Abstract
Background
Developmental dyslexia (DD) and attention deficit/hyperactivity disorder (ADHD) are highly comorbid neurodevelopmental disorders. Individuals with DD or ADHD have both been shown to have deficits in white matter tracts associated with reading and attentional control networks. However, white matter diffusivity in individuals comorbid with both DD and ADHD (DD + ADHD) has not been specifically explored.
Methods
Participants were 3rd and 4th graders (age range = 7 to 11 years; SD = 0.69) from three diagnostic groups ((DD (n = 40), DD + ADHD (n = 22), and typical developing (TD) (n = 20)). Behavioral measures of reading and attention alongside measures of white matter diffusivity were collected for all participants.
Results
DD + ADHD and TD groups differed in mean fractional anisotropy (FA) for the left and right Superior Longitudinal Fasciculus (SLF)-Parietal Terminations and SLF-Temporal Terminations. Mean FA for the DD group across these SLF tracts fell between the lower DD + ADHD and higher TD averages. No differences in mean diffusivity nor significant brain-behavior relations were found.
Conclusions
Findings suggest that WM diffusivity in the SLF increases along a continuum across DD + ADHD, DD, and TD.
Similar content being viewed by others
Background
Although reading occurs relatively easily for most children, the 5–17% of children who cannot learn to read proficiently may be affected by developmental dyslexia (DD; [1,2,3]). DD is a neurodevelopmental disorder that is defined by difficulty in processing phonological information [2, 4] along with deficits in rapid automatic naming [5]. DD is also associated with poor reading fluency and comprehension in comparison to typically developing peers. Many of these deficits found within DD are linked to impairments in the well-defined left-hemisphere language and reading network [1, 6]. Other deficits commonly related to DD include poor short-term and working memory [7], difficulties with visual information processing [8], and slowed processing speed [9], all of which are seen in children with other neurodevelopmental disorders.
Research indicates that approximately 40% of children with DD also meet criteria for at least one additional disability [10, 11], with attention deficit/hyperactivity disorder (ADHD) being the most commonly co-occurring impairment, diagnosed in 25–40% of children with DD [11]. A majority of these comorbid ADHD cases fall within the inattentive subtype of ADHD (ADHD-I; 11). The prevalence of comorbid inattentive behaviors is significantly higher than would be expected by chance, with up to 26% of individuals with DD also meeting criteria for ADHD-I [11, 12]. Moreover, genetic overlaps have been reported between DD and ADHD-I [13,14,15,16]. Inattention in these children has also been shown to negatively impact behavior and academic performance [17, 18], and those students with DD plus co-morbid ADHD-I may suffer from deficits in attention that additionally impede their reading development [17, 19]. Children experiencing greater inattention typically perform poorly on math and reading achievement tests, even after controlling for intelligence [12, 20,21,22]. Furthermore, a strong relationship between attention and the development of pre-reading skills in preschoolers may later impact the development of word identification abilities [23]. The combination of DD and ADHD disorders therefore may impact a child’s reading development above that of either DD or ADHD when diagnosed exclusively [9, 24,25,26].
Neural correlates
Although much research has characterized DD and ADHD as two separate and distinct disorders [27, 28], DD and ADHD may share components across their underlying neural systems, which may account for their higher rate of comorbidity. Research has identified a complex neural reading network, consisting of a predominantly left–hemisphere system that encompasses the inferior frontal, temporoparietal, and occipitotemporal cortical regions [29, 30]. Three distinct neural pathways, or subsystems, have been shown to work in parallel to accomplish fluent and proficient reading [2, 31]. The reading network’s dorsal system is comprised of left temporoparietal areas including the angular gyrus, supramarginal gyrus, and posterior portions of the superior temporal gyrus, which are thought to play a role in mapping orthographic information to the phonological and semantic properties of written words [32]. The ventral system is associated with the left ventral occipitotemporal cortex extending into the middle and inferior temporal gyrus, which facilitates processing of the orthographic features of written language that is necessary for automatic word recognition [33, 34]. The anterior system is focused within the left inferior frontal gyrus (IFG) and adjacent frontal gyri and is important for several processes such as phonological recoding and semantic integration [35,36,37]. Individuals with DD show reduced functional [38] and network connectivity [39] within the ventral subsystem. Moreover, network connectivity within the ventral system improves with reading skills over time, yet dorsal system network connectivity decreases over time with improved reading skills [39].This suggests an increased reliance on the automatic ventral subsystem and reduced reliance on the phonological dorsal subsystem with improved reading.
Research has deemed adequate attentional control as necessary for efficient executive functioning [40, 41], and children with DD show impairments in selective attention and the executive functions of inhibition and working memory [42]. In the same vein, neuroimaging research connected to attention and ADHD has identified a cingulo–fronto–parietal attentional control network, which is further associated with the fronto–striatal and fronto–parietal pathways [43]. Indeed, this attentional control network consists of connections between the lateral frontal pole, anterior cingulate cortex, dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, inferior parietal lobe, and various subcortical regions [44]. As a primary substrate for attention and executive functioning [43], this network is thought to facilitate goal–directed processes and provides for the ability to respond to changing task demands [44]. Individuals with attentional deficits, such as those associated with ADHD, show decreased activation within the attentional control network [45, 46]. Specifically, individuals with deficits in attentional control have shown hypoactivation in brain areas associated with both attention and executive function such as the anterior cingulate cortex, the parietal cortex, the ventrolateral prefrontal cortex, and the dorsolateral prefrontal cortex.
These distinct, yet overlapping, neural systems attributed to DD and ADHD may at times share a range of neural deficits between the two disorders, leading to the commonly occurring co-morbid DD and ADHD (DD + ADHD). Indeed, individuals with both a reading disability (encompassing DD) and ADHD have shown gray matter differences within regions of the frontal-striatal pathway partly comprising the attentional control network [47] and the reading network [48], which have been functionally associated with executive functioning and reading ability, respectively [49]. Likewise, a meta-analysis found similar regional deficits within the frontal-striatal pathways in DD and ADHD populations [50]. Moreover, research shows overlapping gray matter correlates within the attentional control network for ADHD and comorbid reading disability and ADHD [47] and within the reading network for reading disability and comorbid reading disability and ADHD [48, 49]. Therefore, it may be expected that those children who show co-morbid DD + ADHD attributes would show increasing levels of deficits among those shared components of the neural systems.
