Abstract
Background
The procedure described in The Organisation for Economic Co-operation and Development (OECD) test guideline (TG) 216 is used to assess the effects of chemicals on microbial nitrogen transformation in soil, and the results are considered in regulatory risk assessments. We investigated the suitability of this method to characterize the effects of two silver nanomaterials and a soluble silver salt. We applied three different test procedures: (i) nitrogen transformation using the complex organic nitrogen source lucerne meal (OECD TG 216), (ii) nitrogen transformation using the inorganic nitrogen source (NH4)2SO4 (following OECD TG 216), and (iii) ammonium oxidation (ISO 15685). The results were compared with substrate-induced respiration (OECD TG 217).
Results
The standard nitrogen transformation test using lucerne meal suggested that the test materials had no effect, whereas significant effects were identified with the other two test procedures. The absence of effects with lucerne meal probably reflected the sorption of silver ions to the additional organic nitrogen source, thus reducing its bioavailability, or blocking the silver nanomaterial oxidation sites by sorption of organic matter.
Conclusions
This common test used in the context of chemical registration is therefore unsuitable for the detection of potential effects caused by silver nanomaterials and soluble silver salts because it can yield false negative results. We instead recommend the use of an inorganic nitrogen source. The observed effects were not specific to nanomaterials. The time course of the effect in the nitrogen transformation test based on (NH4)2SO4 and the potential ammonium oxidation test varied according to the test substance, indicating different kinetic behaviors of ion release.
Similar content being viewed by others
Background
Microorganisms play an important role in the breakdown and transformation of organic matter in fertile soils with many species contributing to different aspects of soil fertility. Several guidelines are currently used to investigate the effects of chemicals on selected microbial activities, as outlined in chapter R7c of the ECHA guidance for the implementation of Regulation (EC) 1907/2006 - the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) [1]. Two Organisation for Economic Co-operation and Development (OECD) test guidelines are available to detect chemical effects on soil microflora, i.e., the nitrogen and carbon transformation tests [2],[3]. These are designed to determine the long-term adverse effects of substances on nitrogen or carbon transformation in aerobic soils over at least 28 days. According to the guidance listed above [1], the nitrogen transformation test is considered sufficient for most non-agrochemical substances. Further ISO-standard methodologies are available, but in the absence of corresponding OECD guidelines, these methods are used less often than the two microbial assays mentioned above. One of the ISO methods evaluates the potential activity of the nitrifying population by measuring the accumulation of nitrite over a short incubation period of 6 h [4].
The quantity of nanosilver produced and utilized worldwide is estimated to be 55 tons per annum (median value), 5.5 tons per annum in Europe and in a range of 2.8 to 20 tons per annum in the USA [5]. Nanosilver is often applied in coatings to take advantage of its antimicrobial properties, but it is also found in paints, cleaning agents, consumer electronics, cosmetics, and textiles [6],[7]. The antimicrobial mechanisms of silver nanomaterials have recently been reviewed [8] and may include the formation of pits on the cell surface, the generation of free radicals that damage the cell membrane, the release of silver ions - which affect Gram-positive and Gram-negative bacteria [7] - and the interaction between silver ions and the thiol and phosphate groups of enzymes and other organic molecules.
Several studies have already been carried out to monitor the potential adverse effects of nanosilver on microorganisms in the environment. One such study showed that nanosilver was toxic to bacteria in sewage sludge, especially nitrifying bacteria [9], but that the toxicity was strongly influenced by the ambient medium, e.g., the sulfur content [10]. Another study showed that a commercially-available spray containing nanosilver, which promotes the growth of plants according to the manufacturer, had a negative impact on microbial biomass in the soil and enhanced basal respiration [11]. The latter study did not state which test guidelines were used. However, another study in which tests were carried out in accordance with OECD and ISO guidelines showed that nanosilver applied to soil as pristine material or adsorbed to sewage sludge had a negative impact on nitrifying bacteria, microbial biomass, and soil exoenzymes [12], supporting earlier reports that nanosilver inhibits soil exoenzymes [13]. Studies have also been carried out to determine the effect of nanosilver on other soil organisms, although the observed effect concentrations were higher than those recorded for soil microbes [14]-[16].
