Abstract
Background
We aimed to evaluate the role of plasma fibrinogen and D-dimer as prognostic biomarkers in patients with non-muscle invasive bladder cancer (NMIBC).
Methods
The prospective study included 35 patients (30 males) with newly diagnosed NMIBC with no history of thromboembolic event or anti-coagulant intake or active infection and underwent complete trans-urethral resection between September 2020 and December 2021. Patients with deranged hepato-renal functions, refractory hypertension or diagnosed with COVID-19 infection with in one-month before surgery or routine follow-up were excluded. Follow-up was done as per NCCN guidelines. Fibrinogen and D-dimer levels were measured with in seven days of surgery or follow-up and analyzed for recurrence-free survival (RFS) and progression-free survival (PFS). Cox regression analyses were adopted to assess the influence of these two parameters on RFS and PFS.
Results
The mean age was 53.9 years with a median follow-up of 9-months. Nine had recurrence of which six had progression. The cut-off values of fibrinogen and D-dimer were 402.5 mg/dl and 0.55 µg/ml, respectively. Kaplan–Meier analysis demonstrated that high fibrinogen and D-dimer levels were significantly related to poor RFS and PFS (p < 0.001). On multivariate analysis only fibrinogen and D-dimer retained their significance for RFS (p = 0.026 and 0.014, respectively) and PFS (p = 0.027 and 0.042, respectively). High levels of fibrinogen and D-dimer were also present in patients who had recurrence or progression at follow-up visits compared to rest of the patients.
Conclusions
High levels of fibrinogen and D-dimer may indicate worse prognosis in patients with NMIBC, suggesting that these two can be used as prognostic biomarkers.
Similar content being viewed by others
1 Background
Bladder cancer is second most prevalent tumors of the genitourinary tract worldwide. Its incidence in developed countries (9.5 per 100,000) is almost three times higher than that in less developed countries (3.3 per 100,000) [1]. Approximately 70% of patients initially diagnosed with UCB have their tumor localized to mucosa and submucosa, known as non-muscle invasive bladder carcinoma (NMIBC) [2]. Such patients usually have a good prognosis, but there is risk of recurrence in 48–70% of the cases and progression to muscle invasiveness in about 27–61% of the cases [3, 4]. The standard therapeutical protocol that is followed for these patients includes transurethral resection of bladder tumor (TURBT), eventually combined with intravesical instillation of chemotherapeutic agents based upon risk stratification [5]. Owing to frequent relapse and high progression rates, active cancer surveillance using standard methods of diagnosis is important, which makes bladder cancer one of the most expensive cancer to manage. Several prognostic factors have been reported that predict the recurrence and progression in patients with NMIBC, such as serum cholinesterase, neutrophil to lymphocyte ratio, albumin to-globulin ratio, etc., to identify patients with high or low risk and tailor further management accordingly. However, till date, none of these factors have been able to accurately predict the risk of progression or recurrence, in patients with bladder cancer [6,7,8]. Various risk stratification systems have been developed such as EORTC risk tables, the Club Urológico Espanol de Tratamiento Oncológico (CUETO) scoring model, etc., to predict the probability of recurrence and progression but lacks independent external validation [9]. These scoring models use clinical and pathological variables to estimate the risk of recurrence and progression. But evaluation of some of these variables is subjective such as tumor size and number. All these inaccuracies may lead to an incorrect tumor classification, which makes the process of validation complicated [9,10,11]. Therefore, it is essential that some simple prognostic markers in NMIBC be identified so that better evaluation of tumor aggressiveness and its prognosis can be done, and accordingly accurate individualized treatment could be provided.
Several studies have confirmed that parameters of the coagulation/fibrinolysis system are abnormal in various cancers [12, 13]. Cao et al. reported that preoperative D-dimer levels can predict survival in patients with resectable pancreatic cancer [14]. Hou et al. observed that the number of elevated preoperative coagulation factors may have a significant effect on progression-free survival and could be used to predict the prognosis of non-small cell lung carcinoma patients after surgery [15]. The exact molecular mechanism of plasma fibrinogen and D-dimer by which they promote disease progression remains controversial. It has been observed that fibrinogen gets deposited around malignant tumor cells to form a protective shield against endogenous defense mechanisms. It also promotes angiogenesis, metastasis, and proliferation as well as potentiate the effect of growth factors such as the vascular endothelial growth factor and the fibroblast growth factor, on tumor cells [16]. It has also been reported that tumor cells themselves could promote conversion of fibrinogen into fibrin, thereby increasing their ability of vascular growth, proliferation, and invasion [17]. D-dimer is a specific cleavage product of fibrin and its high levels in plasma represents hyperfibrinolysis. High levels of plasma D-dimer have also been reported to indicate the existence of circulating tumor cells or micro-metastases and help in predicting high-risk pathological features of tumors [18].
