Abstract
Background
Aerococcus species are Gram-positive cocci, with negative catalase and oxidase activities and growth characteristics similar to viridans streptococci. They rarely cause infection in humans. However, there are reports of bacteremia, meningitis, septic arthritis, and endocarditis due to this pathogen in the literature. Herein we report a rare case of pyelonephritis due to A. viridans.
Case presentation
A 31-year-old-female patient with type 1 diabetes mellitus was presented with left loin pain, fever, nausea, and anorexia for 3 days. She had a history of obstructive nephropathy due to sloughed necrotic papillae 3 months earlier, mandating bilateral JJ stent insertion. She was treated with a 2 weeks course of doxycycline (100 mg, twice daily) based on the antibiotic susceptibility profile of her urine culture and responded well.
Conclusion
This case highlights the possibility of complicated urinary tracts infection due to a rare human pathogen.
Similar content being viewed by others
1 Background
Pyelonephritis is a renal tubulointerstitial infection that causes inflammation of the renal pelvis and parenchyma, resulting from bacteriuria and with the consequence of renal scarring. Nephropathy is a major complication of type 1 (insulin-dependent) diabetes mellitus [1]. Patients with uncontrolled hyperglycemia have an increased susceptibility to infection, with the urinary tract being the second most frequent site having an incidence of 12.9% in females and 3.9% in males [2, 3]. Urinary tract infection (UTI) is one of the most common human infections that can cause critical morbidity and subsequent mortality [4].
In diabetic pyelonephritis, the most common pathogens usually belong to the family Enterobacteriaceae, whereas UTIs due to Gram-positive bacteria are less common but remain important [5, 6]. The Aerococcus spp. are rarely implicated as pathogens in humans and can cause an invasive and diverse spectrum of infections. However, they have low virulence and are mostly pathogenic in patients who are in vulnerable conditions [7]. A. viridans is a microaerophilic Gram-positive coccus, first described in 1953, with negative catalase and oxidase activities, α-hemolytic features, and only highly rarely implicated in UTI [8, 9]. The organism has many growth characteristics similar to enterococci and streptococci, thus routinely misidentified as α-hemolytic streptococci [10]. This study emphasizes the unusual occurrence of isolated A. viridans pyelonephritis in a young female patient with type 1 diabetes mellitus.
2 Case presentation
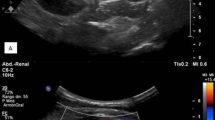
A 31-year-old married female patient with type 1 diabetes mellitus was presented with left loin pain, fever, nausea, and anorexia for the last three days. Further questioning revealed a history of poorly controlled diabetes and recurrent urinary tract infections. Four months prior, she underwent emergency bilateral ureteroscopy for bilateral obstructive nephropathy (serum creatinine 8.4 mg/dL and blood urea 160 mg/dL) resulting from ureteral obstruction secondary to sloughed renal papillae. She was discharged with a baseline serum creatinine of 1.4 mg/dL and advised to observe a low-protein and -carbohydrate diet. On presentation, she had tachycardia (110 BPM), tachypnea (22 cycles/min), fever (39.4 °C), and normal blood pressure. An abdominal examination revealed a soft abdomen, but she had a tender left loin. Blood investigations showed leukocytosis, blood urea 57 mg/dL, serum creatinine 2.5 mg/dL, and CRP 415 mg/dL. US scan showed bilateral grade 1 increased parenchymal echogenicity with left pelvicalyceal dilatation and urothelial thickening. Microscopic analysis of the urine sample revealed pyuria and bacteriuria. Urine culture demonstrated significant growth of Gram-positive cocci with negative catalase and oxidase activities (105 CFU, colony-forming unit). The isolate was subjected to identification using the fully automated instrument BD Phoenix™ M50, USA yielding A. viridans. Antibiotic susceptibility testing was performed using the Kirby–Bauer disc diffusion method following the susceptibility guidelines of the National Committee for Clinical Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [11, 12]. The isolate was sensitive to tetracycline, doxycycline, and clindamycin but resistant to levofloxacin, ciprofloxacin, norfloxacin, penicillin, piperacillin, erythromycin, trimethoprim-sulfamethoxazole, cefepime, and cefotaxime. After sending the urine sample for culturing, the patient was admitted and treated with intravenous fluid, analgesics, empiric meropenem (500 mg, twice daily), and insulin according to the blood glucose level. She responded to the medical management and was discharged home after three days on oral doxycycline (100 mg, twice daily). The new urinalysis showed normal parameters two weeks later, and her serum creatinine was 1.4 mg/dL. Since then, she has been under close observation.
