Abstract
Background
Bladder cancer (BC) is the tenth most common cancer worldwide with urothelial carcinoma (UC) being the main histologic subtype. Survivin is an apoptosis inhibitor that is associated with tumor proliferation and invasion. P27 is a cyclin-dependent kinase inhibitor that negatively regulates cell proliferation. The expression of both proteins was variable among different solid tumors including UC.
Methods
We aimed to investigate the expression of survivin and P27 in UC of urinary bladder and correlate their expressions with histopathological parameters in an attempt at studying the possibility of their use as targeted therapies. The investigation was performed through immunohistochemical staining for both proteins on sections belonging to 60 UCs and 12 mild chronic cystitis cases (controls). Immunopositivity (number of positive cases) and expression score (percentage of positive urothelial cells) were evaluated.
Results
Both survivin and P27 were absent in urothelial cells of mild chronic cystitis lesions while expressed in 60% and 43.3% of UCs, respectively. High score of survivin and low score of P27 were associated with poor prognostic factors of UC (solid pattern, high grade, and deep tumors). By logistic regression test, survivin expression can be a predictive risk factor associated with solid pattern and high-grade UC, while P27 expression can be a predictive risk factor associated low-grade UC.
Conclusion
High survivin and low P27 expression scores were associated with the studied prognostic factors of UC. Both proteins may play a role in UC progression and can have a value as prognostic and/or diagnostic markers of UC, as well as targeted therapies.
Similar content being viewed by others
1 Background
Bladder cancer (BC) is the tenth most frequent cancer globally and is the eighth leading cause of cancer-related deaths, being the fourth most prevalent cancer in men and the twelfth most frequent cancer in women, with mortality rates almost four times higher in men than in women [1]. In Egypt, it is the fourth most common cancer and the fifth cause of cancer-related deaths [2]. Urothelial carcinoma (UC) is considered the most common histologic subtype of BC [3].
Clinicopathological variables, such as grading and staging systems, have long been recognized as prognostic factors in UC patients; however, more recent insights into molecular pathogenesis of UC had identified potential markers that can predict the progression and behavior of UC. It was observed that UCs of the same grade and stage can behave differently, in addition to that, there is variability in the likelihood of progression, recurrence, and survival [4].
Survival, growth, and proliferation of malignant cells are affected by changes in both pro-apoptotic and anti-apoptotic signaling pathways. Survivin belongs to the family of inhibitors of apoptosis proteins (IAPs) that plays a pivotal role in mitotic progression, apoptosis inhibition, angiogenesis, progression of cancer, and invasion [5]. Survivin gene is expressed during embryonic life but not in terminally differentiated adult tissues. It is expressed in cancer cells during the G2/M phase of the cell cycle and counteracts apoptosis activation during mitosis by interfering with caspase activity [6].
The interactions of cyclins, cyclin-dependent kinases (CDKs), and their inhibitors regulate cell cycle progression. P27 is a cyclin-dependent kinase inhibitor (CKI) that controls protein synthesis during the cell cycle. In a quiescent state, it negatively regulates cell proliferation through the inhibition of the G1 to S phase transition to allow repair of DNA damages [7]. P27 is inactivated in cancer cells by various mechanisms including impaired synthesis, accelerated degradation, and mislocalization. Its expression in UC is contradictory, as it can exhibit reduced or increased expression and distinct nuclear or cytoplasmic location [8].
In the present study, we investigated the significance of survivin and P27 expressions in UC of the urinary bladder, by correlating their expression with clinicopathological features, including tumor pattern (papillary or solid), grade of differentiation, and stage of invasion (non-muscle or muscle-invasive).
2 Methods
2.1 Study design
This retrospective study enrolled sixty specimens of UC that were selected from archival blocks at the Pathology Department, Theodor Bilharz Research Institute in the period from 2020 to2021. Specimens were obtained either as radical cystectomy or transurethral bladder tumor resections. In all cases, we took only samples with histologically identifiable detrusor muscle. None of the patients had received radiation or chemotherapy before sampling.