However, the potential prognostic value of coagulation parameters, plasma fibrinogen and D-dimer, in predicting recurrence-free (RFS) and progression-free survival (PFS) in patients with NMIBC remains unknown. So, the purpose of our study was to evaluate prognostic significance of plasma fibrinogen and D-dimer in patients with NMIBC.
2 Methods
All patients who presented for the first time with bladder mass between September 2020 and December 2021 were included in this prospective observational study after obtaining approval from the Institutional Ethics committee (803/Ethics/2020. Reference code: 101st ECM II B/P48). Informed written consent was taken from all the patients included in this study after explaining the nature of study. The selected patients met the following inclusion criteria: (1) presenting for first time with bladder mass; (2) the post-operative pathological results after TURBT consistent with NMIBC; (3) results of plasma fibrinogen and D-dimer levels available from within seven days before surgery and on follow-up; (4) a minimum follow-up duration of six months. The excluded patients had following characteristics: (1) incomplete resection (2) perioperative death (3) thromboembolic event within three months or congenital thrombophilia (4) anticoagulant or pro-coagulant therapy within the past eight-weeks (5) patients with deranged liver or renal functions (6) active infection, endocarditis, pregnancy, inflammatory bowel disease or serious refractory hypertension (7) patients who were diagnosed with COVID-19 infection within one-month before surgery or routine follow-up.
2.1 Methodology
Clinical characteristics of patients, including age, sex, BMI, tobacco exposure and general physical examination were recorded. Patient’s fasting blood samples were collected within seven days before surgery and on follow-up to determine the levels of plasma fibrinogen and D-dimer. Biomarker levels were measured using an automatic analyzer (Stago STA Satellite®, France). Plasma fibrinogen and D-dimer were measured using the solidification and immune-turbidimetry method, respectively.
All patients underwent TURBT and intra-operative findings were recorded. The pathological diagnosis of tumor was established by a single pathologist, with > ten-years of experience in genitourinary malignancies, based on hematoxylin–eosin stained sections combined with immune-histochemical methods. Patient’s pathological characteristics including pathological stage and differentiation grade were recorded according to the UICC-TNM (2018) and WHO/ISUP (2004) classification, respectively.
Patients were followed up as per the NCCN-guidelines. The routine investigations at each follow-up consist of physical examination, blood investigations (including Plasma fibrinogen and D-dimer) and check cystoscopy. Excretory urogram and/or computed tomography (CT) was done to assess the upper urinary tract annually. The authors confirm the availability of, and access to all original data reported in this study.
2.2 Statistical analysis
All prospective data was analyzed using IBM SPSS Statistics Windows, version 24.0 (Armonk, NY: IBM Corp.). The receiver operating characteristic (ROC) curve for preoperative D-dimer and plasma fibrinogen were built to evaluate the optimal cut-off values of the coagulation parameters. The relationship between the clinico-pathologic factors and the coagulation parameters was analyzed using Chi-square test and Fisher’s exact test. The RFS (the interval from primary TURBT until the first reoccurrence of disease) and the PFS (the interval from initial surgical therapy to first progress of disease i.e. any advance in tumor grade or stage) were evaluated by creating Kaplan–Meier curves. Variables showing significant impact on survival in univariate analysis (p ≤ 0.05) were included in the Cox proportional hazard regression models for multivariate analysis. A p < 0.05 was considered to be statistically significant.
3 Results
A total of 66 patients were operated at our center from September 2020 to June 2021, who presented for the first time with a urinary bladder mass. Of these, 35 patients fulfilled the inclusion/exclusion criteria and were included in the study. Thirty were males and the mean age of subjects was 53.91-years (range, 27–81 years). The median follow-up was 9-months (range, 3–15-months). The demographic details are depicted in Table 1. Nine (25.71%) patients had disease recurrence, with progression to higher grade in two (5.7%) and invasion of muscle in four (11.4%).
