Abstract
Background
The recent global pandemic due to severe acute respiratory syndrome coronavirus-2 resulted in a high rate of multi-organ failure and mortality in a large patient population across the world. As such, a possible correlation between acute kidney injury (AKI) and increased mortality rate in these patients has been suggested in literature.
Methods
This is a two-year retrospective study of critically ill adult patients infected with COVID-19 that were admitted to the intensive care unit (ICU) on ventilatory support. Two groups of patients were identified in this study, those who were directly admitted to the ICU or those who were initially admitted to the Medical Floor and were later transferred to the ICU due to either worsening respiratory status or change in their hemodynamic conditions. Within each group, three subgroups were created based on the status of AKI, namely, those who did not develop AKI, those who developed AKI, and those who with previous history of dialysis dependent AKI.
Results
The AKI subgroup had the highest mortality rate in the ICU and Floor patients. Of note, those patients who were directly admitted to the Floor and were later transferred to the ICU for worsening conditions also experienced a higher mortality rate if they had developed AKI during their course of hospital stay.
Conclusions
This study identified a statistically significant higher mortality in patients who developed AKI than those who did not develop AKI among critically ill patients.
Trial registration
Clinicaltrials.gov registration number NCT05964088. Date of registration: July 24 2023.
Similar content being viewed by others
Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), more commonly known as COVID-19 led to a global pandemic in 2020 and 2021. The high rate of mortality and morbidity of the disease led to a global panic and strict quarantine guidelines. The veracity of this virus was in large part secondary to the immunologic and inflammatory response of the host, which resulted in a significant number of critically ill patients [1]. SARS-CoV-2 is an inflammatory respiratory illness via the mechanism of the virus entering type 2 alveolar epithelial cells via the host ACE-2 receptor [2, 3]. Ultimately, complications of the disease process such as acute respiratory distress syndrome, sepsis, disseminated intravascular coagulation, pulmonary embolism, and in particular kidney dysfunction contributed to multiorgan failure and death in many of these individuals [4,5,6].
A possible correlation between acute kidney injury (AKI) and increased mortality rate among the COVID-19 population has been suggested in scientific literature [7, 8]. Acute kidney injury is defined as an increase of serum creatinine (SCr) more than 0.3 milligrams per deciliter (mg/dL) within 48 h, increase in SCr 1.5 times the baseline, or urine volume reduction of less than 0.5 milliliter per kilogram per hour for six hours [9]. The utility of inflammatory markers and co-morbidities in predicting the probability of developing AKI in hospitalized patients infected with COVID-19 have been a topic of recent investigations [10]. Recent studies reported that the proportion of AKI in hospitalized COVID-19 patients can range between 19% and 43% [1]. Additional studies have also examined how risk factors such as hypertension, diabetes mellitus (T2DM), and obesity are directly linked to an increased risk of AKI [11,12,13].
This study examined the incidence of mortality and morbidity associated with AKI in critically ill adult patients infected with COVID-19. These critically ill patients were either directly admitted to the intensive care unit (ICU) from the emergency department (ED) or were initially managed on the Medical Floor and later transferred to the ICU for worsening respiratory status. Furthermore, the investigating team evaluated the correlation of inflammatory markers and common risk factors such as obesity, T2DM, hypertension, and ethnicity to the development of AKI in the target population. We hypothesized that patients with newly developed AKI will have a higher mortality rate than those who did not develop AKI during the hospital stay.
Methods
This is a two-year retrospective study of critically ill adult patients infected with COVID-19 that were admitted to the ICU on ventilatory support. Patients included in this study were admitted to Arrowhead Regional Medical Center (ARMC) from March 1, 2020 to February 12, 2022. ARMC is a 456-bed university-affiliated teaching public hospital that serves as a safety net hospital for the uninsured and underserved population in San Bernardino County, California. San Bernadino County is the largest geographic county in the continental United States with a diverse population of 2.2 million people in 2023. According to the 2023 Census data, the racial demographics of SBC include 56.2% Hispanic or Latino, 24.7% White, and 9.3% African American [14]. The county has a large proportion of low socioeconomic status individuals, with 13.5% of the population living below the poverty line [14].