2.2 Histopathological technique and evaluation
One paraffin-embedded block was selected from each case and cut into 4 μm sections, then stained by hematoxylin and eosin (H&E) stain for routine histopathological examination and diagnosis.
The histological diagnoses, grading, and staging of UCs were based on WHO criteria. UCs were divided according to the grade of differentiation into low (GI-II) and high (GIII) grades, and divided according to the stage of invasion into superficial, non-muscle invasive tumors (Ta, T1) and deep, muscle-invasive tumors (T2-T4) [9]. In addition; 12 specimens with mild chronic cystitis were taken as controls.
The inclusion criteria include cases of primary UC and with properly recorded clinical, radiological, and operative data in their files. Exclusion criteria were cases of non-urothelial malignancy or those with inadequate data in their files.
2.3 Immunohistochemical (IHC) technique
IHC was performed on the formalin fixed-paraffin embedded tissues [10]. The tissue sections were put in the oven at 60 °C for 4 h, deparaffinized in xylene, and rehydrated in a graded ethanol series. Antigen retrieval was performed with 10 ml sodium citrate buffer, pH 6.0, at 90 °C for 30 min. Sections were incubated in 0.03% hydrogen peroxide (EnVision/HRP, Dako) for 10 min at room temperature, to remove endogenous peroxidase activity, then were rinsed in wash buffer. The sections were incubated with survivin antibody (code M3624, Dako, Copenhagen, Denmark) at a dilution of 1:100, and P27 antibody (F-8): sc-1641, Santa Cruz Biotechnology, CA, USA) overnight at 4 °C. Sections were then washed three times for 5 min in PBS. Slides were rinsed in wash buffer and incubated for 30 min in blocking serum (0.04% bovine serum albumin, A2153, Sigma-Aldrich, Shanghai, China, and 0.5% normal goat serum X0907 (EnVision/HRP, Dako). The chromogenic reaction was carried out with 3,3′-diaminobenzidine chromogen solution for 10 min. Finally, sections were counterstained with hematoxylin, dehydrated with graded ethanols, and mounted.
For each setting; positive and negative controls were routinely used. Duodenal tissue was used as a positive control for survivin, while lymphocytes in the used section served as an internal control for P27. Negative controls were carried out in which phosphate-buffered saline was used instead of the primary antibody.
2.4 Scoring and data analysis
The tissue sections were examined by using light microscope (Scope A1, Axio, Zeiss, Germany). Photomicrographs were taken using a microscope camera (AxioCam, MRc5, Zeiss, Germany).
Scoring of survivin and P27 immunostaining was performed in a blind fashion to the patients’ clinicopathological data using × 40 objective.
Both immunopositivity (number of positive cases) and expression score (percentage of positive urothelial cells) were evaluated. Expression in > 10% of urothelial cells was considered positive. Positive expression in 10–50% of urothelial cells = low score, expression in ≥ 50% of urothelial cells = high score [11].
2.5 Statistical analysis
Analyses were performed using SPSS version 23 (IBM Corp., Armonk, New York, USA). The significance of differences in means was calculated using t-test. Fischer’s exact test was used to assess the significance of differences between clinicopathological variables. Logistic regression test was performed to identify prognostic factors in relation to studied clinicopathological variables. Differences were considered statistically significant whenever p < 0.05.
3 Results
Our study sample included 60 specimens of UC belonging to 54 males and 6 females. The majority of them had papillary architecture (55%) and were high-grade (61.6%), whereas half of them were superficial. The mean age of UC patients was 62.47 ± 10.85 years, meanwhile, the mean age of controls was 52.75 ± 14.34 years with a significant difference between them (p < 0.01).
3.1 Survivin immunoreactivity
Positive survivin immunoreactivity was detected as nuclear staining. Table 1 shows that survivin expression was not detected in chronic cystitis cases, while 36/60 (60%) of the studied UCs showed expression.