Here we investigated whether the type of nitrogen source influences nitrite and nitrate accumulation caused by two silver nanomaterials differing in parameters such as size and shape, and by a soluble silver salt, over an incubation period of 28 days. We applied three different procedures addressing the nitrogen mineralization in the nitrogen cycle. Briefly, the nitrogen cycle consists of the following: (i) nitrogen mineralization with the individual steps ammonification (organic nitrogen → ammonium) and nitrification (ammonium → nitrite → nitrate), (ii) nitrogen immobilization and assimilation (nitrate → organic nitrogen; ammonium → organic nitrogen), (iii) denitrification (nitrite → N2), and (iv) nitrogen fixation (N2 → organic nitrogen) [17]. At the beginning of the test, we added the complex insoluble organic nitrogen source lucerne meal according to OECD test guideline (TG) 216 [2] (first approach), or the soluble inorganic nitrogen source (NH4)2SO4 (second approach), and periodically measured the nitrate content/nitrate accumulation during the 28-day incubation period. The addition of the ammonium salt (second approach) was based on a former German test guideline for the investigation of pesticide side effects [18]. In a third approach, we incubated non-augmented soil and measured the transformation of (NH4)2SO4 to nitrite in periodic subsamples [4]. This test has a short incubation period of 6 h and was applied 2 to 3 times during the 28-day incubation period. The three procedures all cover a 28-day exposure of the soil microflora to silver. The measured microbial activity, however, depends on the time point at which the nitrogen source is added. The test on nitrite accumulation provides information on the activity of the respective microorganisms at the time when samples are taken: the nitrogen source is added to a subsample, and microbial activity is determined. The test on nitrate accumulation monitors the microbial activity from test start up to the sampling day since the nitrogen source is added at the start of the 28-day incubation period. The effects on nitrite and nitrate accumulation were compared with the results of the carbon transformation test (soil-induced respiration) [3], in which short-term aerobic respiration after the addition of an easily degradable soluble carbon source (glucose) is indicated by oxygen depletion. Short-term respiration was determined 2 to 3 times during the 28-day incubation period. Analogous to the short-term test on nitrite accumulation, the short-term respiration test provides information on the microbial activity at the sampling time. The selected approaches allowed us to determine the suitability of the current procedure for the assessment for silver nanomaterials in the context of chemical regulation.
Results
Most experiments were repeated. The results were comparable. In the following, the results of one test for each experimental design are presented in detail in Tables 1 and 2 as well as in the Figures. The EC50 values of the second experiment are presented in Table 1 and proof the statements of the first experiments.
Nitrogen transformation test
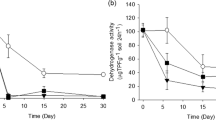
The N-transformation test according to OECD TG 216 monitors the formation of nitrate from a nitrogen source (in our investigation: powdered lucerne or NH4SO4). The test was carried out in the presence of three test substances (Figures 1 and 2). An increase in the soil nitrate concentration due to microbial activity was observed. The nitrate concentration increased in the untreated control regardless of the nitrogen source, but there were clear differences in the observed effects of the test substances depending on which nitrogen source was applied.
Effect of silver nanomaterials and AgNO 3 on nitrate concentration with powdered lucerne as nitrogen source [[2]]. The results for AgNO3 were corrected by the nominal nitrate concentration added with the test substance. Left figure column: mean activity and standard deviation. Right figure column: percentual deviation. Asterisks indicate a statistically significant difference to controls (p ≤ 0.05).
Effect of silver nanomaterials and AgNO 3 on nitrate concentration with (NH4) 2 SO 4 as nitrogen source [[2]]. The results for AgNO3 were corrected by the nominal nitrate concentration added with the test substance. Left figure column: mean activity and standard deviation. Right figure column: percentual deviation. Asterisks indicate a statistically significant difference to controls (p ≤ 0.05).
At the end of the test, there was no statistically significant deviation in the nitrate concentration in the treated soil samples supplemented with the complex organic nitrogen source lucerne meal, although a short-term concentration-dependent effect was detected on day 7 in soils treated with either AgNO3 or NM-300 K (Figure 1). In contrast, applying the inorganic nitrogen source (NH4)2SO4 resulted in a concentration-dependent effect relationship throughout the 28-day incubation period (Figure 2). The highest test concentrations of NM-300 K (15 mg/kg) and NM-302 (100 mg/kg) caused a 65% and 70% decrease of the nitrate concentration by the end of the test, and the highest concentration of AgNO3 (15 mg/kg) caused a 100% inhibition. Lower test concentrations resulted in 0% to 71% decrease of the nitrate concentration compared to the control.
Potential ammonium oxidation test
The potential ammonium oxidation test according to ISO 15685 monitors the transformation of ammonium to nitrite. Concentration-dependent effects were also observed in the potential ammonium oxidation activity test (Figure 3). For technical reasons described in the 'Methods' section, the initial effect determination measurement was taken on day 1 rather than day 0 in this test, at which point it was already clear that both NM-300 K and AgNO3 had a significant inhibitory effect. At the end of the test, the highest test concentrations of all three substances achieved nearly 100% inhibition, whereas lower test concentrations resulted in 0% to 94% inhibition.
Effect of silver nanomaterials and AgNO 3 on potential ammonium oxidation according to ISO 15685 [[4]]. Left figure column: mean activity and standard deviation. Right figure column: percentual deviation. Asterisks indicate a statistically significant difference to controls (p ≤ 0.05).
Carbon transformation test
The test describes changes in size and activity of microbial communities responsible for carbon transformation. Carbon transformation is measured via glucose-induced respiration activity. Inhibition was also detected in the carbon transformation test, although we observed differences in the inhibition profile between the nanomaterials and AgNO3 (Figure 4). Neither of the nanomaterials showed any effects at the start of the test, but a concentration-dependent effect was observed at the next measuring point followed by recovery by the end of the test. AgNO3 had a significant inhibitory effect immediately after application (sampling time 3 h) and also at the 7-day measuring point albeit only at the highest test concentration. After 28 days, there was no statistically significant residual effect on respiration activity.
