Abstract
Background
Mayo Adhesive Probability (MAP) score is based on posterior perinephric fat thickness and perinephric fat stranding and ranges from 0 to 5. We intend to validate the score and identify preoperative factors predictive of Adherent Perinphric Fat (APF) encountered in robotic-assisted partial nephrectomy.
Methods
The retrospective and prospective observational study was done at a single tertiary care hospital after appropriate ethical clearance. Sixty-two patients with clinical stage cT1 renal mass planned for robotic-assisted partial nephrectomy were selected over a study period of 3 years after obtaining informed consent. Data that were collected included demographic details and perioperative details including CT renal angiography which was done in all patients preoperatively. Intraoperative and postoperative data were collected. Associations of patient and tumor characteristics with the presence of APF during RAPN were evaluated by multivariable logistic regression models and using Chi-square test to calculate p value.
Results
Out of total 62 patients included; 24 patients (38.7%) had intraoperative Adhesive Perinephric Fat (APF). Three patients required conversion to open surgery and three patients underwent conversion to radical nephrectomy. Thirty-five patients were males. Mean age was 51.27(20–77) years. We noted an increased likelihood of APF with an increase in age (p = 0.003), higher preoperative creatinine (p = 0.003), greater posterior perinephric fat thickness (p = 0.002), and perirenal fat stranding (p < 0.001). From these four variables, posterior perinephric fat thickness and fat stranding were the most predictive. The combined score given to these two highly predictive factors for APF and the calculated score, termed Mayo Adhesive Probability (MAP) score ranges from 0 to 5. APF was seen in 10.7% of patients with a MAP score of 0, 25% with a score of 1, 50% with a score of 2, 44.4% with a score of 3, 88.8% with a score of 4, and 100% of patients with a score of 5 was found. Our study validates the MAP score given by Davidiuk et al. Smoking, high BMI, Sex of patient, tumor size, lateral perinephric fat thickness do not significantly predict APF in our study.
Conclusion
MAP score can be easily calculated from a CT scan. We validate the MAP score in RAPN. Higher MAP score has higher APF which would be useful to all urologists doing RAPN.
Similar content being viewed by others
1 Background
The incidence of RCC in Indian population is 1.3/100,000, with a mortality of 1.2/100,000 [1]. With the advances in radiological imaging, easy availability of imaging and increase awareness, more asymptomatic renal masses are being detected incidentally to the tune of almost 67% [2]. Recent European Association of Urology Guidelines recommend Partial Nephrectomy for all T1 tumors and T2 tumors with a solitary kidney, CKD patient, where it is technically feasible [3].
The acceptance of robotic NSS has rapidly increased for the treatment of larger, and complicated renal masses [4]. It provides some advantages, such as 3-D vision, helps in removing tremors, provides surgeons with a magnified view and allows delicate movement of instruments with seven degrees of freedom, and fulfills the requirements of effective laparoscopic surgery. The RAPN is technically easy and is associated with less chance of conversion to radical nephrectomy, less blood loss, shorter ischemia times, and early postoperative discharge [5, 6].
For anatomical characteristic identification and preoperative standard anatomical evaluation of renal mass RENAL morphometric score and PADUA score have been developed. This scoring system was based on CT scan findings and they aid in predicting perioperative and postoperative complications of partial nephrectomy [7,8,9]. These scoring systems are useful for selecting the surgical method for a better oncological outcome. The RENAL nephrometry score was developed for treatment decision-making and included tumor-specific factors but did not include patient-specific factors like APF which predict surgical difficulty [10].
MAP score was created based on individual scores for posterior perinephric fat thickness and perinephric fat stranding were then combined to create the MAP score that ranges from 0 to 5. Scores for posterior perinephric fat thickness (< 1 cm assigned 0 points, 1–1.9 cm assigned 1 point, > 2 cm assigned 2 points). Grading of perinephric stranding. None: 0 points. The fat around the kidney demonstrates no stranding and computed tomography images show a completely black color surrounding the kidney. Mild/moderate (type 1): 2 points. The fat around the kidney has some stranding present without thick bars of inflammation. Severe stranding (type 2): 3 points. Thick bar of fat stranding around kidney [11].