Survivin immunopositivity was significantly more frequent in tumors with solid growth pattern (92.6%), high-grade of differentiation (83.8%), and deep tumors (80%) compared to papillary tumors (33.3%), low-grade (21.7%), and superficial tumors (40%), respectively.
High and low expression scores were seen in 64 and 36% of survivin-positive UCs. High expression score was significantly higher in solid tumors (80%) and deep tumors (79.2%) compared to papillary tumors (27.3%) and superficial ones (33.3%). Also, high score was more prevalent in high-grade tumors (67.7%) compared to low-grade ones (40%) without significant difference.
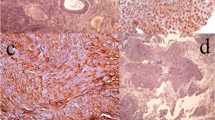
Furthermore, all papillary, low-grade, and superficial UCs had low expression scores (Fig. 1).
Hematoxylin and eosin staining of urothelial carcinoma (a, b) and immunohistochemical expression of survivin (c, d) and P27 (e, f). a Low-grade papillary urothelial carcinoma (× 200), b High-grade urothelial carcinoma (× 400), c Low-grade papillary urothelial carcinoma with low survivin score, nuclear expression (× 200), d High-grade solid urothelial carcinoma with high survivin score, nuclear expression (× 200), e Low-grade papillary urothelial carcinoma with high P27 score, nuclear expression (× 200), f High-grade urothelial carcinoma with high P27 score, cytoplasmic expression (× 200)
Regarding patients’ sex, survivin was detected in 35/60 (64.8%) of UCs in males, 23 of them (65.7%) were of high expression score and the remainder were of low score. Only 1/6 (16.7%) of females was positive for survivin with low expression score.
3.2 P27 immunoreactivity
P27 immunoreactivity was confirmed in 26/60 (43.3%) of UCs which was expressed as nuclear and cytoplasmic staining of UCs cells. However, in chronic cystitis cases, P27 was detected only in scattered nuclei of lymphocytes.
Table 2 shows that P27 immunopositivity was observed in 45.5% of papillary tumors compared to 40.7% of solid ones, without statistical significance. P27 was significantly more frequent in low-grade tumors (74%) and superficial tumors (63.3%) compared to high-grade (24.3%) and deep tumors (23.3%), respectively.
High and low expression scores were seen in 27% and 73% of P27-positive UCs, respectively. High score was predominant in papillary (64.7%), low-grade (64.7%), and superficial (58%) tumors, whereas all solid, high-grade, and deep UCs have low expression scores (Fig. 1).
By considering patients’ sex, P27 was detected in 24/54 (44.4%) of UCs in males, 17 of them (70.8%) were of low score and the remainder 7/24 (29.2%) were of high score, while P27 was detected in 33.3% of females with UCs and were of low score. These relations did not achieve a statistical significance.
3.3 Correlations between survivin and P27 immunoreactivity with clinicopathological variables of the urothelial carcinomas
Coexpression of survivin and P27 was seen in 12/60 (20%) of UCs. Seven out of these 12 cases (58.5%) showed high score of survivin/low score of P27, meanwhile, both proteins were negative in 10/60 (16.7%) of UCs (Table 3). A significant negative correlation was found between survivin and P27 scores (r = -0.336, p = 0.009).
According to Logistic regression test (Table 4); survivin expression showed a significant correlation with solid pattern and high-grade UC, while P27 expression was associated with low-grade UC. Moreover, survivin expression was associated with P27 expression.
4 Discussion
Infiltrating UC is the most common variant of urinary bladder cancer. Muscle-infiltrating tumors affect around 25–30% of UC patients, with a serious risk for further progression, metastasis, and death [12].