Patients were included in the study if they tested positive for SARS-CoV-2 on a real time polymerase chain reaction test (RT-PCR) upon admission to the hospital, respiratory status either on mechanical or non-invasive mechanical ventilation for direct ICU admission. Respiratory status for the patients initially on the Medical Floor included either high flow nasal cannula or nasal cannula and later required non-invasive mechanical ventilation and mechanical ventilation. Patients with any evidence of chronic kidney disease or end stage renal disease on dialysis were excluded. This study was approved by the institutional review board at ARMC with the approval number 22 − 18. This study was also registered on clinicaltrial.gov with the ID NCT05964088. Lastly, the work has been reported in line with the STROCSS criteria [15].
Two groups were created for the analysis. The first group included patients who were directly admitted to the ICU due to their critical respiratory status from the ED (referred to as the ICU group). The second group included patients who were initially admitted to the Medical Floor with respiratory status on high flow nasal cannula or other supplemental oxygens and transferred to the ICU due to either worsening respiratory status or change in their hemodynamic conditions (referred to as the Floor group). Both Floor and ICU groups were treated with steroids according to the RECOVERY trial once the trial was published in 2021 [16]. Prior to the RECOVERY trial, the use of convalescent plasma, Remdesivir, and other monoclonal antibodies were physician dependent. Both groups had close monitoring of oxygenation with pulse oximeter and periodic arterial blood gas assessments.
Three subgroups were created for the ICU and Floor patients separately. The ICU group was further divided into three subgroups: patients who developed AKI while admitted to the ICU (referred to as the ICU-AKI subgroup), patients who did not develop AKI during the admission and were admitted to the ICU for other Covid 19 complications (referred to as the ICU-No-AKI subgroup), and patients with previous history of dialysis dependent AKI who were admitted to the ICU resulting from other Covid 19 complications (referred to as the ICU-Dialysis subgroup). Similarly, the Floor patients were also divided into three subgroups in a similar fashion: patients who developed AKI while admitted to the Medical Floor (referred to as the Floor-AKI subgroup), patients who did not develop AKI during the course of their hospital stay admitted to the Medical Floor (referred to as the Floor-No-AKI subgroup), and patients with known history of dialysis dependent AKI who were admitted into the Medical Floor resulting from other Covid 19 complications (referred to as the Floor-Dialysis subgroup). The primary outcome of this study was mortality. The presence of comorbidities was recorded and used as a secondary endpoint. These comorbidities included obesity, T2DM, hypertension, and body mass index (BMI). All patients included in this study developed new onset AKI either directly on admission to the hospital or during the hospitalization, however for this study the AKI onset time (days) was not differentiated. The AKI was either identified on admission or throughout the hospitalization.
All statistical analyses were conducted using the SAS software for Windows version 9.4 (Cary, North Carolina, USA). Descriptive statistics were presented as means and standard deviations for continuous variables if normally distributed, or median and corresponding first and third quartile for continuous variables if not normally distributed, or frequencies and proportions for categorical variables. Independent t-test were conducted to assess the difference between floor and ICU patients for each of the three AKI subgroups separately. Analysis of Variance tests were conducted to assess the difference of continuous variables among the subgroups. Chi-square tests were conducted to assess the association between categorical variables and the subgroups. All statistical analyses were two-sided. P-value < 0.05 was statistically significant.
Results
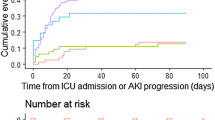
A total of 90 ICU and 173 Floor patients were included in the final analysis. The highest mortality is noted in the AKI subgroups in both the Floor and ICU cohorts. Figure 1 presented the detailed patient flow.
The first analysis was conducted to assess the difference among the three AKI subgroups for the ICU group. Table 1 presents the analysis results. There was a statistical significance among the three subgroups on blood urea nitrogen (BUN, p = 0.0251) and creatinine (p = 0.0086). Though not statistically significant, the ICU-AKI subgroup had the highest mortality rate (97.6%), followed by the ICU-Dialysis subgroup (89.5%).