3.1 Determination of optimal cut-off values of plasma fibrinogen and D-dimer regarding prediction of RFS
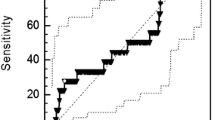
The mean value of pre-operative plasma fibrinogen and D-dimer was 351.19 mg/dl (range, 217–505 mg/dl) and 0.448 µg/ml (range, 0.13–1.40 µg/ml), respectively. Patients who had recurrence of disease, had higher mean value of pre-operative plasma fibrinogen and D-dimer (428.22 mg/dl and 0.72 µg/ml, respectively) compared to patients without recurrence (323.69 mg/dl and 0.36 µg/ml, respectively). ROC curves were drawn to obtain optimal cut-off values for pre-operative plasma fibrinogen and D-dimer, Fig. 1. The optimal cut-off value for plasma fibrinogen was 402.5 mg/dl (area under curve- 0.887, sensitivity 0.667, specificity 1.0) and D-dimer was 0.55 µg/ml (area under the curve 0.949, sensitivity 0.889, specificity 0.962). Patients were divided into two groups on the basis of these cut-off values for further analyses. High levels of plasma fibrinogen and D-dimer were present in six (17.1%) and nine (25.7%) patients, respectively, and low levels were present in 29 (82.9%) and 26 (74.3%) patients, respectively.
3.2 Relationship between clinico-pathologic characteristics and plasma fibrinogen and D-dimer in patients with NMIBC
Pre-operative plasma fibrinogen and D-dimer levels of patients were compared with demographic and clinico-pathological characteristics. Statistically, significantly higher levels of plasma fibrinogen and D-dimer were found in patients with larger tumor size (> 3 cm) compared to those with tumor size ≤ 3 cm (p < 0.001). No significant association was recorded with any other characteristic of patients, including BMI (p = 0.983) or the duration of symptoms (p = 0.839) (Table 2).
3.3 Relationship between plasma fibrinogen, D-dimer and RFS in patients of NMIBC
To assess the potential prognostic value of plasma fibrinogen and D-dimer in predicting RFS, Kaplan–Meier survival analysis was carried out. The results demonstrated that patients with plasma fibrinogen level > 402.5 mg/dl (p < 0.001) and D-dimer level > 0.55 mcg/ mL (p < 0.001) had significantly poorer RFS compared to those with lower levels of both the parameters, Fig. 2. Univariate and multivariate analysis was done using Cox proportional hazards model to find any significance between patient’s demographic characteristics, clinico-pathological parameters, pre-operative plasma fibrinogen and D-dimer, and risk of recurrence or progression of disease. Three parameters, including tumor size, pre-operative plasma fibrinogen and D-dimer, were found to be related to RFS on univariate analysis but on multivariate analysis only two parameters, high levels of pre-operative plasma fibrinogen and D-dimer were found to have independent association with a shorter RFS (p = 0.026 and 0.014, respectively) (Table 3).
3.4 Plasma fibrinogen, D-dimer and progression-free survival (PFS) of NMIBC patients
To assess the potential prognostic value of plasma fibrinogen and D-dimer in predicting PFS, Kaplan–Meier survival analysis was carried out. The results demonstrated that patients with plasma fibrinogen level more than 402.5 mg/dl (p < 0.001) and D-dimer level more than 0.55 mcg/ mL (p < 0.001) had significantly poorer PFS compared to those with lower levels of both the parameters (Fig. 1). Univariate and multivariate analysis was done using Cox proportional hazards model to find any significance between patient’s demographic characteristics, clinico-pathological parameters, coagulation parameters (pre-operative plasma fibrinogen and D-dimer) and risk of recurrence or progression of disease. Three parameters, including, tumor size, pre-operative plasma fibrinogen and D-dimer, were found to be related to PFS on univariate analysis but on multivariate analysis only two parameters, high levels of pre-operative plasma fibrinogen and D-dimer, were found to have independent association with a shorter PFS (p = 0.027 and 0.042 respectively) (Table 4).
