Abstract
Background
Signal transduction mediated by heterotrimeric G proteins, which comprise the α, β, and γ subunits, is one of the most important signaling pathways in rice plants. RGA1, which encodes the Gα subunit of the G protein, plays an important role in the response to various types of abiotic stress, including salt, drought, and cold stress. However, the role of RGA1 in the response to heat stress remains unclear.
Results
The heat-resistant mutant ett1 (enhanced thermo-tolerance 1) with a new allele of the RGA1 gene was derived from an ethane methyl sulfonate-induced Zhonghua11 mutant. After 45 °C heat stress treatment for 36 h and recovery for 7 d, the survival rate of the ett1 mutants was significantly higher than that of wild-type (WT) plants. The malondialdehyde content was lower, and the maximum fluorescence quantum yield of photosystem II, peroxidase activity, and hsp expression were higher in ett1 mutants than in WT plants after 12 h of exposure to 45 °C. The RNA-sequencing results revealed that the expression of genes involved in the metabolism of carbohydrate, nicotinamide adenine dinucleotide, and energy was up-regulated in ett1 under heat stress. The carbohydrate content and the relative expression of genes involved in sucrose metabolism indicated that carbohydrate metabolism was accelerated in ett1 under heat stress. Energy parameters, including the adenosine triphosphate (ATP) content and the energy charge, were significantly higher in the ett1 mutants than in WT plants under heat stress. Importantly, exogenous glucose can alleviate the damages on rice seedling plants caused by heat stress.
Conclusion
RGA1 negatively regulates the thermo-tolerance in rice seedling plants through affecting carbohydrate and energy metabolism.
Similar content being viewed by others
Background
Rice (Oryza sativa L.) is the major staple food for more than half of the world’s population. Rice plants are always exposed to various types of abiotic stress, such as heat, cold, and drought stress. Heat stress always occurs when the temperature exceeds the threshold beyond which plants can tolerate for an extended period (Sehgal et al. 2019; Li et al. 2020; Jajoo and Allakhverdiev 2017; Kumazaki and Suzuki 2019). Under such stress, the thermolabile proteins was inactivated, and that induces the accumulation of reactive oxygen species and programmed cell death (Grover et al. 2013; Liu et al. 2016; Zhang et al. 2016), which causes irreversible damage to crop plants. It has been reported that a large decrease in spikelet fertility, grain yield and quality were showed in those rice plants caused by heat stress at reproductive stage (Zhang et al. 2016, 2017 and 2018; Fu et al. 2015; 2016). Therefore, it is urgent to breed heat-resistant rice cultivar and develop heat-resistant cultivation technique to alleviate yield losses caused by extreme high temperature climates.
These years, several genes have been identified conferring heat tolerance in rice (Li et al. 2015a; Xu et al. 2020; Kan et al. 2022; Zhang et al. 2022). Thermo-tolerance 1 (OsTT1), which encodes the α subunit of the 26S proteasome, plays a role in regulating the thermo-tolerance of African rice by eliminating cytotoxic denatured proteins (Li et al. 2015a). SLENDER GUY 1 (SLG1) encodes the cytosolic tRNA 2-thiolation protein 2 and enhances thermo-tolerance in indica rice (Xu et al. 2020). It has been found that OsTT2, which encodes a Gγ subunit, confers rice thermo-tolerance via the SCT1 (sensing Ca2+ transcription factor 1)-dependent alteration of wax biosynthesis (Kan et al. 2022). Further, OsTT3, consisting of two genes, positive regulator TT3.1 and the negative regulator TT3.2, that interact together to enhance rice thermotolerance (Zhang et al. 2022). However, how these genes affecting energy metabolism in rice plants under heat stress has not been documented in reference. As well-known, heat stress can disturb energy metabolism (Li et al. 2020; Jiang et al. 2020), and thus reduce plant productivity and stress tolerance (Dobrota 2006; Hashida et al. 2009; Chen et al. 2022; Li et al. 2023).
The energy levels of organisms need to be maintained sufficiently high to support their survival and growth. While photosynthesis is the main driver of plant productivity, cellular respiration (glycolysis, citrate cycle, and mitochondrial electron transport) is responsible for converting fixed carbon into energy used for growth and development. Nicotinamide adenine dinucleotide (NAD+) plays a central role in the electron transport chain (De and Van 2011), which is crucial for maintaining the flow of energy. NAD+ can be synthesized via two pathways: de novo synthesis, which involves aspartate and requires a minimum of five adenosine triphosphates (ATPs), and the salvage pathway, which only requires three ATPs at most (Hashida et al. 2009). Overconsumption of NAD+ destabilizes normal cell function, and its re-synthesis depletes the cellular ATP pool. A low energy level limits anabolism and disturbs normal cellular activities, resulting in cell damage and ultimately cell death. Furthermore, the ratio of NAD+/NADH plays a key role in regulating many redox reactions (Noctor et al. 2007). NAD+ and its metabolites can interact with signaling pathways, which is often overlooked but remains an important function of NAD+ and its metabolites (Hunt et al. 2004; Koch-Nolte et al. 2009). The NAD+ content of cells must be maintained at a constant level to maintain normal functioning (Wang et al., 2007). When plants are exposed to oxidative stress, the synthesis of poly (ADP-ribose) polymerases (PARPs) is induced. PARPs are synthesized from NAD+, using large negatively charged chains of ADP-ribose to modify nuclear proteins such as histones and transcription factors. As a result, large numbers of NAD+ molecules are metabolized, which ultimately results in cell death (De et al. 2005). Under stress conditions, energy consumption is sharply increased, and energy production is inhibited; leading to negative effects on energy homeostasis. When energy levels decrease below a certain threshold, growth ceases, damage accumulates, and the organism ultimately dies. Thus, maintaining energy homeostasis is a major challenge for all living organisms, and there is an intimate relationship between energy availability and stress tolerance in plants (De and Van 2011; Dröge-Laser and Weiste 2018; Jiang et al. 2020). Under stress conditions, energy homeostasis can be maintained for a prolonged period by reducing the activity of PARPs, which enhances stress tolerance in Arabidopsis thaliana and Brassica napus (Amor et al. 1998; De et al. 2005).