Although grading and staging systems are the most reliable predictors of bladder cancer development, they are insufficient to predict the course of most invasive tumors [13]. Furthermore, conventional therapies, such as chemotherapy or radiation, are only effective in a minority of individuals due to disease heterogeneity. In this study, we examined the expression patterns of survivin and P27 proteins seeking to find prognostic markers that may be employed in the clinical setting.
Since the discovery of survivin more than two decades ago, its use as a target for cancer therapeutics has been a major goal in cancer research. This is because it is not detected in normal adult tissues [14]. In BC, survivin expression was shown to be an indicator of poor prognosis in some studies, but not in others [15].
In the current study, survivin was not detected in urothelial cells of the mild chronic cystitis lesions, but it was detected in UC cells. We detect survivin positivity in 60% of UCs, although Makboul et al. [16] and Arafat et al. [17] reported positivity in a slightly higher percentage (78 and 71%, respectively). Although each of these studies was conducted on Egyptian patients, however, higher percentage may be related to use of different antibody clones.
We found that the difference in survivin immunopositivity between solid and papillary UCs, low and high grades, as well as superficial and deep UCs was significant. Furthermore, high score of survivin was associated with solid pattern, high-grade and deep tumors with no significant difference between them and their counterparts. Our findings matched those of Arafat et al. [17] and Stec et al. [18]. These findings can reflect the role of survivin in the differentiation and invasiveness of UC and indicate the association between survivin expression with a poor prognosis in patients with UC. Other studies by Srivastava et al. [19] and Lv et al. [20] did not find a statistically significant relationship between survivin immunopositivity and grade or stage of UCs. These conflicting findings indicate the need for more study in this area.
In our study, no P27 expression was detected in the urothelial cells of the mild chronic cystitis lesions, the same finding was reported by Al-Fakhar et al. [21]. This can be explained as P27 is high during quiescence and low during S phase, as cells start to proliferate, so with presence of cystitis; levels of P27 can be decreased or absent.
We found that P27 was positive in 43.3% of UCs, which was close to that reported by Al-Fakhar et al. [21] (50%), while Kami et al. [22] and Kapur et al. [23] reported a greater percentage (83.4 and 71%, respectively). This higher percentage can be attributed to type of specimens examined in both studies, being recurrent or progressing UCs, and adenocarcinomas, respectively.
Papillary, low-grade, and superficial UCs had higher P27 positivity than their counterparts. Furthermore, more than half of papillary, low-grade, and superficial UCs had high P27 scores, whereas all solid pattern, high-grade, and deep stage UCs had low P27 scores. Thus, there was an association between P27 expression and better prognostic factors in UC patients, while its lack was linked to poor prognostic factors. Our findings confirm the results of previous studies [24, 25]. Contrary to our findings, Santos et al. [26] and Doganay et al. [27] found high P27 levels in some highly aggressive bladder carcinoma cases and concluded that there was no correlation between P27 and pathological data in UC of the bladder. These conflicting findings were attributed to the fact that P27 tumor suppressor function is mediated through its association with the cyclin E/CDK2 complex, which inhibits the G1 to S phase transition during the cell cycle. When low molecular weight cyclin E isoforms (which are resistant to P27 inhibition) are overexpressed, P27 can no longer function as a CDK inhibitor [21]. As a result, P27 function can be altered by its interactions with other proteins during the cell cycle.
In this study, we detected P27 as nuclear or cytoplasmic expression. Papillary, low-grade, and superficial UCs exhibited nuclear localization, whereas solid, high-grade, and deep UCs exhibited cytoplasmic expression. According to Serres et al. [28], the cytoplasmic location of P27 prevents it from binding to and inhibiting nuclear cyclin-CDK targets, contributing to tumor development through collaboration with other genes including the Ras oncogene. This can explain why cytoplasmic expression was more common in high-grade and deep UCs in our study.