Effect of silver nanomaterials and AgNO 3 on glucose-induced respiration according to OECD TG 217 [[3]]. Left figure column: mean activity and standard deviation. Right figure column: percentual deviation. Asterisks indicate a statistically significant difference to controls (p ≤ 0.05).
Time course of the effects
Table 1 presents the EC10 and EC50 values (experiment 1) and EC50 values (experiment 2) for every test procedure and test substance on day 28. In the following the results of experiment 1 are presented in detail. The EC50 values of the second experiment proof the statements of the first experiments. If only the results determined after an incubation period of 28 days are considered, the toxicity of NM-300 K and AgNO3 are comparable in terms of potential ammonium oxidation activity and nitrate accumulation when applying an inorganic soluble nitrogen source, but no inhibition of nitrate accumulation was observed when lucerne meal was used as the additional nitrogen source. In experiment 1, the EC values for NM-300 K and AgNO3 for (i) nitrate formation using (NH4)2SO4 as nitrogen source and (ii) the potential ammonium oxidation activity differ by a factor of less than 3 (e.g., EC50 potential ammonium oxidation for NM-300 K and AgNO3 = 1.6 and 2.9 mg/kg, respectively; EC50 nitrate accumulation for NM-300 K and AgNO3 = 7.3 and 2.8 mg/kg, respectively). NM-302 appeared to be slightly less toxic than NM-300 K and AgNO3 in terms of the effect on nitrate accumulation. The EC values differed at least by a factor of 6, but this was a screening approach carried out with three test concentrations and a spacing factor of 10, suggesting the EC values for NM-302 are less reliable. The toxicity of the three silver materials measured in terms of the effect on potential ammonium oxidation appeared to be comparable, with EC50 values of 1.6 to 6 mg/kg. As stated above, even the highest test concentrations of the test substances failed to inhibit nitrate accumulation when lucerne meal was used as the nitrogen source, thus no EC values were calculated for day 28.
The toxicity of the three silver materials measured in terms of the effect on substrate-induced respiration (carbon transformation) is also represented by the EC values for day 28. These values show no toxicity for AgNO3, medium toxicity for NM-302 (EC50 = 68 mg/kg), and relatively high toxicity for NM-300 K (EC50 = 5 mg/kg).
Table 2 shows the time course of the EC50 values of experiment 1 over the incubation period, revealing clear differences between the test substances and the test procedures. When lucerne meal was used as the nitrogen source, we observed either no effects or only short-term intermediate effects on nitrate accumulation with all three test substances. There was no difference in AgNO3 toxicity on days 7 and 28 based on the nitrate concentration applying an inorganic nitrogen source (2.7 and 2.8 mg/kg, respectively), but the toxicity of both NM-302 and NM-300 K increased during the test. For NM-300 K, toxicity equilibrium was achieved during the incubation period with comparable EC50 values recorded on days 21 and 28 (7.1 and 7.3 mg/kg, respectively). The toxicity of NM-302 increased throughout the incubation period (EC50 = 190 mg/kg on day 0 and 44.6 mg/kg on day 28).
The effects of AgNO3 and NM-302 on potential ammonium oxidation activity were similar to the corresponding nitrate accumulation profiles, with comparable values for AgNO3 on both testing dates (potential ammonium oxidation; 3.8 and 2.9 mg/kg) and a significant increase in the toxicity of NM-302 (from >100 mg/kg to 6 mg/kg). The toxicity of NM-300 K to potential ammonium oxidation was comparable at both measuring points (2.3 mg/kg on day 7 and 1.6 mg/kg on day 28).
The microflora involved in glucose-induced respiration recovered by the end of the test. The effect was more obvious with NM-300 K based on test concentrations (Figure 4) instead of EC50 values (Table 2). This nanomaterial caused about 50% inhibition on day 7 at test concentrations of 0.6, 1.7, and 5 mg/kg. On day 28 however, although there was still about 50% inhibition at the test concentration of 5 mg/kg, only 10% inhibition was recorded in soils treated with 0.6 and 1.7 mg/kg of the test substance (Figure 4).
Discussion
Comparison: nitrite and nitrate accumulation - carbon transformation
Tests according to OECD TGs 216 and 217 are used for the assessment of chemicals as required, e.g., in the context of regulation. OECD TG 216 describes a laboratory test that is used to investigate the long-term effects of chemicals on the microbial nitrogen transformation resulting in nitrate accumulation after a single exposure. The measured nitrate concentration reflects the sum of several microbial activities, such as ammonification, nitrification, denitrification, and formation of microbial biomass. Denitrification is expected to be of minor relevance due to the aerobic test conditions. Sieved soil is supplemented with powdered plant meal (e.g., lucerne meal). According to the OECD test guideline, for non-agrochemicals, the nitrate concentrations in treated and control samples are measured at the start of the test and after incubation for 28 days; for agrochemicals additional sampling points are requested. We applied this test to two silver nanomaterials and the soluble silver salt AgNO3, and by considering only the start and end point, we found no evidence of toxicity towards soil-nitrifying bacteria. However, the same concentrations of test materials showed clear concentration-effect curves in the carbon transformation test (substrate-induced respiration) [3]. For strongly biocidal compounds, dose-related inhibition of substrate-induced respiration combined with a stimulated N-mineralization is typical [19]. In the following chapters, we discuss/consider the unusual sensitivity of both test systems (effects in C-transformation test; no effect in N-transformation test) when investigating Ag-NM and soluble AgNO3.