White matter tracts of interest
These complex reading and attentional networks require effective and rapid communication between their connected regions to function effectively. Therefore, at a basic structural level, it is important to understand the quality and role of these network’s connecting white matter tracts and their relationship with specific behavioral outcomes in children with DD. Diffusion tensor imaging (DTI), which allows for the quantification of diffusion properties for white matter [51], permits such an examination of the structural pathways that underlie the reading and attentional networks. Quantitative analysis yields many different diffusivity-based measures of white matter with both fractional anisotropy (FA) and mean diffusivity (MD) being widely used [52]. FA utilizes eigenvalues that quantify diffusivity parallel and perpendicular to tract fibers to measure the fraction of the “magnitude” of anisotropic diffusion, which quantifies the degree of directionality for diffusivity [52, 53]. MD is the average of principal diffusivities parallel and perpendicular to the axon [54] with higher MD reflecting higher diffusivity along the axon. As markers of white matter diffusivity, FA and MD are useful quantities to compare across subjects as they provide information about directional architecture and axonal myelination [55].
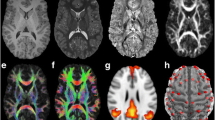
Four primary white matter tracts (Fig. 1) – namely, the superior longitudinal fasciculus (SLF), the inferior longitudinal fasciculus (ILF), the uncinate fasciculus (UF), and regions of the corpus callosum (CC) – have been closely studied within both the reading [2] and attentional control [43] networks. The SLF is a large lateral associative white matter bundle generally connecting the temporoparietal area (TPA) with the frontal, parietal and temporal areas and may segmented into three branches: (1) the dorsal SLF, a direct segment running medially, connecting the TPA with the middle (MFG) and superior frontal gyri; (2) the ventral SLF, the lateral anterior segment linking the IFG and MFG to the inferior parietal lobule (IPL); (3) the posterior SLF, linking the TPA with the IPL via the lateral indirect posterior segment [56]. The ventral and dorsal SLF, subsuming portions of the arcuate fasciculus (AF), are typically thought to constitute the SLF temporal bundle (SLFt), whereas the posterior SLF constitutes the SLF parietal bundle (SLFp; [57]. Children and adults with poor word reading ability have shown decreased white matter diffusivity in the left SLFt [6] and SLFp [58] as indicated by lower FA values. Moreover, Chinese children with DD have been shown to have lower FA values in the left SLFt, associated with phonological processing skills [59]. Individuals with ADHD have also been shown to have reduced FA in the right SLF [60] and increased MD in the left SLF [61] compared to non-impaired controls.
The ILF is a ventral associative tract consisting of long and short fibers that directly connect the occipital and anterior temporal lobes [62]. Research has suggested relations between the left ILF and measures of word reading fluency [63, 64] and reading comprehension [63, 65]. Decreased FA in the left ILF has been reported in Chinese children with DD and this was related to semantic skills as appropriate for the Chinese logographic alphabet [66]. Reduced diffusivity in the ILF, bilaterally, has also been shown in children with ADHD [67]. Moreover, reduced FA in the left ILF has been observed in adults with ADHD, whereas MD in the left ILF has shown a negative association with attentional performance [68].
The UF is a ventral association bundle that connects the anterior temporal lobe with the orbitofrontal cortex, including the IFG [69]. It is thought to play a role in language functions such as lexical retrieval and semantic associations [70] with research implicating a role in reading comprehension [65, 71]. DD has been associated with reduced white matter connectivity in the UF [72]. Conversely, increased FA [61, 73] and MD [61] values in the bilateral UF have been observed in adults with the combined inattentive and hyperactive ADHD subtype (ADHD-C).
The CC is a major commissure that connects the left and right hemispheres of the brain and is mainly associated with interhemispheric connectivity [74]. Based on a multivariate machine learning approach, the CC is one of the most discriminative features classifying DD [75], and research has shown that children’s reading skill is negatively correlated with FA within the posterior callosum across typical and impaired readers [76]. For individuals with ADHD, measures of inattention and hyperactivity have been negatively correlated with a reduced cortical thickness of the CC in older adults [77] and lower FA values in the CC for children [78]. Most notably, a meta-analysis of white matter diffusivity in children and adults with ADHD found lower FA in the right forceps minor of the CC in comparison to those without ADHD [79].
Current study
Researchers have investigated the relations between white matter tract diffusivity of the CC, ILF, SLF, and UF as they relate to groups of individuals diagnosed with either DD or ADHD independently but have not evaluated their role in comorbid subjects who have DD + ADHD. The primary aim of this study was to explore potential differences in white matter tract diffusivity of the CC, ILF, SLF, and UF in children with DD only, DD + ADHD, and compared to typically developing, unimpaired readers (TD). We also sought to investigate the relations between measures of white matter diffusivity and behavioral measures of reading ability and attentional control in these groups. We hypothesized that white matter diffusivity for the tracts of interest would be significantly reduced in DD + ADHD compared to both DD and TD groups due to their shared underlying neural deficits, and based on the previous literature, that the DD only group would show reduced white matter diffusivity compared to the TD group. Furthermore, we hypothesized that white matter tract diffusivity would be positively correlated with measures of reading proficiency and attentional control.
Methods
Participants
Participants were recruited from public and charter elementary schools in the greater Atlanta area as part of a longitudinal study of reading intervention approved by the Georgia State University/Georgia Tech Center for Advanced Brain Imaging Institutional Review Board. All parents/students provided informed consent/assent before any participation in the study. Participants included 3rd and 4th grade students from 7 to 11 years old (mean age = 9.32; SD = 0.69; please see Table 1 for age statistics separated by group) who completed baseline behavioral/cognitive assessments and an MRI scan (including DWI sequences) as part of participation in the larger study. Participants were assigned to one of three groups based on their reading disability and ADHD comorbidity status: DD, DD + ADHD, or TD. Children identified with DD (n = 40) scored at least one standard deviation below age-norm expectations on any of the following: Woodcock Johnson 3rd Edition (WJ-3; [80]) Broad Reading Cluster subtests or the composite, the WJ-3 Basic Reading Cluster subtests or composite, or Test of Word Reading Efficiency 2nd Edition (TOWRE-2; [81]) subtests. DD + ADHD readers (n = 22) met the same criteria for DD and also exhibited high ADHD symptomology as defined by the Strengths and Weaknesses of ADHD symptoms and Normal Behavior (SWAN; [82]) and the Disruptive Behavior Rating Scale (DBRS; [83]) as rated by a guardian and teacher. Guardians and classroom teachers were asked to complete both the SWAN and DBRS rating scales on all participants in the study. A composite of these scores and individual symptom ratings on these scales were used to identify subjects in the DD + ADHD group using current DSM-5 criteria for both the Combined and Inattentive types. In the rare case that data was not returned from one rater (i.e., parent or teacher) or rating scale, available scores were used. TD readers (n = 20) were recruited from the same schools but did not meet criteria for either DD or ADHD. All participants had a verbal and/or performance intelligence standard score at or above 80 on one of the subtests of the Wechsler Abbreviated Scale of Intelligence—II (WASI-II; [84]) in order to rule out intellectual disabilities. All children in the study completed screening materials for diagnostic criteria and were native English speakers. Children with chronic absenteeism (> 15 absences per year), hearing impairment (< 20/40), serious emotional/psychiatric disturbance, chronic medical/neurological condition, or MRI contraindicative according to guardian report were excluded.