In the current study, 20% of UC patients co-expressed survivin and P27, allowing them to benefit from targeted therapies targeting both proteins, whereas 16.7% of UC patients were negative for both proteins, preventing them from benefiting from such therapies. Survivin score had a significant positive correlation with UC grade and stage of UCs, while P27 score had a significant negative correlation with them. Similar to our results, Chen et al. [29] found a close relation between survivin immunopositivity and UC grade and stage, and Yang et al. [30] found a significant negative correlation between P27 score with UC grade and stage.
Data in our study revealed that high survivin scores and low P27 scores dominate in elderly patients (≥ 60 years). Age can affect the immune response against a therapeutic protein and the pharmacokinetic response to drugs [31]. According to Johnson et al. [32]. Within precision medicine, age must be considered for therapies to be tailored to a person's specific genetic and molecular composition.
Our work has the advantage of demonstrating survivin and P27 expressions in the same patients with UC, which might lead to more significant results; nevertheless, the study was limited by the small number of cases investigated.
5 Conclusion
Both survivin and P27 were upregulated in UCs but not in mild chronic cystitis lesions. High score of survivin and low score of P27 predominate in UCs with poor prognostic factors (solid pattern, high grade, and deep tumors), while low score of survivin and high score of P27 predominate in UCs with better prognostic factors (papillary, low-grade, and superficial UCs). High survivin score and low P27 score dominate in elderly cases (≥ 60 years). By logistic regression test, survivin expression is a predictive risk factor associated with solid pattern and high-grade UC, while P27 expression is a predictive risk factor associated low-grade UC.
Thus, survivin and P27 can be useful prognostic and/or diagnostic tumor markers that can be used to predict UC progression in conjunction with traditional grading and staging systems, as well as may be employed as targeted therapies.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BC:
-
Bladder cancer
- UC:
-
Urothelial carcinoma
- IAPs:
-
Inhibitors of apoptosis proteins
- CDKs:
-
Cyclin-dependent kinases
- CKI:
-
Cyclin-dependent kinase inhibitor
- IHC:
-
Immunohistochemical
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30. https://doi.org/10.3322/caac.21590
Globoscan-The Global Cancer Observator (2020) available at: https://gco.iarc.fr/today/data/factsheets/populations/818-egypt-fact-sheets.pd. Updated on December 2020
Moussa S, El-Sheshtawy WH (2017) Pathological pattern of urinary bladder cancer: data from a single egyptian institute. Res Oncol 13:14–17. https://doi.org/10.21608/RESONCOL.2017.869.1023
Hutchinson R, Lotan Y (2018) Diagnostic, prognostic and predictive biomarkers on bladder tissue and blood. In: Hansel DE, Lerner SP (eds) Precision molecular pathology of bladder cancer, 1st edn. Springer, Cham and Switzerland
Pennati M, Folini M, Zaffaroni N (2008) Targeting survivin in cancer therapy. Expert Opin Ther Targets 12:463–476. https://doi.org/10.1517/14728222.12.4.463
Jang TJ, Le KS (2009) The expression of cyclooxygenase-2 and survivin in urinary bladder transitional cell carcinoma. J Pathol Transl Med 43:206–211. https://doi.org/10.4132/KoreanJPathol.2009.43.3.206
Satoh T, Kaida D (2016) Upregulation of P27 cyclin-dependent kinase inhibitor and a C-terminus truncated form of P27 contributes to G1 phase arrest. Sci Rep 6:27829. https://doi.org/10.1038/sreP27829