Influence of nitrogen source (refer to 'Nitrogen transformation test' and 'Potential ammonium oxidation test' sections)
Usually, the effects of chemicals on nitrite and nitrate accumulation determined in tests using an inorganic nitrogen source are more obvious than in tests investigating the effects on soil respiration [20]-[22]. The different sensitivities of the endpoints may reflect the diversity of the microflora responsible for the different activities and hence their potential for recovery. This means that the ability to respire using easily degradable carbon sources such as glucose (the principle of the carbon transformation test) is more widespread than the ability to transform ammonium to nitrite and nitrate. When we replaced the powdered plant meal in the N-transformation test according to OECD TG 216 with (NH4)2SO4 as a form of inorganic nitrogen, the same tests revealed clear concentration-effect curves for each test substance, and similar results were obtained when we tested the potential ammonium oxidation activity of the soil. These tests specifically monitored the transformation of ammonium to nitrate and ammonium to nitrite and did not require the introduction of complex organic matrices into the soil that might interfere with the bioavailability of the test substances. Indeed, organic materials such as sewage sludge have already been shown to interact with silver nanomaterials, with sorption rates greater than 90% for uncoated particles [23]-[25] and lower rates for functionalized particles [26]. Studies with biofilm communities and pure cultures have also shown that organic matter such as exopolysaccharides and humic acids can protect microorganisms and reduce the toxicity of silver nanomaterials [27]-[29]. The toxicity of ZnO nanomaterials towards Folsomia candida was found to be dependent on the soil pH and the proportion of organic matter [30]. These data, together with our results in the present investigation, suggest that silver nanomaterials and the ions released therefrom interfere with organic matter which reduces their long-term toxic effects on the sum of microbial activities resulting in nitrate accumulation in soil. The sorption of ions can also explain the missing effect of the soluble silver salt on day 28. The suggested mechanisms for the inhibition of silver nanomaterial dissolution are through surface adsorption of natural organic matter which in turn will block silver nanomaterial oxidation sites or released silver ions reducing back to silver through reaction with humic/fulvic acids [31]. This demonstrates that the ecotoxicity of such substances can be underestimated using the nitrogen transformation test recommended for the hazard and risk assessment of chemicals. Hazard assessment as required in the context of regulation can result in false negative conclusions. In the first step of a risk assessment, conservative conditions have to be simulated and refinements are foreseen only in case a risk cannot be excluded under these conditions [32]. As a consequence, for nanomaterials, an inorganic nitrogen source should be used in the first step. If a risk cannot be excluded, a complex organic nitrogen source could be considered, which reflects more natural conditions.
The toxicity of silver nanomaterials among other things depends on the release of free ions [33],[34]. The impact of soluble metal ions in aquatic and terrestrial ecotoxicological tests is usually demonstrated by comparing the effect of nanomaterials against the corresponding soluble salts [16],[29],[35]. For aquatic environments, comparable results are achieved if the test is based on ion concentrations because dissolution is the principal exposure mechanism [33],[36]. However, both dissolution and sorption must be considered when testing soils. For example, the toxicity of Zn2+ in F. candida exceeds that of Zn nanomaterials if the calculations are based on the total zinc concentration [37]-[39], but is less than that of zinc nanomaterials if calculations are based on the pore water Zn2+ concentration [39]. However, different results were observed when testing the toxicity of silver in earthworms: there was no significant difference in toxicity based on the total concentration of silver nanomaterials/soluble silver salts and based on the amount of free Ag+[16]. This discrepancy has yet to be explained, but it may depend on nanomaterial-specific factors (differences between zinc and silver, size of nanomaterials, and velocity of ion release), differences in the time of ion determination (directly after spiking [39]; at test end (56 days) [16]), or differences in species habitat: F. candida lives in air-filled soil pores and earthworms in bulk soil. We did not measure the pore water Ag+ concentration in our investigation, but based on the total silver concentration, our results suggest there is no significant difference in the toxicity of soluble silver salts and NM-300 K at the end of the test.
Time course of effects
Although there was only a negligible difference in the effect of the test substances on the sum of microbial activities resulting in a nitrate accumulation after 28 days, the effect profile changed during the test in different ways for each substance. AgNO3 showed immediate toxicity, whereas the small spherical nanoparticles showed the rapid development of toxicity followed by toxicity equilibrium and the larger rod-shaped nanomaterials became toxic more slowly but toxicity was still increasing at the end of the test. These differences may depend on the surface area of the nanomaterials, with the smaller particles having a larger surface area and thus a higher release of ions and a more rapid effect. These effect profiles may indicate the stability of the nanomaterials, the dynamic release of ions, and/or the overall toxicity, but this cannot be addressed specifically without further experiments involving prolonged incubation periods that achieve equilibrium of toxicity, thus allowing various relationships such as velocity and duration or shape and velocity to be characterized in more detail. An influence of the morphology of NM has also been demonstrated in other studies [40],[41].