Behavioral measures
Reading measures
The composite score of the Sight Word Efficiency and Phonological Decoding Efficiency subtests of the TOWRE-2 was used as a measure of word reading fluency (TOWRE-CST). The TOWRE-2 requires participants to read aloud words and pseudowords, respectively, as quickly and as accurately as possible. A higher TOWRE-CST reflects better word reading efficiency.
Attentional and Executive Function measures
The Behavior Rating Inventory of Executive Function (BRIEF) measured executive function via an 86-item questionnaire answered by a parent or guardian [85]. The Global Executive Composite (GEC) T Score is a combined measure of all sub-scales produced by BRIEF and provides a measure of executive function (GEC-T). A higher GEC-T reflects poorer executive function. The Behavioral Regulation Index (BRI) T Score is a combined measure of the Inhibit, Shift, and Emotional Control subscales and provides a measure of behavioral attention (BRI-T). A higher BRI-T reflects poorer behavioral attention.
Magnetic resonance imaging
Data acquisition MRI
Images were acquired using a 3 T Siemens scanner located at the GSU/GaTech Center for Advanced Brain Imaging in Atlanta, Georgia. The site scanner was upgraded from a Trio (12-channel head coil) to a PRIMSA-Fit (20-channel) during the final year of data collection (n = 13). Data acquisition and scan parameters were kept consistent throughout the duration of the study and processed data was harmonized to account for inter-scanner differences (see Imaging data preprocessing section below). All included subjects completed diffusion-weighted imaging data, collected in two separate sequences with reverse phase encoding (anterior-to-posterior and posterior-to-anterior) via the following parameters: FoV: 220 × 220 mm; slice thickness: 2 mm; repetition time TD/TE: 8900/97 ms; slices: 64; b:1000, 4* b:0; 32 gradient directions; voxel size isotropic: 2 mm. Total DWI acquisition time for collection of both sequences was approximately 10 min total. T2*-weighted images were acquired in an axial-oblique orientation parallel to the intercommissural line (32 slices; 4 mm slice thickness; no gap) using single-shot echo planar imaging (matrix size = 64 × 64; voxel size = 3.438 × 3.438 × 4 mm; FoV = 220 mm; TD = 2000 ms; TE = 30 ms; flip angle = 80°). Anatomical scans were collected in the same orientation (MPRAGE; matrix size = 256 × 256; voxel size = 1 × 1 × 1 mm; FoV = 256 mm; TD = 2530 ms; TE = 2.77 ms; flip angle = 7°).
Imaging data preprocessing
After visual and automated quality assurance for all image data, DWI data were preprocessed with TORTOISE [86] using a T2* structural file and a MPRAGE reorientation file. DIFF_PREP was used for motion and eddy current distortion with computed B-matrix of gradient tables [87]. DR-BUDDI corrected susceptibility induced EPI distortions via blip-up and blip-down data (AP/PA co-registration), and DWIs were reoriented into target space with B-matrices [88].
The FreeSurfer 6.0 image analysis suite was used to process anatomical data [89]. This automated procedure contained segmentation of cortical and subcortical white matter, tessellation of gray matter/white matter boundaries, inflation of the folded surface tessellation patterns [90, 91], and automatic correction of topographical defects [92]. Manual intervention was performed by a trained technician consistent with FreeSurfer protocol, when necessary.
Automated reconstruction of white matter tracts of interest was carried out via FreeSurfer’s TRACULA pipeline using global probabilistic tractography [57]. Specifically, the combination of FreeSurfer’s cortical parcellation and subcortical segmentation with TRACULA’s anatomical atlas provided the automated reconstruction of 18 major white matter tracts. TRACULA utilizes FSL’s bedpostx to fit the ball-and-stick model to DWI data and reconstruct pathways. Out of the 18 tracts, we extracted the FA and MD values for the following white matter tracts of interest (L = left; R = right): the SLFt (SLF temporal bundle, consisting of components of ventral and dorsal SLF and AF), the SLFp (SLF parietal bundle; SLF-posterior), the ILF, the UF, the fminor (anterior CC; forceps minor), and fmajor (posterior CC; forceps major).
To account for differences between data collected before and after scanner upgrade, DTI data were harmonized using ComBat [93]. ComBat assumes the imaging feature measurements can be modeled as a linear combination of the biological variables with the scanner effects as an error term that includes a multiplicative scanner-specific scaling factor. It has been shown to effectively reduce inter-scanner variation in DTI data while effectively preserving biological associations [94, 95].
Statistical analyses
A separate one-way between subjects analysis of variance compared the TOWRE-CST, BRI-T, and GEC-T on three levels: DD + ADHD, DD, and TD. Likewise, separate one-way between subject analysis of variance models compared the harmonized means for FA and MD for each of the tracts of interest individually on three levels: DD, DD + ADHD, and TD. Mean FA and MD data were assessed for extreme outliers via box-and-whisker plots, which resulted in the removal of two outliers that were present in more than one white tract of interest. Participants section details our total sample size (n = 82) after outlier removal and for all data analyses. The Tukey–Kramer post hoc test was used to test for significant group differences for all analyses. Pearson bivariate correlations were run for mean FA and MD of the tracts of interest and the following behavioral measures: TOWRE-CST, BRI-T, and GEC-T. The False Discovery Rate (FDR) was applied to all correlations to correct for multiple comparisons.
Results
Behavioral comparisons between groups
The one-way analysis of variance revealed significant differences between groups on all behavioral measures of reading, attention, and intelligence (Tables 1 and 2): WJ-3 Basic (F(2,79) = 88.9, p < 0.001), WASI-II (F(2,79) = 31.0, p < 0.001), TOWRE-CST (F(2,79) = 93.5, p < 0.001), BRI-T (F(2,79) = 7.42, p < 0.001), and GEC-T (F(2,79) = 23.3, p < 0.001). For diagnostic measures, TD significantly differed from DD and DD + ADHD on the WJ-3- Basic (p < 0.001) and WASI-II (p < 0.001) showing the highest reading and intelligence scores, respectively; however, DD and DD + ADHD showed no significant differences. Likewise, TD significantly differed from DD and DD + ADHD on the TOWRE-CST (p < 0.001) showing the highest reading score; however, DD and DD + ADHD showed no significant differences. Moreover, DD + ADHD significantly differed from both TD (p < 0.001) and DD (p = 0.026) on the BRI-T showing the lowest behavioral attentional score; however, TD and DD showed no significant difference. On the GEC-T, TD significantly differed from DD (p = 0.014) and DD + ADHD (p < 0.001), while DD significantly differed from DD + ADHD ( p < 0.001) with DD + ADHD showing the lowest and TD showing the highest executive functioning score.
