize to rats. Food Nutr China 23(06):12–17
Wang XJ, Wang J, Liu HL et al (2012) Subchronic toxicity test of rice containing transgenic HJC-1 and G6-EPSPS in rats. J Environ Health 29(11):970–976. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.019
Wang Y (2011) Food safety assessment of genetically modified milk with human lactoferrin gene. MS Thesis, Tianjin Medical University, Tianjin, China
Wang Y, Lai WQ, Chen JG et al (2000) Toxicity of anti-herbicide gene (BAR) transgenic rice. J Hyg Res 29(03):141–142
Wu JH (2013) The nutritional, edible safety and efficacy assessment of genetically modified rice with human lactoferrin gene and its purified protein and the nutritional assessment of genetically modified wheat expressing GmDREB/TaDREB4 genes with drought-resistance. Ph.D. Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Wu P, Su YL, Zhang J et al (2003) Safety evaluation of transgenic tomato against Cucumber Mosaic Virus. J Capital Univ Med Sci 24(03):254–258
Xu YJ (2012) The forage safety assessment of genetically modified organism corn to weaning piglets. M.S. Thesis, Fujian Agriculture and Forestry University, Fujian, China
Yu T, Liu Y, Wang JW et al (2017) Teratogenic test of recombinant human lactoferrin in rats. J Toxicol 31(03):247–250
Yuan JQ (2015) The detection of the transgenic GTS40-3-2 related genes and their products of livestock products sold and toxicology study of Sprague-Dawley rats (Rattus norvegicus). Ph.D. Thesis, Shanxi Agricultural University, Shanxi, China
Zhang L, Cheng C, He N et al (2011) Study on subchronic toxicity of transgenic soybean with high oleic acid on rats. J Toxicol 25(05):391–394. https://doi.org/10.16421/j.cnki.1002-3127.2011.05.012
Zhang L, Wang J, Jiang SQ et al (2016) Subchronic toxicity of genetically modified corn with Cry1Ab/Cry2Aj and G10evo (EPSPS) genes in rats. J Environ Health 33(07):585–589
Zhang LL (2018) The Unintended Effects of Long-Term Intergenerational Feeding Transgenic Maize Diets to Pure Line White Leghorn Chickens on the Intestinal Health. M.S. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Zhang M, Zhuo Q, Tian Y et al (2012) Study on chronic toxicity of genetically modified rice expressing human lactoferrin. Chin J Food Hyg 24(06):391–394. https://doi.org/10.13590/j.cjfh.2012.06.008
Zhang Q (2014) Production of GH transgenic goat by somatic cell nuclear transfer. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China
Zhang WP, Li YY, Wang WG et al (2009) Detection of mutagenicity of chitinase and -1, 3 dextran gene in maize. Chin Remed Clin 9(05):405–407
Zhang ZY, Liu LJ, Zhang L et al (2010) Subchronic toxicity of Bt transgenic rice to mice. J Toxicol 24(02):126–129. https://doi.org/10.16421/j.cnki.1002-3127.2010.02.016
Zhao L, Zhang L, Zhang YY et al (2009) Immunotoxicological evaluation of transgenic soybean oil. China Health Care Nutr 11:5
Zhi Y, Liu HB, Di GY et al (2013) Genetic toxicity of transgenic human α-lactalbumin powdered milk. Carcinogen Teratogen Mutagen 25(02):124–133. https://doi.org/10.3969/j.issn.1004-616x.2013.02.010
Zhi Y, Liu HB, Di GY et al (2011) Study on sub-chronic toxicity of powered milk containing transgenic human α-lactalbumin. J Hyg Res 40(04):426–430. https://doi.org/10.19813/j.cnki.weishengyanjiu.2011.04.004
Zhong F (2013) Subchronic feeding study of transgenic BADH alfalfa on rabbits. M.S. Thesis, Shandong Agricultural University, Shandong, China
Zhou GL, Lu MJ, Chen YX et al (2007) Antisense LeETR1 transgenic tomato rats were fed for 4 weeks. J Toxicol 21(02):160–161
Zhou H (2012) Safety aeeseement of the phytase transgenic corn in the broiler diet. M.S. Thesis, Fujian Agriculture and Forestry University, Fujian, China
Zhou LG (2009) Research of high lysine transgenic paddy on broiler feed security. M.S. Thesis, Yangzhou University, Jiangsu, China
Zhou WL, Yang XF, Ping A et al (2014) Effects of transgenic Dwarf-mosaic-resistant maize on learning and memory abilities of rats. J Shanxi Agric Univ Nat Sci Ed 34(02):129–131. https://doi.org/10.13842/j.cnki.issn1671-8151.2014.02.006
Zhou XH (2012) Toxicological studies on the food safety of two transgenic rice. Ph.D. Thesis, Jiangsu University, Jiangsu, China
Zhou ZW, Wang DZ, Shen H et al (2012) Comprehensive evaluation on functions & safety of imported GM soybean using BDI-GS system. Soybean Sci 31(05):822–826