The toxicity of each test substance was greater when measured as an impact on potential ammonium oxidation [4] than when measured as an impact on nitrate accumulation using (NH4)2SO4 as the nitrogen source in the N-transformation test [2]. There are several differences between the tests that may explain this phenomenon. Only the transformation step from ammonium to nitrite is investigated in the first test, whereas the whole process from ammonium to nitrate is covered by the second, i.e., that further to the transformation of ammonium to nitrate this test includes additional transformation steps of the nitrogen cycle (release of ammonium from organic nitrogen sources and nitrogen immobilization). The potential ammonium oxidation test provides information on the current activity of the respective microorganisms at the sampling time point, whereas the effect on nitrogen transformation is based on the total nitrate concentration per test concentration and sampling day.
Conclusions
The procedure described in OECD TG 216 is often used to assess chemical effects on the soil microflora responsible for nitrate accumulation under the framework of the REACH regulation and the resp. Guidance Document [1]. We found that the test is unsuitable for the detection of effects caused by silver nanomaterials and soluble silver salts, suggesting that the deficiency relates not to the testing of nanomaterials but substances that release soluble ions in significant amounts that are the basis of the observed toxicity. We therefore recommended an incubation period of the soil of at least 28 days according to OECD TG 216 and the use of an inorganic nitrogen source instead of organic nitrogen or the periodical measurement of potential ammonium oxidation instead of nitrogen transformation [4]. Finally the effect development time course in the nitrogen transformation test with (NH4)2SO4 and the potential ammonium oxidation activity test may provide a useful indicator for the stability of silver nanomaterials, although additional experiments are required to characterize this relationship in more detail.
Methods
Test soil
The experiments were carried out using the reference soil RefeSol 01A [42] (sieved ≤2 mm) which is a loamy, medium-acidic and lightly humic sand (Table 3). RefeSol soils were selected as reference soils by the German Federal Environment Agency (Umweltbundesamt, UBA) and are known to be suitable for testing the influence of substances on the habitat function of soils (bioavailability, effects on organisms). RefeSol 01A matches the properties stated in various OECD terrestrial ecotoxicological guidelines (e.g., tests with plants and soil microflora). The soils were sampled in the field and stored in high-grade stainless steel basins with drainage and ground contact at the Fraunhofer IME in Schmallenberg. Red clover was sown on the stored soils to preserve microbial activity. No pesticides were used. Soil was sampled 1 to 4 weeks before the test. If the soil was too wet for sieving, it was dried at room temperature to 20% to 30% of the maximum water-holding capacity (WHCmax) with periodic turning to avoid surface drying. If the tests did not start immediately after sieving, the soil was stored in the dark at 4°C under aerobic conditions [43].
Test substances
We used one silver nanomaterial with the code NM-300 K and a silver material with the code NM-302 containing nanostructures but also larger particles. Both materials had been selected for the OECD Sponsorship Programme on nanomaterials and are referred to as nanomaterials [44]. NM-300 K is a colloidal silver dispersion with a nominal silver concentration of 10% (w/w). The primary particles of the nanomaterial have a size of approximately 20 nm, measured on TEM images. The general morphology of the primary subunits of the NM is nearly equiaxed and rounded, or slightly elongated. Their suggested 3D structure is spherical or slightly ellipsoidal (determinations performed by EM-service of CODA-CERVA, Brussels, Belgium). NM-300 K is a mixture of a stabilizing agent (NM-300 K DIS) comprising 4% (w/w) each of polyoxyethylene glycerol trioleate and polyoxyethylene sorbitan monolaurate (Tween-20) and silver nanomaterials. NM-302 comprises silver nanomaterial (purity ≥99%, thickness 100 to 200 nm, and length 0.6 to 12 μm) dispersed in an aqueous solution containing (according to the manufacturer) silver nanowires (8.6% w/w), polyvinylpyrrolidone (<1% w/w), acrylic/acrylate copolymer (<2% w/w), and polycarboxylate ether (<2% w/w). The general morphology of the primary subunits of the NM is longitudinal or rounded, but slightly elongated. Their suggested 3D structures are rod-like or poly-angular (determinations performed by EM-service of CODA-CERVA, Belgium). AgNO3 was purchased from Merck, Darmstadt, Germany.
Spiking of soil and incubation
The procedure used to mix the test materials with the soil has been described [45]. All the values below refer to the concentration of silver (regardless of the test substance) and the dry weight of the soil. Both NM-300 K and AgNO3 were tested at concentrations of 0.56, 1.67, 5.0, and 15.0 mg/kg. NM-302 was tested at concentrations of 1, 10, and 100 mg/kg. NM-300 K and NM-302 consisted of silver nanomaterials in dispersant. For NM-300 K, the pure dispersant was tested in amounts comparable to the amounts present in the NM-300 K applications. For NM-302, only the highest concentration of dispersant was investigated. Neither of the applied tests on nitrogen transformation, potential ammonium oxidation, and on carbon transformation revealed statistically significant differences between the untreated control and soil spiked with dispersant (data not shown).