White matter comparisons between groups
The one-way analysis of variance revealed significant differences between groups for the mean FA values in the following tracts of interest (Tables 3 and 4): L SLFp (F(2,79) = 3.90, p = 0.024), L SLFt (F(2,79) = 3.13, p = 0.003), R SLFp (F(2,79) = 3.33, p = 0.041), and R SLFt (F(2,79) = 3.72, p = 0.029). There were significant differences in the mean FA of the L SLFp (p = 0.018), L SLFt (p = 0.002), R SLFp (p = 0.036), R SLFt (p = 0.029), such that the DD + ADHD group had significantly lower FA than the TD group in all tracts (Fig. 2). There were no significant differences for DD compared to DD + ADHD or between DD compared to TD. There were no significant differences between groups for mean MD for all tracts of interest.
Box and whisker plots for tracts of interest showing significant differences in mean FA values between groups
Note: * = p < .05; ** = p < .01; DD = developmental dyslexia; DD + ADHD = developmental dyslexia comorbid with attention deficit/hyperactivity disorder; TD = typical developing, unimpaired readers; FA = Fractional Anisotropy
Brain—behavior relations
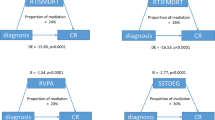
Pearson bivariate correlations revealed relations between behavioral measures and measures of white matter diffusivity for tracts of interest. Non-corrected significant correlations with mean FA included the following: TOWRE-CST and L SLFp (r (80) = 0.220, p = 0.047) and L SLFt (r (80) = 0.269, p = 0.015); BRI-T and L UF (r (80) = 0.229, p = 0.036); GEC-T and L SLFp (r (80) = -0.240, p = 0.030), L SLFt (r (80) = -0.232, p = 0.036), and R SLFp (r (80) = -0.263 p = 0.017). For mean MD, correlations were found between the GEC-T and L UF (r (80) = -0.233, p = 0.035); BRI-T and L UF (r (80) = -0.234, p = 0.034). However, no significant correlations were maintained after correction for multiple comparisons (Table 5) (Fig. 3).
Scatterplots for brain behavior relations of significance before correcting for multiple comparisons within tracts of interest showing significant differences in mean FA values between groups
Note: DD = developmental dyslexia; DD + ADHD = developmental dyslexia comorbid with attention deficit/hyperactivity disorder; TD = typical developing, unimpaired readers; GEC-T = Global Executive Composite T Score taken from the Behavior Rating Inventory of Executive Function; TOWRE-CST = Test of Word Reading Efficiency 2nd Edition Composite Score; FA = Fractional Anisotropy; L = Left; R = Right; SLFp = superior longitudinal fasciculus parietal bundle; SLFt = superior longitudinal fasciculus temporal bundle
Discussion
The current study investigated white matter tracts associated with reading and attentional/executive functioning between three groups: DD + ADHD, DD, and TD. As expected, measures of reading and attentional control were significantly different between groups, with TD showing the highest scores on all measures, while DD and DD + ADHD showed no difference in reading but differed significantly in attentional control (Tables 1 and 2). Mean FA in bilateral temporal and parietal portions of the SLF differed between the DD + ADHD and TD groups, with TD showing the highest mean FA (Tables 3 and 4). However, there were no significant differences in FA between DD and DD + ADHD, nor between DD and TD, within the bilateral SLF, ILF, UF, or CC. Although previous literature has shown positive associations between ADHD and MD in the left SLF, left ILF, and bilateral UF [61, 68, 79], no significant group differences were observed in any tract of interest for mean MD.
As expected, our results support the TD group as superior readers with the strongest attentional control and highest white matter diffusivity bilaterally in the parietal and temporal regions of the SLF [6, 58]. The DD + ADHD group on the other hand displayed the lowest scores on behavioral measures of attentional control and the lowest FA results in bilateral parietal and temporal regions of the SLF. The DD group’s results fell between the DD + ADHD and TD groups on attentional control as well as FA bilaterally in both regions of the SLF, while at the same time showing more similar reading deficits to the DD + ADHD group. Although there were significant differences in reading and attentional control between diagnostic groups, only trends for brain-behavior correlations were observed once corrected for multiple comparisons. TOWRE-CST was signficantly different between groups and there were trends for postive relations between TOWRE-CST and mean FA in the left temporal and parietal regions of the SLF across groups. Furthermore, there was a trend for negative relations between GEC-T, with a higher score indicating worse exectuive funtion, and mean FA in the left temporal and parietal regions and the right parietal regions of the SLF across groups. Taken together, our results begin to suggest that as mean FA in the SLF decreases, so does performance on reading and attentional control measures, proposing a continuous effect of the underlying SLF white matter diffusivity on behavior. It is important to note that this possible continuum effect is only reflected in the effects on brain structure, not on behavioral outcomes as our data did not show significant differences in reading between the DD and DD + ADHD groups.
Accordingly, previous research also has suggested that children with comorbid DD and ADHD may exist as a third phenotype independent from sole DD or ADHD conditions [13,14,15,16]. Results from the current study support the suggestion of a dual or specific role of the SLF as potentially underlying such comorbid DD and ADHD symptomatology, whether considered as a dual diagnosis or an independent phenotype. In regards to the tracts of interest investigated within this study, only the SLF has been identified as a primary white matter tract associated with both the dorsal phonological system of the reading network [6] and attentional control network [43]; therefore, the main underlying factor within the comorbid group may be due to top-down and bottom-up effects of attentional control interacting with the external learning environment at hand. Indeed, distractors in high-difficult tasks have shown to be positively associated with reliance on bottom-up processing, whereas distractors in low-difficult tasks have been positively associated with reliance on top-down processing for individuals with ADHD, indicating a detrimental association between distractibility and task difficulty [96]. Moreover, reading ability may rely on the balanced integration of top-down and bottom-up processing. In comparison to typical readers, individuals with DD showed reduced functional connectivity between the neural substrates of top-down and bottom-up processing [97] and reduced activation of frontal and parietal cortical areas associated with the attentional control network during reading tasks [98]. Likewise, individuals with comorbid reading disability and ADHD have shown specific deficits in frontal regions within the frontal-striatal pathway [48], which has been functionally related to impairments in executive functioning [47, 49], an ability central to the attentional control network. Nonetheless, Langer and colleagues [49] have further associated specific grey matter deficits within the reading network to a reduced reading ability in comorbid reading disability and ADHD individuals.