In this study, we intended to validate MAP (Mayo Adhesive Probability) score and preoperative factors to predict adherent perinephric fat in robotic-assisted partial nephrectomy with reference to Indian population.
2 Methods
This retrospective and prospective observational study was conducted in a single center after achieving appropriate ethical clearance from the institutional ethical committee (Ref No. EC/635/2020). The study was done in accordance with the Declaration of Helsinki and as per Good Clinical Practice guidelines. Written informed consents were obtained from the patients undergoing the procedure in the study. A total of 62 patients with a diagnosis of a renal mass planned for a RAPN was selected for the study. The study period was 3 years from March 2018 to February 2021. All the patients above 18 years of age who were diagnosed with renal mass and amenable to undergo partial resection on preoperative evaluation, irrespective of sex were included in the study. Patient who are unwilling or unable to give informed consent and patients who had not undergone CT scan of Abdomen were excluded from the study.
2.1 Variables
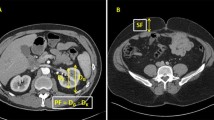
After having obtained informed consent from the patient, detailed demographic data were obtained in the form of patient name, age, sex, and BMI. A detailed past and present history of the patient was sought including a history of comorbidities if any history of smoking, and any family history of renal malignancy. Physical examination of the patient was for any palpable renal mass. All the patients presenting with renal mass had to undergo all the baseline preoperative investigations such as serum creatinine, complete blood count, viral markers, coagulation profile, urine routine and microscopy. Radiological investigations such as CT renal angiography were done to determine the location and size of the mass. Posterior fat thickness, lateral fat thickness, perinephric fat stranding of all patients were calculated from the CT scans. From the CT scan images, the posterior and lateral perinephric fat thickness was measured at the level of the renal vein as similarly described by Eisner et al. [12]. Lateral perinephric fat thickness was measured from the renal capsule to the sidewall of the patient in parallel to the renal vein at the level of a renal vein; posterior fat thickness was measured as a direct vertical line posteriorly from the renal capsule to the posterior abdominal wall (Fig. 1). The perinephric fat stranding was defined as a linear area of soft-tissue attenuation in the perinephric space. If perinephric fat stranding was found on a CT scan, it was graded according to severity. The stranding was graded as 0 (no stranding; Fig. 2A), type 1 (thin rim like mild stranding; Fig. 2B), or type 2 (diffuse, thick-banded severe stranding; Fig. 2C), as described by Kim et al. [13]. All the tumors were scored preoperatively using the MAP score.
Grading of perinephric fat stranding. A None = 0 points. The fat around the kidney demonstrates no stranding in the CT scan. On this CT image, the tissue around the kidney is completely black. B Mild/moderate (Type 1) = 2 points. The perinephric fat around the kidney has some image-dense stranding present, but thick bars of inflammation were absent. C Severe stranding (Type 2) = 3 points. Image shows severe stranding around the kidney with thick image-dense bars of inflammation
2.2 Surgical technique
The surgery was performed by three surgeons with a minimum experience of 50 cases each of RNSS.
The main steps are as follows. Placement of a ureteric catheter. Patient positioning in modified lateral decubitus position. Creation of pneumoperitoneum. Trocar placement as standard. Docking of Robot.
Medial mobilization of the bowel. Hilar identification and dissection. Tumor identification. Hilar clamping. Tumor excision. Renal reconstruction. Specimen extraction and closure.
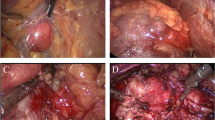
Intraoperative we have observed whether perinephric fat was adhesive or nonadhesive. We have defined as adhesive perinephric fat if one or more of the following things observed during RAPN.
-
1.
Difficulty in peeling of perinephric fat from the kidney.
-
2.
Oozing of blood during dissection of perinephric fat from kidney.
-
3.
Subcapsular dissection to separate perinephric fat.
-
4.