The heterotrimeric G proteins, comprising the α, β, and γ subunits, perceive extracellular stimuli through cell surface receptors; they then transmit signals to effectors to initiate downstream biological processes (Gilman 1987). Unactivated G protein binds to the Gα subunit of GDP and combines with the Gβγ subunit to yield a dimer; when G protein is activated, the Gα subunit dissociates from Gβγ and interacts with downstream effectors until Gα is hydrolyzed to GDP by GTPase (Assmann 2002; Jones and Assmann 2004; Perfus-Barbeoch et al. 2004; Urano et al. 2013; Ferrero-Serrano et al. 2018; Yu et al. 2018). These processes include responses to hormones, drought, pathogens, and other developmental events (Wettschureck and Offermanns 2005). A large heterotrimeric G protein family exists in the mammalian genome; for example, the human genome encodes 23 Gα, 5 Gβ, and 12 Gγ proteins (Jones and Assmann 2004; Bisht et al. 2015); in plants, the heterotrimeric G protein repertoire in plants is much less diverse (Trusov et al. 2012). The rice genome contains a common Gα gene, RGA1 (Ishikawa et al. 1995); a Gβ gene, RGB1 (Ishikawa et al. 1996); four Gγ subunit genes, RGG1, RGG2, GS3, and DEP1 (Kato et al. 2004; Fan et al. 2006; Huang et al. 2009; Zhang et al. 2019); and a fifth related Gγ gene or pseudogene OsGGC2 (Botella 2012). Kan et al. (2022) reported that OsTT2, which encodes a Gγ subunit, confers rice thermo-tolerance through an SCT1 (sensing Ca2+ transcription factor 1)-dependent alteration of wax biosynthesis. Rice RGA1 plays an important role in the response to various types of abiotic stress including salt, drought, and cold stress (Ferrero-Serrano and Assmann 2016; Jangam et al. 2016). However, the role that RGA1 plays in sensing heat stress remains unclear.
In our previous study, a dwarf mutant that is sensitive to low-light was screened and identified; gene mapping and sequencing revealed that it is a new RGA1 allelic mutant (Li et al. 2023). However, we found that this mutant is more resistant to extreme heat stress, which has not been reported before. Here, we compared the agronomic characters of this mutant, named ett1(enhanced thermo-tolerance 1), with wild-type (WT, Zhonghua11) plants. RNA sequencing (RNA-seq) was conducted to clarify the molecular mechanism by which ett1 responds to heat stress. The malondialdehyde (MDA) content, the maximum fluorescence quantum yield of photosystem II (Fv/Fm), antioxidant capacity, carbohydrate content, and energy status were characterized to clarify the thermo-tolerance and mechanism underlying the response of ett1 mutants to heat stress.
Materials and Methods
Plant Materials and Growth Conditions
The dwarf and rounded grain mutant was obtained from mutagenesis of the WT cultivar Zhonghua11 using ethane methyl sulfonate (Li et al. 2023). This mutant was selfed for more than six generations, and the target trait has been stably expressed under greenhouse and field conditions in Hangzhou, Zhejiang, China. Gene mapping and sequencing revealed that ett1 is a new RGA1 allelic mutant (Additional file 5: Table S1 and Additional file 1: Fig. S1). We compared the agronomic characters of ett1 and wild-type (WT, Zhonghua11) plants (Additional file 2: Fig. S2). The ovD1 (over-expressed D1 in ett1 mutant) plant was selected from the over-expression lines and the original name was OE-1 (Li et al. 2023). To evaluate the thermo-tolerance of the mutant, the seeds of the WT and ett1 mutants were directly sown in pots (10 cm in height and 10 cm in diameter) in a plant growth chamber at 30/23 ºC (day/night) under natural sunlight and 70–80% relative humidity until the seedlings reached the fifth- to sixth-leaf stage. The plants were then divided into two groups. One group was subjected to heat stress conditions (45 ºC) for 36 h, followed by recovery for 7 d; the other group was exposed to control conditions. After 12 h of heat stress treatment, the second expanded leaves were sampled, immediately frozen in liquid nitrogen, and stored at − 80ºC for analyzing RNA sequencing, Fv/Fm, MDA, antioxidant capacity, carbohydrates content, and energy status.
According to the previous results, sugar signaling might play a key role in mediating the heat response between WT and ett1 seedling plants. Therefore, 0.1% glucose was sprayed onto rice seedling plants about 30 min before heat stress conducted. After 12 h of heat stress treatment, the second expanded leaves were sampled to determine the Fv/Fm, MDA, and the energy status.
Maximum Fluorescence Quantum Yield of Photosystem II (Fv/Fm)
Fv/Fm measurements were conducted following a previously described method (Zhao et al. 2017). After 12 h of heat stress treatment, the leaves were adapted to the dark for 30 min. A portable chlorophyll fluorometer, PAM-2500 (Walz Heinz GmbH, Effeltrich, Germany), was then used to measure Fv/Fm.
RNA Sequencing and Analysis
Total RNA was extracted from 100 mg leaves using the Trizol reagent (Invitrogen, CA, USA) following the manufacturer's protocol. Paired-end sequencing was conducted on an Illumina Hiseq 4000 system (LC Sciences, USA) per the manufacturer’s protocol. Prior to assembly, low-quality reads were removed. A total of 77.57 G of cleaned paired-end reads were produced. FPKM was calculated to evaluate the expression levels of genes. Differentially expressed genes (DEG) were identified according to the following criteria using the R package Ballgown: log2(fold change) > 1 or log2(fold change) < − 1 and p-value < 0.05. Gene Ontology (GO) analysis, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis, and Gene Set Enrichment Analysis (GSEA) 4.1.0, https://www.gsea-msigdb.org/gsea/ index.jsp) were conducted to analyze the sequencing data.
Quantitative RT-PCR (qRT-PCR) Analysis
qRT-PCR was conducted using the Step One Plus™ Real-Time PCR system (Thermo Fisher Scientific Biotechnology, Shanghai, China). The primers used are listed in Additional file 6: Table S2. PCR analysis and detection were performed as described previously (Feng et al. 2013). The 2−∆∆CT method was used to analyze relative gene expression levels of the mean values of three replicates.