For the carbon transformation [3] and potential ammonium oxidation activity [4] tests, the soil was incubated without nutrients. For the nitrogen transformation test with soil microflora [2], sieved and spiked soil was supplemented with 5 g powdered plant material (lucerne meal) per kg of dry soil or with 1 g (NH4)2SO4 per kg of dry soil, corresponding to 212 mg nitrogen per kg. The (NH4)2SO4 was added in a 20-mL aqueous solution.
The soil was then adjusted to 50% ± 5% WHCmax, transferred to glass vessels (500 g for the nitrification and ammonium oxidation activity tests and 1,500 g for the carbon transformation tests), and incubated under aerobic conditions at 20 C ± 2°C for 4 weeks. Every 7 days, evaporated water was replaced. The incubation corresponded to the procedure described in OECD TGs 216 and 217. According to the OECD test guidelines sampling times for non-agrochemicals are mandatory on days 0 (3 h after spiking) and 28. Additional samples were analyzed on days 7 and/or 21. The ammonium oxidation activity was determined over a period of 6 h on day 1 instead of day 0 because spiking, incubation for 3 h, and testing is not possible within 1 day.
Ecotoxicological determinations
Nitrate levels were measured as an indicator of the soil nitrification activity after mixing with lucerne meal or (NH4)2SO4 as a nitrogen source. Nitrate was extracted by shaking soil samples (20 g dm) with 0.1 M KCl (100 mL minus the water content of the soil sample) for 60 min at 150 rpm. Three replicates for the control and every test concentration were sampled. The mixtures were filtered and nitrate in the filtrate was measured using a Spectroquant® NOVA 400 spectrophotometer (Merck, Darmstadt, Germany) at 370 nm immediately after preparation. For the determinations we used a test kit (nitrate cell test; Spectroquant 1.14542.0001; measuring range 0.5 to 18.0 mg/L NO3-N; Merck, Darmstadt, Germany). The determination is based on the reaction of nitrate ions with benzoic acid derivates in the presence of sulfuric acid resulting in a red nitro compound that is determined photometrically. Commercially available nitrate standard solutions CRM (Spectroquant, 2.5 and 15 mg/L; Merck, Darmstadt, Germany) were used for checking the procedure. Soil extracts with nitrate concentrations exceeding the measuring range were diluted with deionized water.
The potential ammonium oxidation activity [4] was measured in a slurry of 25 g dry soil matter in 100 mL mineral test medium (0.56 mM KH2PO4, 1.44 mM K2HPO4, 5 mM NaClO3, 1.50 mM (NH4)2SO4). The slurries were incubated on an orbital shaker at 25°C ± 2°C, and 10-mL samples were removed after 2 and 6 h. The samples were mixed with 10 mL 4 mol/L KCl, and after filtration, the nitrite levels in the filtrate were measured with sulfanilamide and N-(1-naphthyl)-ethylene-diamine dihydrochloride giving a red-violet diazo dye. The absorbance of the diazo dye was measured using a Cary 300 Scan UV-VIS spectrophotometer (Varian Deutschland GmbH, Darmstadt, Germany) at 530 nm. NaNO2 was used for the calibration curve (0.01 to 1.0 NO2-N/mL).
Substrate-induced respiration [3] was measured in three 500-mL Erlenmeyer flasks per treatment filled with 100 g dry soil matter and 400 mg glucose, mixed homogenously. The vessels were incubated in darkness at 20°C ± 1°C for 24 h. Respiration was measured during incubation with an OxiTop Sensomat system (Aqualytic/WTW, Weilheim, Germany). Oxygen was consumed during respiration and the resulting low pressure was measured. The CO2 generated was bound to KOH to prevent interference with the measurement. The microbial respiration rate was calculated over a linear time scale.
Statistical analysis
In the ecotoxicological tests, probit analysis was used to estimate the EC10 and EC50 values and the dose-response curves. The significance of differences between treatments in the long-term tests was determined using Student's t-test. Statistical analysis was carried out using ToxRat® Pro v2.10 software (Alsdorf, Germany) for ecotoxicity response analysis [46].
Authors' contributions
KHR and KS designed the studies, headed the test performance, and drafted the manuscript. Both authors read and approved the final manuscript.
References
Guidance for the Implementation of REACH - Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.7c: Endpoint Specific Guidance. European Chemicals Agency, Helsinki; 2012.
Guideline 216: OECD Guideline for the Testing of Chemicals. Soil Microorganisms: Nitrogen Transformation Test. Organisation for Economic Co-operation and Development, Paris; 2000.
Guideline 217: OECD Guideline for the Testing of Chemicals. Soil Microorganisms: Carbon Transformation Test. Organisation for Economic Co-operation and Development, Paris; 2000.