Remaining of fat globules over kidney after dissection of perinephric fat from kidney.
-
5.
Surgeon's difficulty during dissection to isolate the renal tumor or open conversion due to adhesion (Fig. 3).
2.3 Statistical analysis
Statistical analysis was done as per the established guidelines [14]. It consisted of following two steps. First, evaluation of data distribution was done by using Shapiro–Wilk test. Then medians and interquartile ranges or frequencies and proportions were reported for continuous or categorical variables, respectively. Secondly, associations of patient and tumor characteristics with the presence of APF during RAPN were evaluated by multivariable logistic regression models, where odds ratios and 95% CI were estimated. For easy interpretation of results, continuous variables were categorized like (age, preoperative creatinine, size of renal mass, posterior perinephric fat thickness, and lateral perinephric fat thickness) or predefined cutoffs of interest (in BMI and RENAL score). We have used Chi-square test to calculate p value. All the statistical analysis was performed using SPSS v20 software (IBM SPSS statistics, Armonk, NY: IBM Corp., USA) and statistical significance was set at p value less than or equal to 0.05.
3 Results
There was a total of 62 patients (n = 62) included in the study. Twenty-four patient (38.7%) had intraoperative APF. Three patients converted to open NSS (two due to adhesion and one due to endophytic tumor) and three patients converted to radical nephrectomy (two due to adhesion and one due to bleeding).
The mean age of all the patients in the study was 51.27 years with a minimum of 20 years and a maximum of 77 years. The median age was 51.50 years. Table 1 suggests an increase in age was significantly associated with intraoperative adhesive perinephric fat. As shown in Table 2, increase in creatinine was significantly associated with intraoperative adhesive perinephric fat.
The mean posterior perinephric fat thickness of all the patients in our study was 1.07 cm with a minimum of 0.12 cm and a maximum of 3.60 cm. The median thickness was 0.84 cm. Table 2 suggests that an increase in perinephric fat thickness was significantly associated with intraoperative adhesive perinephric fat. Also, as can be seen, perinephric fat stranding was significantly associated with intraoperative adhesive perinephric fat. In our study perinephric fat stranding and posterior perinephric fat thickness were most significantly associated to predict intraoperative adherent perinephric fat. We give score 0 to posterior perinephric fat thickness < 1 cm, score 1 to posterior perinephric fat thickness 1–1.9 cm, score 2 to posterior perinephric fat thickness \(\ge\) 2 cm, score 0 to no perinephric fat stranding, score 2 to type-1 perinephric fat stranding, score 3 to type-2 perinephric fat stranding. We combined the score of these two parameters to calculate Mayo Adhesive Probability (MAP) score and ranging from 0 to 5. We observed APF in 10.71% of patients with a MAP score of 0, 25% with a score of 1, 50% with a score of 2, 44.44% with a score of 3, 88.88% with a score of 4, and 100% of patients with a score of 5 as shown in Table 3.
4 Discussion
APF is non-surgeon-friendly fat and it can cause difficulty during RAPN during dissection surrounding the tumor and may result in tear of the renal capsule [15]. Senior Urologists, experts in RAPN have felt the necessity of models or scores to predict intraoperative APF and the complexity of the surgery, preoperatively [10]. Davidiuk et al. [11] did a study to know the relationship between the patient-related factors and intraoperative APF and give MAP score. This score was on the image (CT scan) based. Although the MAP score appears promising, because of the small sample size its validation in other centers and in the Asian population is required.
Recent studies suggest a higher MAP score associated with an increase in intraoperative adhesive perinephric fat and difficulty in performing dissection and adverse intraoperative outcomes like increased estimated blood loss, operation time. Most of the urologists use RENAL, PADUA scores to decide RAPN based on tumor factor and do not include patient factors. Urologist do not use MAP scores nowadays routinely to predict surgical difficulty during RAPN. Thus, we decided to study to validate MAP (Mayo Adhesive Probability) score and preoperative factors to predict intraoperative adherent perinephric fat in robotic-assisted partial nephrectomy.
