Abstract
Background
Primary squamous cell carcinoma (SCC) of the kidney is a rare entity which tends to be associated with nephrolithiasis, chronic irritation, and infection. Due to its rarity and the non-specific clinical signs and symptoms as well as radiological findings, it is often not suspected preoperatively. Patients with SCC of the renal pelvis typically present with advanced stage disease and have a poor outcome. Most of our current knowledge of SCC of the renal pelvis has been derived from case reports or limited case series, and there are no standard treatment guidelines. The clinical, radiological, and histopathological findings of this unusual neoplasm are described herein.
Case presentation
A 61-year-old female presented with left flank pain and sepsis. A computerized tomography (CT) scan showed renal calculi and hydronephrosis, and a mercapto-acetyl triglycine (MAG-3) scan showed a left-sided non-functioning kidney. She underwent a nephrectomy for an infected, non-functioning kidney. Histopathological examination revealed an invasive, moderately differentiated squamous cell carcinoma.
Conclusion
The significance of this case is highlighted by the unusual location of such a tumour, and while rare, it is an important consideration in the differential diagnoses of renal tumours. The present case report may assist patient management of this rare tumour by highlighting and documenting treatment and clinical outcome of our patient.
Similar content being viewed by others
1 Background
Primary squamous cell carcinoma (SCC) of the renal pelvis is a rare occurrence, accounting for only 0.5–7.0% of upper urinary tract tumours [1,2,3,4,5]. Due to its rarity together with the association with nephrolithiasis and chronic inflammation, it may present a diagnostic challenge to the urologist. Patients present with non-specific signs and symptoms and radiological investigations may not help to distinguish it from other neoplastic or inflammatory conditions of the kidney. This case discusses squamous cell carcinoma of the renal pelvis incidentally diagnosed after nephrectomy for a chronically infected, non-functioning kidney.
2 Case presentation
A 61-year-old female patient was referred to the Department of Urology from a private general practitioner (GP) with a one-month history of intermittent, localized, dull left flank pain, which was then associated with nausea, vomiting, fever, and rigors. She did not have macroscopic haematuria and had no other urinary symptoms. There was no history of previous carcinoma of the bladder or uterine cervix to suggest possible metastatic disease. An abdominal ultrasound (U/S) had been performed at the general practitioner which showed a 10.2 cm left kidney with multiple calculi and a large, complex cyst measuring 6.7 × 6.6 cm. There was no evidence of hydronephrosis on this U/S.
On admission, physical examination revealed left renal angle tenderness. There was no abdominal distension, and there were no palpable masses. Urine dipstick analysis showed 3 + leucocytes and 1 + protein. No bacteria were grown on urine culture. Haematological investigations revealed a white cell count (WCC) of 4.3 × 109/L (3.9–12.6 × 109/L) and haemoglobin (Hb) of 10.2 g/dL (11.6–16.4 g/dL). She had normal electrolytes, but she had raised urea and creatinine levels of 21.3 mmol/L (2.1–7.1 mmol/L) and 193 umol/L (49–90 umol/L), respectively. She had an estimated Glomerular Filtration Rate (eGFR) of 23 mL/min/1.73m2 (> 60 mL/min/1.73 m2). Her C-reactive protein (CRP) was elevated at 94 mg/L (normal < 10 mg/L).
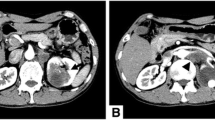
In light of the patient’s renal dysfunction and the sonographic report of renal calculi, a non-contrast computerized tomography (CT) scan of the abdomen and pelvis was requested which demonstrated three left-sided calculi within the renal pelvis, as well as a complex para-pelvic cyst, hydronephrosis, and hydroureter. In addition, peri-renal fatty stranding and multiple enlarged lymph nodes of varying size were identified. The largest lymph node measured approximately 1.8 × 1.5 cm. Following fluid rehydration and correction of the patient’s renal dysfunction, a contrast-enhanced CT intravenous pyelogram (IVP) was performed which showed a heterogeneous soft tissue density mass extending along the left renal pelvis and the superior ureter with associated renal caliectasis and renal calculi (Figs. 1 and 2). There was no contrast excretion from the left kidney to provide any further anatomical detail of the left ureter. As noted on the initial CT scan, peri-renal fatty stranding and renal lymphadenopathy was identified, with the largest lymph node measuring 1.5 × 1.6 cm.
The left kidney was suspected to be non-functional and a MAG-3 scan was performed, which revealed only a minimal rim of activity in the expected anatomical position of the left kidney, thus confirming the diagnosis of a non-functioning left kidney. The soft tissue mass and lymphadenopathy were thought to be reactive and in response to chronic inflammation. Thus, the clinical diagnosis at this stage was that of xanthogranulomatous pyelonephritis (XGP).