Soil Quality - Determination of Potential Nitrification and Inhibition of Nitrification - Rapid Test by Ammonium Oxidation. International Organization for Standardization, Geneva; 2012.
Piccinno F, Gottschalk F, Seeger S, Nowack B: Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res 2012, 14(1109: 1–1109):11.
Chaloupka K, Malam Y, Seifalian AM: Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol 2010, 28: 580–588. 10.1016/j.tibtech.2010.07.006
Yuan G, Cranston R: Recent advances in antimicrobial treatments of textiles. Text Res J 2008, 78: 60–72. 10.1177/0040517507082332
Prabhu S, Poulose E: Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett 2012, 2(32:1–32):10.
Choi O, Hu Z: Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol 2008, 42: 4583–4588. 10.1021/es703238h
Choi O, Clevenger TE, Deng B, Surampalli RY, Ross L Jr, Hu Z: Role of sulfide and ligand strength in controlling nanosilver toxicity. Water Res 2009, 43: 1879–1886. 10.1016/j.watres.2009.01.029
Hánsch M, Emmerling C: Effects of silver nanoparticles on the microbiota and enzyme activity in soil. J Plant Nutr Soil Sc 2010, 173: 554–558. 10.1002/jpln.200900358
Schlich K, Klawonn T, Terytze K, Hund-Rinke K: Hazard assessment of a silver nanoparticle in soil applied via sewage sludge. Environ Sci Eu 2013, 25: 17. 10.1186/2190-4715-25-17
Shin YJ, Kwak JI, An YJ: Evidence for the inhibitory effects of silver nanoparticles on the activities of soil exoenzymes. Chemosphere 2012, 88: 524–529. 10.1016/j.chemosphere.2012.03.010
Shoults-Wilson WA, Reinsch BC, Tsyusko OV, Bertsch PM, Lowry GV, Unrine JM: Role of particle size and soil type in toxicity of silver nanoparticles to earthworms. Soil Sci Soc Am J 2011, 75: 365–377. 10.2136/sssaj2010.0127nps
Yang Y, Chen Q, Wall JD, Hu Z: Potential nanosilver impact on anaerobic digestion at moderate silver concentrations. Water Res 2012, 46: 1176–1184. 10.1016/j.watres.2011.12.024
Schlich K, Klawonn T, Terytze K, Hund-Rinke K: Effects of silver nanoparticles and silver nitrate in the earthworm reproduction test. Environ Toxicol Chem 2013, 32: 181–188. 10.1002/etc.2030
van Beelen P, Doelman P: Significance and application of microbial toxicity tests in assessing ecotoxicological risk of contaminants in soil and sediment. Chemosphere 1997, 34(3):455–499. 10.1016/S0045-6535(96)00388-8
Biologische Bundesanstalt für Land- und Forstwirtschaft: Auswirkungen auf die Aktivität der Bodenmikroflora. Richtlinien für die amtliche Prüfung von Pflanzenschutzmitteln Teil VI, 1–1: 1990.
Malkomes H-P: Microbiological-ecotoxicological soil investigations of two herbicidal fatty acid preparations used with high dosages in weed control. UWSF - Z Umweltchem Ökotox 2006, 18: 13–20. 10.1065/uwsf2005.10.107
Hund-Rinke K, Simon M: Terrestrial ecotoxicity of eight chemicals in a systematic approach. J Soils Sediments 2005, 5: 59–65. 10.1065/jss2004.10.123
Agnihotri VP: Persistence of captan and its effects on microflora, respiration, and nitrification of a forest nursery soil. Can J Microbiol 1971, 17: 377–383. 10.1139/m71-063
Remde A, Hund K: Response of soil autotrophic nitrification and soil respiration to chemical pollution in long-term experiments. Chemosphere 1994, 29: 391–404. 10.1016/0045-6535(94)90112-0
Wang Y, Westerhoff P, Hristovski KD: Fate and biological effects of silver, titanium dioxide, and C60 (fullerene) nanomaterials during simulated wastewater treatment processes. J Hazard Mater 2012, 201-202: 16–22. 10.1016/j.jhazmat.2011.10.086
Tiede K, Boxall ABA, Wang X, Gore D, Tiede D, Baxter M, David H, Tear SP, Lewis J: Application of hydrodynamic chromatography-ICP-MS to investigate the fate of silver nanoparticles in activated sludge. J Anal At Spectrom 2010, 25: 1149–1154. 10.1039/b926029c
Kiser MA, Ryu H, Jang H, Hristovski K, Westerhoff P: Biosorption of nanoparticles to heterotrophic wastewater biomass. Water Res 2010, 44: 4105–4114. 10.1016/j.watres.2010.05.036
Kiser MA, Ladner DA, Hristovski KD, Westerhoff PK: Nanomaterial transformation and association with fresh and freeze-dried wastewater activated sludge: implications for testing protocol and environmental fate. Environ Sci Technol 2012, 46: 7046–7053. 10.1021/es300339x
Sheng Z, Liu Y: Effects of silver nanoparticles on wastewater biofilms. Water Res 2011, 45: 6039–6050. 10.1016/j.watres.2011.08.065
Wirth SM, Lowry G, Tilton RD: Natural organic matter alters biofilm tolerance to silver nanoparticles and dissolved silver. Environ Sci Technol 2012, 46: 12687–12696. 10.1021/es301521p
J-y R, Sim SJ, Yi J, Park K, Chung KH, Ryu D-y, Choi J: Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ Sci Technol 2009, 43: 3933–3940. 10.1021/es803477u
Waalewijn-Kool PL, Rupp S, Lofts S, Svendsen C, van Gestel CAM: Effects of soil properties on the toxicity of ZnO nanoparticles to Folsomia candida in a comparsion of four natural soils. In Ecotoxicological Assessment of ZnO Nanoparticles to Folsomia candida. Edited by Waalewijn-Kool PL. The Netherlands: PhD thesis, VU University Amsterdam, Department of Ecological Science; 2013. Waalewijn-Kool PL, Rupp S, Lofts S, Svendsen C, van Gestel CAM: Effects of soil properties on the toxicity of ZnO nanoparticles toFolsomia candidain a comparsion of four natural soils. In Ecotoxicological Assessment of ZnO Nanoparticles to Folsomia candida. Edited by Waalewijn-Kool PL. The Netherlands: PhD thesis, VU University Amsterdam, Department of Ecological Science; 2013.