In our study, we found four factors include increased perirenal fat stranding, posterior perinephric fat thickness, increase in age, and increased creatinine was significantly associated with APF. Smoking, high BMI, Sex of patient, tumor size, and lateral perinephric fat thickness do not significantly predict APF in our study. From four significant factor, perinephric fat stranding and posterior perinephric fat stranding were most significantly predicted APF. We combined the score of these two factors to create a MAP score. Our study validates the MAP score given by Davidiuk et al. [11], who had done a study of 100 patient from which 30 patient (30%) had APN.
We included patients above 18 years of age. The mean age of this patient in our study was 51.2 + 14.1 years with a maximum of 77 years and a minimum of 20 years. The median age was 51.5 years. The median age in study by Davidiuk et al. [11] was 63 years. In our study age is less in comparison. In our study increase in age is associated with significant intraoperative adhesive perinephric fat, but in the study by Davidiuk et al. [11], there was no significant intraoperative adhesive perinephric fat with an increase in age.
Our study suggests a significant correlation between intraoperative adherent perinephric fat and an increase in creatinine (p = 0.003). In the study by Davidiuk et al. [11], they did not suggest a significant correlation between intraoperative adherent perinephric fat and creatinine. The reasons could be due to ascertainment bias or bias due to small sample population. A theoretical probable explanation is that increased serum creatinine levels due to chronic pyelonephritis may have resulted in APF.
In our study, we found a significantly increased likelihood of APF in patients who had a greater posterior perinephric fat thickness (< 1.0 cm: 21.6%; 1.0–1.9 cm: 56.25%; > = 2.0 cm: 77.7%; p = 0.002), and patients who had greater perinephric stranding (no stranding: 14.7%, type 1: 46.7%, type 2: 92.3%; p < 0.001). We could not find any significant correlation between APF and lateral perinephric fat thickness. The study by Davidiuk et al. [11] showed that there was a significantly increased APF in patients who had a greater posterior perirenal fat thickness (< 1.0 cm: 5%; 1.0–1.9 cm: 23%; > = 2.0 cm: 66%; p < 0.001), patients who had a greater lateral perirenal fat thickness (< 1.5 cm: 5%; 1.5–2.4 cm: 30%; > = 2.5 cm: 64%; p < 0.001), and patients who had greater perirenal stranding on CT scan (no stranding: 14%, type 1: 80%, type 2: 92%; p < 0.001).
From the four significant factors associated with APF in our study, perinephric fat stranding and posterior perinephric fat thickness were the most significant predictors. We combined the score of these two factors to create a Mayo Adhesive Probability (MAP) score that ranges from 0 to 5, to predict the presence of APF. We observed APF in 10.71% of patients with a MAP score of 0, 25% with a score of 1, 50% with a score of 2, 67% with a score of 3–4, and 100% of patients with a score of 5 in our study (n = 62) Davidiuk et al. [11], also calculated MAP score by a combination of score given to the two most significant factors (posterior perirenal fat thickness and perirenal fat stranding). He observed APF in 6% of patients with a MAP score of 0, 16% with a score of 1, 31% with a score of 2, 73% with a score of 3–4, and 100% of patients with a score of 5. MAP score of both studies was comparable in percentage of the patients having APF with same MAP score and thus our study validate the MAP score given by Davidiuk et al. [11].
Kawamura et al. [16] did a study in the Asian population that underwent partial nephrectomy (n = 237). He observed APF in forty patients (17%). He found male patient has a significantly increase in APF. His result show MAP score was valid to predict intraoperative APF. Ji et al. [17], in their study showed posterolateral and medial perirenal fat thickness and DM associated with MAP score. Tumor type (malignant vs. benign) was not statistically different. Haehn et al. [18], in their study, involved 100 open partial nephrectomy by single surgeon and found 43 patients had intraoperative APF and his result validated MAP score for open partial nephrectomy The MAP score had the ability to predict APF in open partial nephrectomy (95% CI). APF was found in 6% of patients with a MAP score of 0–1, 27% with score 2, 52% with score 3, 75% with score 4, and 90% with score 5.