Measurements of AMP, ADP, ATP, NAD(H), and NADP(H) Contents
Frozen leaves (0.2 g) were homogenized with 2 mL of perchloric acid in an ice bath and centrifuged for 10 min at 8,000 × g. Next, 2 mL of NaOH was added to the supernatant and centrifuged for 10 min at 8,000 × g. The supernatant was collected and placed in an ice bath for analysis. The ATP and ADP content was determined using the ATP and ADP assay kits, respectively, according to the manufacturer’s instructions (Comin Biotechnology Co., Ltd., Suzhou, China). The energy charge was calculated according to the formula: EC = (ATP + 0.5ADP)/(ATP + ADP + AMP).
NAD+, NADH, NADP+, and NADPH were extracted with 1 mL of 0.1 M HCl or 0.1 M NaOH following the method of Matsumura and Miyachi (1983). The NAD+ and NADH content was determined using an assay kit, and different kits were used to determine the NADP+ and NADPH content per the manufacturer’s instructions (Comin Biotechnology Co., Ltd., Suzhou, China).
Measurement of Carbohydrate Content
The total soluble sugar content was determined using the sulfuric acid–anthrone colorimetric method with modifications (DuBois et al. 1956). Leaf samples (0.2 g) were homogenized with 10 ml of deionized water and then boiled for 30 min three times. The extract was filtered and treated with anthrone and 98% sulfuric acid; the mixture was then incubated in boiling water for 10 min. After cooling to room temperature, the absorbance was measured at 620 nm using a spectrophotometer. The starch content was determined using the sulfuric acid–anthrone colorimetric method (DuBois et al. 1956). The sediment of the extract filtered with the sugar was dried, weighed, and boiled with deionized water at 9.2 M and 4.6 M perchloric acid; the supernatant was used to determine the starch content. The total content of non-structural carbohydrates (NSCs) was calculated as the sum of the content of soluble sugars and starch. The method of glucose extraction was identical to that for total soluble sugar, and the content of glucose was determined following a previously described method with minor modifications (Zhang et al. 2018).
Measurement of Antioxidant Enzyme Activities and MDA Content
Frozen leaves (0.2 g) were ground in liquid nitrogen and then homogenized in 5 mL of 50 mM sodium phosphate buffer (pH = 7.0). The homogenate was centrifuged at 10,000 × g for 15 min at 4 °C, and the supernatant was stored in aliquots at -20ºC for further analysis. Superoxide dismutase (SOD) activity was measured according to the degree of inhibition of the photoreduction of nitroblue tetrazolium as described by Giannopolitis and Ries (1977). Peroxidase (POD) activity was measured according to the conversion of guaiacol to tetraguaiacol, which was monitored at 470 nm, as previously described by Maehly and Chance (1955). Catalase (CAT) activity was measured following the previously described method of Aebi (1983) with some modifications. Ascorbate peroxidase (APX) activity was measured following the method of Bonnecarrère et al. (2011). The content of MDA was estimated by measuring the concentration of thiobarbituric acid-reactive substances (Dhindsa et al. 1981). Frozen leaves (0.2 g) were homogenized in 2 mL of 5% trichloroacetic acid. The absorbance was measured at 450, 532, and 600 nm. MDA was calculated using following the formula: C (μM) = 6.45(A532-A600) − 0.56A450.
Results
Alterations in Carbohydrate Metabolism and the Energy Supply Confer ett1 with Thermo-tolerance
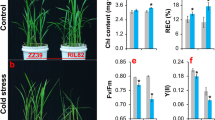
After rice seedling plants were exposed to 45 ºC for 36 h and allowed to recover for 7 d, most of the WT and ovD1 leaves were withered, while most of leaves in the ett1 mutants were still green (Fig. 1A a). Heat stress significantly decreased the values of Fv/Fm, in which the highest reduction was showed in ovD1 plants, the next was WT plants, while the lowest reduction were presented in ett1 mutants (Fig. 1A b). Accordingly, the highest survival rate was found in ett1 mutants, followed by WT, and ovD1 plants. This suggested that the gene ett1 negatively regulated thermal tolerance in rice seedling plants (Fig. 1A c).
The performance of WT plants and ett1 mutants under HS and the results of RNA-seq analysis. A a, Plant performance under HS; Fv/Fm value (b) and survival rate (c) under HS; B GSEA of the RNA-seq data of WT plants and the ett1 mutant. C GO enrichment. D KEGG enrichment. E Simple descriptive model of the response of ett1 mutants to HS. Fv/Fm, maximum fluorescence quantum yield of photosystem II; GSEA, Gene Set Enrichment Analysis; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; The arrow mark “→” indicates induction. Vertical bars denote standard deviations (n = 3). T-tests were conducted to evaluate the significance of differences among plants exposed to HS and control conditions. Different letters indicate significant differences under HS and control conditions (P < 0.05)
To clarify the mechanism underlying the heat response of ett1, RNA-seq was conducted under control conditions and heat stress treatment with WT and ett1 seedling plants. The results of quality control analyses, DEG analyses, volcano plots, and heat map hierarchical clustering analysis are shown in the (Additional file 7: Table S3, Additional file 3: Fig. S3 and Additional file 4: S4). GSEA showed that the expression of genes involved in carbohydrate metabolic process, glucose metabolic process, glycogen metabolic process, NAD metabolic process, and energy reserve metabolism was down-regulated in WT plants under heat stress. The expression of genes involved in these five processes was less down-regulated in ett1 mutants than in WT plants; in some cases, the expression of genes was up-regulated in ett1 mutants (Fig. 1B). GO analysis was conducted on the four groups of plants. The GO terms response to heat and ATP binding were significantly enriched (Fig. 1C). Significantly enriched pathways according to KEGG analysis were protein processing in endoplasmic reticulum (ko04141), endocytosis (ko04144), RNA transport (ko03013), carbon metabolism (ko01200), and amino sugar and nucleotide sugar metabolism (ko00520) (Fig. 1 D). According to these findings, we speculate that carbohydrate metabolism and energy metabolism might be responsible for the significant differences in the response of the two rice seedling plants to heat stress (Fig. 1E).