3 Discussion
Diabetes mellitus is a common cause of bacteriuria, cystitis, pyelonephritis, rare complicated UTIs, and renal papillary necrosis [13]. Bilateral pyelonephritis has predominantly been seen among diabetic patients. Obstructive nephropathy causes high intrapelvic pressure in the kidney and subsequent sepsis resulting from biofilm formation by the causative microorganisms. It is a severe condition requiring emergency medical attention from nephrologists [14]. Ultrasonography is the initial diagnostic imaging tool used in patients with pyelonephritis [15]. The ultrasound of the current case revealed bilateral grade one increased parenchymal echogenicity with left pelvicalyceal dilatation and urothelial thickening.
Classic associated symptoms of pyelonephritis include fever, chills, and unilateral or bilateral flank pain with common laboratory findings of pyuria, leukocytic casts, positive urine culture, raised inflammatory markers, and leukocytosis with a neutrophilic shift [16]. The current case had poor glycemic control with the classic symptoms of upper urinary tract infection such as fever, nausea, and anorexia, and the laboratory findings of pyuria, leukocytosis, and elevation of C-reactive protein as markers of inflammation.
Increasingly, rare and antibiotic-resistant microorganisms are found as the causative agents of UTIs in diabetic patients [14]. We isolated the A. viridans strain in significant numbers (> 105 CFU/mL) from the urine culture of the current case.
A. viridans are Gram-positive cocci, and the microscopic appearance shows arranged tetrads and irregular clusters. This appearance is similar to that of staphylococci, while the growth characteristics of the organism are similar to those of streptococci and enterococci, often leading to incorrect diagnosis [8]. Apart from A. viridans, the first subspecies of Aerococcus, two other subspecies were later isolated as rare human pathogens causing infective endocarditis and urinary tract infections, named A. urinae and A. sanguinicola [7]. According to the available literature, the first reported case of A. viridans bacteremia was in Korea [9].
The pathogenicity and virulence of A. viridans have not been fully described. Infections by this organism are seemingly due to previously damaged tissue or nosocomial in origin due to prolonged hospitalization, invasive procedures, neutropenic and diabetic states, or antibiotic treatment [9]. Mohan et al. reported the first case of A. viridans in nosocomial UTI in India [17].
Antimicrobial susceptibility patterns reveal a guide for choosing appropriate antibiotic treatment. Studies from clinical cases and specimens have demonstrated that Aerococcus spp. is generally susceptible to β-lactam antimicrobials (penicillin, ampicillin, amoxicillin-clavulanic acid), and vancomycin, as well as other groups of antibiotics [18, 19]. Schuur et al. reported that 100% of all the Aerococcus spp. were susceptible to penicillin, amoxicillin, and nitrofurantoin [20]. In contrast, Augustine et al. reported a case of endocarditis caused by A. viridans that was resistant to penicillin, ampicillin, cefotaxime, and quinolones [21]. This agrees with the current case, as the isolated A. viridans strain was susceptible to tetracycline, doxycycline, and clindamycin while being resistant to levofloxacin, ciprofloxacin, norfloxacin, penicillin, piperacillin, erythromycin, trimethoprim-sulfamethoxazole, cefepime, and cefotaxime.
4 Conclusion
Despite the rare implication of A. viridans in human infections, the organism is capable of causing severe UTIs, with several penicillin-resistant strains that can complicate the infection further, especially in diabetic patients.
Availability of data and materials
Not applicable.