Zatonski T, Ciesielska U, Nowinska K, Ratajczak-Wielgomas K, Kobierzycki C, Pula B et al (2016) Expression of cell cycle-related proteins p16, p27, p53 and ki-67 in HPV-positive and -negative samples of papillomas of the upper respiratory tract. Anticancer Res 36:3917–3924
Grignon DJ, Al-Ahmadie H, Algaba F, Amin MB, Comperat E, Dyrskjot L et al (2016) Infiltrating urothelial carcinoma. In: WHO Classification of Tumors of the Urinary System and Male Genital Organs. Moch H, Humphrey PA, Ulbright TM, Reuter VE, editors. 4th edn. Lyon: International Agency for Research on Cancer
Kim SW, Roh J, Park CS (2016) Immunohistochemistry for pathologists: protocols, pitfalls, and tips. J Pathol Transl Med 50:411–418. https://doi.org/10.4132/jptm.2016.08.08
AbdElazeez TA, Ael-L E-B, Khalil MM, El-Tabye MM, Abdul-Halim H (2011) Prognostic significance of P27 (Kip 1) and MUC1 in papillary transitional cell carcinoma of the urinary bladder. Urol Ann 3:8–13. https://doi.org/10.4103/0974-7796.75857
Bellmunt J, Teh BT, Tortora G, Rosenberg JE (2013) Molecular targets on the horizon for kidney and urothelial cancer. Nat Rev Clin Oncol 10:557–570. https://doi.org/10.1038/nrclinonc.2013.155
Nedjadi T, Al-Ammari A, Khayyat D, Salem N, Al-Sayyad A, Hussain S et al (2016) The cell cycle regulator P27Kip1 is associated with urothelial bladder cancer invasion. Int J Clin Exp Pathol 9:10515–10521
Li F, Aljahdali I, Ling X (2019) Cancer therapeutics using survivin BIRC5 as a target: what can we do after over two decades of study? J Exp Clin Cancer Res 38:368. https://doi.org/10.1186/s13046-019-1362-1
Gradilone A, Petracca A, Nicolazzo C, Gianni W, Cortesi E, Naso G et al (2010) Prognostic significance of survivin-expressing circulating tumour cells in T1G3 bladder cancer. BJU Int 106:710–715. https://doi.org/10.1111/j.1464-410X.2009.09130.x
Makboul R, Refaiy AE, Badary FA, Abdelkawi IF, Merseburger AS, Mohammed RA (2015) Expression of survivin in squamous cell carcinoma and transitional cell carcinoma of the urinary bladder: a comparative immunohistochemical study. Korean J Urol 56:31–40. https://doi.org/10.4111/kju.2015.56.1.31
Arafat W, Ashry MSE, Alrazek MAA, Matta CA, El Aleem E, Kamel EM et al (2017) The relation between survivin gene expression and urinary bladder cancer disease. Hos Pal Med Int J 1:26–33. https://doi.org/10.15406/hpmij.2017.01.00007
Stec R, Cierniak S, Lubas A, Brzóskowska U, Syryło T, Zieliński H et al (2020) Intensity of nuclear staining for Ki-67, p53 and survivin as a new prognostic factor in non-muscle invasive bladder cancer. Pathol Oncol Res 26:1211–1219. https://doi.org/10.1007/s12253-019-00678-1
Srivastava AK, Singh PK, Srivastava K, Singh D, Dalela D, Rath SK et al (2013) Diagnostic role of survivin in urinary bladder cancer. Asian Pac J Cancer Prev 14:81–85. https://doi.org/10.7314/apjcp.2013.14.1.81
Lv S, Turlova E, Zhao S, Kang H, Han M, Sun HS (2014) Prognostic and clinicopathological significance of survivin expression in bladder cancer patients: a meta-analysis. Tumour Biol 35:1565–1574
Al-Fakhar SA, Ali SH, Al-Zuwaid AA, Al-Alwany SH (2016) Over expression of P27 protein from CDKN1B gene in patients with invasive bladder transitional cell carcinoma. Pharma Innovation J 5:107–111
Kamai T, Takagi K, Asami H, Ito Y, Oshima H, Yoshida KI (2001) Decreasing of p27(Kip1) and cyclin E protein levels is associated with progression from superficial into invasive bladder cancer. Br J Cancer 84:1242–1251. https://doi.org/10.1054/bjoc.2000.1736