Zhu H, Zhu LY, Guo JY et al (2014) Safety evaluation of BT-799 corn on Wistar rats. Food Nutr China 20(09):63–67
Zhuo Q, Chen XP, Piao JH et al (2004) Experimental study on converting cowpea trypsin inhibitor rice for 90 days. J Hyg Res 33(02):176–179
Zhuo Q, Chen XP, Piao JH et al (2004) Study on teratogenic effect of cowpea trypsin inhibitor rice. J Hyg Res 33(01):74–77
Sakamoto Y, Tada Y, Fukumori N et al (2008) A 104-week feeding study of genetically modified soybeans in F344 rats. Shokuhin Eiseigaku Zasshi 49(4):272–282. https://doi.org/10.3358/shokueishi.49.272
Sakamoto Y, Tada Y, Fukumori N et al (2007) A 52-week feeding study of genetically modified soybeans in F344 rats. Shokuhin Eiseigaku Zasshi 48(3):41–50. https://doi.org/10.3358/shokueishi.48.41
Keshani P, Sharifi MH, Heydari MR et al (2020) The effect of genetically modified food on infertility indices: a systematic review study. Sci World J. https://doi.org/10.1155/2020/1424789
Edge MS, Kunkel ME, Schmidt J et al (2018) Evidence analysis library systematic review on advanced technology in food production. J Acad Nutr Diet 118(6):1106–1127. https://doi.org/10.1016/j.jand.2017.08.005
Dunn SE, Vicini JL, Glenn KC et al (2017) The allergenicity of genetically modified foods from genetically engineered crops: a narrative and systematic review. Ann Allergy Asthma Immunol 119(3):214–222. https://doi.org/10.1016/j.anai.2017.07.010
de Vos CJ, Swanenburg M (2018) Health effects of feeding genetically modified (GM) crops to livestock animals: a review. Food Chem Toxicol 117:3–12. https://doi.org/10.1016/j.fct.2017.08.031
Ricroch AE, Boisron A, Kuntz M (2014) Looking back at safety assessment of GM food/feed: an exhaustive review of 90-day animal feeding studies. Int J Biotechnology 13(4):230–256
Domingo Roig JL, Gómez Arnáiz M (2000) Riesgos sobre la salud de los alimentos modificados genéticamente: una revisión bibliográfica [Health risks of genetically modified foods: a literature review]. Revista espanola de salud publica 74(3):255–261
Domingo JL (2007) Toxicity studies of genetically modified plants: a review of the published literature. Crit Rev Food Sci Nutr 47(8):721–733. https://doi.org/10.1080/10408390601177670
Domingo JL, Giné Bordonaba J (2011) A literature review on the safety assessment of genetically modified plants. Environ Int 37(4):734–742. https://doi.org/10.1016/j.envint.2011.01.003
Teshima R, Watanabe T, Okunuki H et al (2002) Effect of subchronic feeding of genetically modified corn (CBH351) on immune system in BN rats and B10A mice. J Food Hyg Soc Jpn 43:273–279
Dubois AEJ, Pagliarani G, Brouwer RM et al (2015) First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1-silenced apples cause fewer allergy symptoms than the wild-type cultivar. Allergy 70:1406–1412
Acknowledgements
We appreciate Yi-Zhen Li for participating in screening the titles and abstracts.
Funding
This work was supported by Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202006). Prof. Nicola Robinson (visiting professor of Beijing University of Chinese Medicine) was funded by the International development and capacity enhancement of evidence-based Chinese medicine Project, Ministry of Science and Technology of the People's Republic of China, G20200001187.
Author information
Authors and Affiliations
Contributions
All work was done by the authors. JPL and YTF conceived the study and revised the manuscript. CS contributed to data searching, screening and extraction, analysis of the data, drafted and revised the paper and approved the final version to be submitted. XCY, BYJ, JP, XHC, JXR, JL, XWZ, HDL, WBH and MF participated in identifying or screening the titles, abstracts and full-text screening and data extraction. XL, NR and JPL advised on the analysis of the data and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Appendix S1.
Search strategy applied in English language databases. Appendix S2. Funding sources or sponsors. Appendix S3. Adverse events/effects—other biomarkers.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shen, C., Yin, XC., Jiao, BY. et al. Evaluation of adverse effects/events of genetically modified food consumption: a systematic review of animal and human studies. Environ Sci Eur 34, 8 (2022). https://doi.org/10.1186/s12302-021-00578-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-021-00578-9