3.5 Relationship between RFS, PFS and coagulation parameter’s level at follow up
It was also observed that mean plasma fibrinogen and D-dimer values were significantly higher at 3-month and 6-month follow-up in patients who had recurrence (p < 0.001) or progression (p < 0.001) of disease compared to those who had neither recurrence nor progression (Table 5).
4 Discussion
Urothelial carcinoma accounts for more than 90% of all the bladder tumors [19]. Majority of new patients, who present with bladder mass are initially diagnosed with NMIBC, which frequently recurs [3, 4]. Also, the patients who progress to muscle invasive disease have a poorer prognosis compared to those who had MIBC at initial presentation [20]. Thus, it is important to identify factors that can predict high risk disease in NMIBC patients, so that appropriate risk adapted treatment and surveillance strategies could be employed in such patients for early detection and timely management.
A total of 66 new patients with bladder mass were operated in our institute from September 2020 to June 2021, but only 35 patients were eligible for inclusion into the study. Similar to other large studies there was a clear male preponderance (30 versus 5) [21, 22].
Age is a strong and independent risk factor for the development of UCB [23]. The mean age at presentation was 53.91-years (SD ± 12.2; range, 27–81). No significant difference was noticed in the histological grade of tumor among patients of different age groups.
Various demographic and clinico-pathological characteristics including smoking, increased BMI, high tumor grade, large size (> 3 cm), multiple number and T-stage have been associated with increased risk of bladder tumor recurrence and progression [4, 24, 25]. In our study, only tumor size (> 3 cm) was found to be significantly associated with increased risk of recurrence and progression on univariate analysis (p = 0.007 and 0.043, respectively); however, on multivariate analysis, it failed to sustain independent association. No statistically significant relationship was found among rest of the demographic or clinico-pathological characteristics with the risk of recurrence or progression. The reason could be small sample size.
Numerous studies have demonstrated that the coagulation/fibrinolytic system is initiated in vivo in patients with various cancers and the levels of these parameters can be used to predict tumor load and prognosis [14, 15]. In another study, Chen et al. reported that elevated plasma D-dimer levels (> 0.36 mg/L) were significantly associated with poorer survival in patients with upper tract urothelial carcinoma. They also reported that pre-operative D-dimer levels can help in improving the risk stratification and a more accurate prediction of recurrence and survival. This can help in decision making for follow-up scheduling, administration of adjuvant therapies, and precise individualized treatment for patients [26]. In another study on prognostic value of preoperative inflammation-based predictors in patients with bladder carcinoma by Gui et al., they reported that high plasma fibrinogen levels are associated with a more aggressive clinical stage and plasma fibrinogen levels may be used to predict survival outcome of patients with bladder carcinoma [27].
In our study, we found that high levels of pre-operative plasma fibrinogen (> 402.5 mg/dl) and D-dimer (> 0.55 µg/ml) were present in 13 (37.1%) and 10 (28.6%) patients and low levels were present in 22 (62.9%) and 25 (71.6%) patients, respectively. When compared with patient’s demographic and clinic-pathological characteristics, statistically significant higher levels of pre-operative plasma fibrinogen (> 402.5 mg/dl) and D-dimer (> 0.55 µg/ml) were found in patients with larger tumor size (> 3 cm) compared to those with smaller tumor size (p < 0.001). Higher levels of pre-operative plasma fibrinogen (> 402.5 mg/dl) and D-dimer (> 0.55 µg/ml) were not found to be significantly associated with any other demographic or clinico-pathological characteristic of patient.
On multivariate analysis, it was also observed that patients with recurrent disease and those who had progression, had significantly higher levels of the pre-operative plasma fibrinogen (> 402.5 mg/dl; p = 0.026 and 0.027, respectively) and D-dimer (> 0.55 µg/ml; p = 0.014 and 0.042, respectively) compared to those who had no recurrence or progression of disease within the follow-up period. To assess the potential prognostic value of these two coagulation parameters (plasma fibrinogen and D-dimer) in predicting RFS and PFS, Kaplan–Meier survival analysis was performed. The results demonstrated that patients with higher levels of plasma fibrinogen (> 402.5 mg/dl) and D-dimer (> 0.55 mcg/ mL) had significantly shorter RFS (p < 0.001) and PFS (p < 0.001) compared to those with lower levels of both the parameters. These results were similar to the study by Li et al. that demonstrated that NMIBC patients who had high plasma fibrinogen (> 3.56 g/L) and D-dimer (> 0.48 mg/mL) levels, had worse pathologic features (T-stage, tumor size and multiplicity of tumor) as well as significantly poor RFS (p = 0.029 and 0.001, respectively) and PFS (p = 0.023 and 0.003, respectively) [28].
