Abstract
Background
Ewing sarcoma represents a spectrum of aggressive malignancies with the poor outcome. Primary renal Ewing sarcoma is rare and accounts for less than 1% of renal masses.
Case presentation
We present a case of a 45-year-old male presented in OPD with complaint of right flank pain and hematuria. He underwent a contrast-enhanced CT abdomen which depicted a right renal mass with liver lesions. He underwent Robotic Right Radical Nephrectomy and a pathological diagnosis of Ewing Sarcoma was made after which he was started on chemotherapy.
Conclusions
Owing to presentation in younger age group with poor prognosis, an integrated analysis including radiological imaging, histopathology, and immune-histological staining is essential for early detection of renal Ewing sarcoma.
Similar content being viewed by others
1 Background
Ewing sarcoma also called as primitive neuroectodermal tumors (PNET) is a group of small round cell tumor that are typically encountered in the bone and soft tissue of young adults and children. They rarely present as a primary renal tumor [1]. Primary renal Ewing sarcomas/PNET were first reported by Seemayer and colleagues in 1975 [2]. These lesions are often silent and are discovered once the tumor enlarges to cause mass effect, pain, swelling or sometimes hematuria.
These tumors are characterized by an aggressive clinical course with local recurrence and early metastasis, resulting in a very poor prognosis, and thus, early recognition of these are essential [3].
2 Case presentation
A 45-year-old male presented to the surgical oncology department with the complaints of pain in right flank and hematuria. The general physical was unremarkable. Chest examination revealed normal air entry bilaterally. Neurological examination was also unremarkable. Renal function tests were normal.
Patient underwent a contrast-enhanced computed tomography of the abdomen which revealed an ill-defined hypo-enhancing infiltrative mass predominantly involving the middle and lower pole of the right kidney. It was reaching up to the renal hilum with evidence of tumor thrombus in the right renal vein, extending into the infra hepatic portion of IVC. No areas of internal hemorrhage/calcification or fat density were seen within the mass. Multiple hypodense lesions were seen in both lobes of the liver. Based on the imaging findings, a provisional diagnosis of renal cell carcinoma with liver metastasis was made.
3 Management and outcome
Patient underwent Robotic Right Radical Nephrectomy with IVC thrombectomy. Light microscopy showed multiple small round blue cells with a high nucleocytoplasmic ratio, arranged in sheets. On immunohistochemistry, tumor cells were positive for NKX2.2 and CD99 and were negative for synaptophysin, INSM1, CK, CK7, 2SC, AMACR with absent rhabdoid features. Final opinion of Ewing sarcoma was made. Patient was started on VAC-based chemotherapy including vincristine, actinomycin and cyclophosphamide.
4 Discussion
Approximately 90% malignant neoplasms of the kidney are renal cell carcinoma out of which clear cell carcinomas are the most common. Primary renal sarcomas are rare mesenchymal tumors with a reported incidence < 1% of all renal malignancies [4]. Renal PNET mainly affects younger age group, with mean presentation aged 28–34 years and having slightly male predominance [5] Sarcomas of the kidney are usually asymptomatic but when they become enlarged they can produce nonspecific symptoms such as pain, palpable masses and hematuria. The imaging characteristics of Ewing sarcomas are similar to that of RCC [5].
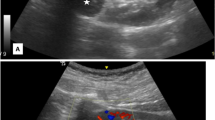
On ultrasound, renal Ewing’s sarcoma/PNET may be hypoechoic, or hyperechoic to the adjacent renal parenchyma with areas of necrosis. These are vascular tumors and thus demonstrate increased vascularity on color Doppler imaging [5]. On post-contrast CT, these appears heterogeneous density mass with necrotic or hemorrhagic areas and have heterogeneous enhancement [5]. Calcification may be present in some cases. Similar to renal cell carcinoma renal vein & IVC involvement can be seen in the Ewing sarcoma [5] On MRI, the tumor masses can appear lobulated with an isointense or hypointense appearance on T1-weighted images with a heterogeneous to hyperintense appearance on T2-weighted images. On post-gadolinium T1-weighted images, this tumor shows heterogeneous enhancement. Similar imaging features may be seen in the other renal tumors, including renal cell carcinoma, Wilms’s tumor, neuroblastoma, lymphoma, renal sarcomas, and metastatic carcinoma [6]. Few imaging features of a renal EWS differentiating it from advanced RCC include the presence of multiple irregular septa seen in both necrotic as well as solid portions of the tumor which are not seen in the advanced RCC [6, 7]. A low enhancement in both the arterial and venous phase favors diagnosis of EWS over large conventional RCCs, which show marked enhancement on the arterial phase and low enhancement on the venous phase. However, low enhancement cannot differentiate with other type of RCC like papillary type and a collecting duct carcinoma [6, 7]. In our case, low enhancement on the arterial and venous phase were seen. It has also been reported that compared to other renal tumors with similar imaging characteristics, the areas of necrosis and hemorrhage seen in renal Ewing's sarcoma ⁄PNET are more commonly located along the periphery of the tumor [8].