The second analysis was conducted to assess the difference among the three AKI subgroups for the Floor group. Table 2 presents the analysis results. There was a statistical significance difference among the three AKI subgroups on mortality (p = 0.0008), age (p = 0.0286), BUN (p = 0.0036), creatinine (p = 0.0004) and ICU length of stay (LOS) (p = 0.0084). The Floor-AKI and Floor-Dialysis subgroups have almost equal likelihood of mortality (98.5% and 97.5% for Floor-AKI and Floor-Dialysis subgroups, respectively). In this analysis, the Floor-Dialysis subgroup had the highest value in BUN and creatinine.
Discussion
This study examined the mortality and morbidity of critically ill patients infected with COVID-19. Our data revealed a statistically significant association between mortality and the presence of AKI. For the Floor patients, the mortality was nearly 25% higher in AKI and Dialysis subgroups, when compared to the No-AKI subgroup. For the ICU patients, though not statistically significant (p = 0.0889), the mortality among ICU patients was on average 11–19% higher in AKI and Dialysis subgroup than the No-AKI subgroup. The current findings were consistent with prior publications indicating a possible robust immunological response with effects beyond the respiratory tract in critically ill COVID-19 patients [11, 17,18,19]. The mechanism of immunologically mediated multiorgan dysfunction appears to be a causative factor in the high incidence of AKI, along with higher mortality rate among COVID-19 patients [20]. Medeiros et al. reported that COVID-19 patients with AKI development have significantly higher serum concentration of growth factors, chemokines, proinflammatory and anti-inflammatory cytokines [21].
This study also noted an association between T2DM and all three subgroups amongst ICU patients. In the ICU patients, the prevalence of T2DM was lower amongst those with no AKI . Furthermore, the difference in prevalence of T2DM between AKI and No-AKI subgroup was nearly 20%. This results is more noteworthy than the reported 8% difference by Gupta and colleagues [22]. Moreover, the current study noted increased mortality rates in comparison to other published report [1, 23]. These differences may be attributed to the unique patient population in this hospital with more than 75% of patients being Hispanic. Given the lower socioeconomic status based on the US Census, there is concern for lower levels of literacy, vaccination rate, comorbidities, and other disparities that could have limited the patient population in completing preventative care measures. [24]
The current study also examined the prevalence of hypertension in the target population. There was no statistical significance in the prevalence of hypertension among the three AKI subgroup comparisons for ICU and Floor patients separately. This finding may seem paradoxical, as hypertension has been known as a risk factor for developing kidney disease [25]. Dylewska and colleagues reported that the role of hypertension in the development of AKI remains unclear as AKI is described as multifactorial, and largely dependent upon the etiology [26]. Given the results in this study, hypertension and AKI association does not appear to be correlated but this result could be also limited by the number of patients in each group. Future studies are warranted to explore the association between hypertension and AKI among Covid-19 patients.
The generalizability of this study was subjected by several limitations. First, as a retrospective study, the study depends on information retrieval from electronic health records system in one hospital. However, the research team made their utmost efforts to retrieve complete data from records. Secondly, the size of the patient cohorts may have been underestimated if AKI was not documented in progress notes during the hospitalization. Lastly, during the time period of this study, treatments for SARS-CoV-2 were developed and released as well as vaccines. Increasing vaccination rates in the second half of the study may have had an effect on the results.
Conclusion
This study noted higher mortality in the AKI and the Dialysis-dependent patients in comparison to the no-AKI subgroup. These results may be used to help improve the quality of care in patients developing AKI with SARS-CoV-2 infection.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Chan L, Coca SG. Acute kidney injury in the time of COVID-19. Kidney360. 2020;1(7):588.
Amirfakhryan H. Outbreak of SARS-CoV2: Pathogenesis of infection and cardiovascular involvement. Hellenic J Cardiol. 2021;62(1):13–23.
Parasher A. COVID-19: current understanding of its pathophysiology, clinical presentation and treatment. Postgrad Med J. 2021;97(1147):312–20.
Chen Y-T, Shao S-C, Lai EC-C, Hung M-J, Chen Y-C. Mortality rate of acute kidney injury in SARS, MERS, and COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24(1):1–4.