In view of a non-functioning, obstructed kidney in the presence of urolithiasis and sepsis, the patient underwent a left nephrectomy via a left sub-costal incision. Intra-operatively, the kidney was adherent to the spleen and descending colon but was dissected free of these organs. The renal pelvis was aspirated and drained 160 mL of pus. Due to the extensive adhesions and lack of preoperative suspicion of malignancy, fibrotic tissue that was suspected to be inflammatory in nature was not excised with the kidney and lymph node dissection was not performed. The excised kidney was submitted for histopathological assessment.
Macroscopically, the specimen consisted of a kidney with surrounding peri-renal adipose tissue which weighed 351 g. The kidney measured 110 × 70 × 65 mm. The renal capsule was intact. On cut section of the kidney, the renal parenchyma was replaced by an unencapsulated yellow tumour which measured 90 × 88 × 55 mm. The tumour occupied the pelvi-calyceal system and renal hilum. A haemorrhagic, necrotic cyst was noted in the upper pole of the kidney which measured 75 × 70 × 70 mm.
Microscopic examination demonstrated renal parenchyma with areas of metaplastic stratified squamous epithelium together with dysplastic squamous mucosa, arising from which there was an invasive, moderately differentiated squamous cell carcinoma arranged in clusters and nests. The tumour was composed of markedly pleomorphic cells which had raised nuclear to cytoplasmic ratios. Intercellular bridges were seen, and keratin pearl formation was evident (Fig. 3). Large areas of tumour necrosis were identified. Definitive lymphovascular and perineural infiltration were noted in sections from the vascular resection margin (Fig. 4). Tumour was identified in sections from the ureteric resection margin and extended into peri-renal adipose tissue. While multiple soft tissue tumour deposits were documented, there were no identifiable lymph nodes present for assessment. Sections from the renal pelvis showed a squamous metaplastic lining epithelium with areas of dysplasia. Immunohistochemistry demonstrated positive p63 and CK5/6 staining in both the dysplastic squamous epithelium and the invasive tumour with reduced to absent staining of GATA-3 and S100P in the mucosa and invasive tumour (Fig. 5). The residual renal parenchyma showed marked chronic inflammation with thyroidization-type changes. A final diagnosis of an invasive, moderately differentiated squamous cell carcinoma, TNM Stage IV (T4 Nx M0) (according to the Union for International Cancer Control (UICC), TNM Classification of malignant tumours, 8th edition, 2017), was rendered.
a Haematoxylin and eosin (H&E) photomicrograph showing dysplastic squamous mucosa (star) with islands of squamous cell carcinoma within the stroma (long thin arrows). b P63 immunohistochemical stain is positive in dysplastic squamous mucosa (star) and in the invasive tumour (long thin arrows). c CK5/6 immunohistochemical stain is positive in dysplastic mucosa (star) and in the invasive carcinoma (long thin arrows). d S100P immunohistochemical stain is positive in free-lying urothelial cells (long thin arrows) but is negative in dysplastic mucosa (star) and in the invasive tumour. e GATA-3 immunohistochemical stain is positive in lymphocytes in the stroma (long thin arrows) but is negative in dysplastic mucosa (star) and in the invasive carcinoma (original magnification × 200)
Postoperatively, the patient was admitted to the Intensive Care Unit (ICU) for three days. Her postoperative recovery was complicated by urosepsis, for which she received ertapenem and standard postoperative analgesia for three days. Nine days following surgery, she was clinically stable and was discharged from the hospital while awaiting her histopathology result. She was scheduled for a follow-up visit two weeks after discharge for review of her wound.
She was re-admitted one month later with generalized weakness, weight loss and confusion. Physical examination revealed that she was alert but disorientated. Her vital signs were stable, and her abdomen was soft with no palpable masses. An abdominal CT scan revealed a multi-loculated, enhancing cystic lesion in the left renal bed with no plane of separation from the spleen. Multiple hypodense liver lesions suggestive of metastases were seen, which were not present on the initial scans. Due to her poor functional status, adjuvant chemoradiation was not offered and during this hospital admission, approximately 6 weeks after surgery, she died. A postmortem examination was not performed.
3 Discussion
Tumours arising from the urothelium of the renal pelvis and ureter are relatively rare neoplasms, comprising 5–10% of all urothelial tumours [1,2,3, 6]. Approximately, 85–90% of these are urothelial carcinomas, while pure squamous cell carcinomas (SCC) are even rarer, accounting for only 0.5–7.0% of upper urinary tract cancers [1,2,3,4,5,6,7]. Patients tend to be between the fifth to seventh decades at presentation with various studies documenting differing gender predilection [1, 4, 5].