Liu J, Hurt R: Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ Sci Technol 2010, 44: 2169–2175. 10.1021/es9035557
Guidance for the Implementation of REACH - Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.19: Uncertainty Analysis. European Chemicals Agency, Helsinki; 2012.
Kim J, Kim S, Lee S: Differentiation of the toxicities of silver nanoparticles and silver ions to the Japanese medaka ( Oryzias latipes ) and the cladoceran Daphnia magna . Nanotoxicology 2011, 5: 208–214. 10.3109/17435390.2010.508137
Yin L, Cheng Y, Espinasse B, Colman BP, Auffan M, Wiesner M, Rose J, Liu J, Bernhardt ES: More than the ions: the effects of silver nanoparticles on Lolium multiflorum . Environ Sci Technol 2011, 45: 2360–2367. 10.1021/es103995x
Asghari S, Johari S, Lee J, Kim Y, Jeon Y, Choi H, Moon M, Yu I: Toxicity of various silver nanoparticles compared to silver ions in Daphnia magna . J Nanobiotechnol 2012, 10(14):1–14. 11 11
Lee Y-J, Kim J, Oh J, Bae S, Lee S, Hong IS, Kim S-H: Ion-release kinetics and ecotoxicity effects of silver nanoparticles. Environ Toxicol Chem 2012, 31: 155–159. 10.1002/etc.717
Kool PL, Ortiz MD, van Gestel CAM: Chronic toxicity of ZnO nanoparticles, non-nano ZnO and ZnCl 2 to Folsomia candida (Collembola) in relation to bioavailability in soil. Environ Pollut 2011, 159: 2713–2719. 10.1016/j.envpol.2011.05.021
Waalewijn-Kool P, Diez Ortiz M, Gestel CM: Effect of different spiking procedures on the distribution and toxicity of ZnO nanoparticles in soil. Ecotoxicology 2012, 21: 1797–1804. 10.1007/s10646-012-0914-3
Waalewijn-Kool PL, Ortiz MD, Lofts S, van Gestel CAM: The effect of pH on the toxicity of zinc oxide nanoparticles to Folsomia candida in amended field soil. Environ Toxicol Chem 2013, 32: 2349–2355. 10.1002/etc.2302
Chithrani BD, Ghazani AA, Chan WCW: Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 2006, 6: 662–668. 10.1021/nl052396o
Pal S, Tak YK, Song JM: Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli . Appl Environ Microbiol 2007, 73: 1712–1720. 10.1128/AEM.02218-06
Refesol. , [http://www.refesol.de/english/] Refesol.
Soil Quality - Guidance on Long and Short Term Storage of Soil Samples. International Organization for Standardization, Geneva; 2007.
Safety of Manufactured Nanomaterials - Sponsorship Programme for the Testing of Manufactured Nanomaterials. 2007.
Hund-Rinke K, Schlich K, Klawonn T: Influence of application techniques on the ecotoxicological effects of nanomaterials in soil. Environ Sci Eur 2012, 24(12):1–30. 12 12
ToxRat Professional - Software for Ecotoxicity Response Analysis. Alsdorf, Germany: Online Version 2.10. ToxRat® Solutions GmbH. ToxRat Professional - Software for Ecotoxicity Response Analysis. Alsdorf, Germany: Online Version 2.10. ToxRat® Solutions GmbH.
Acknowledgements
The authors would like to thank the MARINA Framework 7 project for providing the funding for this study and the EM-service of CODA-CERVA, Belgium, for the characterization of the nanomaterials.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hund-Rinke, K., Schlich, K. The potential benefits and limitations of different test procedures to determine the effects of Ag nanomaterials and AgNO3 on microbial nitrogen transformation in soil. Environ Sci Eur 26, 28 (2014). https://doi.org/10.1186/s12302-014-0028-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-014-0028-z