The literature shows with an increase in visceral obesity surgery become difficult. In 2010 Morris et al. [19], noted intraabdominal fat (IAF) better predicted intraoperative complication and dissection difficulty as compared to higher BMI and outer abdominal fat thickness. One study by House et al. [20], also suggests that retro renal fat more than 2 cm was associated with higher complications in pancreaticoduodenectomy. Anderson et al. [21] noted longer operation duration in laparoscopic donor nephrectomy inpatients having increased posterior renal fat thickness and in men. Intraabdominal fat-like perinephric fat thickness was better than BMI in the prediction of dissection difficulty, OT time, postoperative complications, and blood loss in patients undergoing PN in two studies [22, 23]. It was thought that non-surgeon-friendly fat may complicate surgery on patients with increased visceral fat. Bylund et al. [23] first sought to conclude that increased thickness of perinephric fat, male sex, and stranding was associated with the finding of APF intraoperatively during PN in a cohort of 29 patients.
From our study, we can conclude that perinephric fat stranding and more fat thickness were responsible for intraoperative APF and difficulty in dissection in RAPN. We validate the MAP score in RAPN. So, based on the MAP score, we can know the APF or the hostile fat preoperatively and anticipate surgical difficulty and decide the best surgical option. This is based on CT scan only which is done routinely preoperatively and no other imaging is required to derive on this score. This helps the novice surgeon with less laparoscopic or robotic experience to choose alternative options like open partial nephrectomy. While at the same time, we cannot comment based on our study whether MAP score can be applied to open partial nephrectomy or not.
Limitations of the study include the relatively small population under study. We did not study the postoperative outcomes in relation to APF.
5 Conclusions
MAP score can be easily calculated preoperatively from a CT scan. We validate the MAP score in RAPN. With an increase in MAP score, adhesive perinephric fat or hostile fat increases and this increases the surgical difficulty. This helps in deciding surgical difficulty for all Urologists doing this surgery.
Availability of data and materials
The datasets generated in the current study are available from the corresponding author on reasonable request.
Abbreviations
- MAP score:
-
Mayo Adhesive Probability score
- APF:
-
Adherent perinephric fat
- IAF:
-
Intraabdominal fat
- BMI:
-
Body mass index
- CKD:
-
Chronic kidney disease
- RAPN:
-
Robotic-assisted partial nephrectomy
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Laguna MP, Algaba F, Cadeddu J, Clayman R, Gill I, Gueglio G et al (2014) Current patterns of presentation and treatment of renal masses: a clinical research office of the endourological society prospective study. J Endourol 28(7):861–870
EAU Guidelines on RCC—DISEASE MANAGEMENT - Uroweb [Internet]. Uroweb - European Association of Urology [cited 3 Jan 2023]. https://uroweb.org/guidelines/renal-cell-carcinoma/chapter/disease-management
Delto JC, Paulucci D, Helbig MW, Badani KK, Eun D, Porter J et al (2018) Robot-assisted partial nephrectomy for large renal masses: a multi-institutional series. BJU Int 121(6):908–915
Long JA, Yakoubi R, Lee B, Guillotreau J, Autorino R, Laydner H et al (2012) Robotic versus laparoscopic partial nephrectomy for complex tumors: comparison of perioperative outcomes. Eur Urol 61(6):1257–1262
Choi JE, You JH, Kim DK, Rha KH, Lee SH (2015) Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol 67(5):891–901
Kutikov A, Uzzo RG (2009) The RENAL nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 182(3):844–853
Simmons MN (2011) Morphometric characterization of kidney tumors. Curr Opin Urol 21(2):99–103
Simmons MN, Ching CB, Samplaski MK, Park CH, Gill IS (2010) Kidney tumor location measurement using the C index method. J Urol 183(5):1708–1713