Abbreviations
- UTI:
-
Urinary tract infection
- CLSI:
-
Clinical Laboratory Standards Institute
- EUCAST:
-
European Committee on Antimicrobial Susceptibility Testing
References
Hudson C, Mortimore G (2020) The diagnosis and management of a patient with acute pyelonephritis. Br J Nurs 29(3):144–150
Nabi T, Rafiq N, Rahman MHU, Rasool S, Wani NUD (2020) Comparative study of emphysematous pyelonephritis and pyelonephritis in type 2 diabetes: a single-centre experience. J Diabetes Metab Disord 19(2):1273–1282
Kumar S, Ramachandran R, Mete U, Mittal T, Dutta P, Kumar V et al (2014) Acute pyelonephritis in diabetes mellitus: Single center experience. Indian J Nephrol 24(6):367
Eswarappa M, Suryadevara S, John MM, Kumar M, Reddy SB, Suhail M (2018) Emphysematous pyelonephritis case series from South India. Kidney Int Rep 3(4):950–955
Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG et al (2011) International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the infectious diseases society of America and the European society for microbiology and infectious diseases. Clin Infect Dis 52(5):e103–e120
Nasr AA, Kishk AG, Sadek EM, Parayil SM (2013) A case report of emphysematous pyelonephritis as a first presentation of diabetes mellitus. Iran Red Crescent Med J. https://doi.org/10.5812/ircmj.10384
Sahu KK, Lal A, Mishra AK, Abraham GM (2021) Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct 9(1):18
Ezechukwu I, Singal M, Igbinosa O (2019) Aerococcus viridans: case report, microbiology, and literature review. Am J Case Rep 20:697
Uh Y, Son JS, Jang IH, Yoon KJ, Hong SK (2002) Penicillin-resistant Aerococcus viridans bacteremia associated with granulocytopenia. J Korean Med Sci 17(1):113–115
Tiwari S, Nanda M (2020) Penicillin resistant Aerococcus viridans septicemia in an immunocompetent person: an unusual case report. Am J Clin Microbiol Antimicrob 3(1):1046
CLSI (2018) M100 performance standards for susceptibility testing. Clinical and Laboratory Standards Institute Wayne (PA)
Giske CG, Turnidge J, Cantón R, Kahlmeter G (2021) Update from the European committee on antimicrobial susceptibility testing (EUCAST). J Clin Microbiol JMC. https://doi.org/10.1128/jcm.00276-21
Chiţă T, Timar B, Muntean D, Bădiţoiu L, Horhat F, Hogea E et al (2017) Urinary tract infections in Romanian patients with diabetes: prevalence, etiology, and risk factors. Ther Clin Risk Manag 13:1
Chávez-Iñiguez JS, Navarro-Gallardo GJ, Medina-González R, Alcantar-Vallin L, García-García G (2020) Acute kidney injury caused by obstructive nephropathy. Int J Nephrol. https://doi.org/10.1155/2020/8846622
Wan Y-L, Lee T-Y, Bullard MJ, Tsai C-C (1996) Acute gas-producing bacterial renal infection: correlation between imaging findings and clinical outcome. Radiology 198(2):433–438
Craig WD, Wagner BJ, Travis MD (2008) Pyelonephritis: radiologic-pathologic review. Radiographics 28(1):255–276
Mohan B, Zaman K, Anand N, Taneja N (2017) Aerococcus viridans: a rare pathogen causing urinary tract infection. J Clin Diagn Res JCDR 11(1):DR01
Zhou W, Nanci V, Jean A, Salehi AH, Altuwaijri F, Cecere R et al (2013) Aerococcus viridans native valve endocarditis. Can J Infect Dis Med Microbiol 24(3):155–158
Humphries RM, Hindler JA (2014) In vitro antimicrobial susceptibility of Aerococcus urinae. J Clin Microbiol 52(6):2177–2180
Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG (1997) Urinary tract infections with Aerococcus urinae in the South of The Netherlands. Eur J Clin Microbiol Infect Dis 16:871–875
Augustine T, Vishnu Bhant B, Bhotia BD (1994) Aerococcus viridans endocarditis. Indian Pediatr 31:599–601
Acknowledgements
None to be declared
Funding
There was no particular grant from public, commercial, or non-profit funding agencies for this research.
Author information
Authors and Affiliations
Contributions
“AS” conceived and designed the manuscript. “RB” and FK drafted the manuscript. “SA”, “KH”, “KM”, “RA”, “DH”, “MM”, “BA” and “RS” critically reviewed, revised, and approved the manuscript. All authors have read and approved the manuscript and agreed to the submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for publication of identifiable details like photographs and/or radiology images and/or case history and/or details within the text was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Bapir, R., Ahmed, S.F., Salih, A.M. et al. Aerococcus viridans pyelonephritis in a young age female patient with type 1 diabetes mellitus: a rare case report. Afr J Urol 28, 58 (2022). https://doi.org/10.1186/s12301-022-00327-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-022-00327-1