Guan W-j, Ni Z-y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506.
Cheng Y, Luo R, Wang K, et al. Kidney impairment is associated with in-hospital death of COVID-19 patients. MedRxiv. 2020. p. 2020.02. 18.20023242.
Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–70.
Group K. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1.
McAdams MC, Xu P, Saleh SN, et al. Risk prediction for acute kidney injury in patients hospitalized with COVID-19. Kidney Med. 2022;4(6):100463.
Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–18.
Legrand M, Bell S, Forni L, et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021;17(11):751–64.
Bhasin B, Veitla V, Dawson AZ, et al. AKI in hospitalized patients with COVID-19 and seasonal influenza: a comparative analysis. Kidney360. 2021;2(4):619.
United States Census Bureau. Quickfacts: San Bernardino County, California. 2023 [cited 2023 March 29]; https://www.census.gov/quickfacts/fact/table/sanbernardinocountycalifornia/AFN120217.
Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg Open. 2021;37:100430.
Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Respiratory Med, 2021. 9(12): p. 1419–26.
Chong WH, Saha BK. Relationship between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the etiology of acute kidney injury (AKI). Am J Med Sci. 2021;361(3):287–96.
Ng JH, Bijol V, Sparks MA, Sise ME, Izzedine H, Jhaveri KD. Pathophysiology and pathology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis. 2020;27(5):365–76.
Prasad N, Agrawal SK, behalf of COVID, O. COVID 19 and acute kidney injury. Indian J Nephrol. 2020;30(3):161.
Ahmadian E, Hosseiniyan Khatibi SM, Razi Soofiyani S, et al. Covid-19 and kidney injury: pathophysiology and molecular mechanisms. Rev Med Virol. 2021;31(3):e2176.
Medeiros T, Guimarães GMC, Carvalho FR, et al. Acute kidney injury associated to COVID-19 leads to a strong unbalance of circulant immune mediators. Cytokine. 2022;157:155974.
Gupta S, Coca SG, Chan L, et al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrology: JASN. 2021;32(1):161.
Neves P, Sato VAH, Mohrbacher S, et al. Acute kidney Injury due to COVID-19 in Intensive Care Unit: an analysis from a latin-american Center. Front Med (Lausanne). 2021;8:620050.
U.S. Census bureau. Quick Facts, San Bernardino City, California; San Bernardino County, California. 2022 [cited 2023 May 1]; https://www.census.gov/quickfacts/fact/table/sanbernardinocitycalifornia,sanbernardinocountycalifornia,CA/INC110221.
National Institute of Diabetes and DIgestive and Kidney Diseases. High Blood Pressure & Kidney Disease. [cited 2023 May 1]; https://www.niddk.nih.gov/health-information/kidney-disease/high-blood-pressure#:~:text=urinary%20tract%20system.-,How%20does%20high%20blood%20pressure%20affect%20the%20kidneys,may%20no%20longer%20work%20properly.
Dylewska M, Chomicka I, Malyszko J. Hypertension in patients with acute kidney injury. Wiad Lek. 2019;72:2199–201.
Acknowledgements
Not applicable.
Funding
No funding was used to support this study.
Author information
Authors and Affiliations
Contributions
XM, JM, CCN: literature review, IRB application, chart review, data extraction, manuscript revision. FD: data analysis and manuscript revision. NF, DW, MMN, TP, SA: involved in patient care, literature review, design of the study, data extraction, and manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval to report this study was obtained from Arrowhead Regional Medical Center Institutional Review Board (IRB) with the IRB approval number 22 − 18. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was waived from all participants and from a parent and/or legal guardian due to the retrospective nature of the study.
Consent for publication
Not applicable. This study is a retrospective chart review. Patients’ identifiers were removed before the data analysis. Patients’ data were reported in aggregated format. No individual patient will be identified in this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fischer, N., Miao, X., Weck, D. et al. Mortality and morbidity associated with new onset acute kidney injury in critically ill COVID-19 infection patients. Int J Emerg Med 17, 97 (2024). https://doi.org/10.1186/s12245-024-00666-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12245-024-00666-6