Gorin MA, Mullins JK, Pierorazio PM, Jayram G, Allaf ME (2013) Increased intra-abdominal fat predicts perioperative complications following minimally invasive partial nephrectomy. Urology 81(6):1225–1230
Davidiuk AJ, Parker AS, Thomas CS, Leibovich BC, Castle EP, Heckman MG et al (2014) Mayo Adhesive Probability score: an accurate image-based scoring system to predict adherent perinephric fat in partial nephrectomy. Eur Urol 66(6):1165–1171
Eisner BH, Zargooshi J, Berger AD, Cooperberg MR, Doyle SM, Sheth S et al (2010) Gender differences in subcutaneous and perirenal fat distribution. Surg Radiol Anat SRA 32(9):879–882
Kim S, Choi SK, Lee SM, Choi T, Lee DG, Min GE et al (2013) Predictive Value of Preoperative Unenhanced Computed Tomography During Ureteroscopic Lithotripsy: A Single Institute’s Experience. Kor J Urol 54(11):772–777
Assel M, Sjoberg D, Elders A, Wang X, Huo D, Botchway A et al (2019) Guidelines for Reporting of Statistics for Clinical Research in Urology. J Urol 201(3):595–604
Zheng Y, Espiritu P, Hakky T, Jutras K, Spiess PE (2014) Predicting ease of perinephric fat dissection at time of open partial nephrectomy using preoperative fat density characteristics. BJU Int 114(6):872–880
Kawamura N, Saito K, Inoue M, Ito M, Kijima T, Yoshida S et al (2018) Adherent Perinephric Fat in Asian Patients: Predictors and Impact on Perioperative Outcomes of Partial Nephrectomy. Urol Int 101(4):437–442
Ji C, Tang S, Yang K, Xiong G, Fang D, Zhang C et al (2017) Analysis of Factors Influencing Mayo Adhesive Probability Score in Partial Nephrectomy. Med Sci Monit Int Med J Exp Clin Res 20(23):6026–6032
Haehn DA, Bajalia EM, Cockerill KJ, Kahn AE, Ball CT, Thiel DD (2021) Validation of the Mayo Adhesive Probability score as a predictor of adherent perinephric fat and outcomes in open partial nephrectomy. Transl Androl Urol 10(1):227–235
Morris K, Tuorto S, Gönen M, Schwartz L, DeMatteo R, D’Angelica M, et al (2010) Simple measurement of intra-abdominal fat for abdominal surgery outcome prediction. Arch Surg Chic Ill 1960. 145(11):1069–1073
House MG, Fong Y, Arnaoutakis DJ, Sharma R, Winston CB, Protic M et al (2008) Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg Off J Soc Surg Aliment Tract 12(2):270–278
Anderson KM, Lindler TU, Lamberton GR, Baron PW, Ojogho OK, Baldwin DD (2008) Laparoscopic donor nephrectomy: effect of perirenal fat upon donor operative time. J Endourol 22(10):2269–2274
Macleod LC, Hsi RS, Gore JL, Wright JL, Harper JD (2014) Perinephric fat thickness is an independent predictor of operative complexity during robot-assisted partial nephrectomy. J Endourol 28(5):587–591
Bylund JR, Qiong H, Crispen PL, Venkatesh R, Strup SE (2013) Association of clinical and radiographic features with perinephric “sticky” fat. J Endourol 27(3):370–373
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
RP, ZK, NP did the material preparation, data collection and analysis. Statistical analysis was done by RP. The first draft of the manuscript was written by RP. AS, AG, RS, MD commented on subsequent versions of the manuscript. All authors contributed to the study conception and design. All authors read and approved the final manuscript. The requirements for authorship have been met and each author believes that the manuscript represents honest work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments. The Institutional Ethics Committee gave the approval for the study (MPSRNUEC). Number – EC/635/2020. Written informed written consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed the informed written consent regarding publishing their data and photographs.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Prajapati, R., Pathak, N., Ganpule, A. et al. Validation of MAP (Mayo Adhesive Probability) score and preoperative factors to predict adherent perinephric fat in robotic-assisted partial nephrectomy. Afr J Urol 29, 30 (2023). https://doi.org/10.1186/s12301-023-00362-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-023-00362-6