Five heat shock protein (Hsp) genes were used to validate the RNA-seq data. The expression levels of genes were higher in ett1 mutants than in WT plants, which indicates that the thermo-tolerance of the ett1 mutants was greater than that of WT plants (Fig. 2A a and b). The RNA-seq results were similar to the qRT-PCR results (Fig. 2B).
qRT-PCR validation of the differentially expressed transcripts identified by RNA-seq. Five hsp genes were selected for qRT-PCR analysis for validation. A qRT-PCR analysis of five hsp genes in WT (a) and ett1 (b) plants. B Expression heat map of five hsp genes between WT and ett1 mutants. hsp, heat shock protein; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; Hsp74.8, LOC_Os09g29840; Hsp71.1, LOC_Os03g16860; Hsp58.7, LOC_Os09g31486; Hsp24.1, LOC_Os02g52150; Hsp23.7, LOC_Os12g38180. Vertical bars denote standard deviations (n = 3). T-tests were conducted to evaluate the significance of differences under HS and control conditions within a single genotype. “*” indicates significance difference exist, NS indicate no significance difference exist
Carbohydrate Degradation is Affected by Heat Stress
Sugar metabolism was significantly altered in ett1 mutants under heat stress. The NSC, soluble sugar, starch, and sucrose content was significantly lower under heat stress than under control conditions in both ett1 and WT plants, with the exception of the soluble sugar content in WT (Fig. 3). The NSC, starch, and sucrose content was 38.16%, 24.23%, and 47.52% lower in ett1 mutants under heat stress than under control conditions, respectively; in WT plants, the NSC, starch, and sucrose content was 18.78%, 9.96%, and 13.54% lower under heat stress than under control conditions, respectively (Fig. 3 a, b, and d). Also, the decreases in the soluble sugar content under heat stress were much more pronounced in ett1 mutants than in WT plants (Fig. 3c). Significant decrease in the glucose content was observed in WT plants under heat stress, while no significant difference was observed in glucose content in ett1 mutants between heat stress and control conditions (Fig. 3e). Similar decreases were observed in fructose content under heat stress in ett1 and WT plant (Fig. 3f).
Effects of HS on carbohydrate metabolism in WT plants and ett1 mutants. (a) NSC content. (b) Starch content. (c) Soluble sugar content. (d) Sucrose content. (e) Glucose content. (f) Fructose content. NSC, nonstructural carbohydrate. Vertical bars denote standard deviations (n = 3). T-tests were conducted to evaluate differences in the content of carbohydrates under HS and control conditions within a single genotype. “*” indicate significant differences between HS and control conditions (P < 0.05)
The expression patterns of six genes involved in sucrose metabolism were analyzed. The expression of the key regulatory genes involved in sucrose metabolism, including invertase (INV) and sucrose synthase (SUS), was up-regulated in ett1 mutants under heat stress compared with control conditions (Fig. 4b); however, different patterns of gene expression were observed in WT plants. Specifically, the expression of INV2, SUS1, and SUS7 was up-regulated under heat stress, and the expression of INV3, SUS2, and SUS3 was down-regulated under heat stress (Fig. 4a). Increases in the expression of genes in ett1 mutants were more pronounced than those observed in WT plants (Fig. 4).
Effects of HS on the expression of sucrose synthase and invertase genes in WT plants (a) and ett1 mutants (b). qRT-PCR, quantitative reverse transcription-polymerase chain reaction; SUS, sucrose synthase; INV, invertase; INV2, LOC_Os04g45290; INV3, LOC_Os02g01590; SUS1, LOC_Os03g28330; SUS2, LOC_Os06g09450; SUS3, LOC_Os07g42490; SUS7, LOC_Os04g17650. Vertical bars denote standard deviations (n = 3). T-tests were conducted to evaluate differences in the content of carbohydrates under HS and control conditions within a single genotype. “*” indicate significant differences between HS and control conditions (P < 0.05)
More ATP was Produced in ett1 Mutants Under Heat Stress
The ATP content and energy charge were significantly higher in ett1 mutants under heat stress than under control conditions; while no significant difference was observed in the ATP content and energy charge in WT plants under heat stress and control conditions (Fig. 5a and b). No significant difference was observed in the ratio of NADPH/NADP+ in ett1 mutants and WT plants between heat stress and control conditions (Fig. 5c). The ratio of NADH/NAD+ in ett1 mutants was significantly decreased by heat stress, while no obvious difference between the heat stress and control were showed in WT plants (Fig. 5d).
Effects of HS on energy metabolism in WT plants and ett1 mutants. (a) ATP content. (b) Energy charge. (c) NADPH/NADP+. (d) NADH/NAD+. ATP, adenosine triphosphate; NADP (H), nicotinamide adenine dinucleotide phosphate; NAD(H), nicotinamide adenine dinucleotide. Vertical bars denote standard deviations (n = 3). T-tests were conducted to evaluate the significance of differences in energy metabolism variables under HS and control conditions within single genotypes. Different letters indicate significant differences between HS and control conditions (P < 0.05)
Antioxidant Capacity was not Decreased by Heat Stress in ett1 Mutants
The activities of four antioxidant enzymes were measured. No significant difference was observed in SOD and CAT activities between the control and heat stress in both rice seedling plants (Fig. 6a and c). Under heat stress, notable decreases in POD and APX activity were presented in WT plants; in ett1 mutants, no significant differences in POD and APX activity were observed under control conditions and heat stress (Fig. 6 b and d).
Effects of HS on the activity of antioxidant enzymes in WT plants and ett1 mutants. (a) SOD activity. (b) POD activity. (c) CAT activity. (d) APX activity. SOD, superoxide dismutase; POD, peroxidase; CAT, catalase; APX, ascorbate peroxidase. Vertical bars denote standard deviations (n = 3). T-tests were conducted to evaluate the significance of differences in antioxidant enzyme activities under HS and control conditions within single genotypes. Different letters indicate significant differences under HS and control conditions (P < 0.05)
Exogenous Glucose Application can Alleviate the Damage Induced by Heat Stress
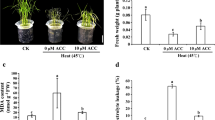
According to the results above, we inferred sugar signaling might play a key role in mediating the response of rice plants to heat stress, thus we conducted an exogenous glucose experiment to evaluate this possibility. Under control conditions, glucose caused little effects on the rice seedling plants compared with those plants treated with H2O; no obvious difference in Fv/Fm, MDA, ATP, and energy charge were found between both treatments in WT and ett1 plants (Fig. 7). Leaves of both ett1 mutants and WT plants sprayed with exogenous glucose were less curly than those sprayed with water (Fig. 7a). Heat stress significantly decreased the values of Fv/Fm of both rice plants, while this inhibition could be reversed by glucose (Fig. 7b and c). The MDA contents were obviously increased by heat stress, in particular for the WT; exogenous glucose significantly reduced the MDA content in either rice plants under such stress condition (Fig. 7d and e). In term of ATP and energy charge, notable enhancements were showed in the glucose treatment compared with the plants treated with H2O under heat stress (Fig. 7f–i).