In our study, we also found that mean plasma fibrinogen and D-dimer value was significantly higher at 3-month and 6-month follow-up in patients who had recurrence (p < 0.001) or progression (p < 0.001) of disease compared to those who had neither recurrence nor progression. But a statistically significant association could not be established for mean plasma fibrinogen values at 9-months and 12-month follow-up because of very small number of patients. After thorough literature search, we could not find any study comparing the levels of plasma fibrinogen and D-dimer at follow-up visits with the risk of recurrence and progression. In our study too, definitive inference could not be drawn due to a very small sample size.
Our study has several limitations. First, the surgeons performing the surgery were heterogeneous with residents in training also performing many TURBT under supervision of trained consultant urologists. Second, the duration of follow-up is very short with a maximal follow-up of 15-months. Lastly, the sample size was relatively small. Therefore, larger prospective studies are needed to further consolidate the position of plasma fibrinogen and D-dimer in predicting disease recurrence and progression in patients with NMIBC and help in making individualized risk-adapted treatment decisions.
5 Conclusions
High levels of plasma fibrinogen and D-dimer may serve as prognostic factors in patients with NMIBC to predict RFS and PFS. Patients with higher risk of recurrence or progression have higher levels of plasma fibrinogen and D-dimer at subsequent follow-up also. These coagulation parameters can help in making individualized risk-adapted treatment decisions and surveillance strategies. A further study with longer follow-up and larger sample size is required for better interpretation of result.
Abbreviations
- AR:
-
Androgen receptor
- ATF:
-
Activating transcription factors
- BC:
-
Bladder cancer
- BMI:
-
Body mass index
- EMT:
-
Epithelial mesenchymal transition
- MIBC:
-
Muscle invasive bladder carcinoma
- MMC:
-
Mitomycin-C
- NLR:
-
Neutrophils to lymphocyte ratio
- NMIBC:
-
Non-muscle invasive bladder carcinoma
- PFS:
-
Progression-free survival
- RFS:
-
Recurrence-free survival
- SCC:
-
Squamous cell carcinoma
- SUO:
-
Society of urologic oncology
- TURBT:
-
Transurethral resection of bladder tumor
- UCB:
-
Urothelial carcinoma of bladder
- UTUC:
-
Upper tract urothelial carcinoma
- WLC:
-
White light cystoscopy
References
Sanli O, Dobruch J, Knowles MA (2017) Bladder cancer. Nat Rev Dis Primers 3(17):22
Burger M, Catto JWF, Dalbagni G (2013) Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 63:234–241
Cheng L, Davison DD, Adams J (2014) Biomarkers in bladder cancer: translational and clinical implications. Crit Rev Oncol Hematol 89:73–111
Montironi R, Lopez-Beltran A (2005) The 2004 WHO Classification of bladder tumors: a summary and commentary. Int J Surg Pathol 13:143–153
Cao J, Fu Z, Gao L (2017) Evaluation of serum D-dimer, fibrinogen, and CA19–9 for postoperative monitoring and survival prediction in resectable pancreatic carcinoma. World J Surg Oncol 15:48–54
Getzler I, Bahouth Z, Nativ O, Rubinstein J, Halachmi S (2018) Preoperative neutrophil to lymphocyte ratio improves recurrence prediction of non-muscle invasive bladder cancer. BMC Urol 18(1):90
Kimura S, Soria F, D’Andrea D (2018) Prognostic value of serum cholinesterase in non-muscle-invasive bladder cancer. Clin Genitourin Cancer 16(6):1123–1132
Quhal F, Pradere B, Laukhtina E (2021) Prognostic value of albumin to globulin ratio in non-muscle-invasive bladder cancer. World J Urol 39(9):3345–3352
Witjes JA, Moonen PM, van der Heijden AG (2006) Review pathology in a diagnostic bladder cancer trial: effect of patient risk category. Urology 67:751–755
Soukup V, Capoun O, Cohen D (2017) Prognostic performance and reproducibility of the 1973 and 2004/2016 World Health Organization grading classification systems in non-muscle-invasive bladder cancer: a European Association of Urology Non-muscle Invasive Bladder Cancer Guidelines Panel systematic review. Eur Urol 72:801–813