SCC of the renal pelvis is associated with renal stones, chronic infection and inflammation, which is thought to lead to squamous metaplasia, dysplasia and eventually SCC [3,4,5,6,7,8,9]. SCC of the kidney is infrequently suspected or diagnosed preoperatively due to its rarity and the non-specific symptoms, signs and radiological findings [4, 5, 8]. Patients may present with microscopic or macroscopic haematuria, flank pain, weight loss, fever or a palpable abdominal mass [5, 7]. SCC has also been associated with paraneoplastic syndromes including hypercalcaemia, leukocytosis and thrombocystosis [8, 10]. Our patient presented with intermittent flank pain, nausea, vomiting and fever but did not have haematuria or paraneoplastic phenomena.
Radiological findings of renal pelvis SCC include a solid renal pelvic or ureteric mass, hydronephrosis, calcifications or regional lymphadenopathy [4, 7, 8]. These are non-specific findings which may be difficult to distinguish from chronic inflammatory conditions such as xanthogranulomatous pyelonephritis (XGP), tuberculosis or other neoplasms of the upper urinary tract [4, 6,7,8]. An enhancing extraluminal or intraluminal mass or exophytic mass may be a helpful finding in cases of SCC [11]. In the present case, the CT scan showed a heterogeneous soft tissue density mass seen to extend along the left renal pelvis and proximal ureter. In addition, multiple calculi, hydronephrosis and renal lymphadenopathy were documented.
The working clinical preoperative diagnosis was that of xanthogranulomatous pyelonephritis. XGP is an uncommon chronic bacterial pyelonephritis which may mimic renal neoplasms [12]. To further confound the diagnosis, the clinical signs and symptoms as well as the radiological findings of XGP have striking similarities to those of renal neoplasms, and specifically that of renal SCC due to the association with nephrolithiasis [4, 8, 12]. Furthermore, the inflammation in XGP may extend beyond the kidney to involve surrounding structures [12].
Microscopically, primary renal SCC resembles squamous cell carcinomas at other sites. In the present case, the tumour was identified in sections of the vascular and ureteric resection margins. Extensive tumour necrosis was documented together with lymphovascular and perineural invasion.
Patients with renal SCC tend to present at an advanced stage, usually at least T3 or higher [1,2,3,4, 7]. The patient in the current case report had stage IV disease. Tumour recurrences have been shown to typically develop rapidly, which was the case in our patient [9, 10]. The overall survival for patients with SCC of the upper urinary tract is much worse in comparison with patients with urothelial carcinoma (UC) [1,2,3,4, 13, 14]. However, when compared stage for stage, there is no disease specific 5-year survival difference between SCC and UC [2, 13].
There is currently no standardized treatment protocol for management of patients with primary renal SCC. The mainstay of treatment has been surgery by either a radical nephrectomy or nephroureterectomy [1, 4,5,6,7,8,9]. Most patients with loco-regional disease do not have accurate lymph node staging [13]. Patients treated with cisplatin-based adjuvant chemotherapy and/or radiotherapy have not demonstrated a survival benefit [1,2,3,4,5,6,7]. Postoperative radiotherapy has not demonstrated a survival benefit in patients with malignancies of the upper urinary tract [13]. Some benefit has, however, been suggested with adjuvant chemotherapy in patients with urothelial carcinoma of the upper urinary tract [13]. However, due to the paucity of cases and poor long-term survival with squamous cell carcinoma, further studies are required to determine whether chemotherapy or radiotherapy will improve patient survival.
Primary renal squamous cell carcinomas are very uncommon tumours which are often not clinically suspected or diagnosed. Commensurate with previous case reports, the patient in the current report presented with advanced disease and died approximately 6 weeks after surgery. Table 1 summarizes the clinical presentation and outcomes of previous reports of patients with renal squamous cell carcinomas.
4 Conclusion
Primary renal squamous cell carcinomas are very uncommon tumours which are often not clinically suspected or diagnosed. These tumours require thorough clinicopathological and radiological correlation together with a high index of suspicion and broad differential diagnosis to ensure timeous, appropriate management of the patient. The need for renal stones to be managed promptly to prevent this rare, but devastating complication, is thus evident.
Availability of data and materials
Not applicable.