Exogenous glucose application alleviates damage caused by HS. (a) Photographs of leaves after spraying with glucose under HS. Fv/Fm value was alleviated by exogenous glucose in WT (b) and ett1 (c). MDA content was alleviated by exogenous glucose in WT (d) and ett1 (e). ATP content was affected by exogenous glucose in WT (f) and ett1 (g). Energy charge was improved by exogenous glucose in WT (h) and ett1 (i). MDA, Malondialdehyde; Fv/Fm, Maximum fluorescence quantum yield of PSII. Vertical bars denote standard deviations (n = 3). T-tests were conducted to evaluate the significance of differences under HS and control conditions within single genotypes. Different letters indicate significant differences under HS and control conditions (P < 0.05)
Discussion
The ett1 mutant is a new allelic mutant of the D1 mutant, which has been largely documented in rice plants (Ishikawa et al. 1995; Fujisawa et al. 1999; Ashikari et al. 1999; Yang et al. 2014). Our previous results indicated that the d1 mutants were susceptible to low light stress at anthesis, in which a large decrease in spikelet fertility were found in d1 mutant plants compared with those of wide-type and over-expression lines caused by low-light stress (Li et al. 2023). Interestingly, this mutant displayed resistant to drought, salt and high light stress (Ferrero-Serrano and Assmann 2016; Ferrero-Serrano et al. 2018; Zait et al. 2021; Peng et al. 2019). Similarly, the d1 plants display heat tolerance compared with its wide-type and over-expression lines (Fig. 1), suggesting that ett1 negatively regulates thermal tolerance in rice plants. This finding was consistent with the results of Jangam et al. (2016), who considered that G-Protein Alpha Subunit played key roles in regulating multiple abiotic stresses such as drought, salinity, heat, and cold through microarray analysis. However, the mechanism underlying ett1 affecting thermal-tolerance in rice plants has not been clarified.
GO and KEGG pathway analyses revealed that genes showing expression differences between the ett1 mutants and WT plants were significantly enriched in carbohydrate and energy metabolism, which was inferred mainly contributing to their different heat tolerance (Fig. 1). As well-known, carbohydrates and energy metabolism not only molecule plant growth and development but also the response to various types of biotic and abiotic stress, including heat stress (Harker et al. 2009; Ruan 2014; Cabello et al. 2014; Li et al. 2015b; Islam et al. 2018). Indeed, RGA1 has been reported to confer low-light resistance through improving the carbohydrate and energy metabolism in pistil of rice (Li et al. 2023). However, a larger decrease in NSC and sucrose were showed in ett1 than WT plants under heat stress compared with their respective controls (Fig. 3). This was inconsistent with results that sucrose content of RGA1 mutant was increased in pistil under low light conditions (Li et al. 2023), suggesting that inhibition on sucrose, rather than carbohydrates deficit contributed to the thermal tolerance showed in ett1 plants (Jiang et al. 2020).
The invertase and sucrose synthesis are reported to play key roles in sucrose metabolism in plants (Sheen et al. 1999). Heat stress could reduce the activities of CWINS and SUS, deplete starch storage, reduce the hexose content in anther and pistil tissues, and ultimately result in pollen abortion (Ruan 2014; Li et al. 2015b). Similar results were found in this experiment, that increase in the relative expression levels of SUS1, SUS2, SUS3, SUS7, INV2 and INV3 were higher than those of WT plants under heat stress (Fig. 4). Therefore, it was inferred that ett1 affected thermal-tolerance in rice plants through alleviating inhibition on expression of INVs and SUSs caused by heat stress. However, different roles in regulating heat response in plants were also found between the invertase and sucrose synthase (Jiang et al. 2020). Compared with heat-tolerant cultivar, lower expression of sucrose phosphate synthase (SPS) and SUS were found in heat-sensitive cultivar, whereas the expression levels cell wall invertase (CWINs) genes in heat–resistant cultivar were significantly lower than those in heat-susceptible line (Zhang et al. 2010). Further, RGA1 can alleviate the inhibition on acid invertase activity in pistil of rice caused by low-light stress, while such effect was not presented in sucrose synthase activity (Li et al. 2023). Interestingly, low light stress obviously inhibited the genes of SUS2, INV2, and CIN2 in pistil of RGA1 mutant plants. Therefore, how ett1 affecting sucrose metabolism in rice plants under heat stress required further researches.
Energy status is important for plants surviving in abiotic stress, since increases in antioxidant capacity and the accumulation of HSPs induced by high temperature and other adverse conditions require large amounts of energy; energy deficiencies and decreases in energy utilization efficiency can increase the stress sensitivity of plants (Zhang et al. 2017; Li et al. 2020). The present results indicated that energy status including ATP and energy charge were obviously increased by heat stress in ett1 seedling plants compared with control, while no obvious difference in WT seedling plants were showed between the control and heat stress. This suggested that ett1 affected the thermal tolerance in rice seedling plants by improving energy status, which could provide more energy for the strong antioxidant capacity and accumulation of heat shock proteins (Fig. 2 and Fig. 5). Indeed, the changes in contents of NADP(H) and NAD(H) also play key roles in regulating energy status in plants (Igamberdiev and Bykova 2023). There was obvious difference in NADPH/NADP+ between the control and heat stress in either rice plants; A notable decrease in NADH/NAD+ were presented in ett1 mutant plants under heat stress compared with control (Fig. 5). This indicated that NADH/NAD+ was an important factor in mediating ett1 to affected the energy homeostasis in plants under heat stress, which has been not reported previously.