Given the profound reading impairment within individuals with DD, the highly demanding task of reading may be further impeded by a dysfunctional attentional control network that moderates top-down and bottom-up processing. This suggests that the SLF, critically employed within both the reading and attentional control networks, may be a main contributor for the successful integration of top-down and bottom-up processing necessary for efficient reading and attentional control. Therefore, one of the primary deficits associated with the comorbid DD and ADHD (particular to ADHD-I) phenotype may be attributed to this additive effect of reduced diffusivity of the SLF, constituting the continuum of FA found within our sample. The consequences of differential white matter integrity in the SLF may be a fundamental factor in attentional control, with DD + ADHD exhibiting poor attentional control and TD exhibiting superior attentional control. Accordingly, a conjunction analysis of studies assessing grey matter differences within ADHD and DD populations found reduced grey matter volume only within the right caudate nucleus of the striatum [50], which compliments the sole finding of reduced grey matter volume within regions of the frontal-striatal pathway for individuals with comorbid reading disability and ADHD [48]. However, as seen in the behavioral measures of reading, the FA differences found within the SLF may not be explicitly additive to reading impairments in DD + ADHD individuals, as the reading network is comprised of several white matter tracts outside the attentional control network [2].
Similarly, white matter diffusivity of the ILF has been associated with both attentional control and reading. Encompassing language systems of logographic phonological alphabets, adults with ADHD [68] and children with DD [59] have been shown to have decreased FA in the ILF compared to controls. In our sample, there was a trend for FA group differences within the ILF similar to the continuum effect shown in the SLF. This may support previous evidence of the effect of ADHD on white matter diffusivity beyond those tracts directly associated with the attentional control network [68]. Although this effect may be detrimental in the case of top-down and bottom-up processing attributed to the attentional control network for individuals with DD and/or ADHD, the suggested third phenotype of comorbid DD + ADHD may show different properties of white matter tract diffusivity within the attentional control network, yet similar diffusivity within the reading network.
Contrary to previous findings concerning white matter diffusivity for individuals with DD [59, 61, 68, 72, 73, 75, 78, 79], mean FA for the ILF, UF, and CC were not significantly different between the DD and TD groups, nor between the DD + ADHD and TD groups. Accordingly, previous research has shown DD to be associated with a decrease in FA within the UF [72], whereas ADHD research has shown an increase in FA within the same tract [61, 73]; however, there were no statistically significant differences between the DD and DD + ADHD groups in the UF in the current study. If there is a continuum effect in the UF that is similar to that observed in the SLF, with the lowest performing readers having decreased white matter diffusivity, this impact may be counteracted in the current sample due to the potential inverse effects of comorbid ADHD on diffusivity properties. Therefore, shared genetic influences between ADHD and DD [14, 15] may impose potential differing effects on white matter within the UF and ILF (i.e., the reading network) yet potential additive effects within the SLF (i.e., the attentional control network) that exist on a continuum, with DD lying in between DD + ADHD and TD.
Limitations
Although decreased white matter diffusivity in the CC has been considered an integral component underlying the DD phenotype [75, 76] and has also been observed in individuals with ADHD [78, 79], no significant results indicating group differences for FA were found. Outside the previously discussed additive and counteracting effects of DD and ADHD on white matter, these results may be mainly due to the tract segmentation methods used in the current study. TRACULA utilizes a broad extraction of whole tract mean FA, yet evidence suggests that differences in white matter diffusivity for individuals with DD may lie in smaller regions of the CC [75]. Following the segmentation of the CC into smaller regions, previous research has shown that individuals with DD have increased mean FA in the splenium [99, 100] and have an abnormally shaped splenium, rostrum, genu, and body of the CC [101]. Therefore, a limitation of the current study may be the use of TRACULA, as it utilizes probabilistic tractography for segmenting tracts of interest and for quantifying white matter diffusivity. It is possible that group differences between our tracts of interest may only be found in smaller segments within the tract; therefore, performing a region of interest analysis may provide different results in tracts such as the ILF, UF, and CC. However, TRACULA utilizes individualized subject-specific anatomical landmarks, which allows for the reliable reconstruction of white matter pathways without manual intervention that potentially decreases researcher bias [102]. This is particularly important in the current sample, as other atlas-based tractography methods use adult standardized templates that may not be appropriate for the developing brain.
Similarly, there were no significant differences observed in MD between groups for any of the tracts of interest. Although changes in MD have been reported in individuals with ADHD, there is less evidence of difference in MD associated with DD. Differences between ADHD and typically developing controls is most frequently reported in adult ADHD populations [61, 68]. These differences may be in part to the limited age range (i.e., 7 to 11 years) represented in the current sample. Although most rapid microstructural changes occur in the first 24 months of age, maturation rates in diffusivity measures have been shown to progress at different rates, with MD changes developing much slower than FA [103,104,105]. This is particularly true for tracts associated with language and cognitive processing such as the SLF and UF. Differences in FA but not MD observed in the current study may have been, in part, due to maturational differences in the diffusion measures used.
Finally, the absence of an ADHD-only comparison group is a potential limitation of the current study. However, our primary aim was to investigate the differences in DD populations with and without ADHD to better understand the differences and similarities of the co-morbid condition in comparison to the’pure’ DD. Due to the nature of the overarching study, we were unable to collect a pure ADHD sample, which prevented us from directly assessing the impact of attentional deficits alone on white matter diffusivity. Future research will benefit from the inclusion of all four potential groups: TD, DD only, ADHD only, and DD + ADHD co-morbid samples. Similarly, future research should further evaluate the contribution of comorbidity to DD observed in ideographic language.
Conclusions
Our results indicate that neurostructural differences in the SLF may occur in children with DD + ADHD in comparison to TD. Results suggest that differences in white matter diffusivity may exist on a continuum, with DD + ADHD having the lowest mean FA compared to DD only or TD groups. Differences in FA in these specific tracts may underlie the severity of specific behavioral impairments seen in comorbid DD + ADHD when compared to those children with only DD exclusively.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ADHD:
-
Attentional deficit/hyperactivity disorder
- ADHD-C:
-
Comorbid inattentive hyperactive subtype of attentional deficit/hyperactivity disorder
- ADHD-I:
-
Inattentive subtype of attentional deficit/hyperactivity disorder
- BRI-T:
-
Behavioral Regulation Index T Score taken from BRIEF
- BRIEF:
-
Behavior Rating Inventory of Executive Function
- CC:
-
Corpus callosum
- DBRS:
-
Disruptive Behavior Rating Scale
- DD:
-
Developmental dyslexia
- DD + ADHD:
-
Developmental dyslexia comorbid with attention deficit/hyperactivity disorder
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- Fmajor:
-
Posterior CC; forceps major
- Fminor:
-
anterior CC; forceps minor
- GEC-T:
-
Global Executive Composite T Score taken from BRIEF
- IFG:
-
Inferior frontal gyrus
- ILF:
-
Inferior longitudinal fasciculus
- IPL:
-
Inferior parietal lobule
- L:
-
Left
- MFG:
-
Middle frontal gyrus
- R:
-
Right
- SLF:
-
Superior longitudinal fasciculus
- SLFp:
-
Superior longitudinal fasciculus parietal bundle
- SLFt:
-
Superior longitudinal fasciculus temporal bundle
- SWAN:
-
Strengths and Weaknesses of ADHD symptoms and Normal Behavior
- TD:
-
Typical developing, unimpaired readers
- TOWRE-CST:
-
Test of Word Reading Efficiency 2nd Edition Composite Score
- TPA:
-
Temporal parietal area
- UF:
-
Uncinate fasciculus
- WASI-II:
-
Wechsler Abbreviated Scale of Intelligence II
- WJ3-Basic:
-
Woodcock Johnson-III Basic Reading Composite Score
References
Peterson RL, Pennington BF. Developmental dyslexia. Annu Rev Clin Psychol. 2015;11(1):283–307.