Kapur P, Lotan Y, King E, Kabbani W, Mitra AP, Mosbah A et al (2011) Primary adenocarcinoma of the urinary bladder: value of cell cycle biomarkers. Am J Clin Pathol 135:822–830. https://doi.org/10.1309/ajcp76kuvotbkqry
Lacoste-Collin L, Gomez-Brouchet A, Escourrou G, Delisle MB, Levade T, Uro-Coste E (2002) Expression of P27(Kip1) in bladder cancers: immunohistochemical study and prognostic value in a series of 95 cases. Cancer Lett 186:115–120. https://doi.org/10.1016/s0304-3835(02)00319-1
Ioachim E, Michael M, Stavropoulos NE, Kitsiou E, Hastazeris K, Salmas M et al (2004) Expression patterns of cyclins D1, E and cyclin-dependent kinase inhibitors p21(Waf1/Cip1) and P27(Kip1) in urothelial carcinoma: correlation with other cell-cycle-related proteins (Rb, p53, Ki-67 and PCNA) and clinicopathological features. Urol Int 73:65–73. https://doi.org/10.1159/000078807
Santos LL, Amaro T, Pereira SA, Lameiras CR, Lopes P, Bento MJ et al (2003) Expression of cell-cycle regulatory proteins and their prognostic value in superficial low-grade urothelial cell carcinoma of the bladder. Eur J Surg Oncol 29:74–80. https://doi.org/10.1053/ejso.2002.1371
Doganay L, Altaner S, Bilgi S, Kaya E, Ekuklu G, Kutlu K (2003) Expression of the cyclin-dependent kinase inhibitor P27 in transitional cell bladder cancers: is it a good predictor for tumor behavior? Int Urol Nephrol 35:181–188. https://doi.org/10.1023/b:urol.0000020301.39181.03
Serres MP, Zlotek-Zlotkiewicz E, Concha C, Gurian-West M, Daburon V, Roberts JM et al (2011) Cytoplasmic P27 is oncogenic and cooperates with Ras both in vivo and in vitro. Oncogene 30:2846–2858
Chen D, Xu J, Zhang Q (2018) Detection of survivin expression in bladder cancer and renal cell carcinoma using specific monoclonal antibodies. Oncol Rep 39:2817–2828. https://doi.org/10.3892/or.2018.6359
Yang CH, Wu CC, Chen WT, Chai CY, Yang SF (2014) Expressions of p16 and P27 in urothelial carcinoma and their prognostic value. Kaohsiung J Med Sci 30:453–458. https://doi.org/10.1016/j.kjms.2014.05.003
Reeve E, Wiese MD, Mangoni AA (2015) Alterations in drug disposition in older adults. Expert Opin Drug Metab Toxicol 11:491–508
Johnson PA, Fitzgerald T, Glynn A, Wood SF, Goldstein JM (2016). Precision Medicine. How Sex and Gender Drive Innovation A Report of the Mary Horrigan Connors Center for Women’s Health & Gender Biology at Brigham and Women’s Hospital. Available at: https://precisionmedicine.bwh.harvard.edu/news-2/
Acknowledgements
Non applicable.
Funding
No funding is to be declared.
Author information
Authors and Affiliations
Contributions
NSH interpreted all data, conceived, designed, and wrote the manuscript, ZO designed the structure and drafted the manuscript, and MM designed and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol of this study was approved by the Institutional Review Board (IRB) of Theodor Bilharz Research Institute, for the protection of human subjects and adopted by the 18th world medical assembly, Helsinki, Finland (2013). The IRB waived the need for informed consent from the participants because the study was performed on stored archival tissue blocks. Personal information of these blocks’ owners was anonymous and cannot reasonably be used by anyone.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Helal, N.S., Omran, Z. & Moussa, M. Assessment of survivin and p27 expression as potential prognostic markers in urothelial cell carcinoma of urinary bladder in Egyptian patients. Afr J Urol 28, 45 (2022). https://doi.org/10.1186/s12301-022-00315-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-022-00315-5