These years, studies have indicated that, similar to classical plant hormones, sugar molecules not only a nutrient, but also can act as signaling molecules that control gene expression, developmental processes and response to abiotic stress in plants (Sheen et al. 1999; Wang et al. 2020; Choudhary et al. 2022). Heat stress significantly decreased the glucose content in wt seedling plants, while no obvious difference in ett1 seedling plants were showed between the control and heat stress, suggesting that glucose signaling might be involved in the process of ett1 regulating thermal tolerance. This hypothesis was confirmed by the exogenous glucose spraying onto rice plants under heat stress (Fig. 7), which might be related its function in enhancing the acid invertase activity as well as inactivating PARP to regulate the energy utilizing efficiency (Jiang et al. 2020).
Conclusion
The ett1 mutant has a dysfunctional RGA1 gene that encodes the Gα subunit of the heterotrimeric G protein, and it negatively regulates thermal tolerance in rice. Under heat stress, the survival rate of ett1 mutants was significantly higher than that of WT plants. According to the transcriptome analysis, the sugar and energy metabolism were involved in mediating ett1 to affect the thermal tolerance. In this process, the ATP content and energy charge was obviously increased by heat stress in ett1 plants, which mainly responsible for the antioxidant capacity and accumulation of HSPs. Exogenous glucose can alleviate heat damages on rice seedling plants through increasing the ATP content and energy charge. Thus, we concluded that ett1 negatively regulated thermo-tolerance in rice by improving carbohydrate metabolism, glucose signaling and energy status.
Availability of Data and Materials
Information on plant materials are included in our previous article (Li et al. 2023). Other datasets supporting the conclusions of this article are included in the article (and its additional files).
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- CWIN :
-
Cell wall invertase
- Fv/Fm:
-
Maximum fluorescence quantum efficiency
- GO:
-
Gene ontology
- GSEA:
-
Gene Set Enrichment Analysis
- Hsp:
-
Heat shock proteins
- INV :
-
Invertase
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- MDA:
-
Malondialdehyde
- NAD:
-
Nicotinamide adenine dinucleotide
- NBT:
-
Nitroblue tetrazolium
- NSC:
-
Non-structural carbohydrates
- PCD:
-
Programmed cell death
- POD:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SPS :
-
Sucrose phosphate synthase
- SOD:
-
Superoxide dismutase
- SUS :
-
Sucrose synthase
- SUT :
-
Sucrose transporters
References
Aebi H (1983). In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic press, London
Amor Y, Babiychuk E, Inzé D, Levine A (1998) The involvement of poly (ADP-ribose) polymerase in the oxidative stress responses in plants. FEBS Lett 440:1–7
Ashikari M, Wu J, Yano M, Sasaki T, Yoshimura A (1999) Rice gibberellin-insensitive dwarf mutant gene dwarf 1 encodes the α-subunit of GTP-binding protein. Proc Nat Acad Sci USA 96:10284–10289
Assmann S (2002) Heterotrimeric and unconventional GTP binding proteins in plant cell signaling. Plant Cell 14:355–373
Bisht NC, Jez JM, Pandey S (2015) An elaborate heterotrimeric G-protein family from soybean expands the diversity of plant G-protein networks. New Phytol 190:35–48
Bonnecarrère V, Borsani O, Díaz P, Capdevielle F, Blanco P, Monza J (2011) Response to photoxidative stress induced by cold in japonica rice is genotype dependent. Plant Sci 180:726–732
Botella JR (2012) Can heterotrimeric G proteins help to feed the world? Trends Plant Sci 17:563–568
Cabello JV, Lodeyro AF, Zurbriggen MD (2014) Novel perspectives for the engineering of abiotic stress tolerance in plants. Curr Opin Biotechnol 26:62–70
Chen TT, Ma JY, Xu CM, Jiang N, Li GY, Fu WM, Feng BH, Wang DY, Wu ZH, Tao LX, Fu GF (2022) Increased ATPase activity promotes heat-resistance, high-yield, and high-quality. Front Plant Sci 13:1035027
Choudhary A, Kumar A, Kaur N, Kaur H (2022) Molecular cues of sugar signaling in plants. Physiol Plant 174(1):e13630
Cui Y, Jiang N, Xu Z, Xu Q (2020) Heterotrimeric G protein are involved in the regulation of multiple agronomic traits and stress tolerance in rice. BMC Plant Biol 20:1–3
De BM, Van LM (2011) Energy efficiency and energy homeostasis as genetic and epigenetic components of plant performance and crop productivity. Curr Opin Plant Biol 14:275–282
De BM, Verduyn C, De BD, Cornelissen M (2005) Poly (ADPribose) polymerase in plants affects energy homeostasis, cell death and stress tolerance. Plant J 41:95–106
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dobrota C (2006) Energy dependent plant stress acclimation. Rev Environ Sci Biotechnol 5:243–251
Dröge-Laser W, Weiste C (2018) The C/S 1 bZIP network: a regulatory hub orchestrating plant energy homeostasis. Trends Plant Sci 23:422–433
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fan C, Xing Y, Mao H, Lu T, Han B, Xu C, Li X, Zhang Q (2006) GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor Appl Genet 112:1164–1171
Feng BH, Yang Y, Shi YF, Shen HC, Wang HM, Huang QN, Xu X, Lv XG, Wu JL (2013) Characterization and genetic analysis of a novel rice spotted-leaf mutant HM47 with broad-spectrum resistance to xanthomonas oryzae pv. oryzae. J Integr Plant Biol 55:473–483
Ferrero-Serrano A, Assmann SM (2016) The alpha-subunit of the rice heterotrimeric G protein, RGA1, regulates drought tolerance during the vegetative phase in the dwarf rice mutant d1. J Exp Bot 67:3433–3443