Accurate diagnosis of Ewing sarcoma is made either on biopsy material or resected specimen (Fig. 1). On light microscopy small, round cells forming rosettes and pseudorosettes, and fascicular, spindle cell component are seen [3]. Immunohistochemical and molecular studies play a major role in differentiating these tumor types. In general, Ewing's family tumors show positivity for CD99 (MIC2), FLI-1, vimentin and NSE and are typically negative for pankeratin, desmin, WT-1, GFAP and PAX2. EWSR1/FLI1 fusion product that results from a t (11;22) (q24/q22; q12) translocation. It is seen in the 90% of cases and unequivocally confirms the diagnosis [9]. Current standards of treatments are chemotherapy including vincristine, doxorubicin, and cyclophosphamide, alternating with ifosfamide and etoposide [10]. Despite aggressive treatment (Fig. 2), the prognosis of patients with metastatic disease is poor [3].
Axial non-contrast A and contrast-enhanced CT B, C images depicting an ill-defined hypoenhancing infiltrative lesion predominantly involving the middle and lower pole of right kidney reaching up to the renal hilum, D Coronal image shows tumor thrombus in the right renal vein, extending into the infrahepatic portion of IVC. (arrow). E, F Axial image showing few hypodense lesions in both lobes of liver. G, H PET axial image shows metabolic active lesion with heterogeneous tracer uptake in the mid & lower pole of the right kidney (SUV = 4.8)
shows a tumor infiltrating the renal parenchyma (A). The tumor shows a nodular pattern of arrangement with surrounding dense fibrous tissue along with entrapment of adjacent renal tubules and glomeruli (right top corner) (B). On high power, the tumor shows a papillary pattern of arrangement with fibro-vascular cores. The tumor cells are small, uniform in size with round nuclei, inconspicuous nucleoli, scant cytoplasm and indistinct cytoplasmic membranes (C, D)
5 Conclusion
Primitive neuro-ectodermal tumors are an infrequent differential diagnosis in urologic malignancies in younger age group. They have aggressive clinical course and early metastasis. So early diagnosis is crucial for initiation of chemotherapy. Thus, imaging have an important role in diagnosis, staging, and treatment monitoring (Fig. 3). The CT and MRI have role in local assessment of resectability as well as detection of metastases.
Availability of data and materials
Not applicable.
Abbreviations
- EWS:
-
Ewing’s sarcoma
- PNET:
-
Primitive neuroectodermal tumor
- CT:
-
Computed tomography
- IVC:
-
Inferior vena cava
- RCC:
-
Renal cell carcinoma
References
Cheng L, Xu Y, Song H et al (2020) A rare entity of Primary Ewing sarcoma in kidney. BMC Surg 20:280
Bilgetekin I, Karaca M, Gönül II, Üner A, Şahinli H, Demir H, Aytekin A, Çiltaû A, Benekli M (2018) Ewing’s sarcoma of kidney in a 60-year-old patient with local recurrence: a rare occurrence. J Cancer Res Ther 14(6):1422–1424
Angel JR, Alfred A, Sakhuja A et al (2010) Ewing’s sarcoma of the kidney. Int J Clin Oncol 15:314–318
Uhlig J, Uhlig A, Bachanek S (2022) Mehmet Ruhi Onur, Sonja Kinner, Primary renal sarcomas: imaging features and discrimination from non-sarcoma renal tumors. Eur Radiol 32(2):981–989
Almeida MF, Patnana M, Korivi BR, Kalhor N, Marcal L, (2014) Ewing sarcoma of the kidney: a rare entity, Case Rep Radiol. 5
Zhang D, Li Z, Gao D, Yang G, Ding Y, Wang G, Dong X (2016) The CT and US features of Ewing’s sarcoma/primary neuroectodermal tumor of the kidney: two case reports and review of literature. Onco Targets Ther 9:1599–2160
Lee H, Cho JY, Kim SH, Jung DC, Kim JK, Choi HJ (2009) Imaging findings of primitive neuroectodermal tumors of the kidney. J Comput Assist Tomogr 33(6):882–886
Wu CF, Wang LJ, Chen CS (2000) Images in clinical urology. Magnetic resonance imaging of primitive neuroectodermal tumor of the kidney. Urology 55(2):284–285
Katkar AS, Vinu-Nair S, Savage J, Chintapalli K (2014) Primary renal ewings sarcoma/primitive neuroectodermal tumor (PNET) of the kidney, can it be diagnosed on imaging? a case report. Austin J Radiol 1(1):4
Grier HE, Krailo MD, Tarbell NJ et al (2003) Addition of if-osfamide and etoposide to standard chemotherapy for Ewing’ssarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 348:694–701
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SG involved in conception & design of work, drafting the work and manuscript preparation. TK performed manuscript reviewing and editing. SP did final review of the work and approval to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Consent was obtained from patient.
Competing interests
All authors have declared that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Gupta, S., Khullar, T. & Puri, S.K. Renal cell carcinoma on imaging unveiled as a primary Ewing’s sarcoma. Afr J Urol 29, 47 (2023). https://doi.org/10.1186/s12301-023-00379-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-023-00379-x