Soukup V (2020) Risk stratification tools and prognostic models in non–muscleinvasive bladder cancer: a critical assessment from the european association of urology non-muscle-invasive bladder cancer guidelines panel. Eur Urol Focus 6(3):479–489
Guo Q, Zhang B, Dong X (2009) Elevated levels of plasma fibrinogen in patients with pancreatic cancer: possible role of a distant metastasis predictor. Pancreas 38(3):75–79. https://doi.org/10.1097/MPA.0b013e3181987d86
Hanna DL, White RH, Wun T (2013) Biomolecular markers of cancer-associated thromboembolism. Crit Rev Oncol Hematol 88(1):19–29
Cao J, Fu Z, Gao L (2017) Evaluation of serum D-dimer, fibrinogen, and CA19-9 for postoperative monitoring and survival prediction in resectable pancreatic carcinoma. World J Surg Oncol 15:48–54
Hou C, Jiang F, Ma H (2019) Prognostic role of preoperative platelet, fibrinogen, and D-dimer levels in patients with non-small cell lung cancer: a multicenter prospective study. Thorac Cancer 10:304–311
Perisanidis C, Psyrri A, Cohen EE (2015) Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev 41:960–970
Hong SK, Ko DW, Park J (2010) Alteration of antithrombin III and D-dimer levels in clinically localized prostate cancer. Korean J Urol 51:25–29
Fukumoto K, Taniguchi T, Usami N (2015) Preoperative plasma D-dimer level is an independent prognostic factor in patients with completely resected non-small cell lung cancer. Surg Today 45:63–67
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Lee CT, Dunn RL, Ingold C (2007) Early-stage bladder cancer surveillance does not improve survival if high-risk patients are permitted to progress to muscle invasion. Urology 69(6):1068–1072
Tan WS, Kelly JD (2018) Intravesical device-assisted therapies for non-muscleinvasive bladder cancer. Nat Rev Urol 15(11):667–685
Pabinger I, Ay C (2009) Biomarkers and venous thromboembolism. Arterioscler Thromb Vasc Biol 29(3):332–336
Garcia-Closas M, Malats N, Silverman D (2005) NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet 366:649–659
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. Available from: https://www.ncbi.nlm.nih.gov/books/NBK179276/13
Sylvester RJ, Van der Meijden AP, Oosterlinck W (2006) Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49(3):466–475
Chen X, Ji H, Wang J, Zhao G, Zheng B (2020) Prognostic value of the preoperative plasma d-dimer levels in patients with upper tract urothelial carcinoma in a retrospective cohort study. Onco Targets Ther 13(8):5047–5055
Gui H, Song Y, Yin Y (2021) Prognostic value of preoperative inflammation-based predictors in patients with bladder carcinoma after radical cystectomy. Open Med (Wars) 16(1):816–825
Li X, Shu K, Zhou J (2019) Preoperative plasma fibrinogen and D-dimer as prognostic biomarkers for non-muscle-invasive bladder cancer. Clin Genitourin Canc 10(25–32):14
Acknowledgements
Not Applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
S conceptualized the design of study and did the literature search, data acquisition, analysis and manuscript preparation. AG performed the data analysis and manuscript review. RL and SRS contributes in acquisition of data. SM performed the histopathological analysis of bladder tissue and assisted in determining the plasma fibrinogen and D-dimer values. SNS contributed by data acquisition and manuscript review. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent for participate
This study was done after obtaining approval from the Institutional Ethics committee King George's Medical University, Lucknow (803/Ethics/2020. Reference code: 101st ECM II B/P48). The date of approval was 01/09/2020.
Consent of publication
All authors have approved the manuscript for submission. The content of the manuscript has not been published, or submitted for publication elsewhere.
Informed consent
Informed written consent was taken from all the patients included in this study after explaining the nature of study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Singla, S., Goel, A., Mishra, S. et al. Role of plasma fibrinogen and D-dimer as prognostic biomarkers in patients with non-muscle invasive bladder cancer. Afr J Urol 29, 18 (2023). https://doi.org/10.1186/s12301-023-00350-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-023-00350-w