Ferrero-Serrano A, Su Z, Assmann SM (2018) Illuminating the role of the Galpha heterotrimeric G protein subunit, RGA1, in regulating photoprotection and photoavoidance in rice. Plant Cell Environ 41:451–468
Fu GF, Zhang CX, Yang YJ, Xiong J, Yang XQ, Zhang XF, Jin QY, Tao LX (2015) Male parent plays more important role in heat tolerance in three-line hybrid rice. Rice Sci 22(3):116–122
Fu GF, Feng BH, Zhang CX, Yang YJ, Yang XQ, Chen TT, Zhao X, Zhang XF, Jin QY, Tao LX (2016) Heat stress is more damaging to superior spikelets than inferiors of rice (Oryza sativa L.) due to their different organ temperatures. Front Plant Sci 7:1637
Fujisawa Y, Kato T, Ohki S, Ishikawa A, Kitano H, Sasaki T, Asahi T, Iwasaki Y (1999) Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc Nat Acad Sci USA 96:7575–7580
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I occurrence in higher. Plants Plant Physiol 59(2):309–314
Gilman AG (1987) G proteins: transducers of receptor-generated signals. Ann Rev Biochem 56:615–649
Grover A, Mittal D, Negi M, Lavania D (2013) Generating high temperature tolerant transgenic plants: achievements and challenges. Plant Sci 205:38–47
Harker KN, O’Donovan JT, Irvine RB, Turkington TK, Clayton GW (2009) Integrating cropping systems with cultural techniques augments wild oat (Avena fatua) management in barley. Weed Sci 57:326–337
Hashida SN, Takahashi H, Uchimyia H (2009) The role of NAD biosynthesis in plant development and stress responses. Ann Bot 103:819–824
Huang X, Qian Q, Liu Z, Sun H, He S, Luo D, Xia G, Chu C, Li J, Fu X (2009) Natural variation at the DEP1 locus enhances grain yield in rice. Nat Genet 41:494–497
Hunt L, Lerner F, Ziegler M (2004) NAD-new roles in signalling and gene regulation in plants. New Phytol 163:31–44
Igamberdiev AU, Bykova NV (2023) Mitochondria in photosynthetic cells: coordinating redox control and energy balance. Plant Physiol 191:2104–2119
Ishikawa A, Tsubouchi H, Iwasaki Y, Asahi T (1995) Molecular cloning and characterization of a cDNA for the α subunit of a G protein from rice. Plant Cell Physiol 36:353–359
Ishikawa A, Iwasaki Y, Asahi T (1996) Molecular cloning and characterization of a cDNA for the rβ subunit of a G protein from rice. Plant Cell Physiol 37:223–228
Islam Md, Feng BH, Chen TT, Tao LX, Fu GF (2018) Role of abscisic acid in thermal acclimation of plants. J Plant Biol 61:255–264
Izawa Y, Takayanagi Y, Inaba N, Abe Y, Minami M, Fujisawa Y, Kato H, Ohki S, Kitano H, Iwasaki Y (2009) Function and expression pattern of the subunit of the heterotrimeric g protein in rice. Plant Cell Physiol 51:271–281
Jangam AP, Pathak RR, Raghuram N (2016) Microarray analysis of rice d1 (RGA1) mutant reveals the potential role of G-protein alpha subunit in regulating multiple abiotic stresses such as drought, salinity, heat, and cold. Front Plant Sci 7:11
Jiang N, Yu PH, Fu WM, Li GY, Feng BH, Chen TT, Li HB, Fu TLX, GF, (2020) Acid invertase confers heat tolerance in rice plants by maintaining energy homeostasis of spikelets. Plant Cell Environ 43:1273–1287
Jones AM, Assmann SM (2004) Plants: the latest model system for G-protein research. EMBO Rep 5:572–578
Kan Y, Mu XR, Zhang H, Gao J, Shan JX, Ye WW, Lin HX (2022) TT2 controls rice thermotolerance through SCT1-dependent alteration of wax biosynthesis. Nat Plants 8:53–67
Kato C, Mizutani T, Tamaki H, Kumagai H, Kamiya T, Hirobe A, Fujisawa Y, Kato H, Iwasaki Y (2004) Characterization of heterotrimeric G protein complexes in rice plasma membrane. Plant J 38:320–331
Koch-Nolte F, Haag F, Guse AH, Lund F, Ziegler M (2009) Emerging roles of NAD+ and its metabolites in cell signaling. Sci Signal 2(57):mr1
Kumazaki A, Suzuki N (2019) Enhanced tolerance to a combination of heat stress and drought in Arabidopsis plants deficient in ICS1 is associated with modulation of photosynthetic reaction center proteins. Physiol Plant 165:232–246
Li X, Lawas LMF, Malo R, Glaubitz U, Erban A, Mauleon R, Heuer S, Zuther E, Kopka J, Hincha DK, Jagadish KSV (2015a) Metabolic and transcriptomic signatures of rice floral organs reveal sugar starvation as a factor in reproductive failure under heat and drought stress. Plant Cell Environ 38:2171–2192
Li XM, Chao DY, Wu Y, Huang XH, Chen K, Cui LG, Su L, Ye WW, Chen H, Chen HG, Dong NQ, Guo T, Shi M, Feng Q, Zhang P, Han B, Shan JX, Gao JP, Lin HX (2015b) Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat Genet 47:827–833
Li GY, Zhang CX, Zhang GH, Fu WM, Feng BH, Chen TT, Peng SB, Tao LX, Fu GF (2020) Abscisic acid negatively modulates heat tolerance in rolled leaf rice by increasing leaf temperature and regulating energy homeostasis. Rice 13:18
Li H, Feng B, Li J, Fu W, Wang W, Chen T, Liu L, Wu Z, Peng S, Tao L, Fu G (2023) RGA1 alleviates low-light-repressed pollen tube elongation by improving the metabolism and allocation of sugars and energy. Plant Cell Environ 46(4):1363–1383
Liu Y, Offler CE, Ruan YL (2016) Cell wall invertase promotes fruit set under heat stress by suppressing ROS-independent plant cell death. Plant Physiol 172:163–180
Maehly AC, Chance B (1955) The assay of catalases and peroxidases. Methods Enzymol 2:764–775
Matsumura H, Miyachi S (1983). In: San Pietro A (ed) Methods in enzymology. Academic Press, New York
Noctor G, De Paepe R, Foyer CH (2007) Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci 12:125–134
Peng P, Gao Y, Li Z, Yu Y, Qin H, Guo Y, Huang R, Wang J (2019) Proteomic analysis of a rice mutant sd58 possessing a novel d1 allele of heterotrimeric G protein alpha subunit (RGA1) in salt stress with a focus on ROS scavenging. Int J Mol Sci 20(1):167
Perfus-Barbeoch L, Jones AM, Assmann SM (2004) Plant heterotrimeric G protein function: insights from Arabidopsis and rice mutants. Curr Opin Plant Biol 7:719–731