Abbreviations
- CRP:
-
C-reactive protein
- CT:
-
computerized tomography
- eGFR:
-
estimated glomerular filtration rate
- GP:
-
general practitioner
- Hb:
-
haemoglobin
- ICU:
-
intensive care unit
- IVP:
-
intravenous pyelogram
- MAG-3:
-
mercapto-acetyl triglycine
- SCC:
-
squamous cell carcinoma
- UC:
-
urothelial carcinoma
- U/S:
-
ultrasound
- WCC:
-
white cell count
- XGP:
-
xanthogranulomatous pyelonephritis
References
Blacher EJ, Johnson DE, Abdul-Karim FW, Ayala AG (1985) Squamous cell carcinoma of renal pelvis. Urology 12(2):124–126
Holmäng S, Lele SM, Johansson SL (2007) Squamous cell carcinoma of the renal pelvis and ureter: incidence, symptoms, treatment and outcome. J Urol 178(1):51–56
Li MK, Cheung WL (1987) Squamous cell carcinoma of the renal pelvis. J Urol 138(2):269–271
Hassan M, Qureshi A (2017) Incidental squamous cell carcinoma of the renal pelvis in a non functioning kidney that was missed on two non-contrast CT-scans. J Ayub Med Coll Abbottabad 29(3):489–492
Jain A, Mittal D, Jindal A, Solanki R, Khatri S, Parikh A et al (2011) Incidentally detected squamous cell carcinoma of renal pelvis in patients with staghorn calculi: case series with review of the literature. ISRN Oncol 2011:620574
Bhaijee F (2012) Squamous cell carcinoma of the renal pelvis. Ann DiagnPathol 16:124–127
Paonessa J, Beck H, Cook S (2011) Squamous cell carcinoma of the renal pelvis associated with kidney stones: a case report. Med Oncol 28:392–394
Palmer CJ, Atty C, Sekosan M, Hollowell CMP, Wille MA (2014) Squamous cell carcinoma of the renal pelvis. Urology 84(1):8–11
Busby JE, Brown GA, Tamboli P, Kamat AM, Dinney CPN, Grossman HB et al (2006) Upper urinary tract tumors with nontransitional histology: a single-center experience. Urology 67(3):518–523
Er Ö, Coşkun HŞ, Altinbaş M, Akgün H, Çetin M, Eser B et al (2001) Rapidly relapsing squamous cell carcinoma of the renal pelvis associated with paraneoplastic syndromes of leukocytosis, thrombocytosis and hypercalcemia. Urol Int 67(2):175–177
Lee TY, Ko SF, Wan YL, Cheng YF, Yang BY, Huang DL et al (1998) Renal squamous cell carcinoma: CT findings and clinical significance. Abdom Imaging 23:203–208
Eastham J, Ahlering T, Skinner E (1994) Xanthogranulomatous pyelonephritis: clinical findings and surgical considerations. Urology 43(3):295–299
Berz D, Rizack T, Weitzen S, Mega A, Renzulli J, Colvin G (2012) Survival of patients with squamous cell malignancies of the upper urinary tract. Clin Med Insights Oncol 6:11–18
Jiang P, Wang C, Chen S, Li J, Xiang J, Xie L (2015) Primary renal squamous cell carcinoma mimicking the renal cyst: a case report and review of the recent literature Urological oncology. BMC Urol [Internet]. 2015 Jul 23 [cited 2020 Jul 7];15. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4511242/
Acknowledgements
Dr. Tumelo Molaoa from the Department of Radiology is thanked for the radiological images.
Funding
No funding was required for this case report.
Author information
Authors and Affiliations
Contributions
NFB contributed to the conception, design, and drafting of manuscript and critically reviewed the manuscript for relevant intellectual content. SB contributed to the histopathological diagnosis, the design, and drafting of manuscript and critically reviewed the manuscript for relevant intellectual content. RW contributed to the histopathological diagnosis, conceptualization, and drafting of the manuscript and critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance (certificate number M190993) was obtained through the Human Research Ethics Committee (Medical) of the University of Witwatersrand, following signed written consent from the patient’s legal guardian for use of the clinical history, clinical photographs, histological tissue sections, photomicrographs, diagnosis, and treatment plan.
Consent for publication
Written and informed consent was obtained from the patient’s legal guardian for use of the clinical history, clinical photographs, radiological images, intra-operative macroscopic photographs, histological tissue sections, photomicrographs, diagnosis, and treatment plan to be used in this case report.
Competing interests
The authors have no conflict of interest to declare.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Brits, N.F., Bulane, S. & Wadee, R. Primary squamous cell carcinoma of the kidney: a case report and review of the literature. Afr J Urol 26, 79 (2020). https://doi.org/10.1186/s12301-020-00088-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-020-00088-9