Vandermosten M, Boets B, Wouters J, Ghesquière P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci Biobehav Rev. 2012;36:1532–52.
Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): what have we learned in the past four decades? J Child Psychol Psychiatry. 2004;45(1):2–40.
Manis FR, Custodio R, Szeszulski PA. Development of phonological and orthographic skill: A 2-year longitudinal study of dyslexic children. J Exp Child Psychol. 1993;56(1):64–86.
Bexkens A, van den Wildenberg WPM, Tijms J. Rapid automatized naming in children with dyslexia: is inhibitory control involved? Dyslexia. 2015;21(3):212–34.
Vandermosten M, Boets B, Poelmans H, Sunaert S, Wouters J, Ghesquière P. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135(3):935–48.
Catts HW. Defining dyslexia as a developmental language disorder. Ann Dyslexia. 1989;39(1):50.
Bucci MP. Visual training could be useful for improving reading capabilities in dyslexia. Appl Neuropsychol Child. 2019;13:1–10.
Willcutt EG, Betjemann RS, McGrath LM, Chhabildas NA, Olson RK, DeFries JC, et al. Etiology and neuropsychology of comorbidity between RD and ADHD: the case for multiple-deficit models. Cortex J Devoted Study Nerv Syst Behav. 2010;46(10):1345–61.
Carroll JM, Maughan B, Goodman R, Meltzer H. Literacy difficulties and psychiatric disorders: evidence for comorbidity. J Child Psychol Psychiatry. 2005;46(5):524–32.
Willcutt EG, Pennington BF. Comorbidity of reading disability and attention-deficit/hyperactivity disorder: Differences by gender and subtype. J Learn Disabil. 2000;33(2):179–91.
Tannock R. ADHD with anxiety disorders. In: ADHD comorbidities: Handbook for ADHD complications in children and adults. Arlington: American Psychiatric Publishing, Inc.; 2009. p. 131–55.
Gialluisi A, Andlauer TFM, Mirza-Schreiber N, Moll K, Becker J, Hoffmann P, et al. Genome-wide association scan identifies new variants associated with a cognitive predictor of dyslexia. Transl Psychiatry. 2019;9(1):77.
Wadsworth SJ, DeFries JC, Willcutt EG, Pennington BF, Olson RK. Genetic etiologies of comorbidity and stability for reading difficulties and ADHD: a replication study. Twin Res Hum Genet Off J Int Soc Twin Stud. 2016;19(6):647–51.
Willcutt EG, Pennington BF, DeFries JC. Twin study of the etiology of comorbidity between reading disability and attention-deficit/hyperactivity disorder. Am J Med Genet. 2000;96(3):293–301.
Willcutt EG, Pennington BF, Boada R, Ogline JS, Tunick RA, Chhabildas NA, et al. A comparison of the cognitive deficits in reading disability and attention-deficit/hyperactivity disorder. J Abnorm Psychol. 2001;110(1):157–72.
Flory K, Milich R, Lorch EP, Hayden AN, Strange C, Welsh R. Online story comprehension among children with ADHD: Which core deficits are involved? J Abnorm Child Psychol. 2006;34:853–65.
Young DJ, Levy F, Martin NC, Hay DA. Attention deficit hyperactivity disorder: a rasch analysis of the SWAN rating scale. Child Psychiatry Hum Dev. 2009;40:543–59.
Pimperton H, Nation K. Suppressing irrelevant information from working memory: evidence for domain-specific deficits in poor comprehenders. J Mem Lang. 2010;62:380–91.
Barriga AQ, Doran JW, Newell SB, Morrison EM, Barbetti V, Dean Robbins B. Relationships between problem behaviors and academic achievement in adolescents: the unique role of attention problems. J Emot Behav Disord. 2002;10(4):233–40.
Grills-Taquechel AE, Fletcher JM, Vaughn SR, Denton CA, Taylor P. Anxiety and inattention as predictors of achievement in early elementary school children. Anxiety Stress Coping. 2013;26(4):391–410.
Roberts G, Rane S, Fall AM, Denton CA, Fletcher JM, Vaughn S. The Impact of Intensive Reading Intervention on Level of Attention in Middle School Students. J Clin Child Adolesc Psychol. 2015;44(6):942–53. https://doi.org/10.1080/15374416.2014.913251.
Dittman CK. Associations between inattention, hyperactivity and pre-reading skills before and after formal reading instruction begins. Read Writ. 2016;29(9):1771–91.
Gooch D, Snowling M, Hulme C. Time perception, phonological skills and executive function in children with dyslexia and/or ADHD symptoms. J Child Psychol Psychiatry. 2011;52(2):195–203.
Rucklidge JJ, Tannock R. Neuropsychological profiles of adolescents with ADHD: effects of reading difficulties and gender. J Child Psychol Psychiatry. 2002;43(8):988–1003.
Willcutt EG, Pennington BF, Boada R, Ogline JS, Tunick RA, Chhabildas NA, et al. A comparison of the cognitive deficits in reading disability and attention-deficit/hyperactivity disorder. J Abnorm Psychol. 2001;110(1):157–72.
De Jong CGW, Van De Voorde S, Roeyers H, Raymaekers R, Oosterlaan J, Sergeant JA. How distinctive are ADHD and RD? Results of a double dissociation study. J Abnorm Child Psychol. 2009;37:1007–17.
Purvis KL, Tannock R. Phonological processing, not inhibitory control, differentiates ADHD and reading disability. J Am Acad Child Adolesc Psychiatry. 2000;39(4):485–94.
Martin A, Schurz M, Kronbichler M, Richlan F. Reading in the brain of children and adults: a meta-analysis of 40 functional magnetic resonance imaging studies: reading in the brain of children and adults. Hum Brain Mapp. 2015;36(5):1963–81.
Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment Retard Dev Disabil Res Rev. 2000;6(3):207–13.
Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, et al. Functional and morphometric brain dissociation between dyslexia and reading ability. PNAS. 2007;104(10):4234–9.
Gaillard WD, Pugliese M, Grandin CB, Braniecki SH, Kondapaneni P, Hunter K, et al. Cortical localization of reading in normal children - An fMRI language study. Neurology. 2001;57(1):47–54.
Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff MA, et al. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307.
Cohen L, Lehéricy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125(5):1054–69.
Perrachione TK, Ghosh SS, Ostrovskaya I, Gabrieli JDE, Kovelman I. Phonological working memory for words and nonwords in cerebral cortex. J Speech Lang Hear Res JSLHR. 2017;60(7):1959–79.
Zhu Z, Hagoort P, Zhang JX, Feng G, Chen HC, Bastiaansen M, et al. The anterior left inferior frontal gyrus contributes to semantic unification. Neuroimage. 2012;60(4):2230–7.
Zhu Z, Feng G, Zhang JX, Li G, Li H, Wang S. The role of the left prefrontal cortex in sentence-level semantic integration. Neuroimage. 2013;1(76):325–31.
Cao F, Yan X, Spray GJ, Liu Y, Deng Y. Brain mechanisms underlying Visuo-orthographic deficits in children with developmental dyslexia. front Hum Neurosci. 2018;12:490.
Wise Younger J, Tucker-Drob E, Booth JR. Longitudinal changes in reading network connectivity related to skill improvement. Neuroimage. 2017;158:90–8.
Perri RL. Is there a proactive and a reactive mechanism of inhibition? Towards an executive account of the attentional inhibitory control model. Behav Brain Res. 2020;13(377):112243.
Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89.
Barbosa T, Rodrigues CC, de Mello CB, de Silva MCSE, Bueno OFA. Executive functions in children with dyslexia. Arq Neuropsiquiatr. 2019;77(4):254–9.
Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):1160–7.
Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacol Rev. 2010;35:278–300.
Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. Trends Cogn Sci. 2012;16(1):17–26.
Dickstein SG, Bannon K, Xavier Castellanos F, Milham MP. The neural correlates of attention deficit hyperactivity disorder: An ALE meta-analysis. J Child Psychol Psychiatry. 2006;47(10):1051–62.
Kibby MY, Dyer SM, Lee SE, Stacy M. Frontal volume as a potential source of the comorbidity between attention-deficit/hyperactivity disorder and reading disorders. Behav Brain Res. 2020;2(381): 112382.
Jagger-Rickels AC, Kibby MY, Constance JM. Global gray matter morphometry differences between children with reading disability, ADHD, and comorbid reading disability/ADHD. Brain Lang. 2018;185:54–66.
Langer N, Benjamin C, Becker BLC, Gaab N. Comorbidity of reading disabilities and ADHD: structural and functional brain characteristics. Hum Brain Mapp. 2019;40(9):2677–98.
McGrath LM, Stoodley CJ. Are there shared neural correlates between dyslexia and ADHD? A meta-analysis of voxel-based morphometry studies. J Neurodev Disord. 2019;11(1):31.
Mori S, Zhang J, Ahrens ET, Laidlaw DH, Readhead C, Brosnan CF, et al. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–39.
Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci MN. 2008;34(1):51–61.
Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson. 2011;213(2):560–70.
Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurother J Am Soc Exp Neurother. 2007;4(3):316–29.
Aung WY, Mar S, Benzinger TL. Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging Med. 2013;5(5):427–40.
Nakajima R, Kinoshita M, Shinohara H, Nakada M. The superior longitudinal fascicle: reconsidering the fronto-parietal neural network based on anatomy and function. Brain Imaging Behav. 2020;14(6):2817–30.
Yendiki A, Panneck P, Srinivasan P, Stevens A, Zöllei L, Augustinack J, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinformatics. 2011;5:23.
Carter JC, Lanham DC, Cutting LE, Clements-Stephens AM, Chen X, Hadzipasic M, et al. A dual DTI approach to analyzing white matter in children with dyslexia. Psychiatry Res. 2009;172(3):215–9.
Su M, Zhao J, de Thiebaut Schotten M, Zhou W, Gong G, Ramus F, et al. Alterations in white matter pathways underlying phonological and morphological processing in Chinese developmental dyslexia. Dev Cogn Neurosci. 2018;31:11–9.
Aoki Y, Cortese S, Castellanos FX. Research review: diffusion tensor imaging studies of attention-deficit/hyperactivity disorder: meta-analyses and reflections on head motion. J Child Psychol Psychiatry. 2018;59(3):193–202.
Konrad A, Dielentheis TF, El Masri D, Bayerl M, Fehr C, Gesierich T, et al. Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. Eur J Neurosci. 2010;31(5):912–9.
Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8–16.
Horowitz-Kraus T, Wang Y, Plante E, Holland SK. Involvement of the right hemisphere in reading comprehension: A DTI study. Brain Res. 2014;1582:34–44.
Lebel C, Shaywitz B, Holahan J, Shaywitz S, Marchione K, Beaulieu C. Diffusion tensor imaging correlates of reading ability in dysfluent and non-impaired readers. Brain Lang. 2013;125(2):215–22.
Feldman HM, Lee ES, Yeatman JD, Yeom KW. Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia. 2012;50(14):3348–62.
Su M, Zhao J, de Thiebaut Schotten M, Zhou W, Gong G, Ramus F, et al. Alterations in white matter pathways underlying phonological and morphological processing in Chinese developmental dyslexia. Dev Cogn Neurosci. 2018;31:11–9.
McAlonan GM, Cheung V, Cheung C, Chua SE, Murphy DGM, Suckling J, et al. Mapping brain structure in attention deficit-hyperactivity disorder: a voxel-based MRI study of regional grey and white matter volume. Psychiatry Res. 2007;154(2):171–80.
Konrad A, Dielentheis TF, Masri DE, Dellani PR, Stoeter P, Vucurevic G, et al. White matter abnormalities and their impact on attentional performance in adult attention-deficit/hyperactivity disorder. Eur Arch Psychiatry Clin Neurosci. 2012;262(4):351–60.
Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94.
Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44(8):953–61.
Nikki Arrington C, Kulesz PA, Juranek J, Cirino PT, Fletcher JM. White matter microstructure integrity in relation to reading proficiency☆. Brain Lang. 2017;174:103–11.
Richards TL, Grabowski TJ, Boord P, Yagle K, Askren M, Mestre Z, et al. Contrasting brain patterns of writing-related DTI parameters, fMRI connectivity, and DTI-fMRI connectivity correlations in children with and without dysgraphia or dyslexia. NeuroImage Clin. 2015;28(8):408–21.
Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brain Mapp. 2009;30(9):2757–65.
Roland JL, Snyder AZ, Hacker CD, Mitra A, Shimony JS, Limbrick DD, et al. On the role of the corpus callosum in interhemispheric functional connectivity in humans. Proc Natl Acad Sci U S A. 2017;114(50):13278–83.
Cui Z, Xia Z, Su M, Shu H, Gong G. Disrupted white matter connectivity underlying developmental dyslexia: a machine learning approach. Hum Brain Mapp. 2016;37(4):1443–58.
Huber E, Henriques RN, Owen JP, Rokem A, Yeatman JD. Applying microstructural models to understand the role of white matter in cognitive development. Dev Cogn Neurosci. 2019;36:100624.
Luders E, Kurth F, Das D, Oyarce DE, Shaw ME, Sachdev P, et al. Associations between corpus callosum size and ADHD symptoms in older adults: the PATH through life study. Psychiatry Res Neuroimaging. 2016;30(256):8–14.
Bessette KL, Stevens MC. Neurocognitive pathways in attention-deficit/hyperactivity disorder and white matter microstructure. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(3):233–42.
van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2012;36(4):1093–106.
Woodcock RW, McGrew KS, Mather N. Examiner’s Manual. Woodcock-Johnson III Tests of Achievement. Itasca: Riverside Publishing; 2001.
Torgesen JK, Rashotte CA, Wagner RK, Pro-Ed (Firm). TOWRE 2: Test of Word Reading Efficiency. Pro-Ed; 2012. Available from: https://books.google.it/books?id=U04RzgEACAAJ.
Swanson JM, Schuck S, Porter MM, Carlson C, Hartman CA, Sergeant JA, et al. Categorical and dimensional definitions and evaluations of symptoms of ADHD: history of the SNAP and the SWAN rating scales. Int J Educ Psychol Assess. 2012;10(1):51–70.
Barkley RA. Defiant children: a clinician’s manual for assessment and parent training. 3rd ed. New York: Guilford Press; 2013. xii. p. 228. (Defiant children: a clinician’s manual for assessment and parent training, 3rd ed.).
Kaufman AS, Kaufman NL. Kaufman brief intelligence test. 2nd ed. Minneapolis: Pearson Assessment (K-BIT-2); 2004.
McCandless S, O’Laughlin L. The Clinical Utility of the Behavior Rating Inventory of Executive Function (BRIEF) in the diagnosis of ADHD. J Atten Disord. 2007;1(10):381–9.
Allouzi R, Irfanoglu A. Development of new nonlinear dynamic response model of reinforced concrete frames with infill walls. Adv Struct Eng. 2018;21(14):2154–68.
Pierpaoli C, Walker L L, Irfanoglu M, Barnett A, Basser P, Chang LC, et al. TORTOISE: an integrated software package for processing of diffusion MRI data. ISMRM 18th Annu Meet. 2010.
Irfanoglu MO, Modi P, Nayak A, Hutchinson EB, Sarlls J, Pierpaoli C. DR-BUDDI (Diffeomorphic Registration for Blip-Up blip-Down Diffusion Imaging) method for correcting echo planar imaging distortions. Neuroimage. 2015;1(106):284–99.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–94.
Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9(2):195–207.
Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–84.
Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–5.
Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostat Oxf Engl. 2007;8(1):118–27.
Fortin JP, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161:149–70.
Fortin JP, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2018;15(167):104–20.
Schneidt A, Jusyte A, Rauss K, Schönenberg M. Distraction by salient stimuli in adults with attention-deficit/hyperactivity disorder: Evidence for the role of task difficulty in bottom-up and top-down processing. Cortex J Devoted Study Nerv Syst Behav. 2018;101:206–20.
Meri R, Farah R, Horowitz-Kraus T. Children with dyslexia utilize both top-down and bottom-up networks equally in contextual and isolated word reading. Neuropsychologia. 2020;147:107574.
Dufor O, Serniclaes W, Sprenger-Charolles L, Démonet JF. Top-down processes during auditory phoneme categorization in dyslexia: a PET study. Neuroimage. 2007;34(4):1692–707.
Frye RE, Hasan K, Xue L, Strickland D, Malmberg B, Liederman J, et al. Splenium microstructure is related to two dimensions of reading skill. NeuroReport. 2008;19(16):1627–31.
Hasan KM, Molfese DL, Walimuni IS, Stuebing KK, Papanicolaou AC, Narayana PA, et al. Diffusion tensor quantification and cognitive correlates of the macrostructure and microstructure of the corpus callosum in typically developing and dyslexic children. NMR Biomed. 2012;25(11):1263–70.
Elnakib A, Casanova MF, Gimel’farb G, Switala AE, El-Baz A. Dyslexia diagnostics by 3-D shape analysis of the corpus callosum. IEEE Trans Inf Technol Biomed Publ IEEE Eng Med Biol Soc. 2012;16(4):700–8.
Yendiki A, Panneck P, Srinivasan P, Stevens A, Zöllei L, Augustinack J, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinformatics. 2011;5:23.
Houston J, Allendorfer J, Nenert R, Goodman AM, Szaflarski JP. White matter language pathways and language performance in healthy adults across ages. Front Neurosci. 2019;13:1185.
Lebel C, Caverhill-Godkewitsch S, Beaulieu C. Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. Neuroimage. 2010;52(1):20–31.
Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340–52.
Acknowledgements
We would like to acknowledge the children, parents, and staff of Atlanta schools for their participation to make this research possible. We would also like to acknowledge Adriane Davis, Eileen Persichetti, Humza Biag, Mykayla Jeter, and Sean Rogers for their aid and support in data collection and processing.
Funding
This research was supported in part under Award P01HD070837 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
RJS conceptualized the project and collected brain and behavioral data in line with the parent study. Moreover, RJS processed, analyzed, and interpreted all data and majorly contributed to writing the manuscript. CNA supervised project conceptualization and administration, supervised brain and behavioral data collection alongside data curation and majorly contributed to writing the manuscript. JM curated brain and behavioral data and contributed to the review and editing of the manuscript. RAS, KRP, and RM acquired funding, designed the parent study, and provided direct supervision in all aspects of data collection and project administration with RM majorly contributing to writing the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Participants were recruited from public and charter elementary schools in the greater Atlanta area as part of a longitudinal study of reading intervention approved by the Georgia State University/Georgia Tech Center for Advanced Brain Imaging Institutional Review Board. All parents/students provided informed consent/assent before any participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Slaby, R.J., Arrington, C.N., Malins, J. et al. Properties of white matter tract diffusivity in children with developmental dyslexia and comorbid attention deficit/hyperactivity disorder. J Neurodevelop Disord 15, 25 (2023). https://doi.org/10.1186/s11689-023-09495-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11689-023-09495-9