Ruan YL (2014) Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Ann Rev Plant Biol 65:33–67
Sehgal A, Sita K, Bhandari K, Kumar S, Kumar J, Vara Prasad PV, Siddique KHM, Nayyar H (2019) Influence of drought and heat stress, applied independently or in combination during seed development, on qualitative and quantitative aspects of seeds of lentil (Lens culinaris Medikus) genotypes, differing in drought sensitivity. Plant Cell Environ 42:198–211
Sheen J, Zhou L, Jang JC (1999) Sugars as signaling molecules. Curr Opin Plant Biol 2(5):410–418
Trusov Y, Chakravorty D, Botella JR (2012) Diversity of heterotrimeric G-protein γ subunits in plants. BMC Res 5:608
Urano D, Chen JG, Botella JR, Jones AM (2013) Heterotrimeric G protein signalling in the plant kingdom. Open Biol 3:120186–120186
Wang G, Pichersky E (2007) Nicotinamidase participates in the salvage pathway of NAD biosynthesis in Arabidopsis. Plant J 49:1020–1029
Wang F, Han T, Song Q, Ye W, Song X, Chu J, Li J, Chen ZJ (2020) The rice circadian clock regulates tiller growth and panicle development through strigolactone signaling and sugar sensing. Plant Cell 32(10):3124–3138
Wettschureck N, Offermanns S (2005) Mammalian G proteins and their cell type specific functions. Physiol Rev 85:1159–1204
Xu YF, Zhang L, Ou SJ, Wang RC, Wang YM, Chu CC, Yao SG (2020) Natural variations of SLG1 confers high-temperature tolerance in indica rice. Nat Commun 11:5441
Yang DW, Zheng XH, Cheng CP, Wang WD, Xing DH, Lu LB, Liu CD, Ye N, Zeng MJ, Ye XF (2014) A dwarfing mutant caused by deactivation function of alpha subunit of the heterotrimeric G-protein in rice. Euphytica 197(1):145–159
Yu YQ, Chakravorty D, Assmann SM (2018) The G Protein b-subunit, AGB1, interacts with FERONIA in RALF1-regulated stomatal movement. Plant Physiol 176:2426–2440
Zait Y, Ferrero-Serrano A, Assmann SM (2021) The alpha subunit of the heterotrimeric G protein regulates mesophyll CO2 conductance and drought tolerance in rice. New Phytol 232(6):2324–2338
Zhang H, Liang WQ, Yang XJ, Luo X, Jiang N, Ma H, Zhang DB (2010) Carbon starved anther encodes a MYB domain protein that regulates sugar partitioning required for rice pollen development. Plant Cell 22:672–689
Zhang CX, Fu GF, Yang XQ, Yang YJ, Zhao X, Chen TT, Zhang XF, Jin QY, Tao LX (2016) Heat stress effects are stronger on spikelets than on flag leaves in rice due to differences in dissipation capacity. J Agron Crop Sci 202:394–408
Zhang CX, Feng BH, Chen TT, Zhang XF, Tao LX, Fu GF (2017) Sugars, antioxidant enzymes and IAA mediate salicylic acid to prevent rice spikelet degeneration caused by heat stress. Plant Growth Reg 83:313–323
Zhang CX, Feng BH, Chen TT, Fu WM, Li HB, Li GY, Jin QY, Tao LX, Fu GF (2018) Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation. Environ Exp Bot 155:718–733
Zhang DP, Zhang MY, Zhou Y, Wang YZ, Shen JY, Chen HYX, Zhang L, Lv B, Liang GH, Liang JS (2019) The rice G protein γ subunit DEP1/qPE9-1 positively regulates grain-filling process by increasing auxin and cytokinin content in rice grains. Rice 12:91
Zhang H, Zhou JF, Kan Y, Shan JX, Ye WW, Dong NQ, Guo T, Xiang YH, Yang YB, Li YC, Zhao HY, Yu HX, Lu ZQ, Guo SQ, Lei JJ, Liao B, Mu XR, Cao YJ, Yu JJ, Lin YS, Lin HX (2022) A genetic module at one locus in rice protects chloroplasts to enhance thermotolerance. Science 376:1293–1300
Zhao X, Chen TT, Feng BH, Zhang CX, Peng SB, Zhang XF, Fu GF, Tao LX (2017) Non-photochemical quenching plays a key role in light acclimation of rice plants differing in leaf color. Front Plant Sci 7:1968
Jajoo A, Allakhverdiev SI (2017) High-temperature stress in plants: Consequences and strategies for protecting photosynthetic machinery. In: Shabala, S. (Ed). Plant Stress Physiology, 138–154. USA Boston, MA: CABI; ISBN 9781780647302.
Acknowledgements
Not applicable.
Funding
This work is supported by Key Pioneer Research Project of Zhejiang Province (2022C02014), Zhejiang Provincial Natural Science Foundation of China (LY22C130003), Sichuan Science and Technology Program (23QYCX0015), Sichuan Key Project of Rice Breeding (2021YFYZ0016), Independent Innovation of Sichuan Province (2022CCZX066), Crop Technology Research Project of Sichuan Finance (2021ZYGG-002), the State Key Laboratory of Rice Biology (20210402), the China National Rice Research Institute Key Research and Development Project (CNRRI-2020-05).
Author information
Authors and Affiliations
Contributions
BF, GF and TC conceived the original research plan and supervised the research work; BF, YX, WF, HL, JL, and WW performed research; BF, GL, LT, and GF analyzed the data and wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
The gene mapping, sequencing results and predicted protein coding of the mutant ett1.
Additional file 2.
The performance of agronomic traits in the mutant ett1 and WT.
Additional file 3.
Number of DEGs in ett1 and WT under heat stress and control condition.
Additional file 4.
The hierarchical clustering analysis of the DEGs of the mutant ett1 and WT under heat stress and control condition.
Additional file 5.
Genetic analysis of the mutant ett1.
Additional file 6.
Primer sequences used in the quantitative RT-PCR.
Additional file 7.
The quality control analysis of the RNA-sequencing results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, B., Xu, Y., Fu, W. et al. RGA1 Negatively Regulates Thermo-tolerance by Affecting Carbohydrate Metabolism and the Energy Supply in Rice. Rice 16, 32 (2023). https://doi.org/10.1186/s12284-023-00649-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12284-023-00649-w